Taking Advantage of the Coordinative Behavior of a Tridentate Schiff Base Ligand towards Pd2+ and Cu2+
Abstract
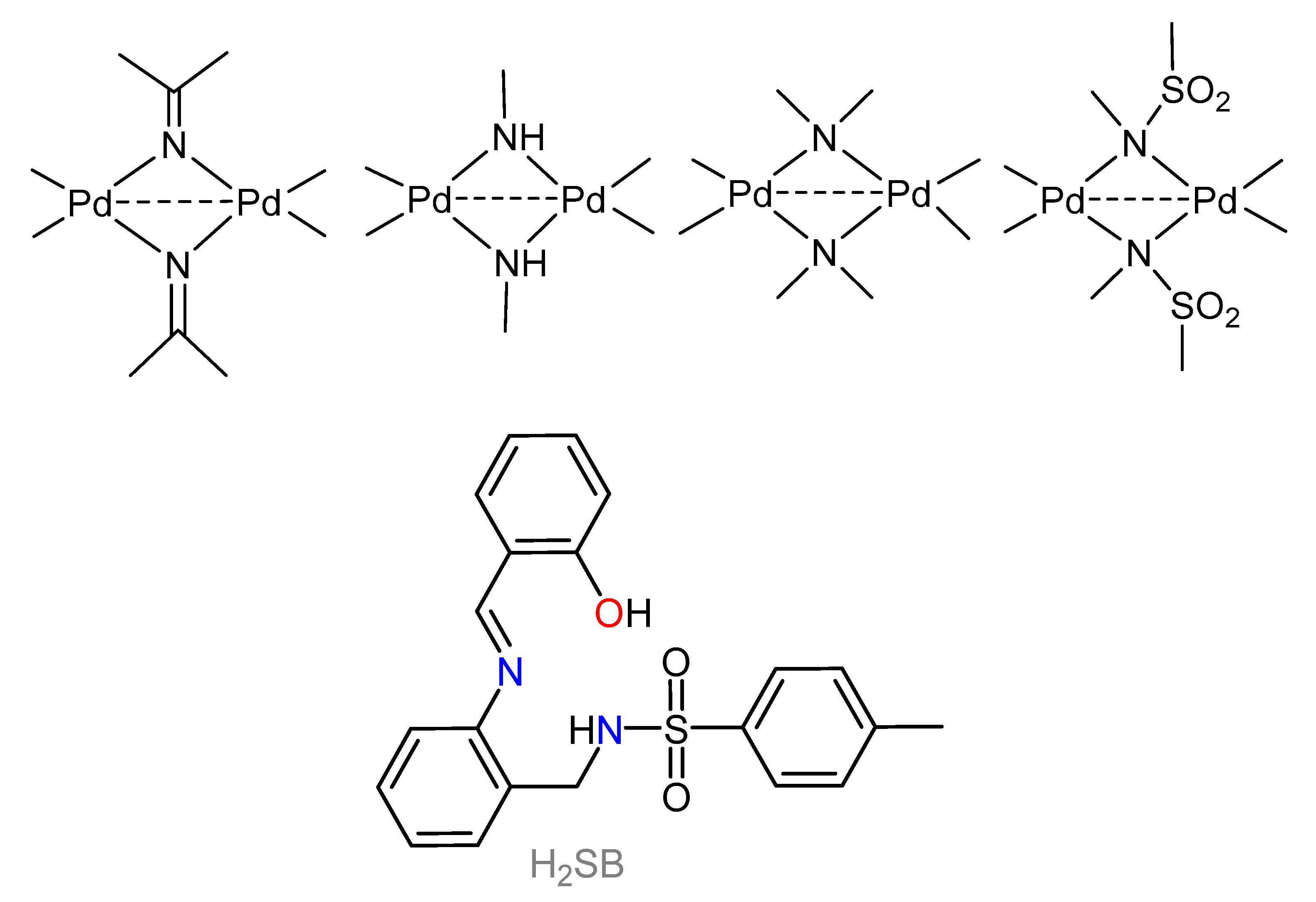
1. Introduction
2. Experiment
2.1. Materials and Methods
2.2. Crystal Structure Analysis Data
2.3. Synthesis of H2SB
2.4. Synthesis of Pd2(SB)2
2.5. Synthesis of Cd2(SB)2
2.6. Synthesis of Zn2(SB)2·4H2O
2.7. Synthesis of Ni2(SB)2·4H2O
2.8. Synthesis of Cu2(SB)2·2MeOH
2.9. Synthesis of Co2(SB)2·4H2O
3. Results and Discussion
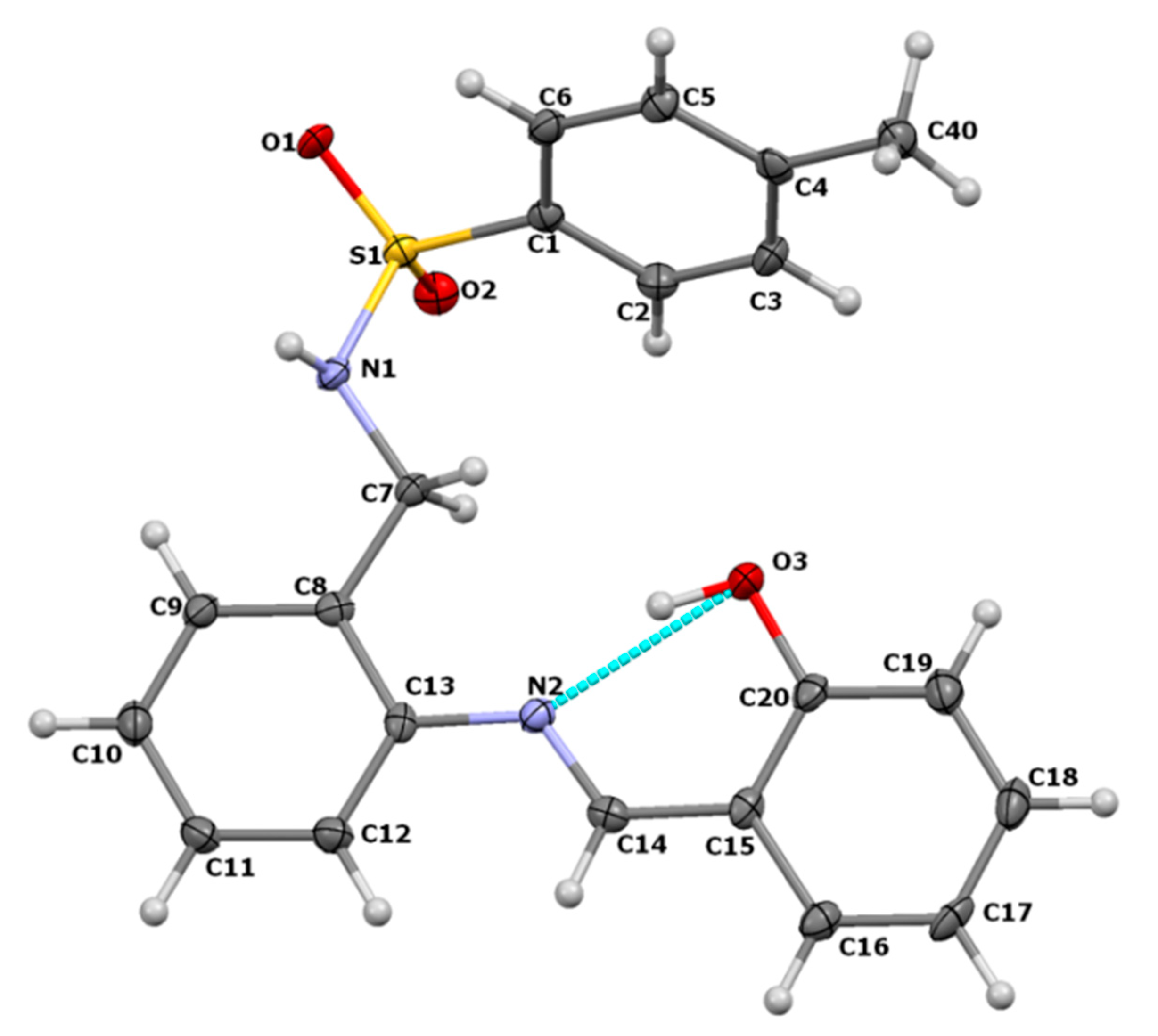
3.1. Crystal Structure of H2SB
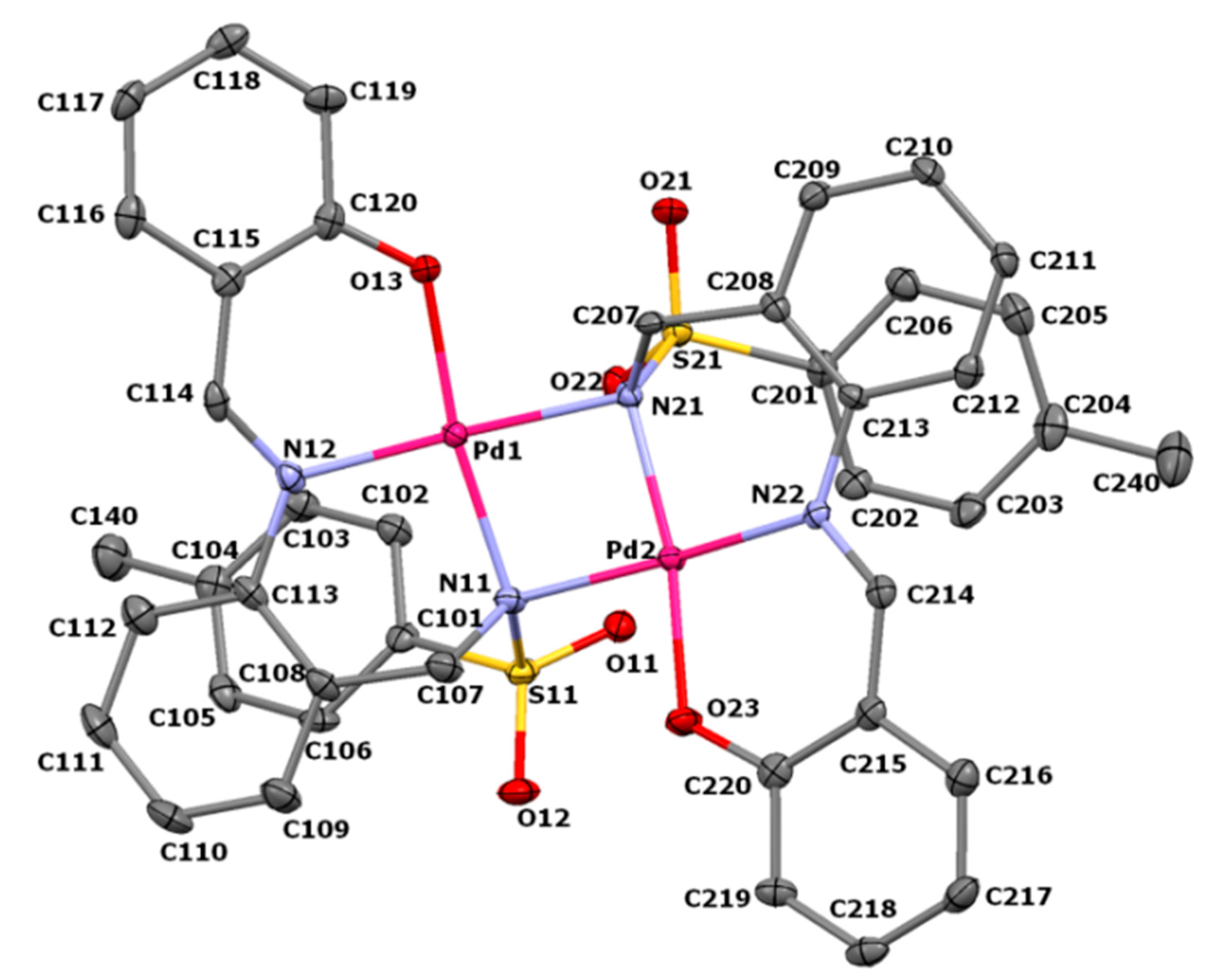
3.2. Crystal Structure of Pd2(SB)2·Me2CO
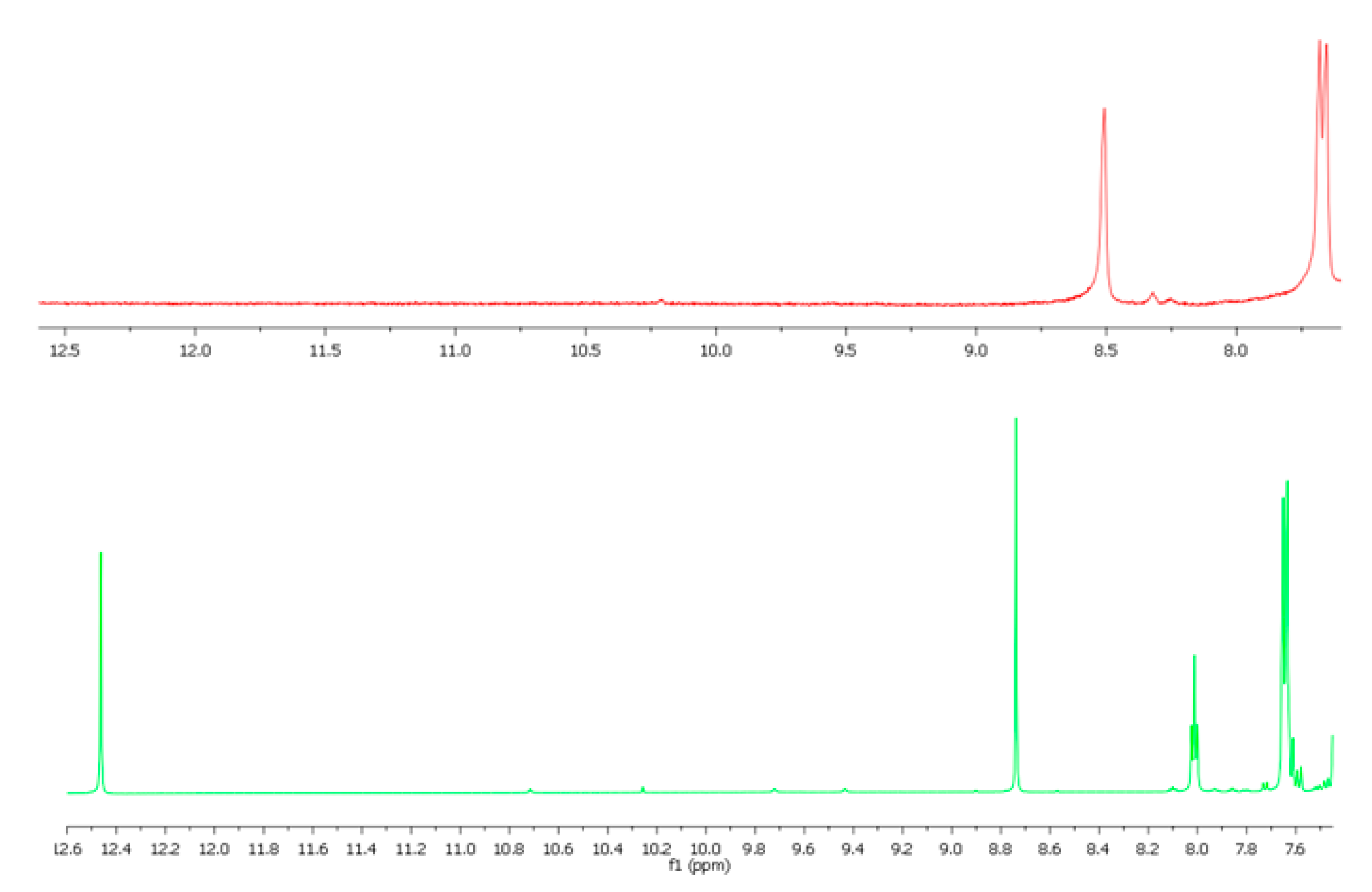
3.3. Spectroscopic Characterization of the Non-Crystalline Complexes
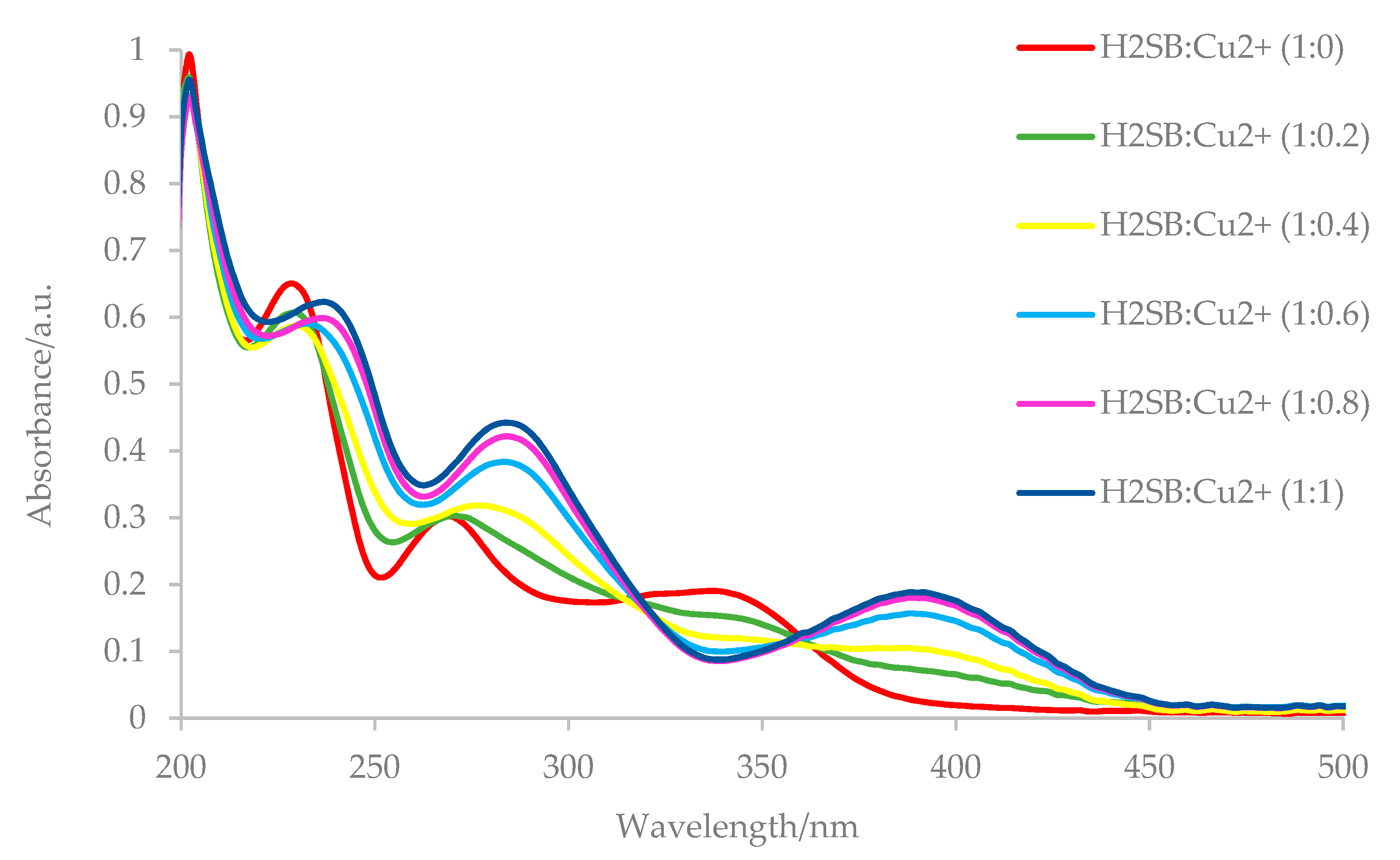
3.4. UV-Vis Studies on the H2SB-M2+ Interaction
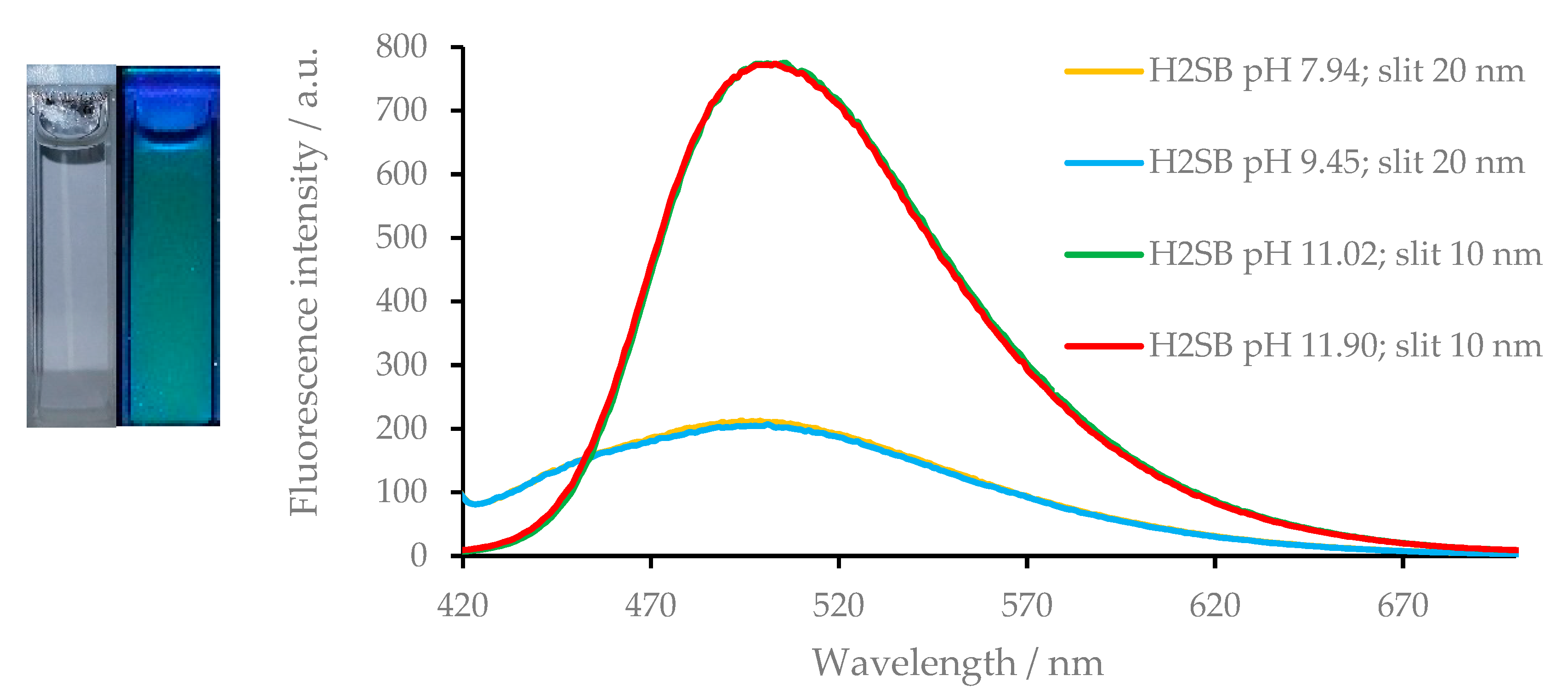
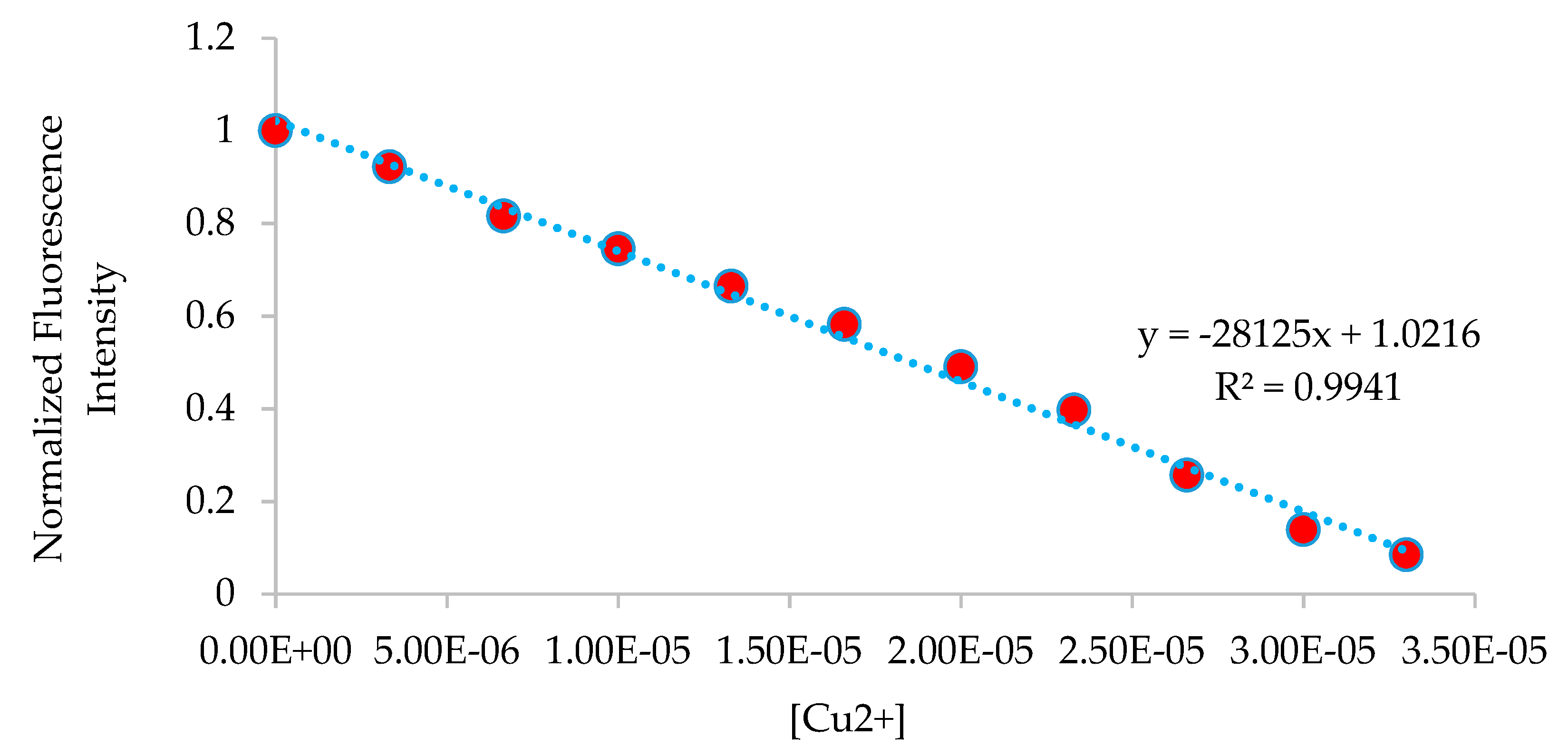
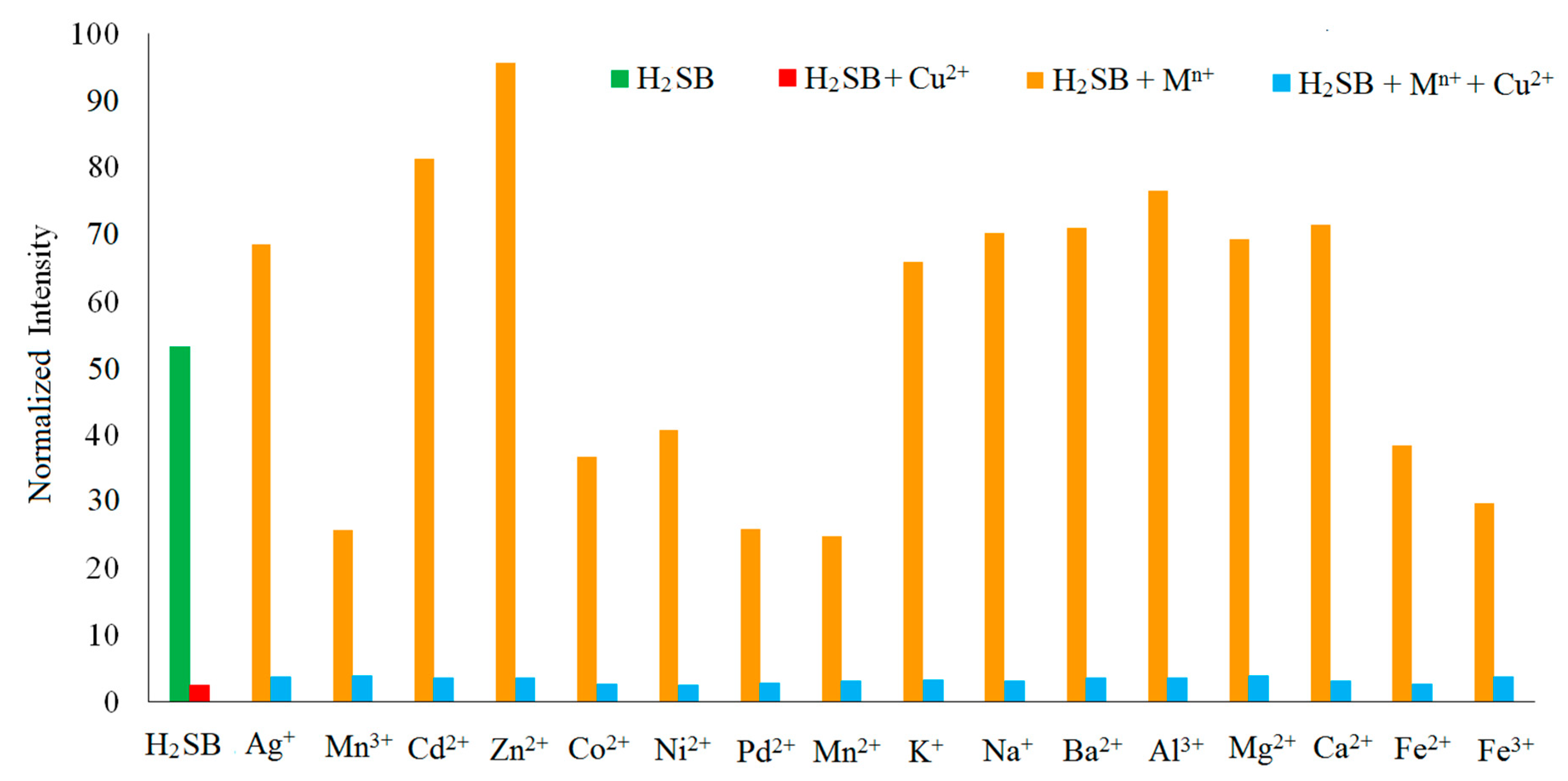
3.5. Fluorescence Studies on the H2SB-Mn+ Interaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Campbell, M.G.; Powers, D.C.; Raynaud, J.; Graham, M.J.; Xie, P.; Lee, E.; Ritter, T. Synthesis and structure of solution-stable one-dimensional palladium wires. Nat. Chem. 2011, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Mitsumi, M.; Goto, H.; Umebayashi, S.; Ozawa, Y.; Kobayashi, M.; Yokoyama, T.; Tanaka, H.; Kuroda, S.-I.; Toriumi, K. A Neutral Mixed-Valent Conducting Polymer Formed by Electron Transfer between Metal d and Ligand π Orbitals. Angew. Chem. Int. Ed. 2005, 44, 4164–4168. [Google Scholar] [CrossRef] [PubMed]
- Finniss, G.M.; Canadell, E.; Campana, C.; Dunbar, K.R. Unprecedented Conversion of a Compound with Metal–Metal Bonding into a Solvated Molecular Wire. Angew. Chem. Int. Ed. Engl. 1996, 35, 2772–2774. [Google Scholar] [CrossRef]
- Givaja, G.; Castillo, O.; Mateo, E.; Gallego, A.; Gómez-García, C.J.; Calzolari, A.; di Felice, R.; Zamora, F. Electrical Behaviour of Heterobimetallic [MM′(EtCS2)4] (MM′ = NiPd, NiPt, PdPt) and MM’X-Chain Polymers [PtM(EtCS2)4I] (M = Ni, Pd). Chem. Eur. J. 2012, 18, 15476–15484. [Google Scholar] [CrossRef] [PubMed]
