Implementing Metal-Organic Frameworks for Natural Gas Storage
Abstract
1. Introduction
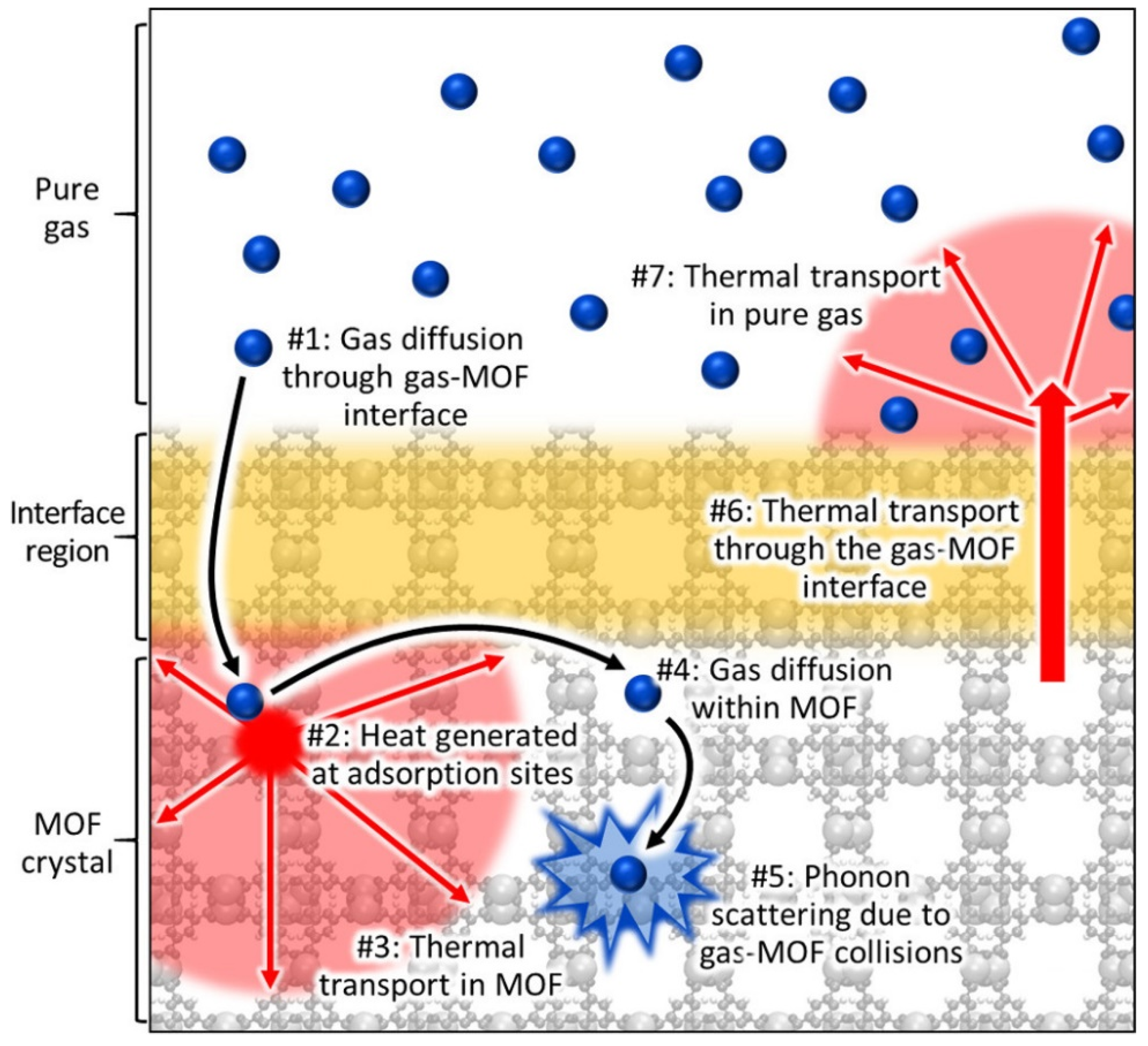
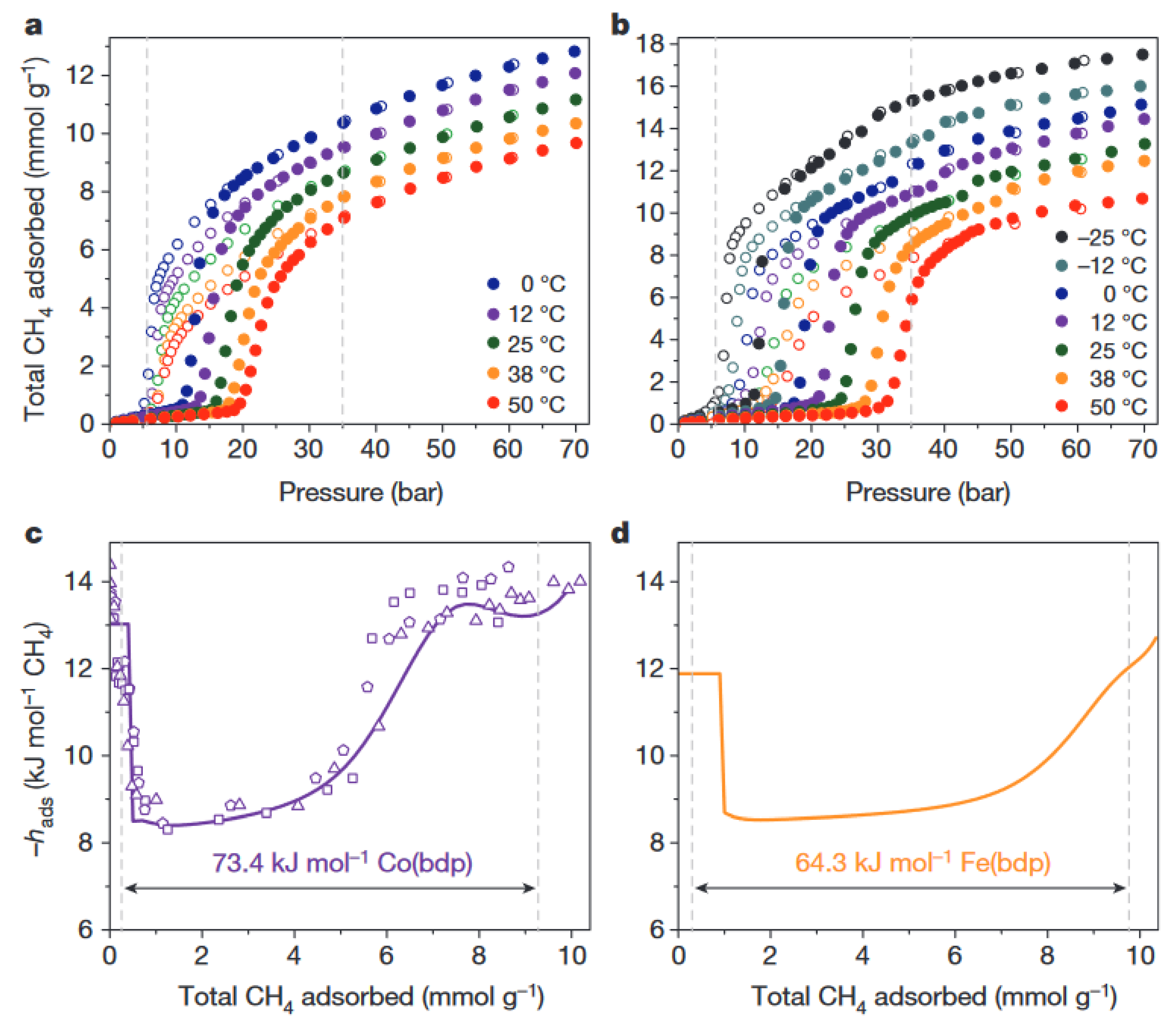
2. Thermal Properties
3. Mechanical Properties
4. Natural Gas Impurities
5. Practical Implementation
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- BP Energy Outlook 2017 Edition. Available online: https://safety4sea.com/wp-content/uploads/2017/01/BP-Energy-Outlook-2017_01.pdf (accessed on 4 August 2019).
- Schoedel, A.; Ji, Z.; Yaghi, O.M. The role of metal–organic frameworks in a carbon-neutral energy cycle. Nat. Energy 2016, 1, 1–13. [Google Scholar] [CrossRef]
- The Outlook for Energy: A View to 2040. Available online: https://cdn.exxonmobil.com/~/media/global/files/outlook-for-energy/2016/2016-outlook-for-energy.pdf (accessed on 4 August 2019).
- IEA. Key World Energy Statistics. Available online: https://www.connaissancedesenergies.org/sites/default/files/pdf-actualites/keyworld_statistics_2015.pdf (accessed on 25 June, 2018).
- BP Statistical Review of World Energy June 2015. Available online: https://www.bp.com/content/dam/bp-country/es_es/spain/documents/downloads/PDF/bp-statistical-review-of-world-energy-2015-full-report.pdf (accessed on 4 August 2019).
- CO2 Emissions (kt) | Data. Available online: https://data.worldbank.org/indicator/EN.ATM.CO2E.KT (accessed on 2 July 2018).
- Zhou, N.; Fridley, D.; McNeil, M.; Zheng, N.; Ke, J.; Levine, M. China’s Energy and Carbon Emissions Outlook to 2050. Ernest Orlando Lawrence Berkeley Natl. Lab. 2011, 1–66. [Google Scholar]
