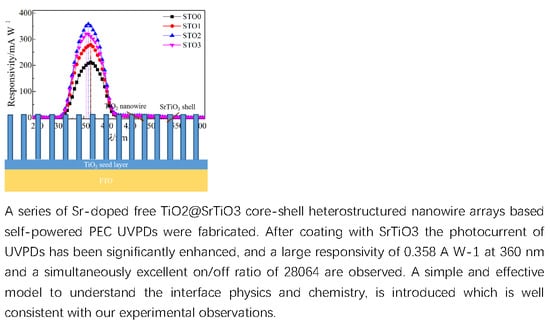
Optimal Sr-Doped Free TiO2@SrTiO3 Heterostructured Nanowire Arrays for High-Efficiency Self-Powered Photoelectrochemical UV Photodetector Applications
Abstract
1. Introduction
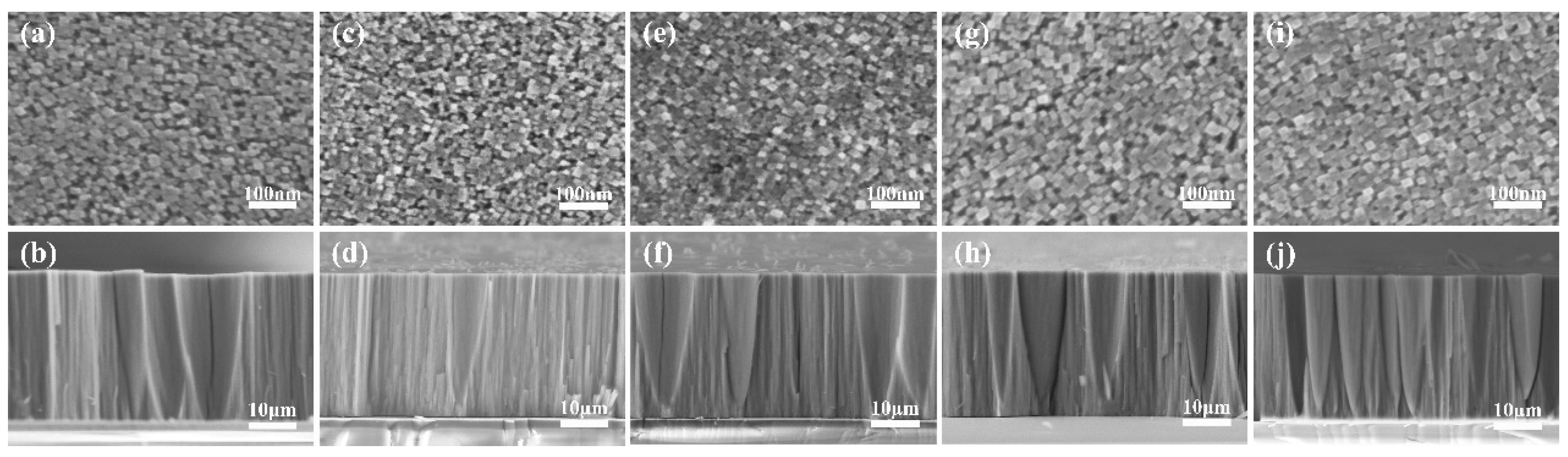
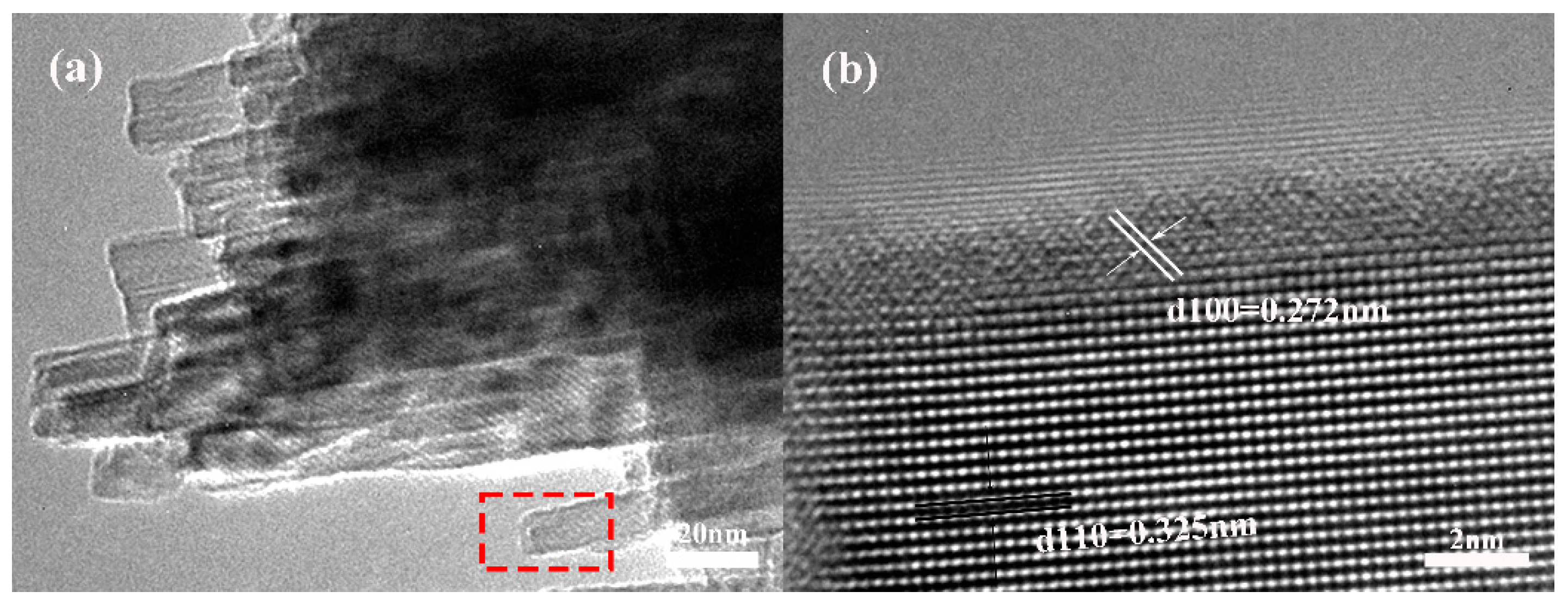
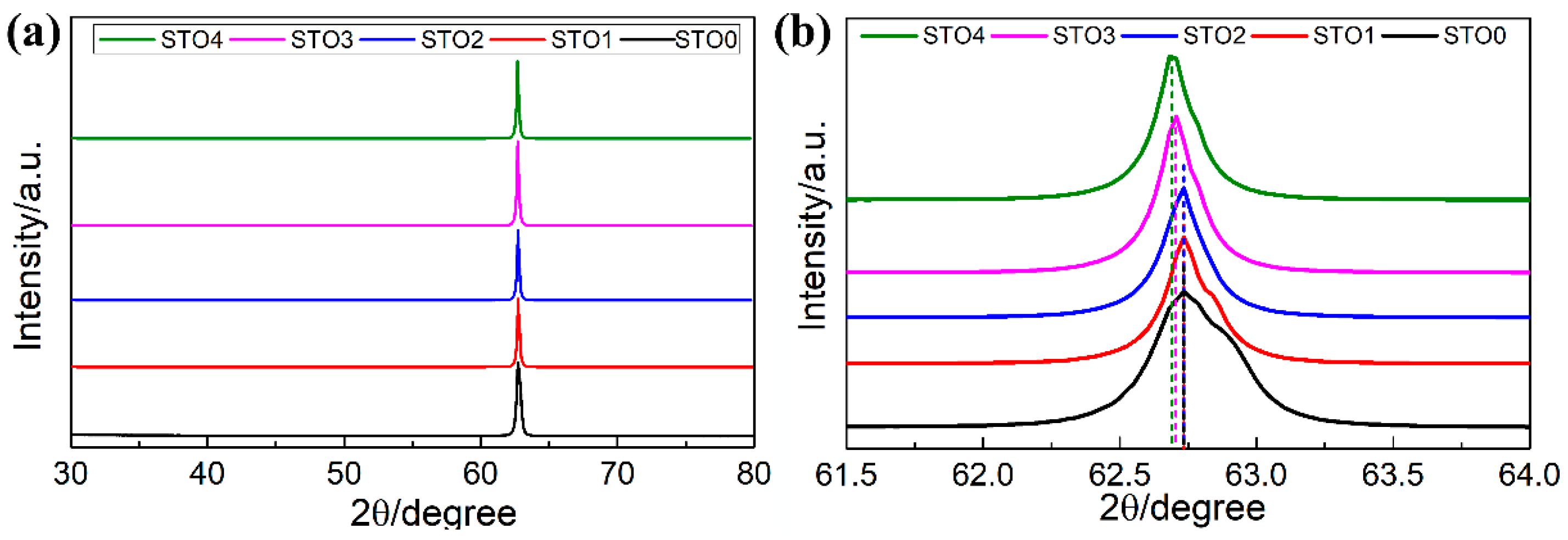
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, Y.; Yu, Q.; Wang, J.; Wang, J.; Yu, C.; Abdalla, J.T.; Zeng, Z.; Jiao, S.; Wang, D.; Gao, S. Plasmon-Enhanced Self-Powered UV Photodetectors Assembled by Incorporating Ag@SiO2 Core−Shell Nanoparticles into TiO2 Nanocube Photoanodes. ACS Sustain. Chem. Eng. 2017, 6, 438–446. [Google Scholar] [CrossRef]
- Zhai, T.; Li, L.; Wang, X.; Fang, X.; Bando, Y.; Golberg, D. Recent Developments in One-Dimensional Inorganic Nanostructures for Photodetectors. Adv. Funct. Mater. 2010, 20, 4233–4248. [Google Scholar] [CrossRef]
- Prades, J.D.; Hernandez-Ramirez, F.; Jimenez-Diaz, R.; Manzanares, M.; Andreu, T.; Cirera, A.; Romano-Rodriguez, A.; Morante, J.R. The Effects of Electron-hole Separation on the Photoconductivity of Individual Metal Oxide Nanowires. Nanotechnology 2008, 19, 465501. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gu, L.; Lou, Z.; Fan, Z.; Shen, G. ZnO Quantum Dots Decorated Zn2SnO4 Nanowire Heterojunction Photodetectors with Drastic Performance Enhancement and Flexible Ultraviolet Image Sensors. ACS Nano 2017, 11, 4067–4076. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Gong, M.; Cook, B.; Ewing, D.; Casper, M.; Stramel, A.; Wu, J. Fused Nanojunctions of Electron-Depleted ZnO Nanoparticles for Extraordinary Performance in Ultraviolet Detection. Adv. Mater. Interfaces 2017, 4, 1601064. [Google Scholar] [CrossRef]