- Palii, A.V.; Reu, O.S.; Ostrovsky, S.M.; Klokishner, S.I.B.; Tsukerblat, S.; Sun, Z.-M.; Mao, J.-G.; Prosvirin, A.V.; Zhao, H.-H.; Dunbar, K.R. A Highly Anisotropic Cobalt(II)-Based Single-Chain Magnet: Exploration of Spin Canting in an Antiferromagnetic Array. J. Am. Chem. Soc. 2008, 130, 14729–14738. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, W.; Prosvirin, A.V.; Whitenack, K.; Dunbar, K.R.; Zubieta, J.A. Thermally and Hydrolytically Stable Microporous Framework Exhibiting Single-Chain Magnetism: Structure and Properties of [Co2(H0.67bdt)3]·20H2O. Angew. Chem. Int. Ed. 2009, 48, 2140–2143. [Google Scholar] [CrossRef] [PubMed]
- Fernández, E.J.; López-de-Luzuriaga, J.M.; Monge, M.; Olmos, M.E.; Pérez, J.; Laguna, A.; Mohamed, A.A.; Fackler, J.P. {Tl[Au(C6Cl5)2]}n: A Vapochromic Complex. J. Am. Chem. Soc. 2003, 125, 2022–2023. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.A.; Connick, W.B.; Lachicotte, R.J.; Gysling, H.J.; Eisenberg, R. Linear Chain Au(I) Dimer Compounds as Environmental Sensors: A Luminescent Switch for the Detection of Volatile Organic Compounds. J. Am. Chem. Soc. 1998, 120, 1329–1330. [Google Scholar] [CrossRef]
- Lee, Y.-A.; McGarrah, J.E.; Lachicotte, R.J.; Eisenberg, R. Multiple Emissions and Brilliant White Luminescence from Gold(I) O,O’-Di(alkyl)dithiophosphate Dimers. J. Am. Chem. Soc. 2002, 124, 10662–10663. [Google Scholar] [CrossRef]
- Matthews, R.C.; Howell, D.K.; Peng, W.J.; Laneman, S.A.; Stanley, G.G. Bimetallic Hydroformylation Catalysis: In Situ Characterization of a Dinuclear Rhodium(II) Dihydrido Complex with the Largest Rh–H NMR Coupling Constant. Angew. Chem. Int. Ed. Engl. 1996, 35, 2253–2256. [Google Scholar] [CrossRef]
- Murahashi, T.; Kurosawa, H. Organopalladium complexes containing palladium-palladium bonds. Coord. Chem. Rev. 2002, 231, 207–228. [Google Scholar] [CrossRef]
- Murahashi, T.; Nagai, T.; Mino, Y.; Mochizuki, E.; Kai, Y.; Kurosawa, H. Reversible Interconversion between Dinuclear Sandwich and Half-Sandwich Complexes: Unique Dynamic Behavior of a Pd−Pd Moiety Surrounded by an sp2-Carbon Framework. J. Am. Chem. Soc. 2001, 123, 6927–6928. [Google Scholar] [CrossRef]
- Werner, H.; Kuhn, A. A General Method for the Synthesis of Sandwich-Type Complexes with a Pd–Pd or Pt–Pt Bond. Angew. Chem. Int. Ed. Engl. 1977, 16, 412–413. [Google Scholar] [CrossRef]