- 2030 Climate & Energy Framework | Climate Action. Available online: https://ec.europa.eu/clima/policies/strategies/2030_en (accessed on 31 July 2019).
- Coren, M.J. Nine Countries Say They’ll Ban Internal Combustion Engines. Available online: https://qz.com/1341155/nine-countries-say-they-will-ban-internal-combustion-engines-none-have-a-law-to-do-so/ (accessed on 31 July 2019).
- Painuly, J.P. Barriers to renewable energy penetration: A framework for analysis. Renew. Energy 2001, 24, 73–89. [Google Scholar] [CrossRef]
- Akella, A.K.; Saini, R.P.; Sharma, M.P. Social, economical and environmental impacts of renewable energy systems. Renew. Energy 2009, 34, 390–396. [Google Scholar] [CrossRef]
- Drennen, T.E. Renewable Energy: Sources for Fuels and Electricity. J. Environ. Qual. 1994, 23, 622. [Google Scholar] [CrossRef]
- ARPA-E MOVE Program Overview. Available online: http://arpa-e.energy.gov/sites/default/files/documents/files/MOVE_ProgramOverview.pdf (accessed on 4 August 2019).
- Current Natural Gas Vehicle Statistics | NGV Global Knowledgebase. Available online: http://www.iangv.org/current-ngv-stats/ (accessed on 31 July 2019).
- Bae, C.; Kim, J. Alternative fuels for internal combustion engines. Proc. Combust. Inst. 2017, 36, 3389–3413. [Google Scholar] [CrossRef]
- Compressed Natural Gas (CNG) in transport | ClimateTechWiki. Available online: https://www.climatetechwiki.org/technology/cng (accessed on 31 July 2019).
