Triple-Ringed Luminescent Heptanuclear Zn(II) Cluster for Efficient Ag(I) Ion Sensing Materials
Abstract
1. Introduction
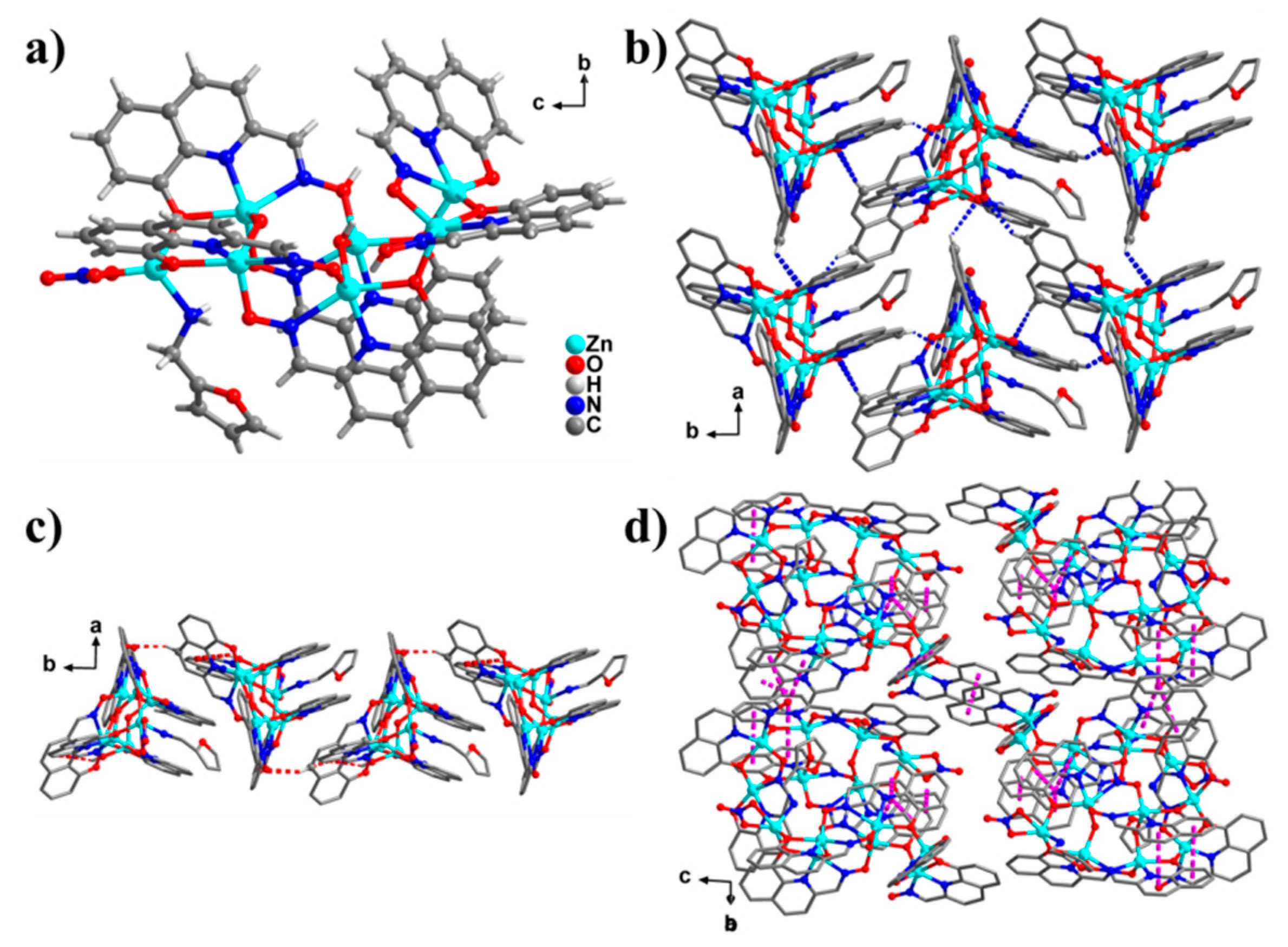
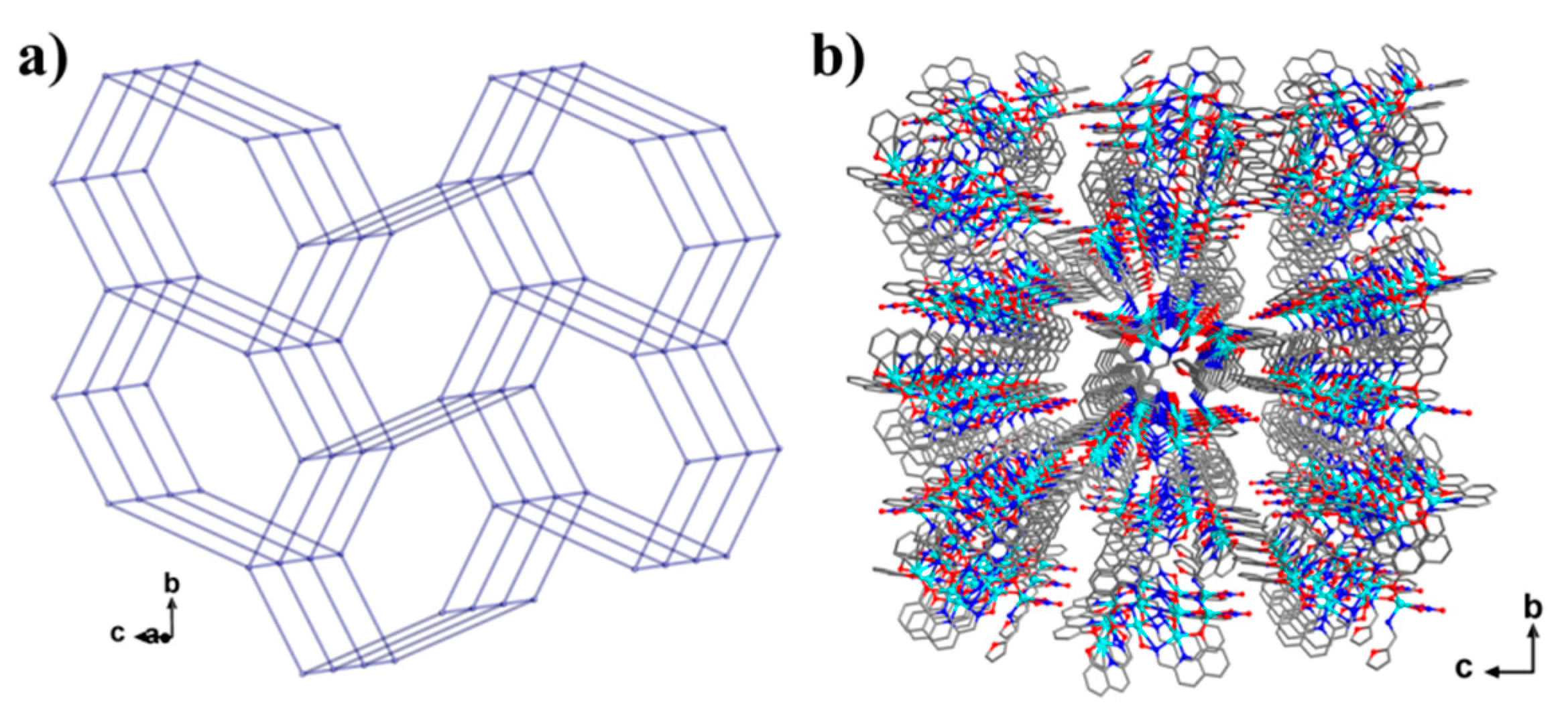
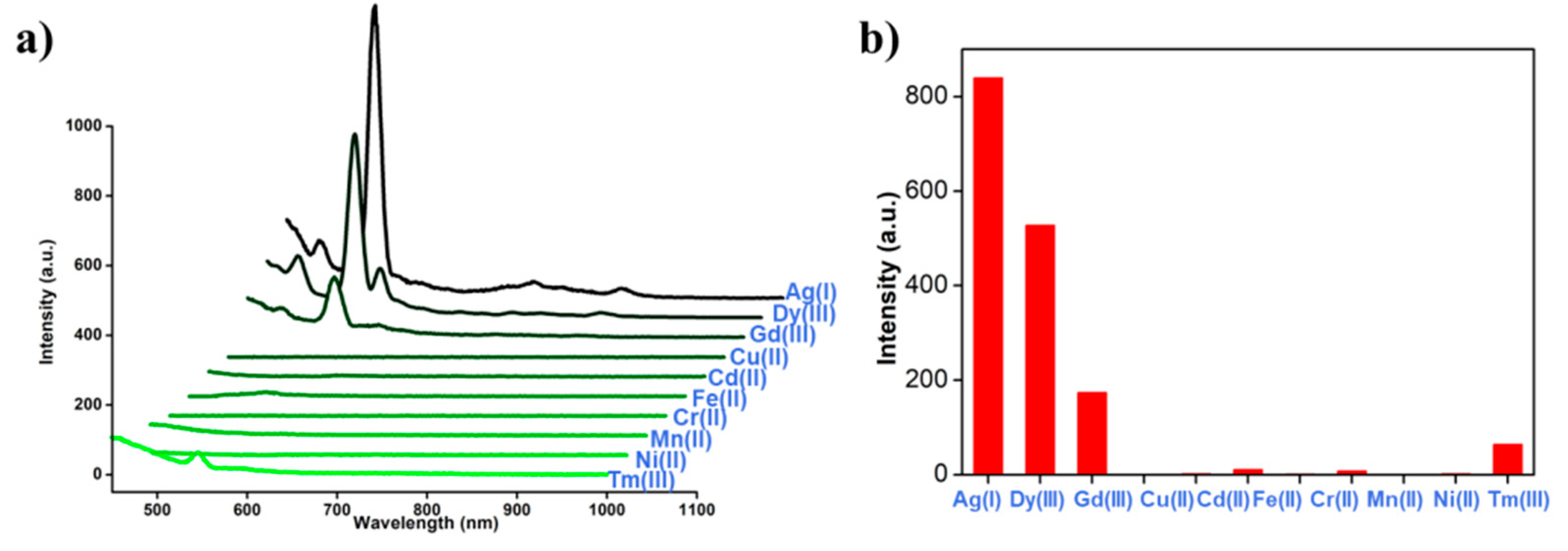
2. Results and Discussion
3. Conclusions
4. Experimental
4.1. Materials and Measurements
4.2. Single-Crystal X-ray Crystallography
4.3. Synthesis of Complex 1
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, J.; Bravo-Suárez, J.J.; Takahashi, A.; Haruta, M.; Oyama, S.T.J. In situ UV–vis studies of the effect of particle size on the epoxidation of ethylene and propylene on supported silver catalysts with molecular oxygen. J. Catal. 2005, 232, 85–95. [Google Scholar] [CrossRef]
- Wu, H.; Kong, D.; Ruan, Z.; Hsu, P.; Wang, S.; Yu, Z.; Carney, T.J.; Hu, L.; Fan, S.; Cui, Y. A transparent electrode based on a metal nanotrough network. Nat. Nanotechnol. 2013, 8, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Chernousova, S.; Epple, M. Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef] [PubMed]
- Barriada, J.L.; Tappin, A.D.; Evans, E.H.; Achterberg, E.P. Dissolved silver measurements in seawater. Trends Anal. Chem. 2007, 26, 809–817. [Google Scholar] [CrossRef]
- Bian, L.; Ji, X.; Hu, W.J. A Novel Single-Labeled Fluorescent Oligonucleotide Probe for Silver(I) Ion Detection in Water, Drugs, and Food. Agric. Food Chem. 2014, 62, 4870–4877. [Google Scholar] [CrossRef]