- Sauthier, M.; Le Guennic, B.; Deborde, V.; Toupet, L.; Halet, J.; Réau, R.F. A Rare Phosphane Coordination Mode: A Symmetrically μ2-Bridging Phosphole in a Dinuclear Palladium(I) Complex. Angew. Chem. Int. Ed. 2001, 40, 228–231. [Google Scholar] [CrossRef]
- Allen, F.H. The Cambridge Structural Database: A quarter of a million crystal structures and rising. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 380–388. [Google Scholar] [CrossRef]
- Lasri, J.; Kopylovich, M.N.; da Silva, M.F.C.G.; Charmier, M.A.J.; Pombeiro, A.J.L. Metal-Free and PdII-Promoted [2+3] Cycloadditions of a Cyclic Nitrone to Phthalonitriles: Syntheses of Oxadiazolines as well as Phthalamide–PdII and Dihydropyrrolyl-iminoisoindolinone–PdII Complexes with High Catalytic Activity in Suzuki–Miyaura Cross-Coupling Reactions. Chem.-Eur. J. 2008, 14, 9312–9322. [Google Scholar] [CrossRef]
- Peng, K.-F.; Chen, C.-T. Synthesis, Structural Studies, and Catalytic Application of Palladium Complexes Containing Anilido-Oxazolinate Ligands. Eur. J. Inorg. Chem. 2011, 5182–5195. [Google Scholar] [CrossRef]
- Cuevas, J.V.; García-Herbosa, G.; Muñoz, A.; García-Granda, S.; Miguel, D. Metal Complexes of Chiral Imidazolin-2-ylidene Ligands. Organometallics 1997, 16, 2472–2477. [Google Scholar] [CrossRef]
- Beck, B.; Schneider, A.; Freisinger, E.; Holthenrich, D.; Erxleben, A.; Albinati, A.; Zangrando, E.; Randaccio, L.; Lippert, B. Inter- and intra-molecular condensation patterns of (en)PdII with trans-[a2PtL2]2+ (a = am(m)ine, L = 2-aminopyridine): PtPd3 and Pt2Pd4 species with multiple amide bridges. Unexpected trapping of a pair of nitrate ions by a Pt2Pd4 double cone. Dalton Trans. 2003, 2533–2539. [Google Scholar] [CrossRef]
- Bruns, H.; Patil, M.; Carreras, J.; Vázquez, A.; Thiel, W.; Goddard, R.; Alcarazo, M. Synthesis and coordination properties of nitrogen(I)-based ligands. Angew. Chem. Int. Ed. 2010, 49, 3680–3683. [Google Scholar] [CrossRef]
- Anandhi, U.; Holbert, T.; Lueng, D.; Sharp, P.R. Platinum and Palladium Imido and Oxo Complexes with Small Natural Bite Angle Diphosphine Ligands. Inorg. Chem. 2003, 42, 1282–1295. [Google Scholar] [CrossRef]