- Dobrota, Đ.; Lalić, B.; Komar, I. Problem of Boil-off in LNG Supply Chain. Trans. Marit. Sci. 2013, 2, 91–100. [Google Scholar] [CrossRef]
- van Biert, L.; Godjevac, M.; Visser, K.; Aravind, P.V. A review of fuel cell systems for maritime applications. J. Power Sources 2016, 327, 345–364. [Google Scholar] [CrossRef]
- Szilágyi, P.Á.; Serra-Crespo, P.; Gascon, J.; Geerlings, H.; Dam, B. The Impact of Post-Synthetic Linker Functionalization of MOFs on Methane Storage: The Role of Defects. Front. Energy Res. 2016, 4, 1–6. [Google Scholar] [CrossRef]
- Liang, C.C.; Shi, Z.L.; He, C.T.; Tan, J.; Zhou, H.D.; Zhou, H.L.; Lee, Y.; Zhang, Y.B. Engineering of Pore Geometry for Ultrahigh Capacity Methane Storage in Mesoporous Metal-Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 13300–13303. [Google Scholar] [CrossRef]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material [Cu3(TMA)2 (H2O)3](n). Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.E.; Wheatley, P.S. Gas storage in nanoporous materials. Angew. Chem. Int. Ed. 2008, 47, 4966–4981. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.C.; Komarneni, S. Porous adsorbents for vehicular natural gas storage: A review. J. Porous Mater. 1998, 5, 43–58. [Google Scholar] [CrossRef]
- Makal, T.A.; Li, J.-R.; Lu, W.; Zhou, H.-C. Methane storage in advanced porous materials. Chem. Soc. Rev. 2012, 41, 7761–7779. [Google Scholar] [CrossRef] [PubMed]
- Hamza, U.D.; Nasri, N.S.; Mohammed, J.; Majid, Z.A. Natural gas adsorption on biomass derived activated carbons: A mini review. MATEC Web Conf. 2016, 60, 1–5. [Google Scholar] [CrossRef]
- Sircar, S.; Golden, T.C.; Rao, M.B. Activated carbon for gas separation and storage. Carbon N. Y. 1996, 34, 1–12. [Google Scholar] [CrossRef]
- Matranga, K.R.; Myers, A.L.; Glandt, E.D. Storage of Natural-Gas By Adsorption on Activated Carbon. Chem. Eng. Sci. 1992, 47, 1569–1579. [Google Scholar] [CrossRef]
- Himeno, S.; Komatsu, T.; Fujita, S. High-Pressure Adsorption Equilibria of Methane and Carbon Dioxide on Several Activated Carbons. J. Chem. Eng. Data 2005, 50, 369–376. [Google Scholar] [CrossRef]
- Noro, S.; Kitagawa, S.; Kondo, M.; Seki, K. A New, Methane Adsorbent, Porous Coordination Polymer [{CuSiF6 (4, 4′-bipyridine)2}n]. Angew. Chemie Int. Ed. 2000, 39, 2081–2084. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.Ö.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.J.; Perman, J.A.; Zaworotko, M.J. Design and synthesis of metal-organic frameworks using metal-organic polyhedra as supermolecular building blocks. Chem. Soc. Rev. 2009, 38, 1400–1417. [Google Scholar] [CrossRef] [PubMed]
- ARPA-E | MOVE. Available online: https://arpa-e.energy.gov/?q=arpa-e-programs/move (accessed on 18 August 2018).
- Guo, Z.; Wu, H.; Srinivas, G.; Zhou, Y.; Xiang, S.; Chen, Z.; Yang, Y.; Zhou, W.; O’Keeffe, M.; Chen, B. A metal-organic framework with optimized open metal sites and pore spaces for high methane storage at room temperature. Angew. Chem. Int. Ed. 2011, 50, 3178–3181. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Konstas, K.; Hill, M.R.; Telfer, S.G. Programmed pore architectures in modular quaternary metal-organic frameworks. J. Am. Chem. Soc. 2013, 135, 17731–17734. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.T.; Furukawa, H.; Gándara, F.; Nguyen, H.T.; Cordova, K.E.; Yaghi, O.M. Selective capture of carbon dioxide under humid conditions by hydrophobic chabazite-type zeolitic imidazolate frameworks. Angew. Chem. Int. Ed. 2014, 53, 10645–10648. [Google Scholar] [CrossRef] [PubMed]
- Almeida Paz, F.A.; Klinowski, J.; Vilela, S.M.F.; Tomé, J.P.C.; Cavaleiro, J.A.S.; Rocha, J. Ligand design for functional metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 1088–1110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Timmons, D.J.; Yuan, D.; Zhou, H.C. Tuning the topology and functionality of metal-organic frameworks by ligand design. Acc. Chem. Res. 2011, 44, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, D.; Lin, W. Metal-organic frameworks as a tunable platform for designing functional molecular materials. J. Am. Chem. Soc. 2013, 135, 13222–13234. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Metal-organic frameworks as efficient materials for drug delivery. Angew. Chem. Int. Ed. 2006, 45, 5974–5978. [Google Scholar] [CrossRef]