- Yu, J.; Tian, N. High Spectrum Selectivity and Enhanced Responsivity of a ZnO Ultraviolet Photodetector Realized by the Addition of ZnO Nanoparticles Layer. Phys. Chem. Chem. Phys. 2016, 18, 24129–24133. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, M. Sustainable energy development: Performance and prospects. Renew. Energy 2006, 31, 571–582. [Google Scholar] [CrossRef]
- Bie, Y.; Liao, Z.; Zhang, H.; Li, G.; Ye, Y.; Zhou, Y.; Xu, J.; Qin, Z.; Dai, L.; Yu, D. Self-Powered, Ultrafast, Visible-Blind UV Detection and Optical Logical Operation based on ZnO/GaN Nanoscale p-n Junctions. Adv. Mater. 2011, 23, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Lin, W.; Hsu, Y.; Wei, K. Conjugated Polymer/Nanocrystal Nanocomposites for Renewable Energy Applications in Photovoltaics and Photocatalysis. Small 2014, 10, 4427–4442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yuan, C.; Zhu, J.; Wang, J.; Zhang, X.; Lou, X. Flexible Films Derived from Electrospun Carbon Nanofibers Incorporated with Co3O4 Hollow Nanoparticles as Self-Supported Electrodes for Electrochemical Capacitors. Adv. Funct. Mater. 2013, 23, 3909–3915. [Google Scholar] [CrossRef]
- Fang, X.; Zhai, T.; Gautam, U.; Li, L.; Wu, L.; Bando, Y.; Golberg, D. ZnS Nanostructures: From Synthesis to Applications. Prog. Mater. Sci. 2011, 56, 175–287. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Xu, C.; Zhu, G.; Wang, Z. High-output Nanogenerator by Rational Unipolar Assembly of Conical Nanowires and Its Application for Driving a Small Liquid Crystal Display. Nano Lett. 2012, 12, 5025–5031. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Fan, F.; Xu, C.; Lin, S.; Wei, M.; Duan, X.; Wang, Z. ZnO Nanotube-based Dye-sensitized Solar Cell and Its Application in Self-powered Devices. Nanotechnology 2010, 21, 405203. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xu, C.; Zhang, Y.; Lin, L.; Snyder, R.; Wang, Z. A Nanogenerator for Energy Harvesting from a Rotating Tire and Its Application as a Self-powered Pressure/Speed Sensor. Adv. Mater. 2011, 23, 4068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yeung, K.; Robbins, J.; Pavlick, R.; Wu, M.; Liu, R.; Sen, A.; Phillips, S. Self-Powered Microscale Pumps Based on Analyte-Initiated Depolymerization Reactions. Angew. Chem. Int. Ed. 2012, 51, 2400–2454. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Jiang, P.; Wang, Z. Nanogenerator as Self-powered Vibration Sensor. Nano Energy 2012, 1, 418–423. [Google Scholar] [CrossRef]
- Li, X.; Gao, C.; Duan, H.; Lu, B.; Pan, X.; Xie, E. Nanocrystalline TiO2 Film Based Photoelectrochemical Cell as Self-powered UV-photodetector. Nano Energy 2012, 1, 640–645. [Google Scholar] [CrossRef]
- Li, X.; Gao, C.; Duan, H.; Lu, B.; Wang, Y.; Chen, L.; Zhang, Z.; Pan, X.; Xie, E. High-performance Photoelectrochemical-type Self-powered UV Photodetector Using Epitaxial TiO2/SnO2 Branched Heterojunction Nanostructure. Small 2013, 9, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Li, X.; Wang, Y.; Chen, L.; Pan, X.; Zhang, Z.; Xie, E. Titanium Dioxide Coated Zinc Oxide Nanostrawberry Aggregates for Dye-sensitized Solar Cell and Self-powered UV-photodetector. J. Power Sources 2013, 239, 458–465. [Google Scholar] [CrossRef]
- Li, Q.; Wei, L.; Xie, Y.; Zhang, K.; Liu, L.; Zhu, D.; Jiao, J.; Chen, Y.; Yan, S.; Liu, G.; et al. ZnO Nanoneedle/H2O Solid-liquid Heterojunction-based Self-powered Ultraviolet Detector. Nanoscale Res. Lett. 2013, 8, 415. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, X.; Wang, Y.; Gao, C.; Zhang, H.; Zhao, B.; Teng, F.; Zhou, J.; Zhang, Z.; Pan, X.; et al. Low-temperature Synthesis of Tin Dioxide Hollow Nanospheres and Their Potential Applications in Dye-sensitized Solar Cells and Photoelectrochemical Type Self-powered Ultraviolet Photodetectors. J. Power Sources 2014, 272, 886–894. [Google Scholar] [CrossRef]
