Floating Zone Growth of Sr Substituted Han Purple: Ba0.9Sr0.1CuSi2O6
Abstract
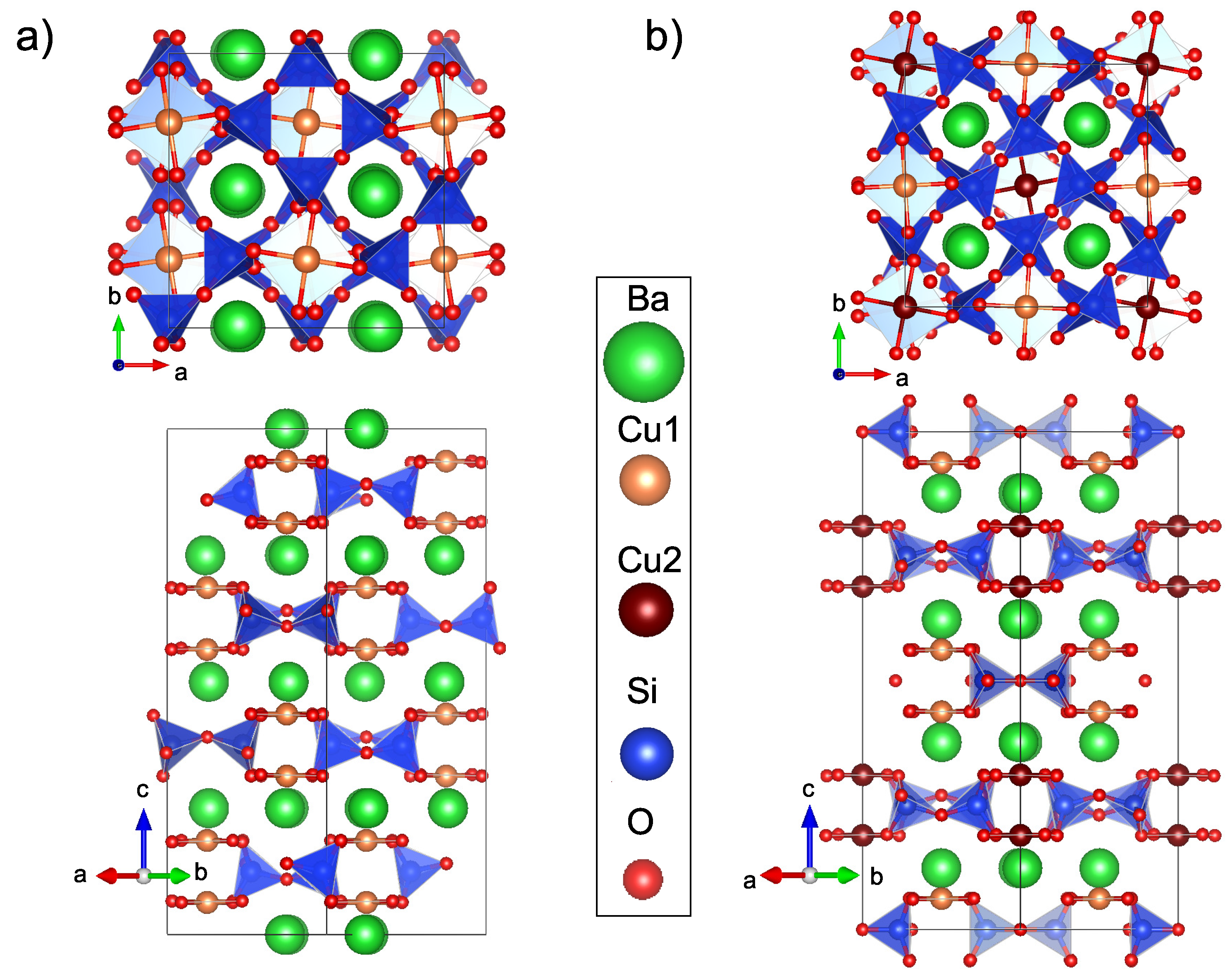
1. Introduction
2. Experimental Details
3. Synthesis
3.1. Atmosphere
3.2. Growth Speed
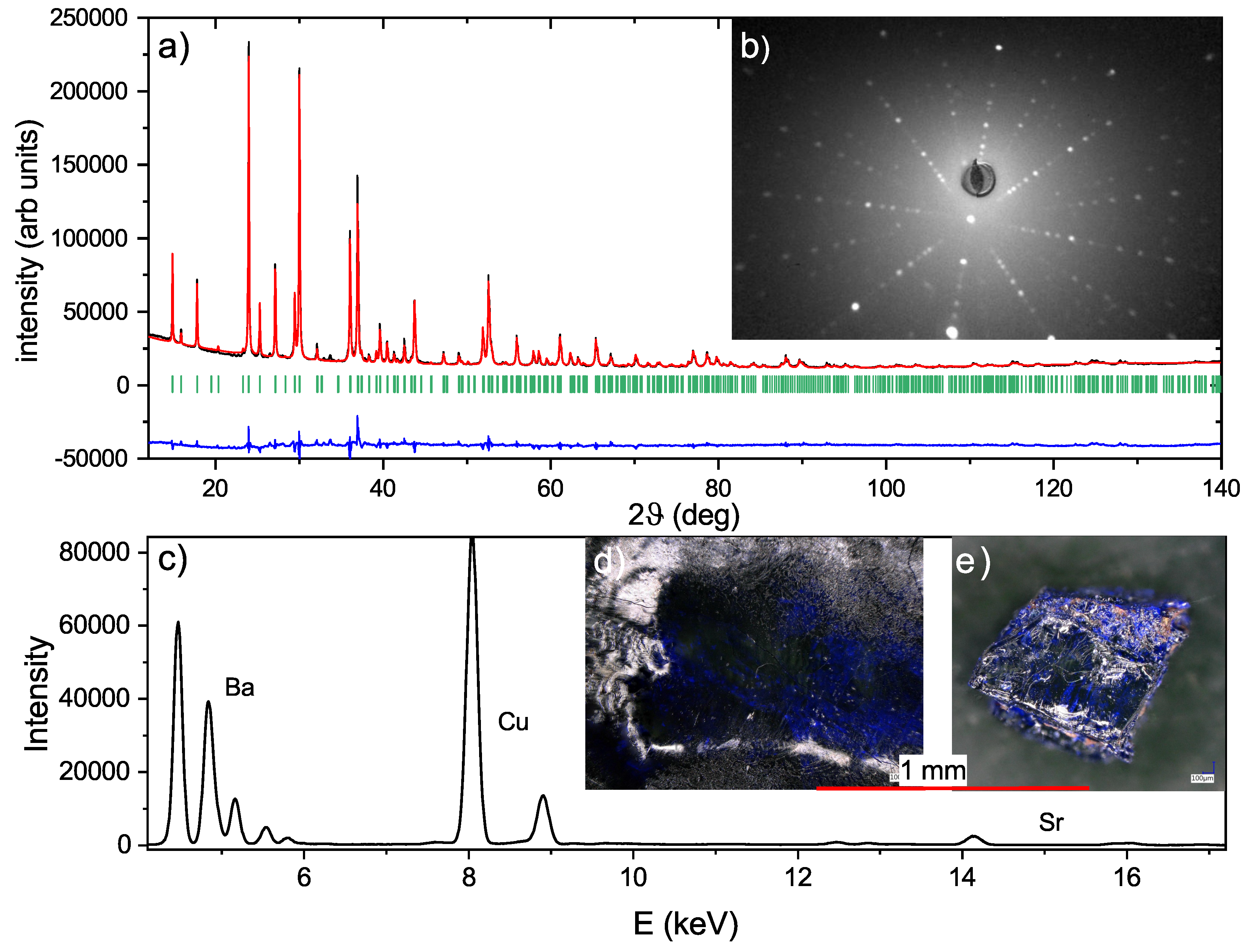
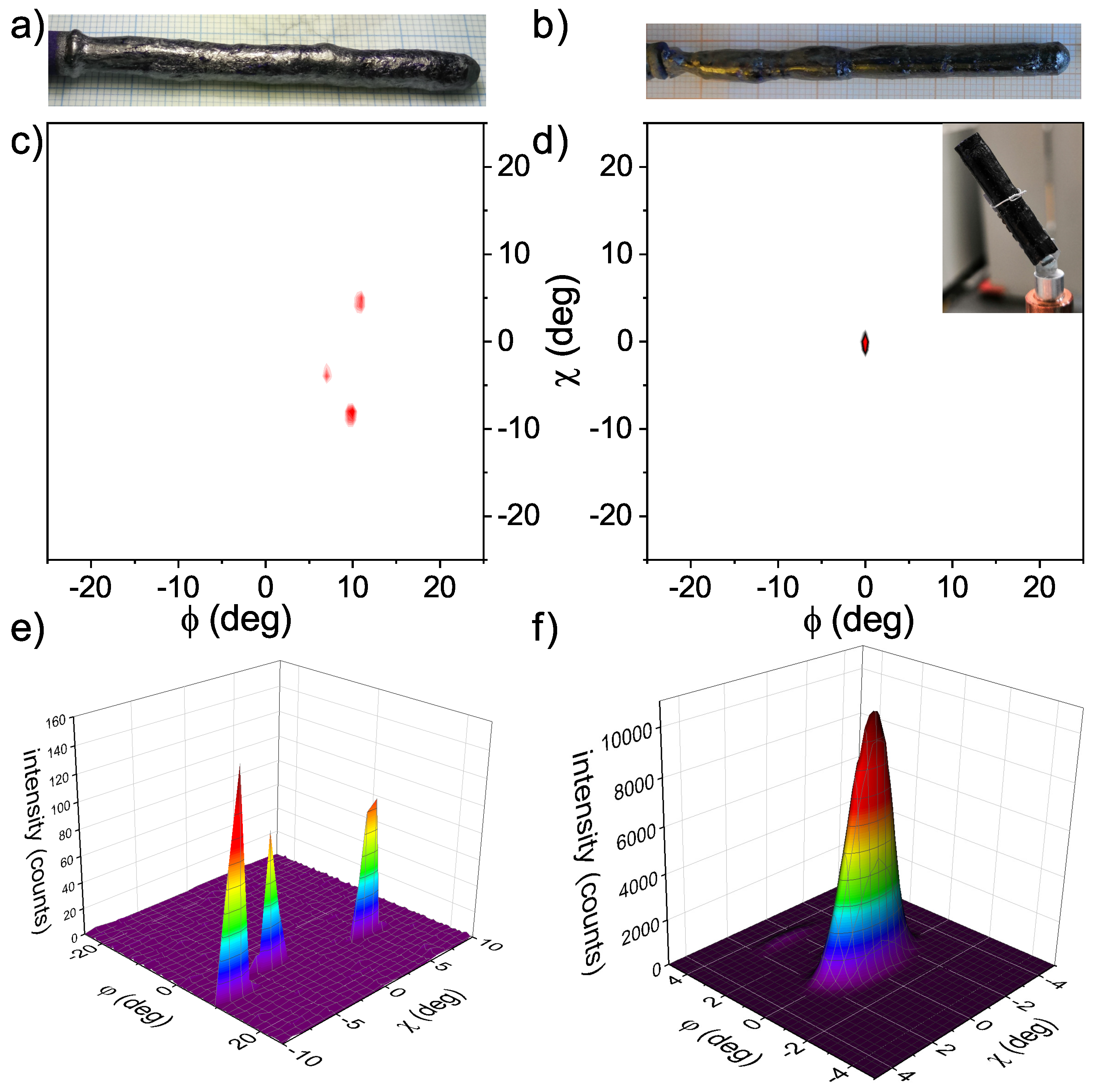
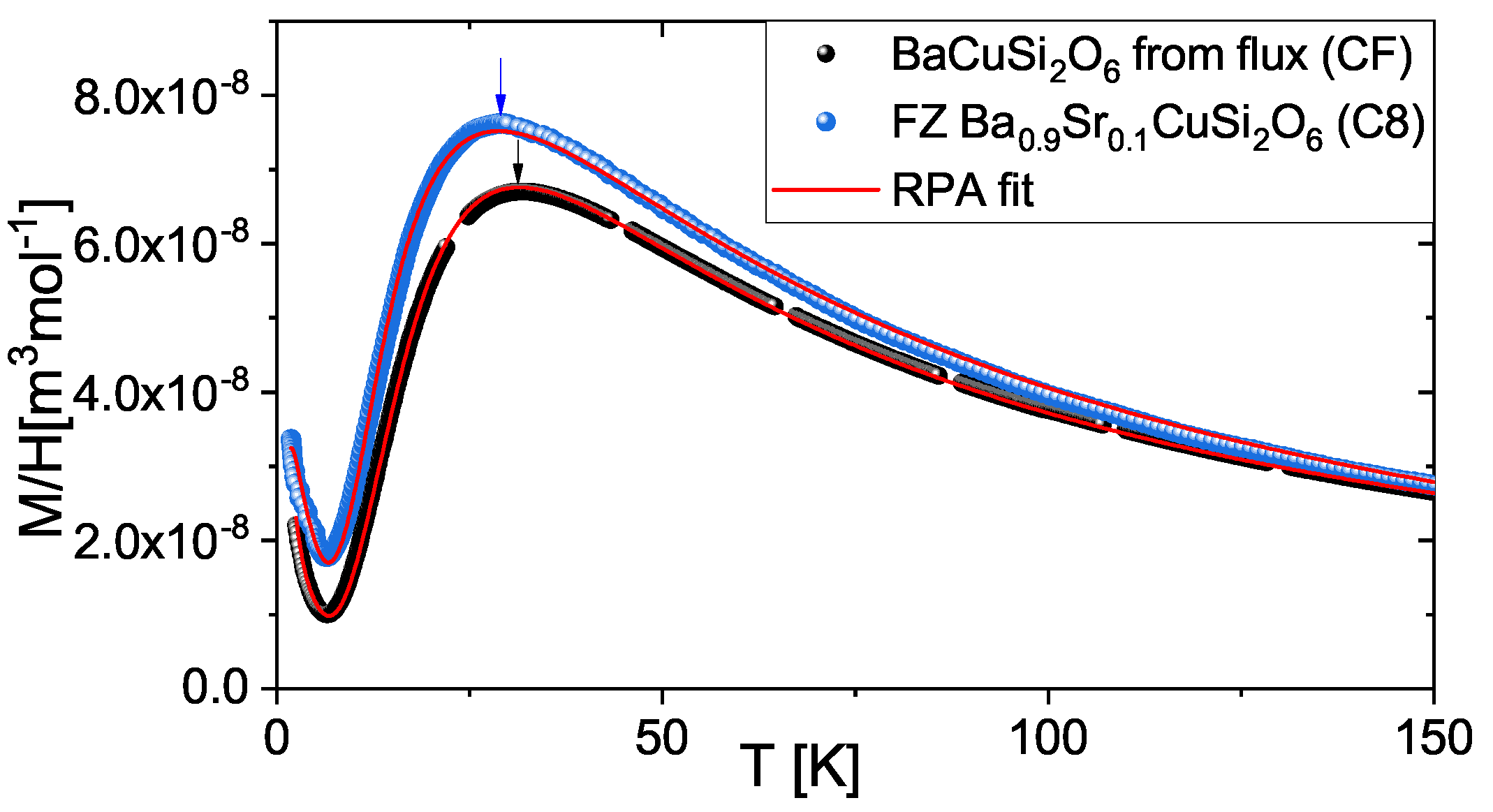
4. Characterization
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berke, H. The invention of blue and purple pigments in ancient times. Chem. Soc. Rev. 2007, 36, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Rieck, B.; Pristacz, H.; Giester, G. Colinowensite, BaCuSi2O6, a new mineral from the Kalahari Manganese Field, South Africa and new data on wesselsite, SrCuSi4O10. Mineral. Mag. 2015, 79, 1769–1778. [Google Scholar] [CrossRef]
- Finger, L.W.; Hazen, R.M.; Hemley, R.J. BaCuSi2O6: A new cyclosilicate with four-membered tetrahedral rings. Am. Mineral. 1989, 74, 952–955. [Google Scholar]