- Taylor, M.K.; Runčevski, T.; Oktawiec, J.; Gonzalez, M.I.; Siegelman, R.L.; Mason, J.A.; Ye, J.; Brown, C.M.; Long, J.R. Tuning the Adsorption-Induced Phase Change in the Flexible Metal-Organic Framework Co(bdp). J. Am. Chem. Soc. 2016, 138, 15019–15026. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Grunder, S.; Cordova, K.E.; Valente, C.; Furukawa, H.; Hmadeh, M.; Gándara, F.; Whalley, A.C.; Liu, Z.; Asahina, S.; et al. Large-pore apertures in a series of metal-organic frameworks. Science 2012, 336, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Krungleviciute, V.; Eryazici, I.; Hupp, J.T.; Farha, O.K.; Yildirim, T. Methane storage in metal-organic frameworks: Current records, surprise findings, and challenges. J. Am. Chem. Soc. 2013, 135, 11887–11894. [Google Scholar] [CrossRef] [PubMed]
- Gándara, F.; Furukawa, H.; Lee, S.; Yaghi, O.M. High methane storage capacity in aluminum metal-organic frameworks. J. Am. Chem. Soc. 2014, 136, 5271–5274. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wen, H.M.; Wang, H.; Wu, H.; Yildirim, T.; Zhou, W.; Chen, B. Porous metal-organic frameworks with Lewis basic nitrogen sites for high-capacity methane storage. Energy Environ. Sci. 2015, 8, 2504–2511. [Google Scholar] [CrossRef]
- Moellmer, J.; Moeller, A.; Dreisbach, F.; Glaeser, R.; Staudt, R. High pressure adsorption of hydrogen, nitrogen, carbon dioxide and methane on the metal-organic framework HKUST-1. Microporous Mesoporous Mater. 2011, 138, 140–148. [Google Scholar] [CrossRef]
- Vikrant, K.; Kumar, V.; Kim, K.H.; Kukkar, D. Metal-organic frameworks (MOFs): Potential and challenges for capture and abatement of ammonia. J. Mater. Chem. A 2017, 5, 22877–22896. [Google Scholar] [CrossRef]
- Yan, Y.; Kolokolov, D.I.; Da Silva, I.; Stepanov, A.G.; Blake, A.J.; Dailly, A.; Manuel, P.; Tang, C.C.; Yang, S.; Schröder, M. Porous Metal-Organic Polyhedral Frameworks with Optimal Molecular Dynamics and Pore Geometry for Methane Storage. J. Am. Chem. Soc. 2017, 139, 13349–13360. [Google Scholar] [CrossRef]
- Gómez-Gualdrón, D.A.; Wilmer, C.E.; Farha, O.K.; Hupp, J.T.; Snurr, R.Q. Exploring the limits of methane storage and delivery in nanoporous materials. J. Phys. Chem. C 2014, 118, 6941–6951. [Google Scholar] [CrossRef]
- Getman, R.B.; Bae, Y.-S.; Wilmer, C.E.; Snurr, R.Q. Review and Analysis of Molecular Simulations of Methane, Hydrogen, and Acetylene Storage in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 703–723. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Long, J.R.; Yaghi, O.M. The pervasive chemistry of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- James, S.L. Metal-organic frameworks. Chem. Soc. Rev. 2003, 32, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M. Postsynthetic methods for the functionalization of metal-organic frameworks. Chem. Rev. 2012, 112, 970–1000. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, W.; Qian, G.; Chen, B. Methane storage in metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 5657–5678. [Google Scholar] [CrossRef] [PubMed]
- Farha, O.K.; Hupp, J.T. Rational design, synthesis, purification, and activation of metal-organic framework materials. Acc. Chem. Res. 2010, 43, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cohen, S.M. Postsynthetic modification of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1315–1329. [Google Scholar] [CrossRef]
- Mason, J.A.; Veenstra, M.; Long, J.R. Evaluating metal–organic frameworks for natural gas storage. Chem. Sci. 2014, 5, 32–51. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.M.; Zhou, W.; Xu, J.Q.; Chen, B. Porous Metal-Organic Frameworks: Promising Materials for Methane Storage. Chem 2016, 1, 557–580. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.C. Recent advances in gas storage and separation using metal–organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Lin, Y.; Kong, C.; Zhang, Q.; Chen, L. Metal-Organic Frameworks for Carbon Dioxide Capture and Methane Storage. Adv. Energy Mater. 2017, 7, 1601296. [Google Scholar] [CrossRef]
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, thermal and mechanical stabilities of metal-organic frameworks. Nat. Rev. Mater. 2016, 1, 1–15. [Google Scholar] [CrossRef]
- Babaei, H.; McGaughey, A.J.H.; Wilmer, C.E. Transient Mass and Thermal Transport during Methane Adsorption into the Metal-Organic Framework HKUST-1. ACS Appl. Mater. Interfaces 2018, 10, 2400–2406. [Google Scholar] [CrossRef]
- Prajwal, B.P.; Ayappa, K.G. Evaluating methane storage targets: From powder samples to onboard storage systems. Adsorption 2014, 20, 769–776. [Google Scholar] [CrossRef]
- Chang, K.J.; Talu, O. Behavior and performance of adsorptive natural gas storage cylinders during discharge. Appl. Therm. Eng. 1996, 16, 359–374. [Google Scholar] [CrossRef]