- Xie, Y.; Wei, L.; Li, Q.; Chen, Y.; Liu, H.; Yan, S.; Jiao, J.; Liu, G.; Mei, L. A High Performance Quasi-solid-state Self-powered UV Photodetector Based on TiO2 Nanorod Arrays. Nanoscale 2014, 6, 9116–9121. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiao, S.; Li, H.; Li, L.; Zhang, X. Band-edge Modulated ZnO Pomegranates-on-paper Photodetector. J. Mater. Chem. C 2015, 3, 3702–3707. [Google Scholar] [CrossRef]
- Wang, X.; Dong, L.; Zhang, H.; Yu, R.; Pan, C.; Wang, Z. Recent Progress in Electronic Skin. Adv. Sci. 2015, 2, 1500169. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qiu, Y.; Yang, S. Branched ZnO Nanostructures as Building Blocks of Photoelectrodes for Efficient Solar Energy Conversion. Phys. Chem. Chem. Phys. 2012, 14, 10872–10881. [Google Scholar] [CrossRef] [PubMed]
- Crossland, E.; Noel, N.; Sivaram, V.; Leijtens, T.; Alexander-Webber, J.A.; Snaith, H.J. Mesoporous TiO2 Single Crystals Delivering Enhanced Mobility and Optoelectronic Device Performance. Nature 2013, 495, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, W.; Jie, J.; Meng, X.; Fan, X.; Lee, S. Photoresponse Properties of CdSe Single-Nanoribbon Photodetectors. Adv. Funct. Mater. 2007, 17, 1795–1800. [Google Scholar] [CrossRef]
- Jie, J.; Zhang, W.; Jiang, Y.; Meng, X.; Li, Y.; Lee, S. Photoconductive Characteristics of Single-crystal CdS Nanoribbons. Nano Lett. 2006, 6, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Yang, X.; Liang, F.; Jie, J.; Li, Q.; Zhu, Z.; Wu, C.; Yu, Y.; Wang, L. Transparent and Flexible Selenium Nanobelt-based Visible Light Photodetector. CrystEngComm 2012, 14, 1942–1947. [Google Scholar] [CrossRef]