- Sasago, Y.; Uchinokura, K.; Zheludev, A.; Shirane, G. Temperature-dependent spin gap and singlet ground state inBaCuSi2O6. Phys. Rev. B 1997, 55, 8357–8360. [Google Scholar] [CrossRef]
- Jaime, M.; Correa, V.F.; Harrison, N.; Batista, C.D.; Kawashima, N.; Kazuma, Y.; Jorge, G.A.; Stern, R.; Heinmaa, I.; Zvyagin, S.A.; et al. Magnetic-Field-Induced Condensation of Triplons in Han Purple PigmentBaCuSi2O6. Phys. Rev. Lett. 2004, 93, 087203. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, S.E.; Harrison, N.; Batista, C.D.; Balicas, L.; Jaime, M.; Sharma, P.A.; Kawashima, N.; Fisher, I.R. Dimensional reduction at a quantum critical point. Nature 2006, 441, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Rüegg, C.; McMorrow, D.F.; Normand, B.; Rønnow, H.M.; Sebastian, S.E.; Fisher, I.R.; Batista, C.D.; Gvasaliya, S.N.; Niedermayer, C.; Stahn, J. Multiple Magnon Modes and Consequences for the Bose-Einstein Condensed Phase inBaCuSi2O6. Phys. Rev. Lett. 2007, 98, 017202. [Google Scholar] [CrossRef] [PubMed]