- Babaei, H.; Wilmer, C.E. Mechanisms of Heat Transfer in Porous Crystals Containing Adsorbed Gases: Applications to Metal-Organic Frameworks. Phys. Rev. Lett. 2016, 116, 025902. [Google Scholar] [CrossRef]
- Kloutse, F.A.; Zacharia, R.; Cossement, D.; Chahine, R. Specific heat capacities of MOF-5, Cu-BTC, Fe-BTC, MOF-177 and MIL-53 (Al) over wide temperature ranges: Measurements and application of empirical group contribution method. Microporous Mesoporous Mater. 2015, 217, 1–5. [Google Scholar] [CrossRef]
- Mu, B.; Walton, K.S. Thermal analysis and heat capacity study of metal-organic frameworks. J. Phys. Chem. C 2011, 115, 22748–22754. [Google Scholar] [CrossRef]
- Babaei, H.; McGaughey, A.J.H.; Wilmer, C.E. Effect of pore size and shape on the thermal conductivity of metal-organic frameworks. Chem. Sci. 2016, 8, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Sezginel, K.B.; Asinger, P.A.; Babaei, H.; Wilmer, C.E. Thermal Transport in Interpenetrated Metal-Organic Frameworks. Chem. Mater. 2018, 30, 2281–2286. [Google Scholar] [CrossRef]
- Mason, J.A.; Oktawiec, J.; Taylor, M.K.; Hudson, M.R.; Rodriguez, J.; Bachman, J.E.; Gonzalez, M.I.; Cervellino, A.; Guagliardi, A.; Brown, C.M.; et al. Methane storage in flexible metal-organic frameworks with intrinsic thermal management. Nature 2015, 527, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, T.; Serre, C.; Huguenard, C.; Fink, G.; Taulelle, F.; Henry, M.; Bataille, T.; Férey, G. A Rationale for the Large Breathing of the Porous Aluminum Terephthalate (MIL-53) Upon Hydration. Chem. Eur. J. 2004, 10, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Boutin, A.; Coudert, F.-X.; Springuel-Huet, M.-A.; Neimark, A.V.; Férey, G.; Fuchs, A.H. The behavior of flexible MIL-53(Al) upon CH 4 and CO 2 adsorption. J. Phys. Chem. C 2010, 114, 22237–22244. [Google Scholar] [CrossRef]
- Huang, J.; Xia, X.; Hu, X.; Li, S.; Liu, K. A general method for measuring the thermal conductivity of MOF crystals. Int. J. Heat Mass Transf. 2019, 138, 11–16. [Google Scholar] [CrossRef]
- Huang, B.L.; Ni, Z.; Millward, A.; McGaughey, A.J.H.; Uher, C.; Kaviany, M.; Yaghi, O. Thermal conductivity of a metal-organic framework (MOF-5): Part II. Measurement. Int. J. Heat Mass Transf. 2007, 50, 405–411. [Google Scholar] [CrossRef]
- Bae, Y.S.; Snurr, R.Q. Optimal isosteric heat of adsorption for hydrogen storage and delivery using metal-organic frameworks. Microporous Mesoporous Mater. 2010, 132, 300–303. [Google Scholar] [CrossRef]
- Rallapalli, P.; Patil, D.; Prasanth, K.P.; Somani, R.S.; Jasra, R.V.; Bajaj, H.C. An alternative activation method for the enhancement of methane storage capacity of nanoporous aluminium terephthalate, MIL-53(Al). J. Porous Mater. 2010, 17, 523–528. [Google Scholar] [CrossRef]
- Bolinois, L.; Kundu, T.; Wang, X.; Wang, Y.; Hu, Z.; Koh, K.; Zhao, D. Breathing-induced new phase transition in an MIL-53(Al)–NH 2 metal–organic framework under high methane pressures. Chem. Commun. 2017, 53, 8118–8121. [Google Scholar] [CrossRef]
- Deng, H.; Doonan, C.J.; Furukawa, H.; Ferreira, R.B.; Towne, J.; Knobler, C.B.; Wang, B.; Yaghi, O.M. Multiple functional groups of varying ratios in metal-organic frameworks. Science 2010, 327, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Wilmer, C.E.; Farha, O.K.; Yildirim, T.; Eryazici, I.; Krungleviciute, V.; Sarjeant, A.A.; Snurr, R.Q.; Hupp, J.T. Gram-scale, high-yield synthesis of a robust metal-organic framework for storing methane and other gases. Energy Environ. Sci. 2013, 6, 1158–1163. [Google Scholar] [CrossRef]
- Peng, Y.; Srinivas, G.; Wilmer, C.E.; Eryazici, I.; Snurr, R.Q.; Hupp, J.T.; Yildirim, T.; Farha, O.K. Simultaneously high gravimetric and volumetric methane uptake characteristics of the metal-organic framework NU-111. Chem. Commun. 2013, 49, 2992–2994. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, W.; Yildirim, T.; Chen, B. A series of metal-organic frameworks with high methane uptake and an empirical equation for predicting methane storage capacity. Energy Environ. Sci. 2013, 6, 2735–2744. [Google Scholar] [CrossRef]
- Wen, H.-M.; Li, B.; Yuan, D.; Wang, H.; Yildirim, T.; Zhou, W.; Chen, B. A porous metal–organic framework with an elongated anthracene derivative exhibiting a high working capacity for the storage of methane. J. Mater. Chem. A 2014, 2, 11516–11522. [Google Scholar] [CrossRef]
