The Photorefractive Response of Zn and Mo Codoped LiNbO3 in the Visible Region
Abstract
:1. Introduction
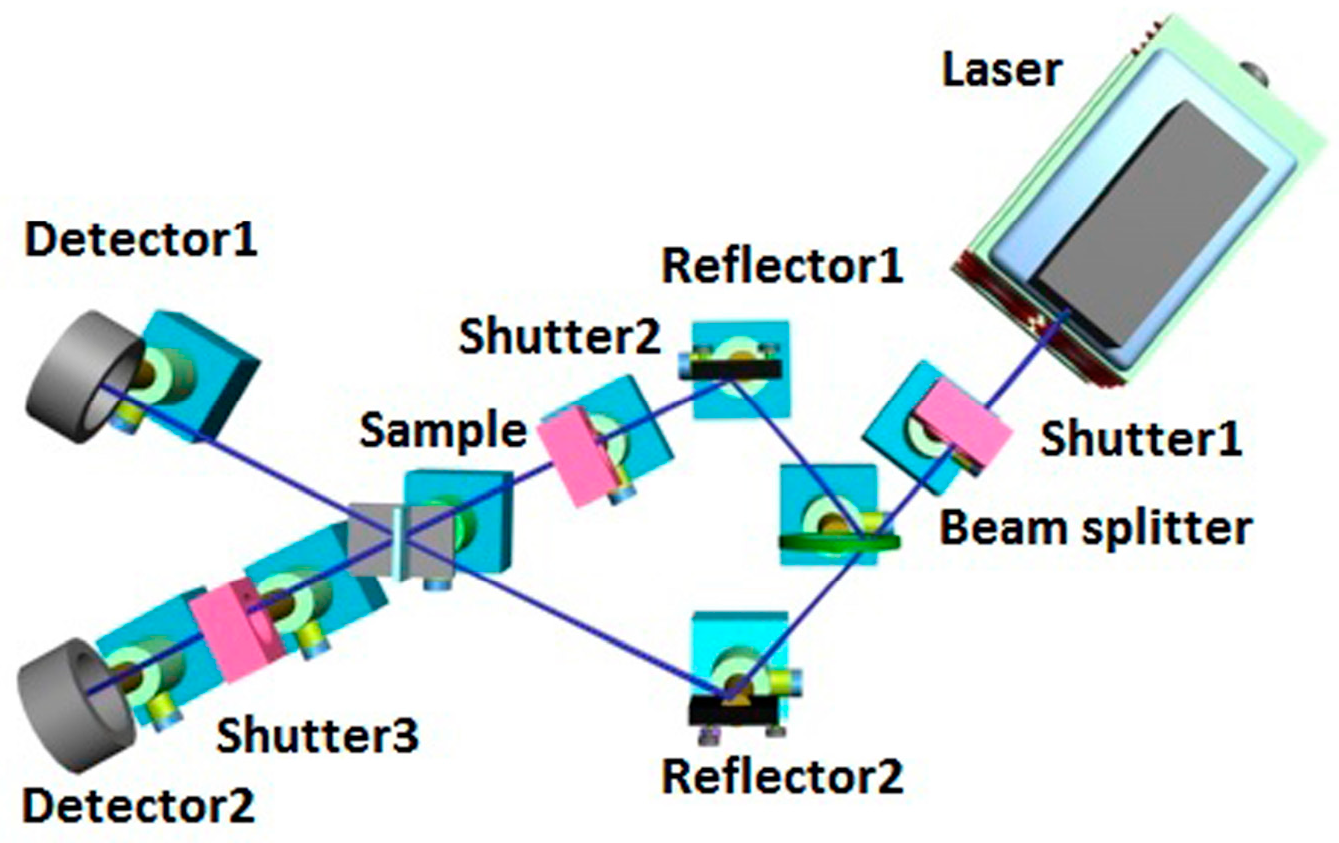
2. Materials and Methods
3. Results
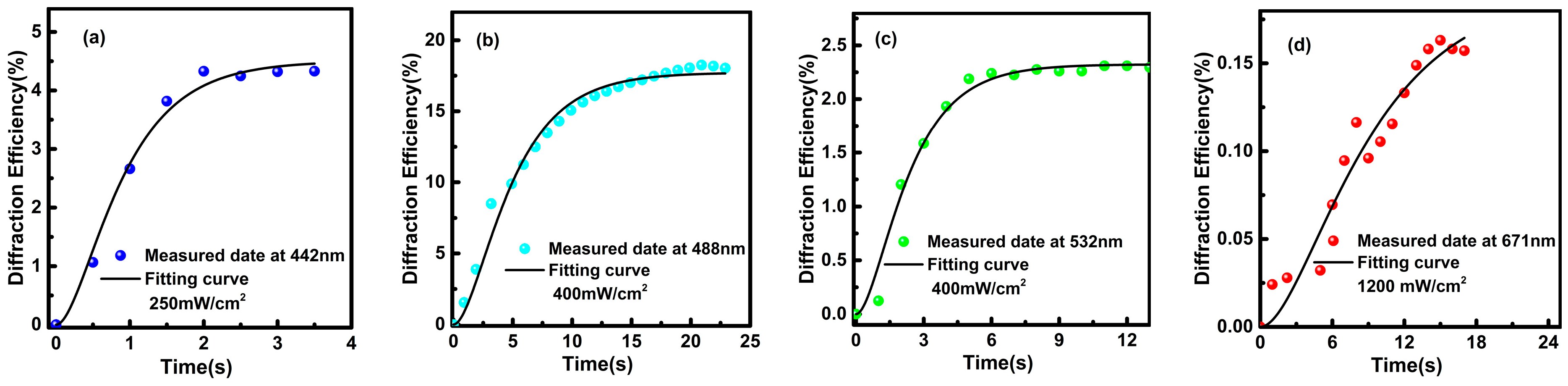
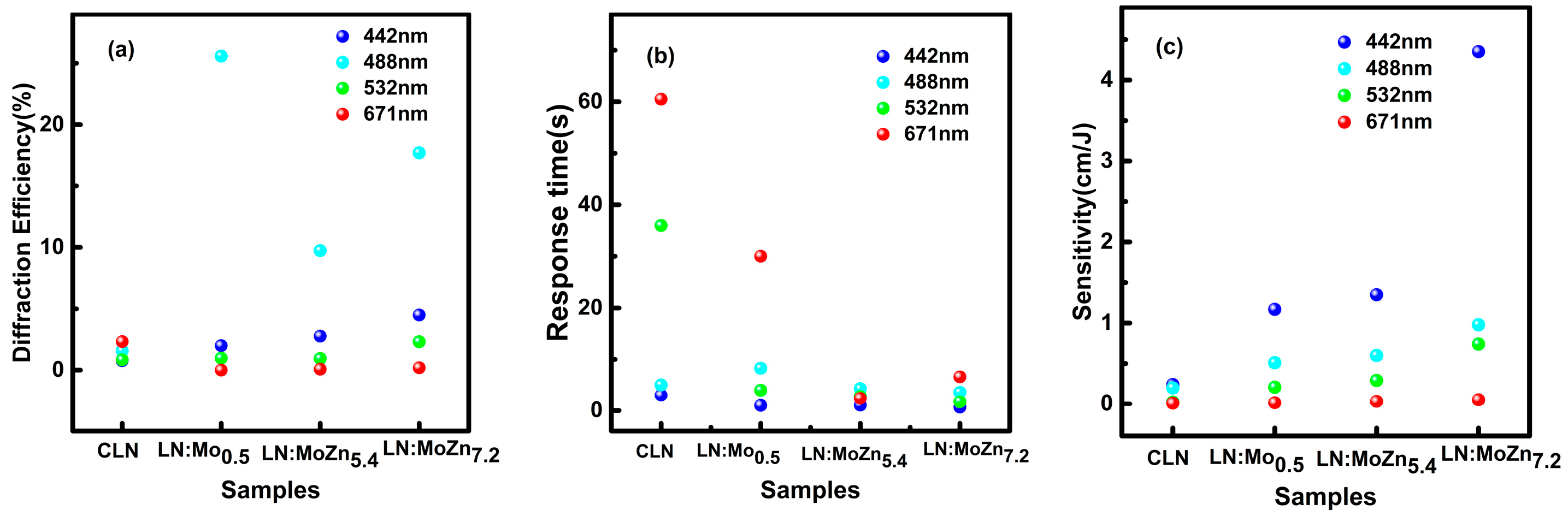
3.1. Photorefractive Properties
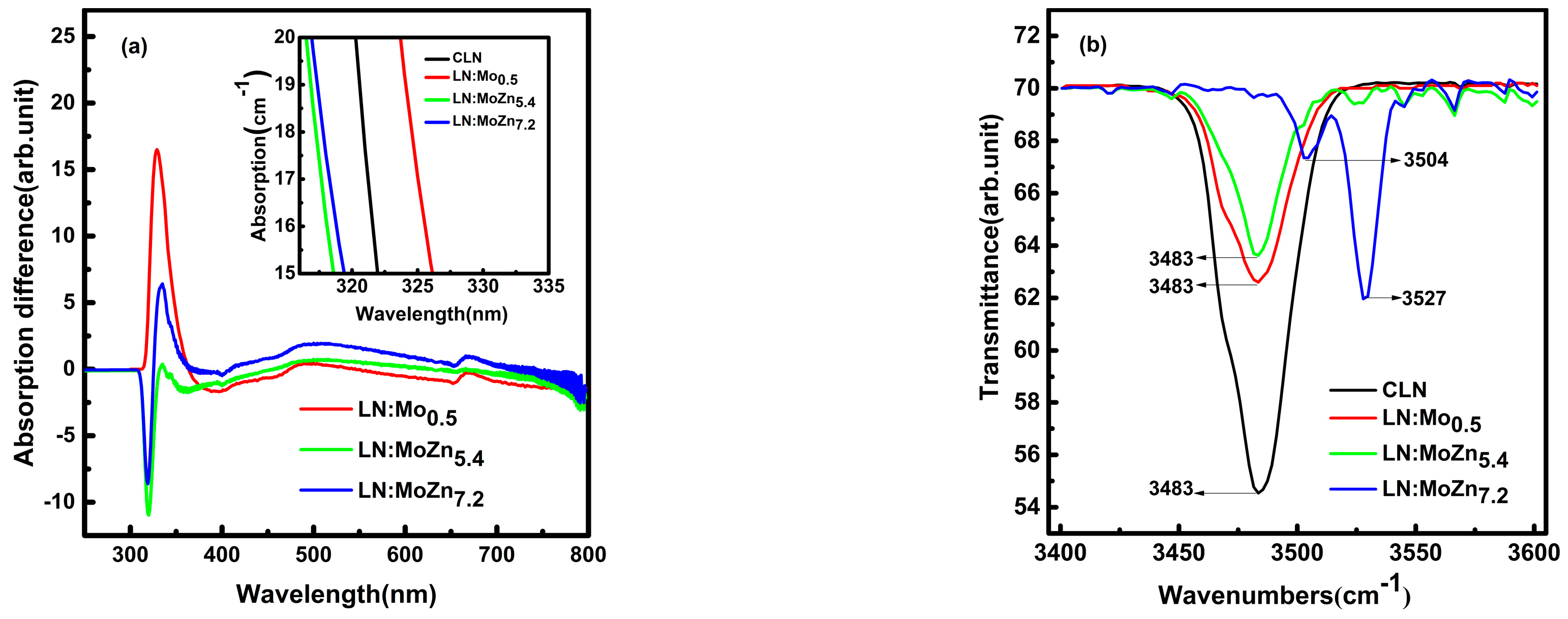
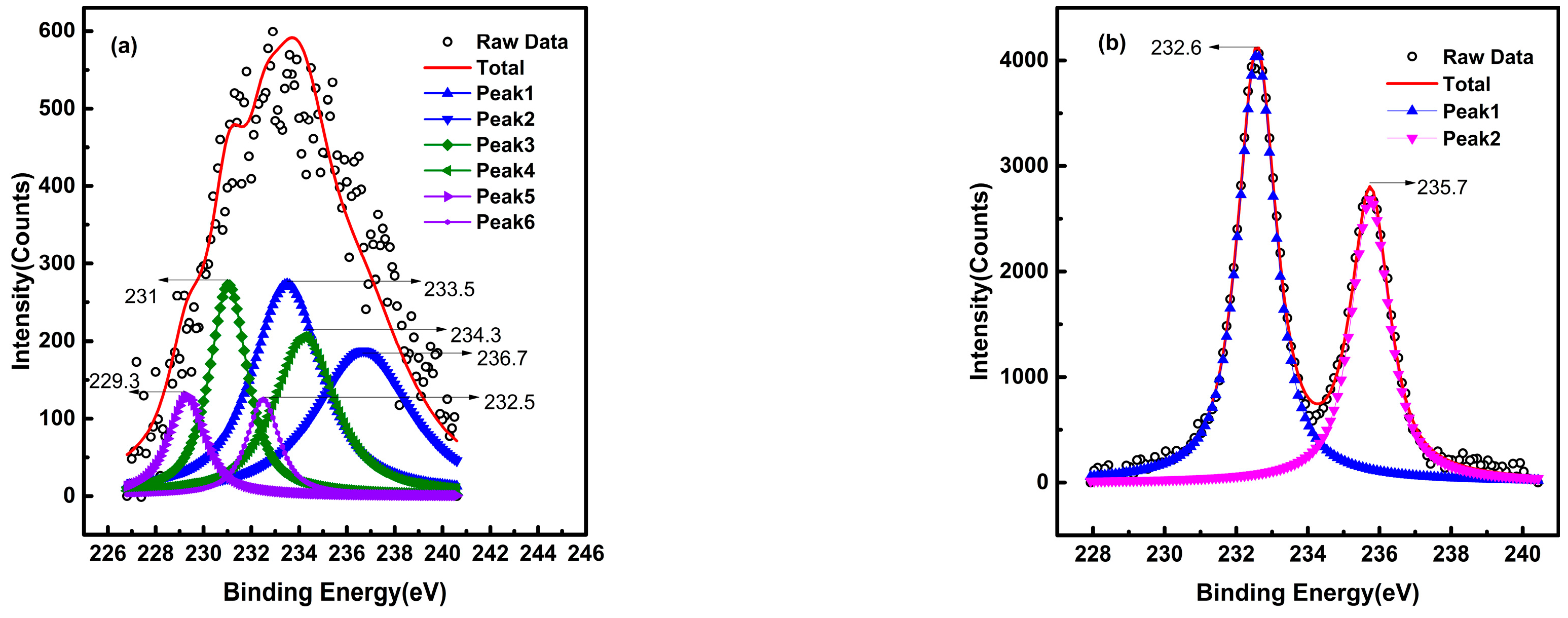
3.2. Spectral Analysis
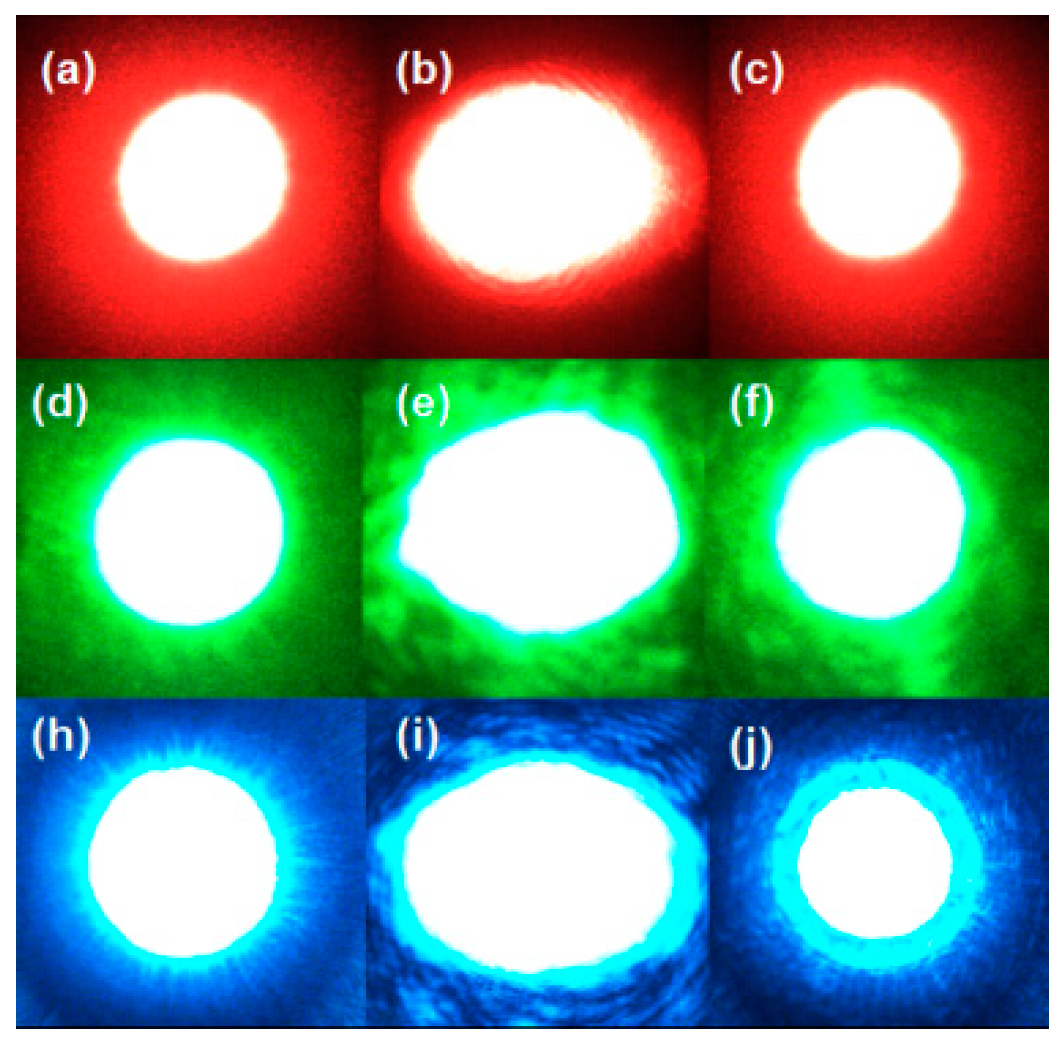
3.3. Optical Damage Resistance Ability
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, C.; Zhang, M.; Stern, B.; Lipson, M.; Lončar, M. Nanophotonic lithium niobate electro-optic modulators. Opt. Express 2018, 26, 1547–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasnokutska, I.; Tambasco, J.J.; Li, X.; Peruzzo, A. Ultra-low loss photonic circuits in lithium niobate on insulator. Opt. Express 2018, 26, 897–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Li, Z.; Kim, M.H.; Xiong, X.; Ren, X.F.; Guo, G.C.; Yu, N.; Lončar, M. Metasurface-assisted phase-matching-free second harmonic generation in lithium niobate waveguides. Nat. Commun. 