Morphological Monte Carlo Simulation for Crystallization of Isotactic Polypropylene in a Temperature Gradient
Abstract
:1. Introduction
2. Mathematical Model and Numerical Method
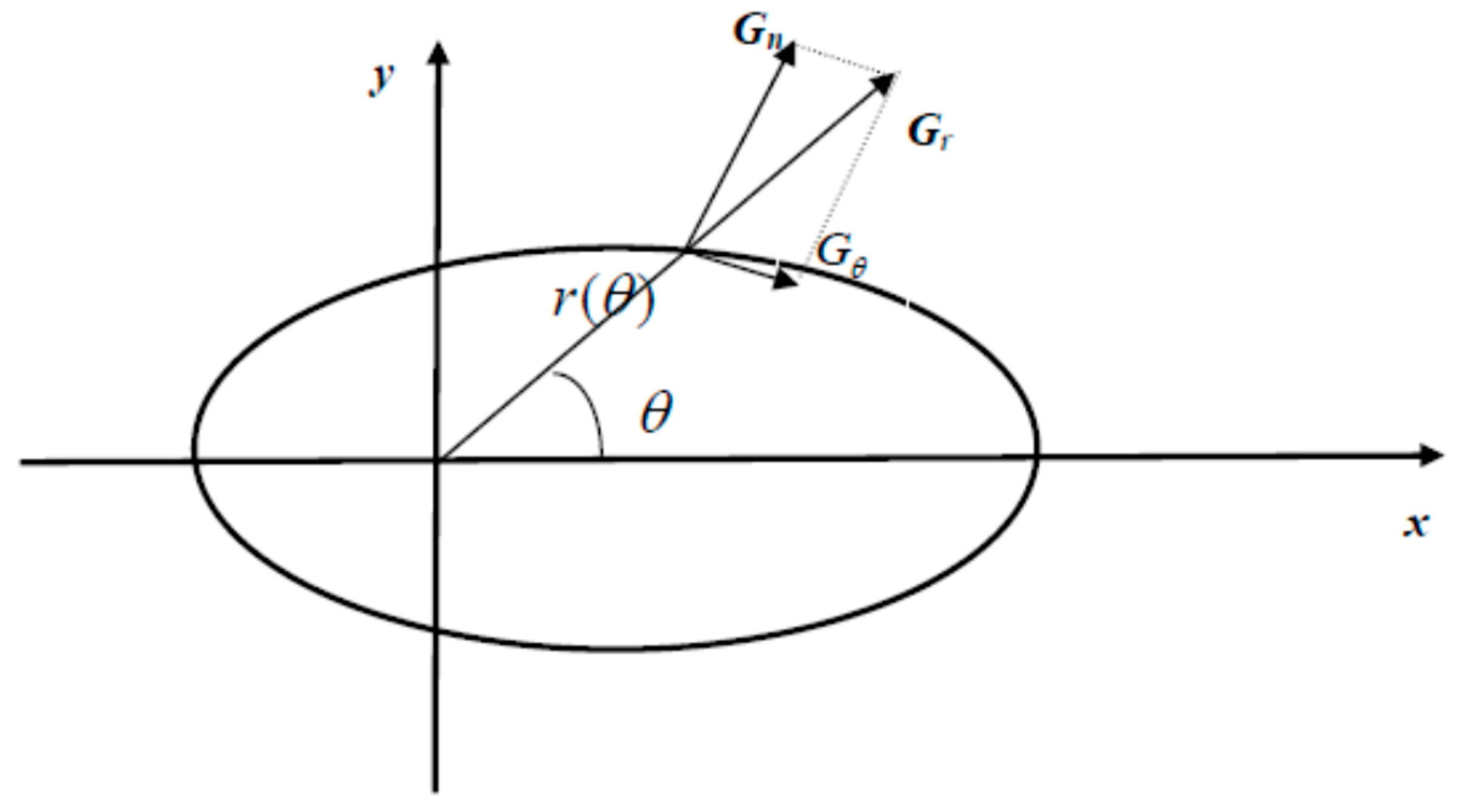
2.1. Mathematical Model
2.2. Numerical Method
3. Results and Discussion
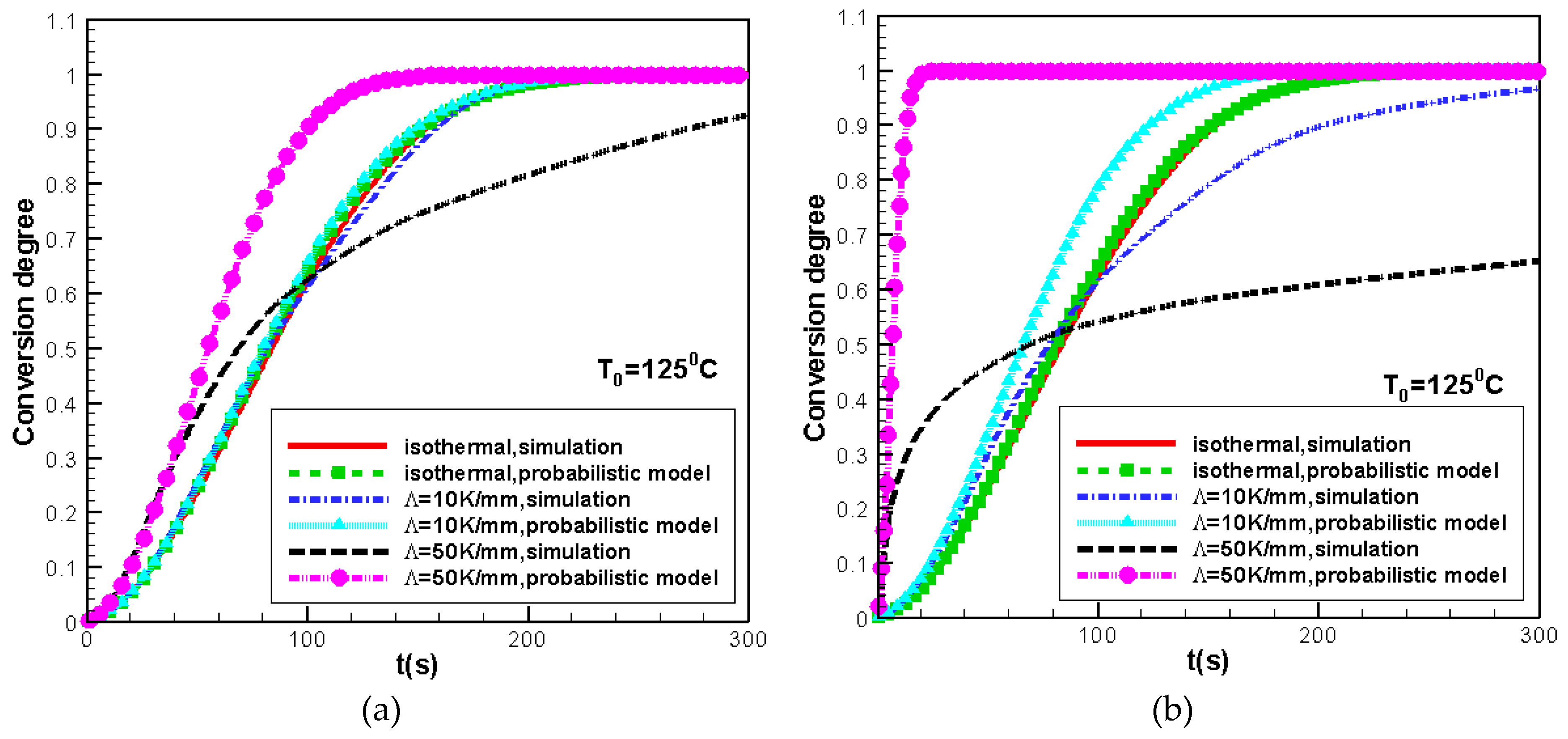
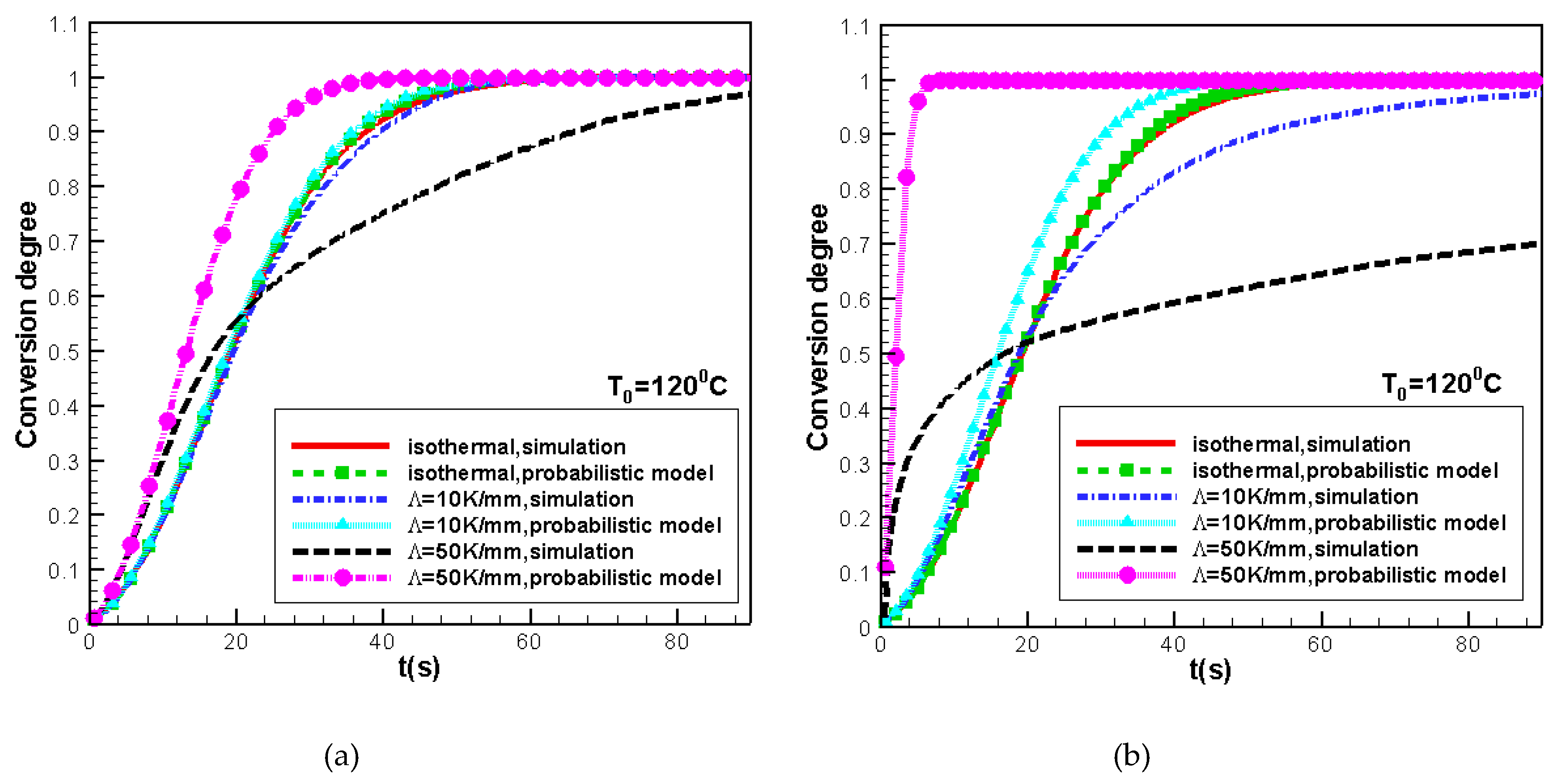
3.1. Validity of the Morphological Monte CarloMethod
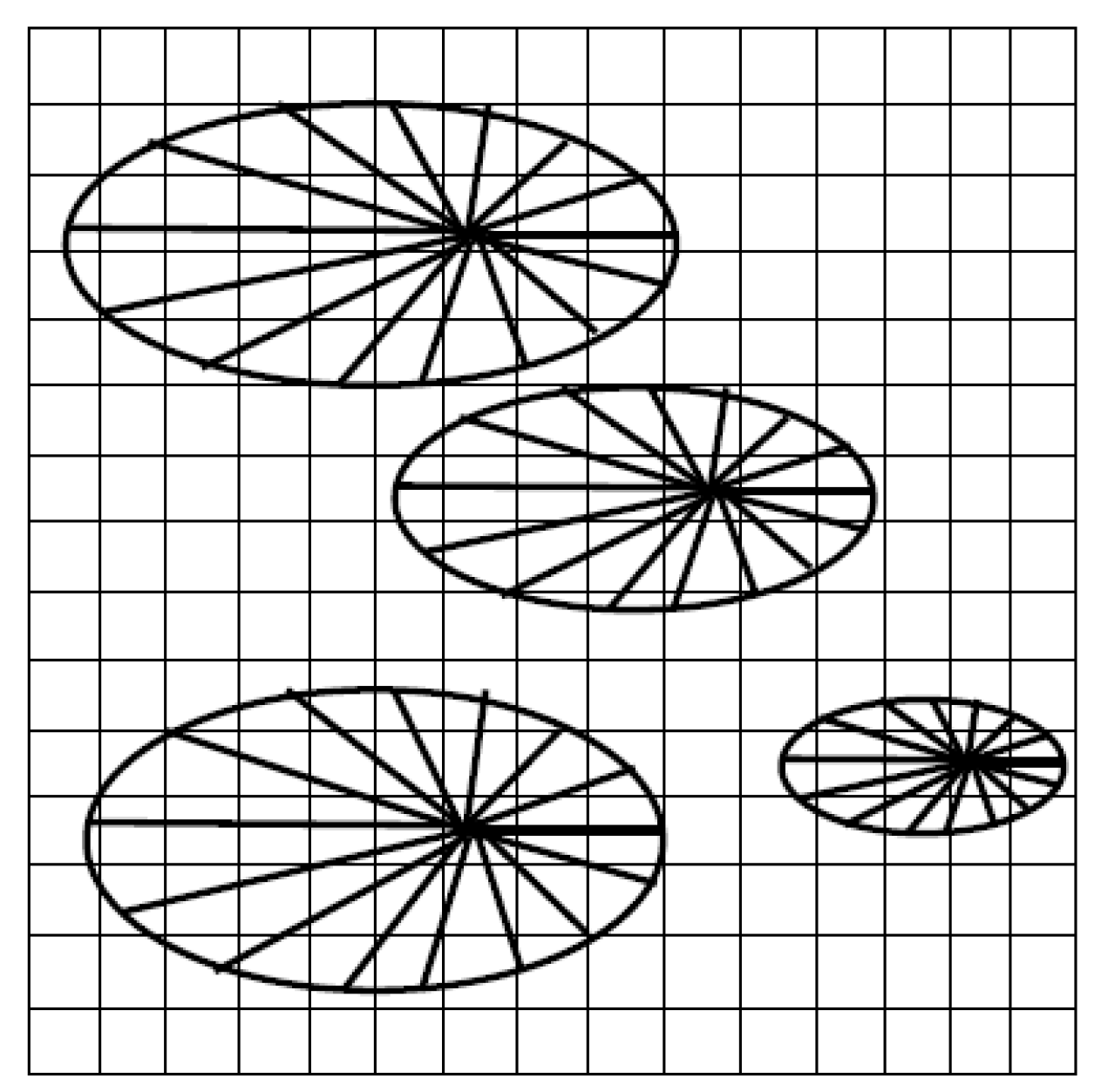
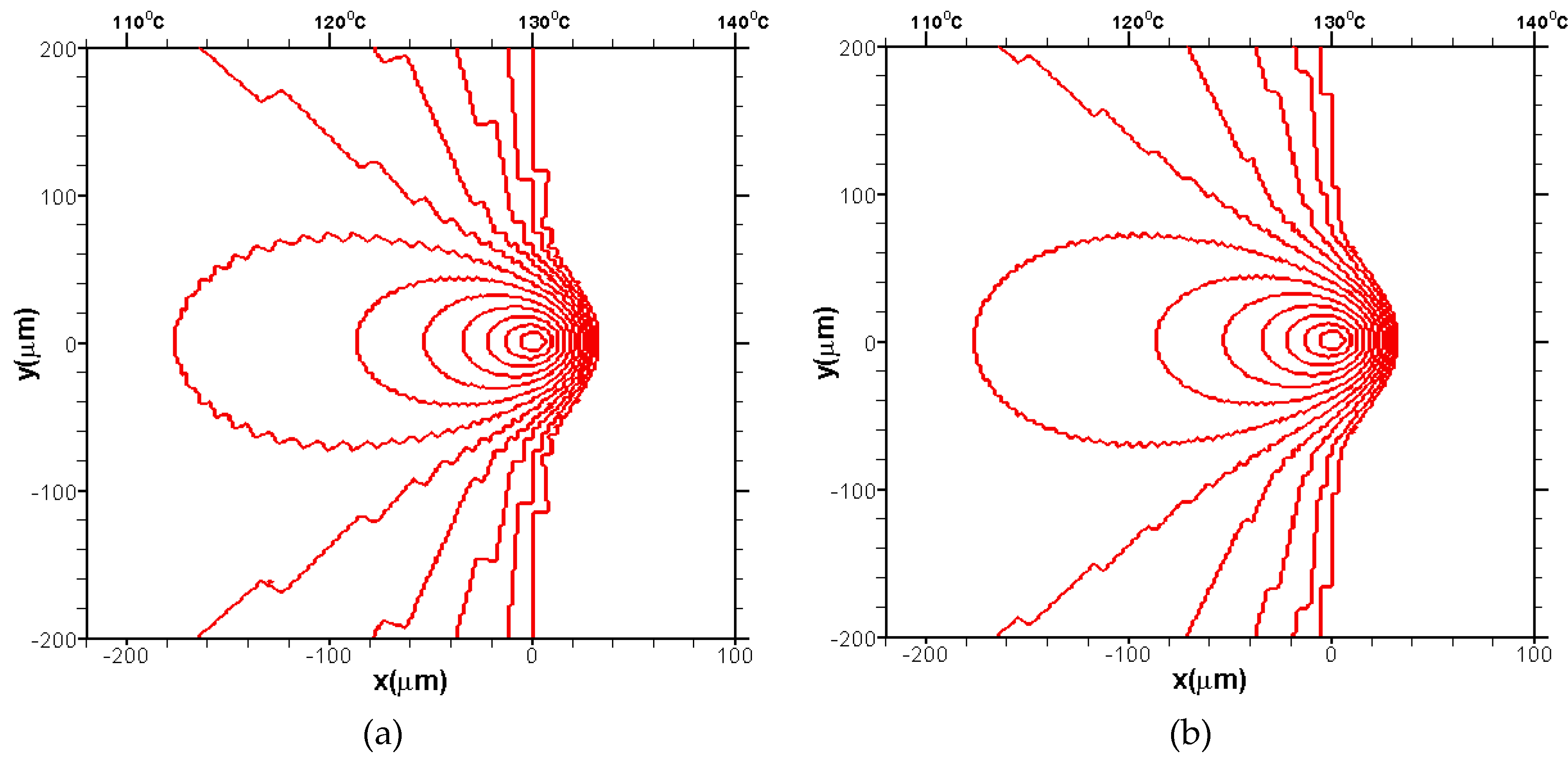
3.1.1. The Growth of a Single Spherulite
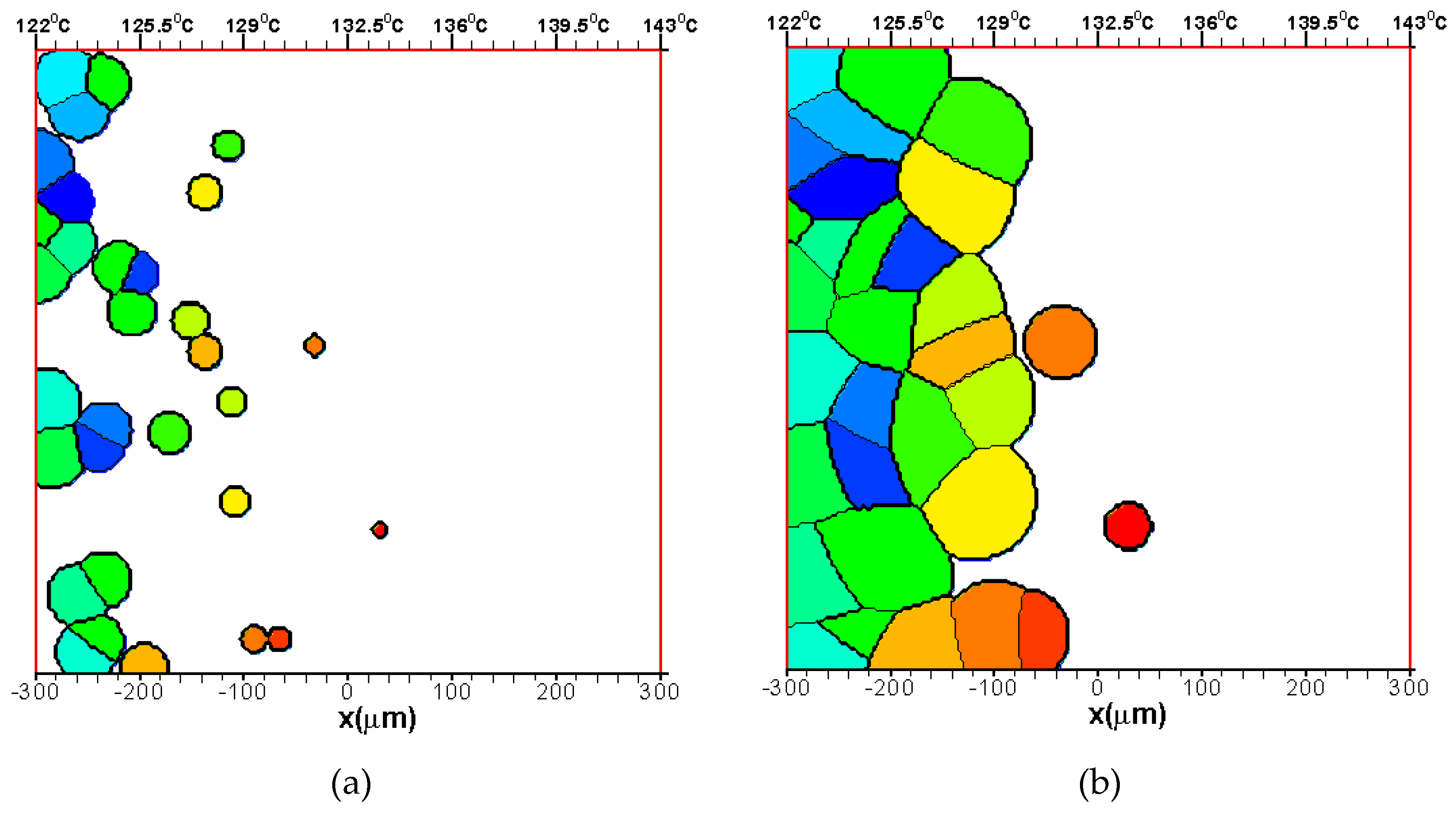
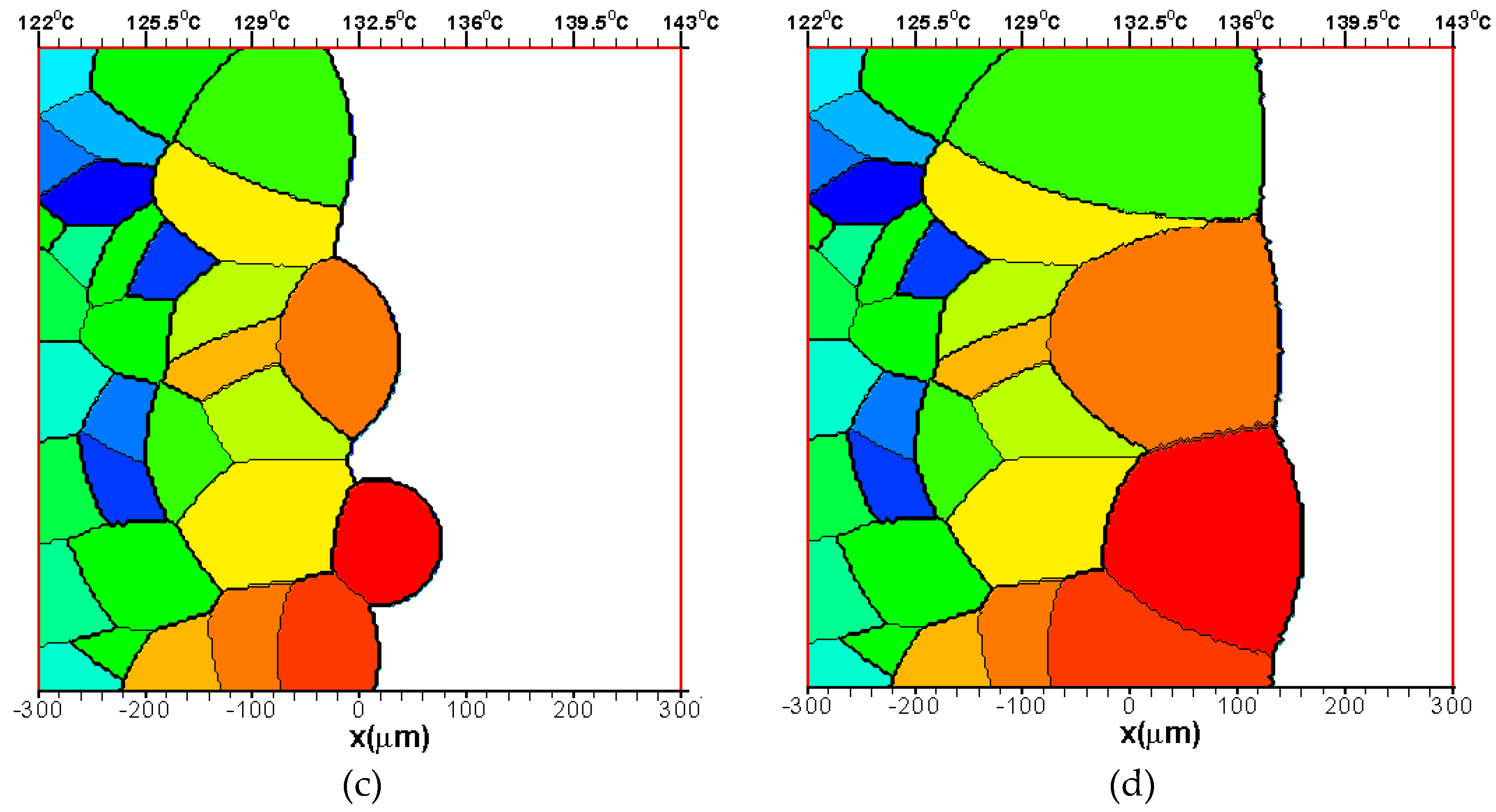
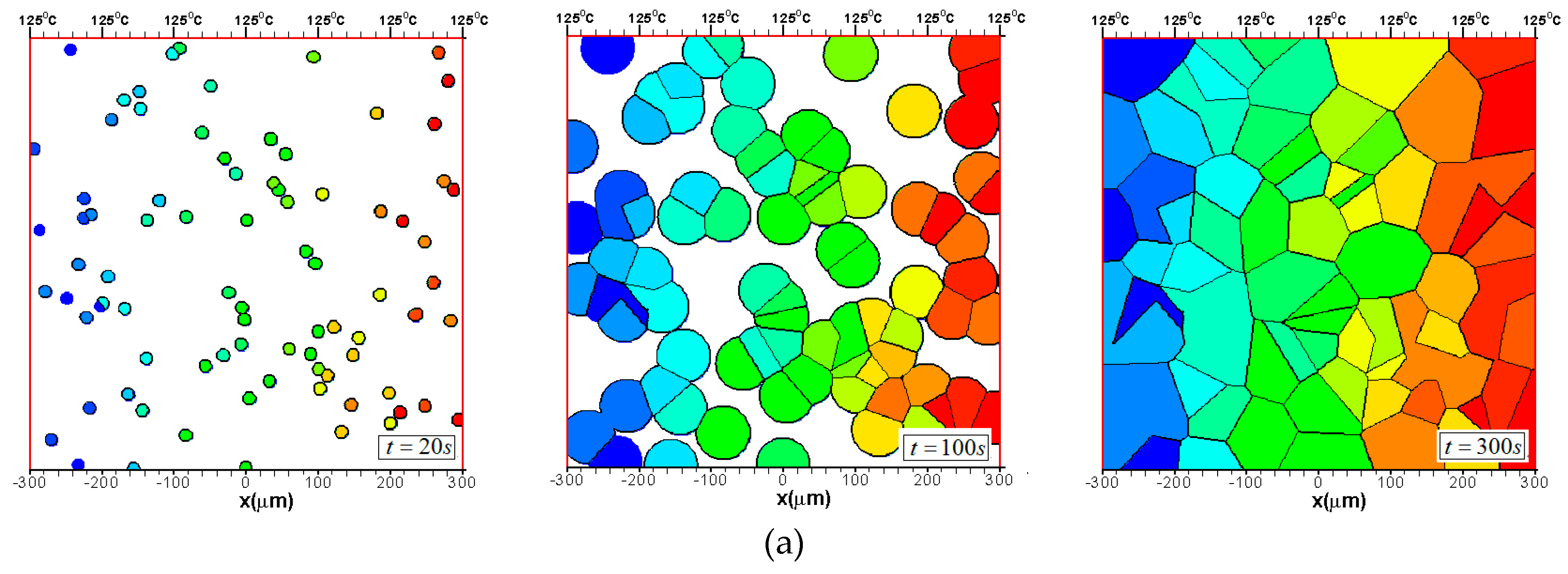
3.1.2. The Growth of Several Spherulites
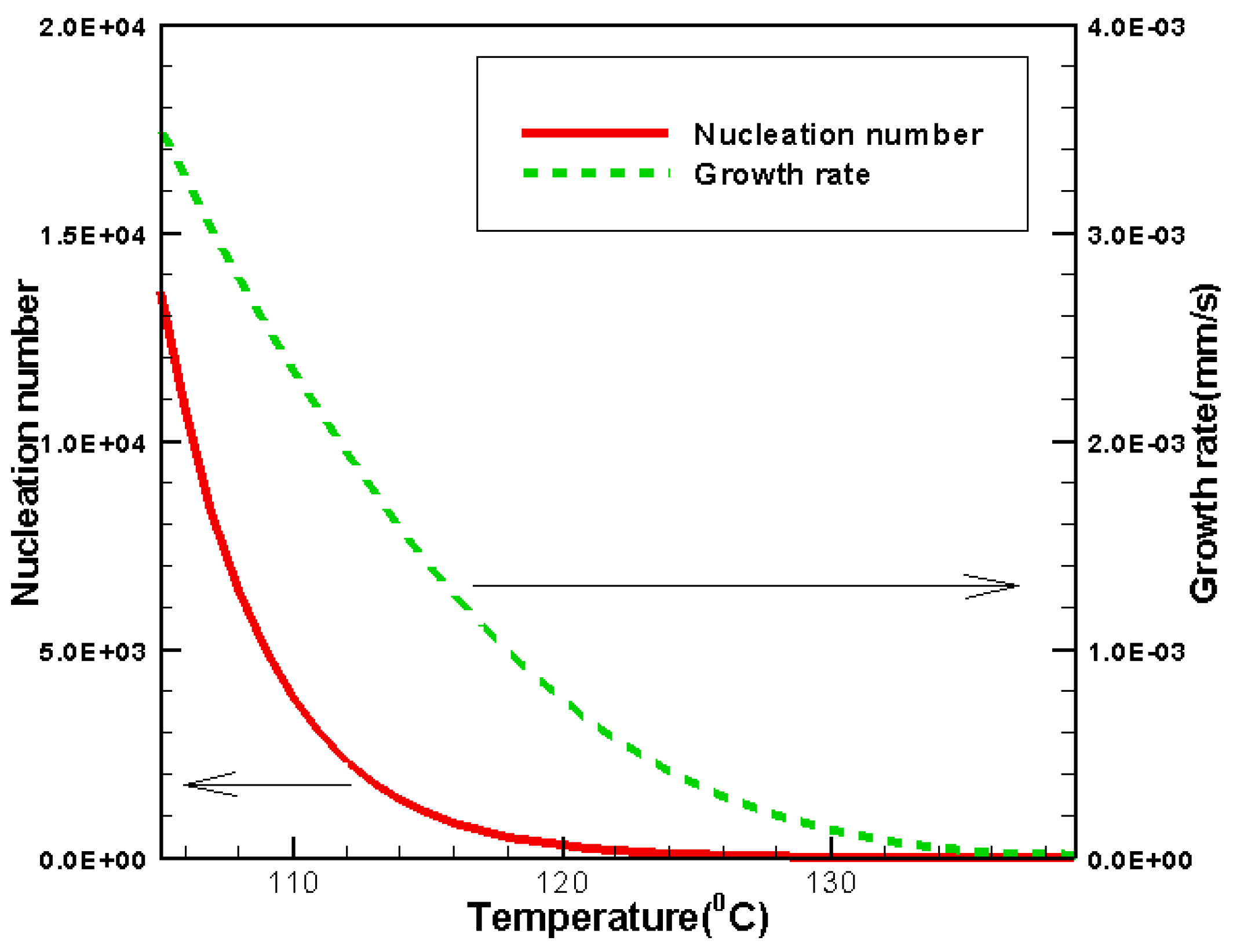
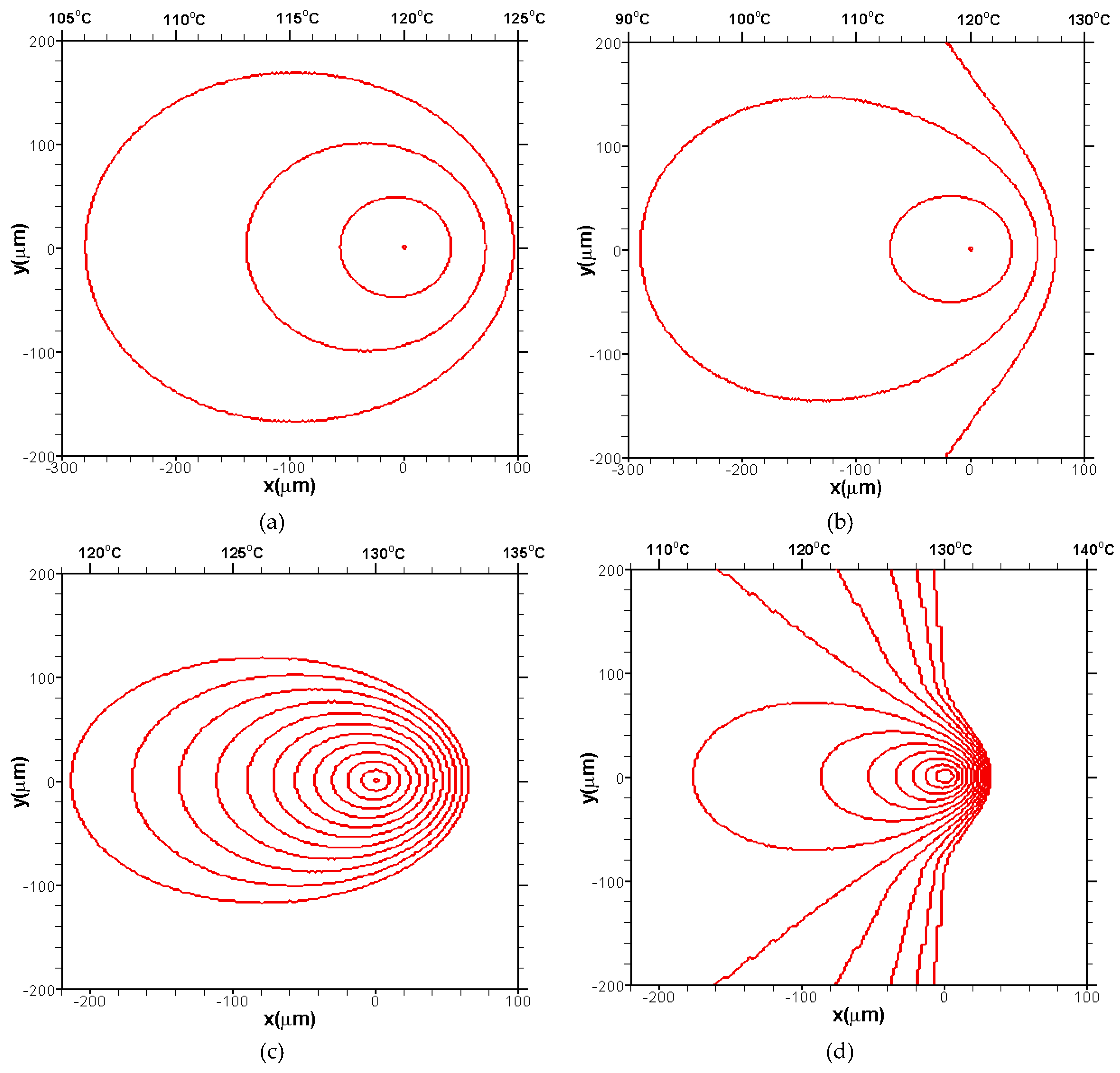