- Li, L.; Tang, S.; Wang, C.; Lv, X.; Jiang, M.; Wu, H.; Zhao, X. High gas storage capacities and stepwise adsorption in a UiO type metal-organic framework incorporating Lewis basic bipyridyl sites. Chem. Commun. 2014, 50, 2304–2307. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Velazquez-Garcia, J.; Bennett, T.D.; Fairen-Jimenez, D. Mechanically and chemically robust ZIF-8 monoliths with high volumetric adsorption capacity. J. Mater. Chem. A 2015, 3, 2999–3005. [Google Scholar] [CrossRef]
- Tagliabue, M.; Rizzo, C.; Millini, R.; Dietzel, P.D.C.; Blom, R.; Zanardi, S. Methane storage on CPO-27-Ni pellets. J. Porous Mater. 2011, 18, 289–296. [Google Scholar] [CrossRef]
- Tian, T.; Zeng, Z.; Vulpe, D.; Casco, M.E.; Divitini, G.; Midgley, P.A.; Silvestre-Albero, J.; Tan, J.C.; Moghadam, P.Z.; Fairen-Jimenez, D. A sol-gel monolithic metal-organic framework with enhanced methane uptake. Nat. Mater. 2018, 17, 174–179. [Google Scholar] [CrossRef]
- Bazer-Bachi, D.; Assié, L.; Lecocq, V.; Harbuzaru, B.; Falk, V. Towards industrial use of metal-organic framework: Impact of shaping on the MOF properties. Powder Technol. 2014, 255, 52–59. [Google Scholar] [CrossRef]
- Rogge, S.M.J.; Waroquier, M.; Van Speybroeck, V. Reliably Modeling the Mechanical Stability of Rigid and Flexible Metal-Organic Frameworks. Acc. Chem. Res. 2018, 51, 138–148. [Google Scholar] [CrossRef]
- Thornton, A.W.; Babarao, R.; Jain, A.; Trousselet, F.; Coudert, F.X. Defects in metal-organic frameworks: A compromise between adsorption and stability? Dalt. Trans. 2016, 45, 4352–4359. [Google Scholar] [CrossRef]
- Dissegna, S.; Vervoorts, P.; Hobday, C.L.; Düren, T.; Daisenberger, D.; Smith, A.J.; Fischer, R.A.; Kieslich, G. Tuning the Mechanical Response of Metal-Organic Frameworks by Defect Engineering. J. Am. Chem. Soc. 2018, 140, 11581–11584. [Google Scholar] [CrossRef]
- Burtch, N.C.; Heinen, J.; Bennett, T.D.; Dubbeldam, D.; Allendorf, M.D. Mechanical Properties in Metal–Organic Frameworks: Emerging Opportunities and Challenges for Device Functionality and Technological Applications. Adv. Mater. 2018, 30, 1704124. [Google Scholar] [CrossRef]
- Dürholt, J.P.; Keupp, J.; Schmid, R. The Impact of Mesopores on the Mechanical Stability of HKUST-1: A Multiscale Investigation. Eur. J. Inorg. Chem. 2016, 2016, 4517–4523. [Google Scholar] [CrossRef]
- Yot, P.G.; Yang, K.; Ragon, F.; Dmitriev, V.; Devic, T.; Horcajada, P.; Serre, C.; Maurin, G. Exploration of the mechanical behavior of metal organic frameworks UiO-66(Zr) and MIL-125(Ti) and their NH2 functionalized versions. Dalt. Trans. 2016, 45, 4283–4288. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Boyd, P.G.; Sarkisov, L.; Smit, B. Improving the Mechanical Stability of Metal-Organic Frameworks Using Chemical Caryatids. ACS Cent. Sci. 2018, 4, 832–839. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Kumar, A.; Seo, Y.; Lee, J.D.; Linga, P. A review of solidified natural gas (SNG) technology for gas storage via clathrate hydrates. Appl. Energy 2018, 262–285. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, C.; Su, W.; Zhou, Y.; Zhou, L. Principles of methane adsorption and natural gas storage. Adsorption 2009, 15, 133–137. [Google Scholar] [CrossRef]
- Zhang, H.; Deria, P.; Farha, O.K.; Hupp, J.T.; Snurr, R.Q. A thermodynamic tank model for studying the effect of higher hydrocarbons on natural gas storage in metal–organic frameworks. Energy Environ. Sci. 2015, 8, 1501–1510. [Google Scholar] [CrossRef]
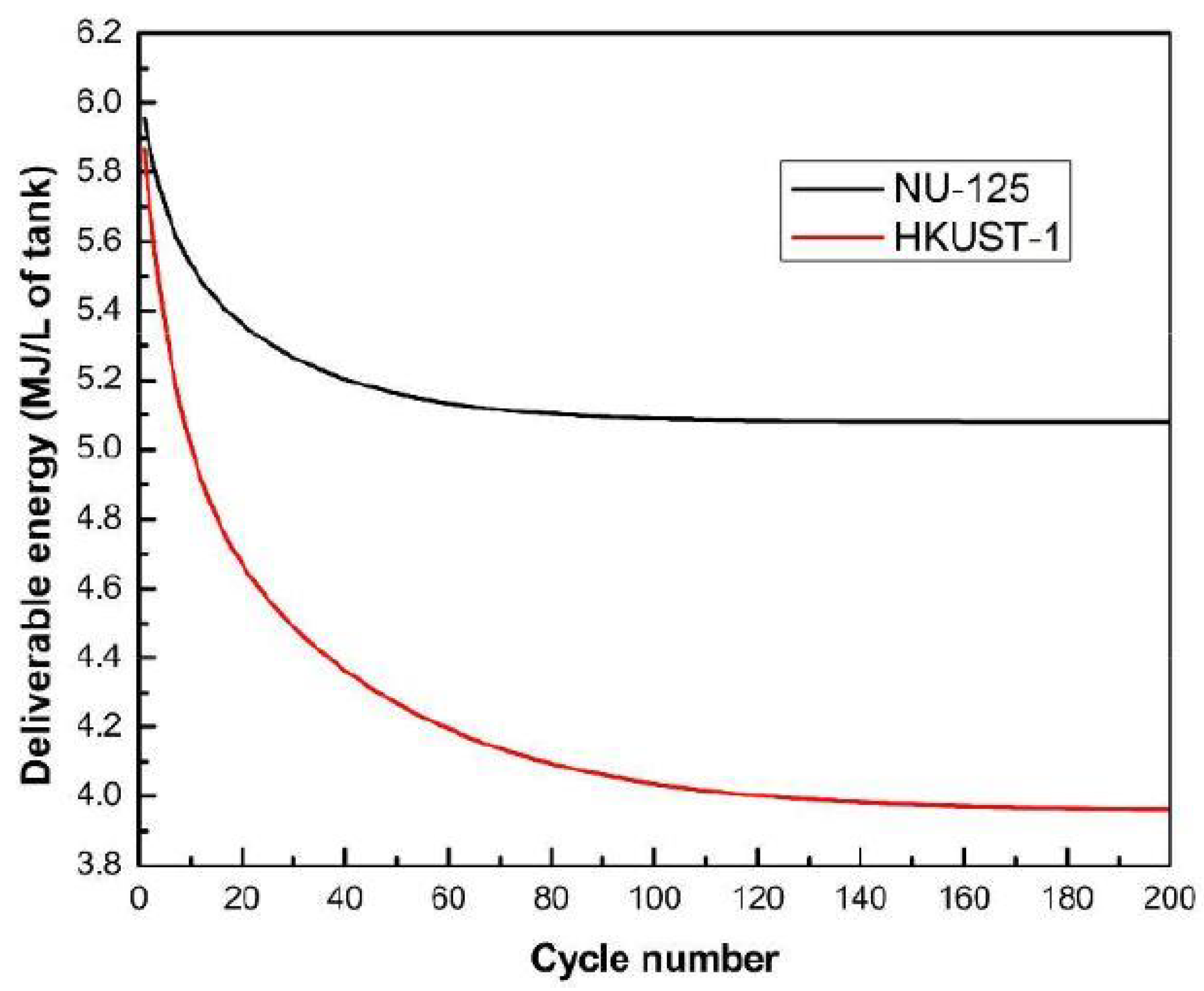
- Wu, Y.; Tang, D.; Verploegh, R.J.; Xi, H.; Sholl, D.S. Impacts of Gas Impurities from Pipeline Natural Gas on Methane Storage in Metal-Organic Frameworks during Long-Term Cycling. J. Phys. Chem. C 2017, 121, 15735–15745. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, W.; Pham, T.; Forrest, K.A.; Liu, W.; He, Y.; Wu, H.; Yildirim, T.; Chen, B.; Space, B.; et al. Fine Tuning of MOF-505 Analogues To Reduce Low-Pressure Methane Uptake and Enhance Methane Working Capacity. Angew. Chem. Int. Ed. 2017, 56, 11426–11430. [Google Scholar] [CrossRef]
- Romanos, J.; Rash, T.; Abou Dargham, S.; Prosniewski, M.; Barakat, F.; Pfeifer, P. Cycling and Regeneration of Adsorbed Natural Gas in Microporous Materials. Energy Fuels 2017, 31, 14332–14337. [Google Scholar] [CrossRef]
- Jacoby, M. Heading to Market with MOFs. Chem. Eng. News 2010, 86, 13–16. [Google Scholar] [CrossRef]
- BASF Metal Organic Frameworks (MOFs): Innovative Fuel Systems for Natural Gas Vehicles (NGVs). Chem. Soc. Rev. 2014, 43, 6173–6174. [CrossRef]
- BASF to Showcase Metal Organic Frameworks (MOFs) for Energy Storage at NGV Americas Conference. Available online: https://www.basf.com/us/en/media/news-releases/2013/11/p-13-452.html (accessed on 1 July 2019).