- Lai, C.Z.; Fierke, M.A.; Costa, R.C.; Gladysz, J.A.; Stein, A.; Bühlmann, P. Highly Selective Detection of Silver in the Low ppt Range with Ion-Selective Electrodes Based on Ionophore-Doped Fluorous Membranes. Anal. Chem. 2010, 82, 7634–7640. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Pomales, G.; Mudalige, T.; Lim, J.; Linder, S.W.J. Rapid Determination of Silver in Nanobased Liquid Dietary Supplements Using. Agric. Food Chem. 2013, 61, 7250–7257. [Google Scholar] [CrossRef]
- Yang, H.; Liu, X.; Fei, R.; Hu, Y. Sensitive and selective detection of Ag+ in aqueous solutions using Fe3O4@Au nanoparticles as smart electrochemical nanosensors. Talanta 2013, 116, 548–553. [Google Scholar] [CrossRef]
- Bian, S.; Shen, C.; Qian, Y.; Liu, J.; Xi, F.; Dong, X. Facile synthesis of sulfur-doped graphene quantum dots as fluorescent sensing probes for Ag+ ions detection. Sens. Actuators B Chem. 2017, 242, 231–237. [Google Scholar] [CrossRef]
- Batista, B.L.; da Silva, L.R.S.; Rocha, B.A.; Rodrigues, J.L.; Berretta-Silva, A.A.; Bonates, T.O.; Gomes, V.S.D.; Barbosa, R.M.; Barbosa, F. Multi-element determination in Brazilian honey samples by inductively coupled plasma mass spectrometry and estimation of geographic origin with data mining techniques. Food Res. Int. 2012, 49, 209–215. [Google Scholar] [CrossRef]
- Song, Q.J.; Greenway, G.M.; McCreedy, T. Interfacing microchip capillary electrophoresis with inductively coupled plasma mass spectrometry for chromium speciation. J. Anal. At. Spectrom. 2003, 18, 1–3. [Google Scholar] [CrossRef]
- Zheng, L.; Chi, Y.; Dong, Y.; Lin, J.; Wang, B. Electrochemiluminescence of Water-Soluble Carbon Nanocrystals Released Electrochemically from Graphite. J. Am. Chem. Soc. 2009, 131, 4564–4565. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.C.; Wang, B.B.; Xu, Z.Q.; He, X.H.; Zou, H.F.; Yang, Q.Q.; Jiang, F.L.; Liu, Y. A novel method for the detection of silver ions with carbon dots: Excellent selectivity, fast response, low detection limit and good applicability. Sens. Actuators B Chem. 2018, 267, 627–635. [Google Scholar] [CrossRef]