2017, 8, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, L.; Orlov, S.S.; Liu, A.; Akella, A.; Lande, D.; Neurgaonkar, R.R. Photorefractive materials for nonvolatile volume holographic data storage. Science 1998, 282, 1089–1094. [Google Scholar] [CrossRef]
- Xu, M.; Kang, H.; Guan, L.; Li, H.; Zhang, M. Facile Fabrication of a Flexible LiNbO3 Piezoelectric Sensor through Hot Pressing for Biomechanical Monitoring. ACS Appl. Mater. Interfaces 2017, 9, 34687–34695. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Ndao, A.; Vila, V.C.; Salut, R.; Courjal, N.; Baida, F.I.; Bernal, M.P. Fano resonance-based highly sensitive, compact temperature sensor on thin film lithium niobate. Opt. Lett. 2016, 41, 1106–1109. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Dong, S.; Xuan, W.; Farooq, U.; Huang, S.; Li, M.; Wu, T.; Jin, H.; Wang, X.; Luo, J. Flexible surface acoustic wave strain sensor based on single crystalline LiNbO3 thin film. Appl. Phys. Lett. 2018, 112, 093502. [Google Scholar] [CrossRef]
- Bai, Y.; Kachru, R. Nonvolatile holographic storage with two-step recording in lithium niobate using cw lasers. Phys. Rev. Lett. 1997, 78, 2944. [Google Scholar] [CrossRef]
- Yoshinaga, H.; Kitayama, K.i.; Oguri, H. Holographic image storage in iron-doped lithium niobate fibers. Appl. Phys. Lett. 1990, 56, 1728–1730. [Google Scholar] [CrossRef]
- Imlau, M.; Brüning, H.; Schoke, B.; Hardt, R.; Conradi, D.; Merschjann, C. Hologram recording via spatial density modulation of NbLi4+/5+ antisites in lithium niobate. Opt. Express 2011, 19, 15322–15338. [Google Scholar] [CrossRef]
- Juodkazis, S.; Mizeikis, V.; Sūdžius, M.; Misawa, H.; Kitamura, K.; Takekawa, S.; Gamaly, E.G.; Krolikowski, W.Z.; Rode, A.V. Laser induced memory bits in photorefractive LiNbO3 and LiTaO3. Appl. Phys. A 2008, 93, 129–133. [Google Scholar] [CrossRef]