- Scott, A. Round Two for MOF Commercialization. Available online: https://cen.acs.org/articles/95/i24/Round-two-MOF-commercialization.html (accessed on 1 August 2019).
- Green Science Alliance Co., Ltd. Has Started the Manufacturing and Custom Synthesis of a Metal Organic Framework (MOF), Porous Coordination Polymers (PCPs). Available online: https://www.prnewswire.com/news-releases/green-science-alliance-co-ltd-has-started-the-manufacturing-and-custom-synthesis-of-a-metal-organic-framework-mof-porous-coordination-polymers-pcps-300644297.html (accessed on 1 August 2019).
- McMahon, M.M. NuMat Brings First MOF-Enabled Gas Storage Product to Market | Institute for Sustainability and Energy at Northwestern (ISEN). Available online: https://isen.northwestern.edu/numat-brings-first-mof-enabled-gas-storage-product-to-market (accessed on 1 August 2019).
- Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-Arndt, K.; Pastré, J. Metal–organic frameworks—prospective industrial applications. J. Mater. Chem. 2006, 16, 626–636. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef]
- DeSantis, D.; Mason, J.A.; James, B.D.; Houchins, C.; Long, J.R.; Veenstra, M. Techno-economic Analysis of Metal–Organic Frameworks for Hydrogen and Natural Gas Storage. Energy & Fuels 2017, 31, 2024–2032. [Google Scholar]
- Rubio-Martinez, M.; Batten, M.P.; Polyzos, A.; Carey, K.C.; Mardel, J.I.; Lim, K.S.; Hill, M.R. Versatile, high quality and scalable continuous flow production of metal-organic frameworks. Sci. Rep. 2014, 4, 1–5. [Google Scholar] [CrossRef]
- Dunne, P.W.; Walton, R.I. Towards scalable and controlled synthesis of metal–organic framework materials using continuous flow reactors. Reaction Chem. Eng. 2016, 1, 352–360. [Google Scholar] [CrossRef]
- Gaab, M.; Trukhan, N.; Maurer, S.; Gummaraju, R.; Müller, U. The progression of Al-based metal-organic frameworks—From academic research to industrial production and applications. Microporous Mesoporous Mater. 2012, 157, 131–136. [Google Scholar] [CrossRef]
- Silva, P.; Vilela, S.M.F.; Tomé, J.P.C.; Almeida Paz, F.A. Multifunctional metal-organic frameworks: From academia to industrial applications. Chem. Soc. Rev. 2015, 44, 6774–6803. [Google Scholar] [CrossRef]
- Julien, P.A.; Mottillo, C.; Friščić, T. Metal-organic frameworks meet scalable and sustainable synthesis. Green Chem. 2017, 19, 2729–2747. [Google Scholar] [CrossRef]
- Witman, M.; Ling, S.; Jawahery, S.; Boyd, P.G.; Haranczyk, M.; Slater, B.; Smit, B. The Influence of Intrinsic Framework Flexibility on Adsorption in Nanoporous Materials. J. Am. Chem. Soc. 2017, 139, 5547–5557. [Google Scholar] [CrossRef]
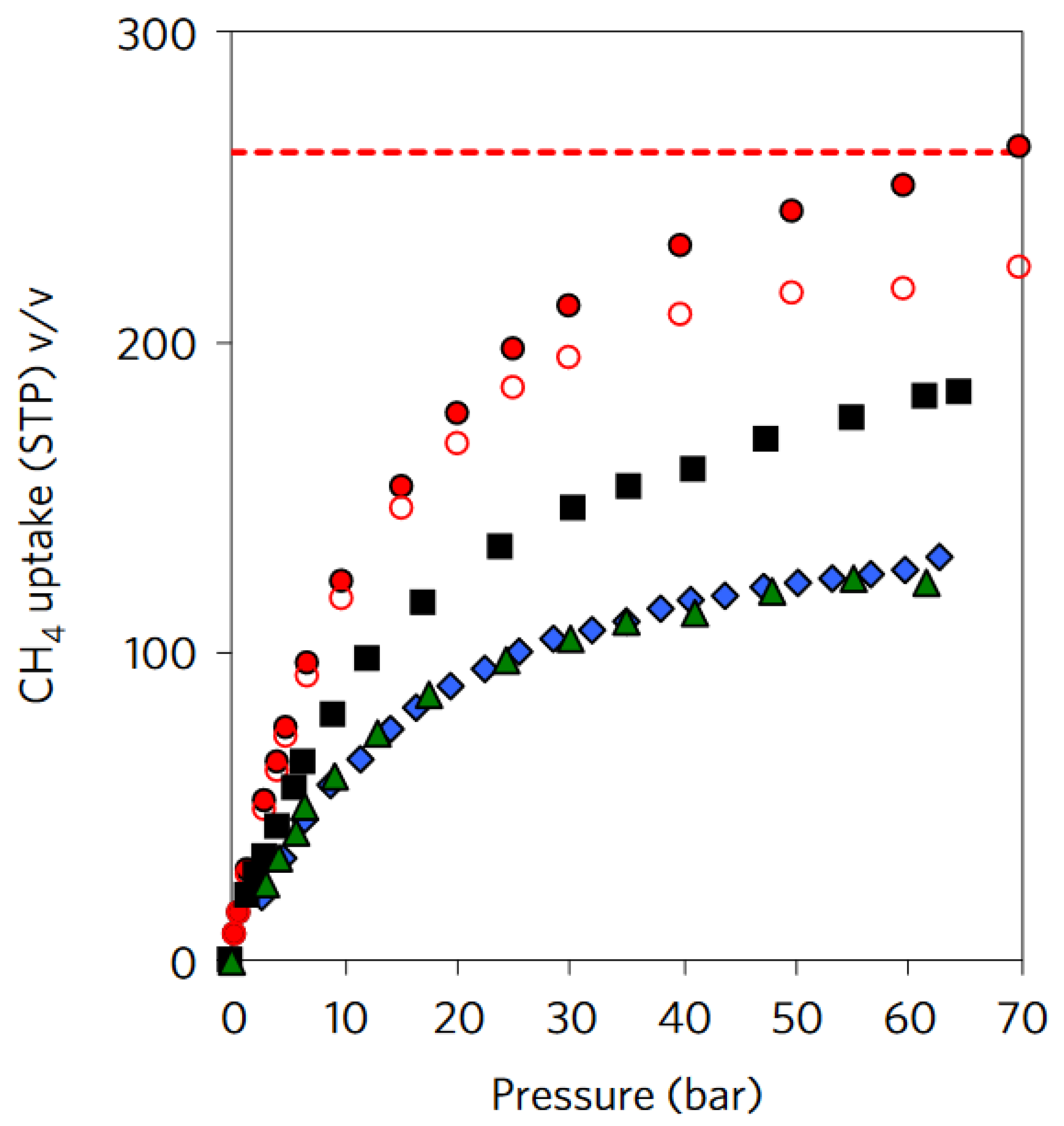