- Ruiz, J.; Rodríguez, V.; Cutillas, N.; Florenciano, F.; Pérez, J.; López, G. First complex containing a Pd2(μ2-N=CPh2)2 functional group. Inorg. Chem. Commun. 2001, 4, 23–25. [Google Scholar] [CrossRef]
- Jess, K.; Baabe, D.; Freytag, M.; Jones, P.G.; Tamm, M. Transition-Metal Complexes with Ferrocene-Bridged Bis(imidazolin-2-imine) and Bis(diaminocyclopropenimine) Ligands. Eur. J. Inorg. Chem. 2017, 412–423. [Google Scholar] [CrossRef]
- Sanmartín-Matalobos, J.; García-Deibe, A.M.; Fondo, M.; Zarepour-Jevinani, M.; Domínguez-González, M.R.; Bermejo-Barrera, P. Exploration of an easily synthesized fluorescent probe for detecting copper in aqueous samples. Dalton Trans. 2017, 46, 15827–15835. [Google Scholar] [CrossRef]
- Sanmartín-Matalobos, J.; Portela-García, C.; Fondo, M.; García-Deibe, A.M.; Llamas-Saiz, A.L. A simple route to dinuclear complexes containing unusual μ-Nsulfonamido bridges. J. Coord. Chem. 2016, 69, 1358–1370. [Google Scholar] [CrossRef]
- Alvarez, S. A cartography of the van der Waals territories. Dalton Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef]
- Sanmartín, J.; Novio, F.; García-Deibe, A.M.; Fondo, M.; Bermejo, M.R. Trimorphism of an asymmetric disulfonamide Schiff base. New J. Chem. 2007, 31, 1605–1612. [Google Scholar] [CrossRef]
- Blessing, R.H. An empirical correction for absorption anisotropy. Acta Crystallogr. Sect. A Fundam. Crystallogr. 1995, A51, 33–38. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, Area-Detector Absorption Correction; Siemens Industrial Automation, Inc.: Madison, WI, USA, 2001. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- García-Deibe, A.M.; Sanmartín-Matalobos, J.; González-Bello, C.; Lence, E.; Portela-García, C.; Martínez-Rodríguez, L.; Fondo, M. Metal-Assisted Ring-Closing/Opening Process of a Chiral Tetrahydroquinazoline. Inorg. Chem. 2012, 51, 1278–1293. [Google Scholar] [CrossRef]
- García-Deibe, A.M.; Portela-García, C.; Fondo, M.; Sanmartín-Matalobos, J. Controlling ring-chain tautomerism through steric hindrance. RSC Adv. 2015, 5, 58327–58333. [Google Scholar] [CrossRef]