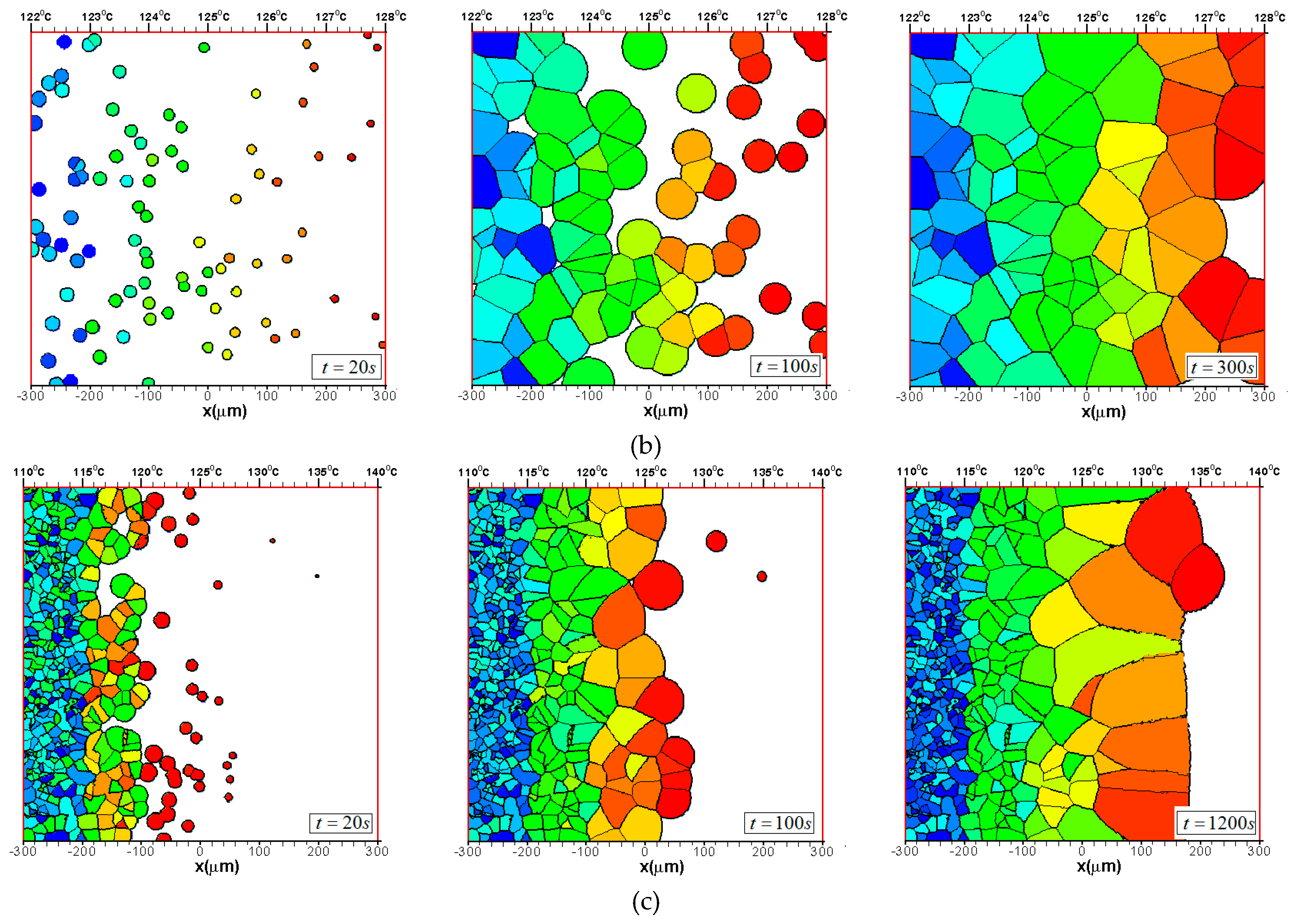
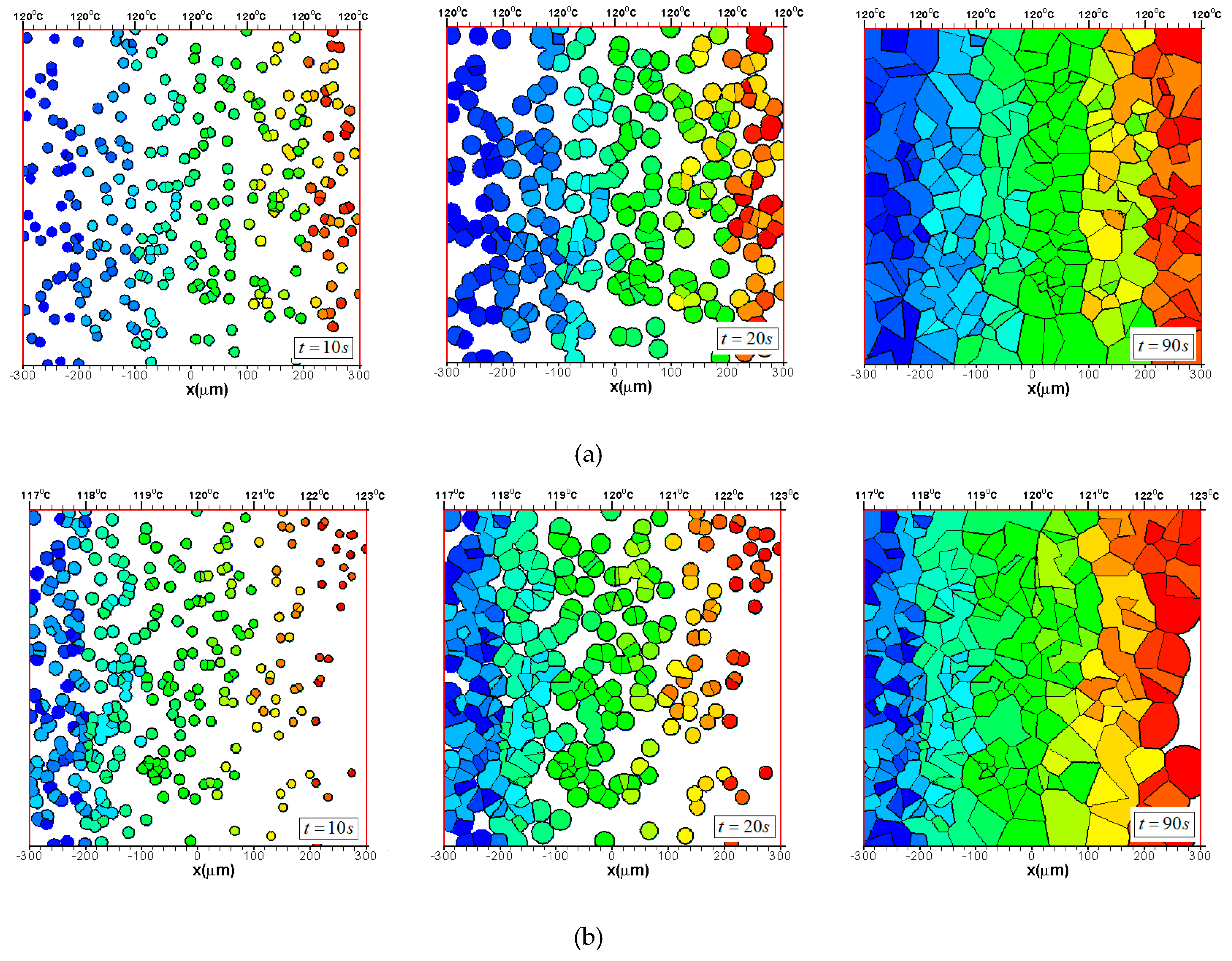
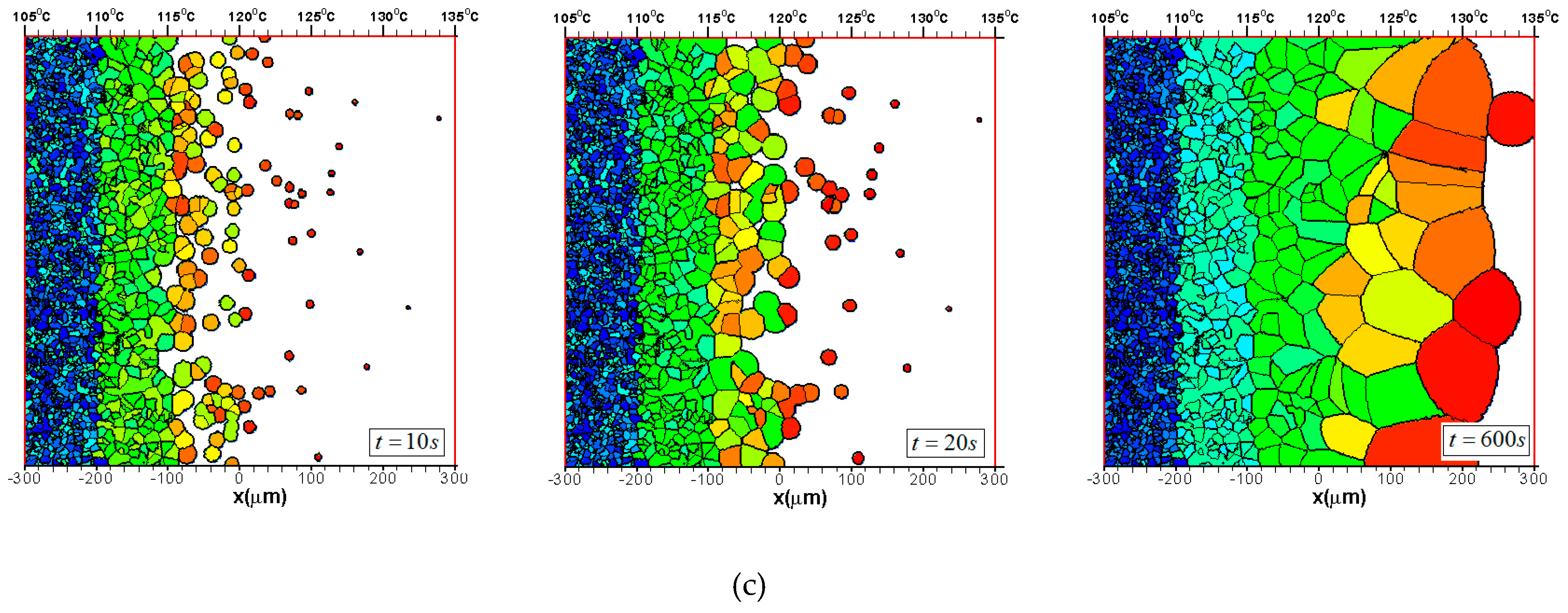
3.2. Effects of CentralTtemperature and Temperature Gradient
3.3. Difference Between IsothermalCcrystallization and Crystallization in a Temperature Gradient
4. Conclusions
Funding
Conflicts of Interest
References
- Kennedy, P.; Zheng, R. Flow Analysis of Injection Molds; Munich Carl Hanser Verlag: Munich, Germany, 2013. [Google Scholar]
- Pantanin, R.; Coccorullo, I.; Speranza, V.; Titomanlio, G. Modeling of morphology evolution in the injection molding process of thermoplastic polymers. Prog. Polym. Sci. 2005, 30, 1185–1222. [Google Scholar] [CrossRef]
- Piorkowska, E.; Galeski, A. Handbook of Polymer Crystallization; Wiley: New Jersey, NJ, USA, 2013. [Google Scholar]