| MOF | VP (cm3g−1) a | BET (m2g−1) | Uptakeb (cm3cm−3) | Deliveryc (cm3cm−3) | T (K) | P (bar) | Uptakeb (cm3cm−3) | Deliveryc (cm3cm−3) | T (K) | P (bar) | QST kJ mol−1 | REF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCN-61 | 1.36 | 3000 | 171 | 127 | 298 | 35 | 219 | 174 | 298 | 65 | - | [45] |
| HKUST-1 | 0.71 | 1555 | 190 | - | 303 | 35 | 254 | - | 303 | 65 | 20.7 | [48] |
| MgMOF-74 | 0.69 | - | 200 | 113 | 298 | 35 | 230 | 142 | 298 | 65 | 18.5 | [60] |
| MOF-5 | 1.4 | - | 150 | 118 | 298 | 35 | 214 | 182 | 298 | 65 | 12.3 | [60] |
| Cu-TDPAT | 0.93 | 1938 | 181 | 122 | 298 | 35 | 222 | 163 | 298 | 65 | - | [43] |
| PCN-14 | 0.83 | 1984 | 202 | 125 | 298 | 35 | 239 | 160 | 298 | 65 | 17.6 | [60] |
| CoMOF-74 | 0.51 | - | 221 | 110 | 298 | 35 | 249 | 136 | 298 | 65 | 19.5 | [60] |
| PCN-61 | 1.36 | 3000 | 171 | 127 | 298 | 35 | 219 | 174 | 298 | 65 | - | [81] |
| MOF-210 | 3.60 | 6240 | 83 | 71 | 298 | 35 | 143 | 131 | 298 | 65 | - | [45] |
| PCN-14 | 0.85 | 2000 | 195 | 122 | 298 | 35 | 230 | 157 | 298 | 65 | 18.7 | [82] |
| NU-111 | 2.09 | 4930 | 138 | 111 | 298 | 35 | 206 | 179 | 298 | 65 | 14.2 | [82] |
| NU-140 | 1.97 | 4300 | 138 | 108 | 298 | 35 | 200 | 170 | 298 | 65 | 14 | [51] |
| NU-125 | 1.29 | 3120 | 181 | 133 | 298 | 35 | 228 | 180 | 298 | 58 | 15.5 | [83] |
| NiMOF-74 | 0.47 | 1218 | 214 | 94 | 298 | 35 | 236 | 116 | 298 | 65 | - | [77] |
| NU-111 | 2.09 | 4930 | 138 | 111 | 298 | 35 | 206 | 179 | 298 | 65 | 15.2 | [84] |
| NOTT-109 | 0.850 | 2110 | 196 | 125 | 300 | 35 | 242 | 170 | 300 | 65 | 17.1 | [85] |
| ZJU-5 | 1.074 | 2823 | 190 | 130 | 300 | 35 | 228 | 168 | 300 | 65 | 15.3 | [66] |
| ZJU-25 | 1.183 | 2124 | 180 | 132 | 300 | 35 | 229 | 181 | 300 | 63 | 15.1 | [86] |
| NU-135 | 1.02 | 2530 | 187 | 127 | 298 | 35 | 230 | 170 | 298 | 65 | 16.6 | [87] |
| NOTT-100 | 0.677 | 1661 | 195 | 104 | 300 | 35 | 230 | 139 | 300 | 65 | 18.1 | [85] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, E.; Ali, L.; El Sayah, A.; Alkhatib, S.A.; Abdulsalam, H.; Juma, M.; Al-Muhtaseb, A.H. Implementing Metal-Organic Frameworks for Natural Gas Storage. Crystals 2019, 9, 406. https://doi.org/10.3390/cryst9080406
Mahmoud E, Ali L, El Sayah A, Alkhatib SA, Abdulsalam H, Juma M, Al-Muhtaseb AH. Implementing Metal-Organic Frameworks for Natural Gas Storage. Crystals. 2019; 9(8):406. https://doi.org/10.3390/cryst9080406
Chicago/Turabian StyleMahmoud, Eyas, Labeeb Ali, Asmaa El Sayah, Sara Awni Alkhatib, Hend Abdulsalam, Mouza Juma, and Ala’a H. Al-Muhtaseb. 2019. "Implementing Metal-Organic Frameworks for Natural Gas Storage" Crystals 9, no. 8: 406. https://doi.org/10.3390/cryst9080406
APA StyleMahmoud, E., Ali, L., El Sayah, A., Alkhatib, S. A., Abdulsalam, H., Juma, M., & Al-Muhtaseb, A. H. (2019). Implementing Metal-Organic Frameworks for Natural Gas Storage. Crystals, 9(8), 406. https://doi.org/10.3390/cryst9080406