- Menabue, L.; Saladini, M. N-(arylsulfonyl)glycines as cyclometalating ligands. Crystal and molecular structures of disodium bis(µ-chloro)bis[µ-N-(phenylsulfonyl)glycinato-O,N,C]bis[µ-N-(phenylsulfonyl)glycinato-O,O’]tetrapalladate(II) hexahydrate and disodium bis(µ-chloro)bis(µ-N-tosylglycinato-O,N,C)bis(µ-N-tosylglycinato-O,O’)tetrapalladate(II)-4.5-water-2-N-tosylglycine. Inorg. Chem. 1991, 30, 1651–1655. [Google Scholar] [CrossRef]
- Banerjee, S.; Dixit, A.; Maheswaramma, K.S.; Maity, B.; Mukherjee, S.; Kumar, A.; Karande, A.A.; Chakravarty, A.R. Photocytotoxic ternary copper (II) complexes of histamine Schiff base and pyridyl ligands. J. Chem. Sci. 2016, 128, 165–175. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Kim, H.M.; Jung, C.; Kim, B.R.; Jung, S.-Y.; Hong, J.H.; Ko, Y.-G.; Lee, K.J.; Cho, B.R. Environment-sensitive two-photon probe for intracellular free magnesium ions in live tissue. Angew. Chem. Int. Ed. 2007, 46, 3460–3463. [Google Scholar] [CrossRef]
- Uyanik, I.; Oguz, M.; Bhatti, A.A.; Uyanik, A.; Yilmaz, M. A New Piperidine Derivatized-Schiff Base Based "Turn-on" Cu2+ Chemo-Sensor. J. Fluoresc. 2017, 27, 791–797. [Google Scholar] [CrossRef]
- Situ, B.; Zhao, J.; Lv, W.; Li, J.; Li, H.; Li, B.; Chai, Z.; Cao, N.; Zheng, L. Naked-eye detection of copper(II) ions by a “clickable” fluorescent sensor. Sens. Actuators B Chem. 2017, 240, 560–565. [Google Scholar] [CrossRef]
- Saleh, S.M.; Ali, R.; Ali, I.A.I. A novel, highly sensitive, selective, reversible and turn-on chemi-sensor based on Schiff base for rapid detection of Cu(II). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 183, 225–231. [Google Scholar] [CrossRef]
- Parthiban, C.; Elango, K.P. Design, synthesis, characterization and cation sensing behavior of amino-naphthoquinone receptor: Selective colorimetric sensing of Cu(II) ion in nearly aqueous solution with mimicking logic gate operation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 174, 147–153. [Google Scholar] [CrossRef]
- Vanlı, E.; Mısır, M.N.; Alp, H.; Ak, T.; Özbek, N.; Ocak, Ü.; Ocak, M. Ion Sensor Properties of Fluorescent Schiff Bases Carrying Dipicolylamine Groups. A Simple Spectrofluorimetric Method to Determine Cu (II) in Water Samples. J. Fluoresc. 2017, 27, 1759–1766. [Google Scholar] [CrossRef]