- Sheptyakov, D.V.; Pomjakushin, V.Y.; Stern, R.; Heinmaa, I.; Nakamura, H.; Kimura, T. Two types of adjacent dimer layers in the low-temperature phase of BaCuSi2O6. Phys. Rev. B 2012, 86, 014433. [Google Scholar] [CrossRef]
- Samulon, E.C.; Islam, Z.; Sebastian, S.E.; Brooks, P.B.; McCourt, M.K.; Ilavsky, J.; Fisher, I.R. Low-temperature structural phase transition and incommensurate lattice modulation in the spin-gap compoundBaCuSi2O6. Phys. Rev. B 2006, 73, 100407. [Google Scholar] [CrossRef]
- Sparta, K.M.; Roth, G. Reinvestigation of the structure of BaCuSi2O6—Evidence for a phase transition at high temperature. Acta Crystallogr. Sect. B Struct. Sci. 2004, 60, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, S.E.; Tanedo, P.; Goddard, P.A.; Lee, S.C.; Wilson, A.; Kim, S.; Cox, S.; McDonald, R.D.; Hill, S.; Harrison, N.; Batista, C.D.; Fisher, I.R. Role of anisotropy in the spin-dimer compoundBaCuSi2O6. Phys. Rev. B 2006, 74, 180401. [Google Scholar] [CrossRef]