- Camarillo, E.; Murrieta, H.; Hernandez, J.M.; Zoilo, R.; Flores, M.C.; Han, T.P.J.; Jaque, F. Optical properties of LiNbO3:Cr crystals co-doped with germanium oxide. J. Lumin. 2008, 128, 747–750. [Google Scholar] [CrossRef]
- Luo, S.; Meng, Q.; Wang, J.; Sun, X. Effect of In3+ concentration on the photorefraction and scattering properties in In:Fe:CU:LiNbO3 crystals at 532nm wavelength. Opt. Commun. 2016, 358, 198–201. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, R.; Wang, B. Growth and holographic storage properties of In:Ce:Cu:LiNbO3 crystal. Mater. Chem. Phys. 2007, 102, 281–283. [Google Scholar] [CrossRef]
- Zhen, X.H.; Li, H.T.; Sun, Z.J.; Ye, S.J.; Zhao, L.C.; Xu, Y.H. Holographic properties of double-doped Zn:Fe:LiNbO3 crystals. Mater. Lett. 2004, 58, 1000–1002. [Google Scholar] [CrossRef]
- Wei, Z.; Naidong, Z.; Qingquan, L. Growth and Holographic Storage Properties of Sc, Fe Co-Doped Lithium Niobate Crystals. J. Rare Earth. 2007, 25, 775–778. [Google Scholar] [CrossRef]
- Xu, C.; Leng, X.; Xu, L.; Wen, A.; Xu, Y. Enhanced nonvolatile holographic properties in Zn, Ru and Fe co-doped LiNbO3 crystals. Opt. Commun. 2012, 285, 3868–3871. [Google Scholar] [CrossRef]