- Roy, N.; Dutta, A.; Mondal, P.; Paul, P.C.; Singh, T.S. Coumarin Based Fluorescent Probe for Colorimetric Detection of Fe3+ and Fluorescence Turn On-Off Response of Zn2+ and Cu2+. J. Fluoresc. 2017, 27, 1307–1321. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, L.; Xu, Y.; Cao, D.; Guan, R.; Liu, Z.; Yu, X. Two coumarin formhydrazide compounds as chemosensors for copper ions. Inorg. Chem. Commun. 2016, 69, 7–9. [Google Scholar] [CrossRef]
- Khaokeaw, C.; Sukwattanasinitt, M.; Rashatasakhon, P. Salicylyl Fluorene Derivatives as Fluorescent Sensors for Cu(II) Ions. J. Fluoresc. 2016, 26, 745–752. [Google Scholar] [CrossRef]
- Thavornpradit, S.; Sirirak, J.; Wanichacheva, N. Turn-on naphthalimide fluorescent sensor with high quantum yield and large Stokes shift for the determination of Cu (II). J. Photochem. Photobiol. A Chem. 2016, 330, 55–63. [Google Scholar] [CrossRef]
- Tümay, S.O.; Okutan, E.; Sengul, I.F.; Özcan, E.; Kandemir, H.; Doruk, T.; Çetin, M.; Çosut, B. Naked-eye fluorescent sensor for Cu (II) based on indole conjugate BODIPY dye. Polyhedron 2016, 117, 161–171. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Yang, X.; He, F.; Huang, J.; Jiang, M.; Zhou, Z.; Chen, H. Design, synthesis and biological evaluation of a novel Cu 2+-selective fluorescence sensor for bio-detection and chelation. RSC Adv. 2015, 5, 80110–80117. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, X.; Tian, X.; Liu, A.; Jia, L. Carboxamidoquinoline–coumarin derivative: A ratiometric fluorescent sensor for Cu (II) in a dual fluorophore hybrid. Sens. Actuators B Chem. 2015, 218, 37–41. [Google Scholar] [CrossRef]
- Aggarwal, K.; Khurana, J.M. Phenazine containing indeno-furan based colorimetric and “on–off” fluorescent sensor for the detection of Cu2+ and Pb2+. J. Lumin. 2015, 167, 146–155. [Google Scholar] [CrossRef]
- Maher, N.J.; Diao, H.; O’sullivan, J.; Fadda, E.; Heaney, F.; McGinley, J. Lower rim isoxazole-calix [4] arene derivatives as fluorescence sensors for copper (II) ions. Tetrahedron 2015, 71, 9223–9233. [Google Scholar] [CrossRef]
- Ganguly, A.; Ghosh, S.; Kar, S.; Guchhait, N. Selective fluorescence sensing of Cu(II) and Zn(II) using a simple Schiff base ligand: Naked eye detection and elucidation of photoinduced electron transfer (PET) mechanism. Spectrochim. Acta A 2015, 143, 72–80. [Google Scholar] [CrossRef]