- Gao, X.; Lu, Y.; Zhang, R.; He, S.; Ju, J.; Liu, M.; Li, L.; Chen, W. One-pot synthesis of carbon nanodots for fluorescence turn-on detection of Ag+ based on the Ag+-induced enhancement of fluorescence. J. Mater. Chem. C 2015, 3, 2302–2309. [Google Scholar] [CrossRef]
- Ran, X.; Sun, H.; Pu, F.; Ren, J.; Qu, X. Ag Nanoparticle-decorated graphene quantum dots for label-free, rapid and sensitive detection of Ag+ and biothiols. Chem. Commun. 2013, 49, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.N.; Yan, B. A water-stable lanthanide-functionalized MOF as a highly selective and sensitive fluorescent probe for Cd2+. Chem. Commun. 2015, 51, 7737–7740. [Google Scholar] [CrossRef]
- Pandey, S.; Kumar, P.; Gupta, R. Polymerization led selective detection and removal of Zn2+ and Cd2+ ions: isolation of Zn- and Cd-MOFs and reversibility studies. Dalton Trans. 2018, 47, 14686–14695. [Google Scholar] [CrossRef]
- Liao, W.M.; Wei, M.J.; Mo, J.T.; Fu, P.Y.; Fan, Y.N.; Pan, M.; Su, C.Y. Acidity and Cd2+ fluorescent sensing and selective CO2 adsorption by a water-stable Eu-MOF. Dalton Trans. 2019, 48, 4489–4494. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Ma, X.F.; Wang, H.L.; Zou, H.H.; Mo, K.Q.; Zhang, Y.Q.; Yang, Q.Z.; Li, B.; Liang, F.P. A triangular Dy3 single-molecule toroic with high inversion energy barrier: magnetic properties and multiple-step assembly mechanism. Inorg. Chem. Front. 2018, 5, 3155–3162. [Google Scholar] [CrossRef]