- Puphal, P.; Sheptyakov, D.; van Well, N.; Postulka, L.; Heinmaa, I.; Ritter, F.; Assmus, W.; Wolf, B.; Lang, M.; Jeschke, H.O.; et al. Stabilization of the tetragonal structure in(Ba1-xSrx)CuSi2O6. Phys. Rev. B 2016, 93, 174121. [Google Scholar] [CrossRef]
- van Well, N.; Puphal, P.; Wehinger, B.; Kubus, M.; Schefer, J.; Rüegg, C.; Ritter, F.; Krellner, C.; Assmus, W. Crystal Growth with Oxygen Partial Pressure of the BaCuSi2O6 and Ba1–xSrxCuSi2O6 Spin Dimer Compounds. Cryst. Growth Des. 2016, 16, 3416–3424. [Google Scholar] [CrossRef]
- Prabhakaran, D.; Boothroyd, A. Single crystal growth of Zn-doped CuO by the floating-zone method. J. Cryst. Growth 2003, 250, 77–82. [Google Scholar] [CrossRef]
- Ito, T.; Yamaguchi, H.; Okabe, K.; Masumi, T. Single-crystal growth and characterization of Cu2O and CuO. J. Mater. Sci. 1998, 33, 3555–3566. [Google Scholar] [CrossRef]
- Zayed, M.; Rüegg, C.; Pomjakushina, E.; Stingaciu, M.; Conder, K.; Hanfland, M.; Merlini, M.; Rønnow, H. Temperature dependence of the pressure induced monoclinic distortion in the spin Shastry–Sutherland compound SrCu2(BO3)2. Solid State Commun. 2014, 186, 13–17. [Google Scholar] [CrossRef]