- Kumar, J.; Bhattacharyya, P.K.; Das, D.K. New duel fluorescent “on–off” and colorimetric sensor for copper (II): Copper (II) binds through N coordination and pi cation interaction to sensor. Spectrochim. Acta A 2015, 138, 99–104. [Google Scholar] [CrossRef]
- Wagh, Y.B.; Kuwar, A.; Sahoo, S.K.; Galluccic, J.; Dalal, D.S. Highly selective fluorimetric sensor for Cu 2+ and Hg 2+ using a benzothiazole-based receptor in semi-aqueous media and molecular docking studies. RSC Adv. 2015, 5, 45528–45534. [Google Scholar] [CrossRef]
- Erdemir, S.; Malkondu, S. Novel “turn on” fluorescent sensors based on anthracene and carbazone units for Cu (II) ion in CH3CN–H2O. J. Lumin. 2015, 158, 86–90. [Google Scholar] [CrossRef]
- Gunduz, Z.Y.; Gunduz, C.; Ozpınar, C.; Urucu, O.A. A novel Schiff-base as a Cu (II) ion fluorescent sensor in aqueous solution. Spectrochim. Acta A 2015, 136, 1679–1683. [Google Scholar] [CrossRef]
- Huang, C.-B.; Li, H.-R.; Luo, Y.; Xu, L. A naphthalimide-based bifunctional fluorescent probe for the differential detection of Hg2+ and Cu2+ in aqueous solution. Dalton Trans. 2014, 43, 8102–8108. [Google Scholar] [CrossRef]
- Fu, Y.; Tian, Q.-F.; Guo, Y.-Q.; Zang, S.-Q. New rhodamine-based turn-on and colorimetric probe for copper(II) ion with high selectivity and sensitivity. Inorg. Chim. Acta 2014, 419, 141–146. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mal, P.; Stoeckli-Evans, H. Hydrazone based luminescent receptors for fluorescent sensing of Cu2+: Structure and spectroscopy. J. Lumin. 2014, 155, 185–190. [Google Scholar] [CrossRef]
- Kao, S.-L.; Lin, W.-Y.; Venkatesan, P.; Wu, S.-P. Colorimetric detection of Cu(II): Cu(II)-induced deprotonation of NH responsible for color change. Sens. Actuators B Chem. 2014, 204, 688–693. [Google Scholar] [CrossRef]
- Kumbhar, H.S.; Yadav, U.N.; Gadilohar, B.L.; Shankarling, G.S. A highly selective fluorescent chemosensor based on thio-β-enaminone analog with a turn-on response for Cu(II) in aqueous media. Sens. Actuators B Chem. 2014, 203, 174–180. [Google Scholar] [CrossRef]