- Pálfalvi, L.; Hebling, J.; Almási, G.; Péter, Á.; Polgár, K.; Lengyel, K.; Szipöcs, R. Nonlinear refraction and absorption of Mg doped stoichiometric and congruent LiNbO3. J. Appl. Phys. 2004, 95, 902. [Google Scholar] [CrossRef]
- Tian, T.; Kong, Y.; Liu, S.; Li, W.; Wu, L.; Chen, S.; Xu, J. Photorefraction of molybdenum-doped lithium niobate crystals. Opt. Lett. 2012, 37, 2679. [Google Scholar] [CrossRef]
- Tian, T.; Kong, Y.; Liu, S.; Li, W.; Chen, S.; Rupp, R.; Xu, J. Fast UV-Vis photorefractive response of Zr and Mg co-doped LiNbO3:Mo. Opt. Express 2013, 21, 128–132. [Google Scholar] [CrossRef]
- Zhu, L.; Zheng, D.; Saeed, S.; Wang, S.; Liu, H.; Kong, Y.; Liu, S.; Chen, S.; Zhang, L.; Xu, J. Photorefractive Properties of Molybdenum and Hafnium Co-Doped LiNbO3 Crystals. Crystals 2018, 8, 322. [Google Scholar] [CrossRef]
- Zhu, L.; Zheng, D.; Liu, H.; Saeed, S.; Wang, S.; Liu, S.; Chen, S.; Kong, Y.; Xu, J. Enhanced photorefractive properties of indium co-doped LiNbO3:Mo crystals. AIP Adv. 2018, 8, 095316. [Google Scholar] [CrossRef]