- Raabe, D. Mesoscale simulation of spherulite growth during polymer crystallization by use of a cellular automaton. Acta Mater. 2004, 52, 2653–2664. [Google Scholar] [CrossRef]
- Raabe, D.; Godara, A. Mesoscale simulation of the kinetics and topology of spherulite growth during crystallization of isotactic polypropylene (iPP) by using a cellular automaton. Modell. Simul. Mater. Sci. Eng. 2005, 13, 733–751. [Google Scholar] [CrossRef]
- Capasso, V. Mathematical Modeling for Polymer Processing; Springer: Berlin, Germany, 2002. [Google Scholar]
- Micheletti, A.; Burger, M. Stochastic and deterministic simulation of nonisothermal crystallization of polymers. J. Math. Chem. 2001, 30, 169–193. [Google Scholar] [CrossRef]
- Ruan, C.; Ouyang, J.; Liu, S. Multi-scale modeling and simulation of crystallization during cooling in short fiber reinforced composites. Int. J. Heat Mass Transfer. 2012, 55, 1911–1921. [Google Scholar] [CrossRef]
- Ruan, C. Kinetics and morphology of flow induced polymer crystallization in 3D shear flow investigated by Monte Carlo simulation. Crystals 2017, 7, 51. [Google Scholar] [CrossRef]
- Ruan, C.; Liang, K.; Liu, E. Macro-micro simulation for polymer crystallization in Couette flow. Polymers 2017, 9, 699. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Liu, C.; Zheng, G. Monte carlo simulation for the morphology and kinetics of spherulites and shish-kebabs in isothermal polymer crystallization. Math. Prob. Eng. 2015, 50624, 1–10. [Google Scholar] [CrossRef]
- Liu, Z.; Ouyang, J.; Ruan, C.; Liu, Q. Simulation of polymer crystallization under isothermal and temperature gradient conditions using praticle level set method. Crystals 2016, 6, 90. [Google Scholar] [CrossRef]