- Puangploy, P.; Smanmoo, S.; Surareungchai, W. A new rhodamine derivative-based chemosensor for highly selective and sensitive determination of Cu2+. Sens. Actuators B Chem. 2014, 193, 679–686. [Google Scholar] [CrossRef]
- Qazi, M.A.; Ocak, Ü.; Ocak, M.; Memon, S. An excellent copper selective chemosensor based on calix [4] arene framework. Anal. Chim. Acta 2013, 761, 157–168. [Google Scholar] [CrossRef]
- Tang, L.; Zhou, P.; Zhang, Q.; Huang, Z.; Zhao, J.; Cai, M. A simple quinoline derivatized thiosemicarbazone as a colorimetic and fluorescent sensor for relay recognition of Cu2+ and sulfide in aqueous solution. Inorg. Chem. Commun. 2013, 36, 100–104. [Google Scholar] [CrossRef]
- Tang, L.; Cai, M.; Huang, Z.; Zhong, K.; Hou, S.; Bian, Y.; Nandhakumar, R. Rapid and highly selective relay recognition of Cu(II) and sulfide ions by a simple benzimidazole-based fluorescent sensor in water. Sens. Actuators B Chem. 2013, 185, 188–194. [Google Scholar] [CrossRef]
- Tang, L.; Zhou, P.; Zhong, K.; Hou, S. Fluorescence relay enhancement sequential recognition of Cu2+ and CN− by a new quinazoline derivative. Sens. Actuators B Chem. 2013, 182, 439–445. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanmartín-Matalobos, J.; Fondo, M.; Zarepour-Jevinani, M.; García-Deibe, A.M. Taking Advantage of the Coordinative Behavior of a Tridentate Schiff Base Ligand towards Pd2+ and Cu2+. Crystals 2019, 9, 407. https://doi.org/10.3390/cryst9080407
Sanmartín-Matalobos J, Fondo M, Zarepour-Jevinani M, García-Deibe AM. Taking Advantage of the Coordinative Behavior of a Tridentate Schiff Base Ligand towards Pd2+ and Cu2+. Crystals. 2019; 9(8):407. https://doi.org/10.3390/cryst9080407
Chicago/Turabian StyleSanmartín-Matalobos, Jesús, Matilde Fondo, Morteza Zarepour-Jevinani, and Ana M. García-Deibe. 2019. "Taking Advantage of the Coordinative Behavior of a Tridentate Schiff Base Ligand towards Pd2+ and Cu2+" Crystals 9, no. 8: 407. https://doi.org/10.3390/cryst9080407
APA StyleSanmartín-Matalobos, J., Fondo, M., Zarepour-Jevinani, M., & García-Deibe, A. M. (2019). Taking Advantage of the Coordinative Behavior of a Tridentate Schiff Base Ligand towards Pd2+ and Cu2+. Crystals, 9(8), 407. https://doi.org/10.3390/cryst9080407