- Dabkowska, H.; Dabkowski, A.; Luke, G.; Dunsiger, S.; Haravifard, S.; Cecchinel, M.; Gaulin, B. Crystal growth and magnetic behaviour of pure and doped SrCu2(11BO3)2. J. Cryst. Growth 2007, 306, 123–128. [Google Scholar] [CrossRef]


| Furnace | Gas | p [bar] | Power [%] | Rate [mm/h] | Comments | Crystallite Size | |
|---|---|---|---|---|---|---|---|
| C1 | FZ1 | O | 0 | 53.7 | - | could not start growth, immense bubbles | 0 |
| C2 | FZ1 | O | 0.5–3 | 54–56.3 | 2-0.5 | neck thinning and bubbles | m |
| C3 | FZ1 | O | 7 | 59 | - | bubbles | 0 |
| C4 | FZ2 | O | 30 | 16–18 | 1 | flowing down of liquid, disconnection | 0 |
| C5 | FZ2 | O | 100 | 22 | 2 | repeated disconnection, phase seperation | 0 |
| C6 | FZ1 | O/Ar | 4.4 | 56 | 1 | stable growth conditions | mm |
| C7 | FZ1 | O/Ar | 7 | 56.3 | 0.5 | stable growth conditions | mm-cm |
| C8 | FZ1 | O/Ar | 5.4 | 54.7 | 0.5 | (seedcrystal) stable growth conditions | cm |
| CF | flux | -- | 2:1 LiBO, °C slow cooling to °C | mm | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puphal, P.; Allenspach, S.; Rüegg, C.; Pomjakushina, E. Floating Zone Growth of Sr Substituted Han Purple: Ba0.9Sr0.1CuSi2O6. Crystals 2019, 9, 273. https://doi.org/10.3390/cryst9050273
Puphal P, Allenspach S, Rüegg C, Pomjakushina E. Floating Zone Growth of Sr Substituted Han Purple: Ba0.9Sr0.1CuSi2O6. Crystals. 2019; 9(5):273. https://doi.org/10.3390/cryst9050273
Chicago/Turabian StylePuphal, Pascal, Stephan Allenspach, Christian Rüegg, and Ekaterina Pomjakushina. 2019. "Floating Zone Growth of Sr Substituted Han Purple: Ba0.9Sr0.1CuSi2O6" Crystals 9, no. 5: 273. https://doi.org/10.3390/cryst9050273
APA StylePuphal, P., Allenspach, S., Rüegg, C., & Pomjakushina, E. (2019). Floating Zone Growth of Sr Substituted Han Purple: Ba0.9Sr0.1CuSi2O6. Crystals, 9(5), 273. https://doi.org/10.3390/cryst9050273