- Liu, Z.; Ouyang, J.; Zhou, W.; Wang, X.D. Numerical simulation of the polymer crystallization during cooling stage by using level set method. Comput. Mater. Sci 2015, 97, 245–253. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, J.; Zhou, W.; Liu, Z. A phase field technique for modeling and predicting flow induced crystallization morphology of semi-crystalline polymers. Polymers 2016, 8, 230. [Google Scholar] [CrossRef]
- Charbon, C.; Swaminarayar, S. A multiscale model for polymer crystallization. II: Solidification of a macroscopic part. Polym. Eng. Sci. 1998, 38, 644–656. [Google Scholar] [CrossRef]
- Swaminarayan, S.; Charbon, C. A multiscale model for polymer crystallization. I. growth of individual spherulites. Polym. Eng. Sci. 1998, 38, 634–643. [Google Scholar] [CrossRef]
- Thananchai, L. Mathematical modeling of solidification of semi-crystalline polymers under quiescent non-isothermal crystallization: Determination of crystallite’s size. Sci. Asia. 2001, 27, 127–132. [Google Scholar]
- Piorkowska, E. Modeling of polymer crystallization in a temperature gradient. J. Appl. Polym. Sci 2002, 86, 1351–1362. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Miller, R.L. Kinetics of crystallization from the melt and chain folding in polyethylene fractions revisited: theory and experiment. Polymer 1997, 38, 3151–3212. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. I. General theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar]
- Mark, H. Introduction to Numerical Methods in Differential Equations; Springer: Berlin, Germany, 2011. [Google Scholar]