- Dischler, B.; Herrington, J.R.; Räuber, A.; Kurz, H. Correlation of the photorefractive sensitivity in doped LiNbO3 with chemically induced changes in the optical absorption spectra. Solid State Commun. 1974, 14, 1233–1236. [Google Scholar] [CrossRef]
- Li, X.; Kong, Y.; Liu, H.; Sun, L.; Xu, J.; Chen, S.; Zhang, L.; Huang, Z.; Liu, S.; Zhang, G. Origin of the generally defined absorption edge of non-stoichiometric lithium niobate crystals. Solid State Commun. 2007, 141, 113–116. [Google Scholar] [CrossRef]
- Grim, S.O.; Matienzo, L.J. X-Ray Photoelectron Spectroscopy of Inorganic and Organometallic Compounds of Molybdenum. Inorg. Chem. 1975, 14, 1014–1018. [Google Scholar] [CrossRef]
- Kong, Y.; Xu, J.; Zhang, W.; Zhang, G. The site occupation of protons in lithium niobate crystals. J. Phys. Chem. Solids 2000, 61, 1331. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, L.; Liu, H.; Zheng, D.; Saeed, S.; Wang, X.; Tian, T.; Zhu, L.; Kong, Y.; Liu, S.; Chen, S.; et al. The Photorefractive Response of Zn and Mo Codoped LiNbO3 in the Visible Region. Crystals 2019, 9, 228. https://doi.org/10.3390/cryst9050228
Xue L, Liu H, Zheng D, Saeed S, Wang X, Tian T, Zhu L, Kong Y, Liu S, Chen S, et al. The Photorefractive Response of Zn and Mo Codoped LiNbO3 in the Visible Region. Crystals. 2019; 9(5):228. https://doi.org/10.3390/cryst9050228
Chicago/Turabian StyleXue, Liyun, Hongde Liu, Dahuai Zheng, Shahzad Saeed, Xuying Wang, Tian Tian, Ling Zhu, Yongfa Kong, Shiguo Liu, Shaolin Chen, and et al. 2019. "The Photorefractive Response of Zn and Mo Codoped LiNbO3 in the Visible Region" Crystals 9, no. 5: 228. https://doi.org/10.3390/cryst9050228
APA StyleXue, L., Liu, H., Zheng, D., Saeed, S., Wang, X., Tian, T., Zhu, L., Kong, Y., Liu, S., Chen, S., Zhang, L., & Xu, J. (2019). The Photorefractive Response of Zn and Mo Codoped LiNbO3 in the Visible Region. Crystals, 9(5), 228. https://doi.org/10.3390/cryst9050228