- Benelli, C.; Gatteschi, D. Magnetism of Lanthanides in Molecular Materials with Transition-Metal Ions and Organic Radicals. Chem. Rev. 2002, 102, 2369–2387. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
| Complex | 1 |
|---|---|
| Formula | C65H46N14O18Zn7 |
| Formula weight | 1768.75 |
| T (K) | 293.15 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| a (Å) | 10.8123(4) |
| b (Å) | 18.0040(8) |
| c (Å) | 34.376(3) |
| α (°) | 90.00 |
| β (°) | 95.493(4) |
| γ (°) | 90.00 |
| V (Å3) | 6661.1(6) |
| Z | 4 |
| Dc (g cm–3) | 1.764 |
| μ (mm–1) | 2.561 |
| Reflns coll. | 38035 |
| Unique reflns | 12360 |
| Rint | 0.1095 |
| aR1 [I ≥ 2σ(I)] | 0.0879 |
| bwR2 (all data) | 0.2473 |
| GOF | 1.047 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Q.-J.; Chen, M.; Chen, D.-C.; Zhu, Z.-H.; Zou, H.-H. Triple-Ringed Luminescent Heptanuclear Zn(II) Cluster for Efficient Ag(I) Ion Sensing Materials. Crystals 2019, 9, 374. https://doi.org/10.3390/cryst9070374
Deng Q-J, Chen M, Chen D-C, Zhu Z-H, Zou H-H. Triple-Ringed Luminescent Heptanuclear Zn(II) Cluster for Efficient Ag(I) Ion Sensing Materials. Crystals. 2019; 9(7):374. https://doi.org/10.3390/cryst9070374
Chicago/Turabian StyleDeng, Qian-Jun, Min Chen, Dong-Chu Chen, Zhong-Hong Zhu, and Hua-Hong Zou. 2019. "Triple-Ringed Luminescent Heptanuclear Zn(II) Cluster for Efficient Ag(I) Ion Sensing Materials" Crystals 9, no. 7: 374. https://doi.org/10.3390/cryst9070374
APA StyleDeng, Q.-J., Chen, M., Chen, D.-C., Zhu, Z.-H., & Zou, H.-H. (2019). Triple-Ringed Luminescent Heptanuclear Zn(II) Cluster for Efficient Ag(I) Ion Sensing Materials. Crystals, 9(7), 374. https://doi.org/10.3390/cryst9070374