- Pawlak, A.; Piorkowska, E. Crystallization of isotactic polypropylene in a temperature gradient. Colloid. Polym. Sci. 2001, 279, 939–946. [Google Scholar] [CrossRef]
- Ziabicki, A. Crystallization of polymers in variable external conditions. Colloid. Polym. Sci. 1996, 274, 705–716. [Google Scholar] [CrossRef]
| 125 °C | 2.1% | 15 K/mm | 5.1% | 27 K/mm |
| 120 °C | 1.6% | 18 K/mm | 5.0% | 30 K/mm |
| 115 °C | 1.5% | 30 K/mm | 5.0% | 45 K/mm |
| 110 °C | 1.5% | 31 K/mm | 5.0% | 48 K/mm |
| 105 °C | 2.2% | 40 K/mm | 3.8% | 50 K/mm |
| 125 °C | 15 K/mm | 8.8% | 27 K/mm | 14.2% |
| 120 °C | 18 K/mm | 3.5% | 30 K/mm | 6.6% |
| 115 °C | 30 K/mm | 3.0% | 45 K/mm | 4.2% |
| 110 °C | 31 K/mm | 2.4% | 48 K/mm | 3.0% |
| 105 °C | 40 K/mm | 2.0% | 50 K/mm | 2.5% |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, C. Morphological Monte Carlo Simulation for Crystallization of Isotactic Polypropylene in a Temperature Gradient. Crystals 2019, 9, 213. https://doi.org/10.3390/cryst9040213
Ruan C. Morphological Monte Carlo Simulation for Crystallization of Isotactic Polypropylene in a Temperature Gradient. Crystals. 2019; 9(4):213. https://doi.org/10.3390/cryst9040213
Chicago/Turabian StyleRuan, Chunlei. 2019. "Morphological Monte Carlo Simulation for Crystallization of Isotactic Polypropylene in a Temperature Gradient" Crystals 9, no. 4: 213. https://doi.org/10.3390/cryst9040213
APA StyleRuan, C. (2019). Morphological Monte Carlo Simulation for Crystallization of Isotactic Polypropylene in a Temperature Gradient. Crystals, 9(4), 213. https://doi.org/10.3390/cryst9040213