- Zhai, T.; Li, L.; Ma, Y.; Liao, M.; Wang, X.; Fang, X.; Yao, J.; Bando, Y.; Golberg, D. One-dimensional Inorganic Nanostructures: Synthesis, Field-emission and Photodetection. Chem. Soc. Rev. 2011, 40, 2986–3004. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, N.; Sun, J.; Liu, C.; Brittman, S.; Andrews, S.; Lim, J.; Gao, H.; Yan, R.; Yang, P. 25th Anniversary Article: Semiconductor Nanowires-synthesis, Characterization, and Applications. Adv. Mater. 2014, 26, 2137–2184. [Google Scholar] [CrossRef] [PubMed]
- Barth, S.; Hernandez-Ramirez, F.; Holmes, J.; Romano-Rodriguez, A. Synthesis and Applications of One-dimensional Semiconductors. Prog. Mater. Sci. 2010, 55, 563–627. [Google Scholar] [CrossRef]
- Guo, P.; Jiang, J.; Shen, S.; Guo, L. ZnS/ZnO Heterojunction as Photoelectrode: Type II Band Alignment towards Enhanced Photoelectrochemical Performance. Int. J. Hydrogen Energy 2013, 38, 13097–13103. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical Cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Tiwana, P.; Docampo, P.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. Electron Mobility and Injection Dynamics in Mesoporous ZnO, SnO2, and TiO2 Films Used in Dye-sensitized Solar Cells. ACS Nano 2011, 5, 5158–5166. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Hou, Y.; Xing, J.; Zhang, B.; Tian, F.; Yang, X.H.; Yang, H.G. Ultrathin SnO2 Scaffolds for TiO2-Based Heterojunction Photoanodes in Dye-Sensitized Solar Cells: Oriented Charge Transport and Improved Light Scattering. Chem. Eur. J. 2013, 19, 9366–9370. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Kong, X.; Liu, Z.; Liu, C.; Zhou, J.; Chen, W.; Ruan, S.; Xu, Q. TiO2 Based Metal-Semiconductor-Metal Ultraviolet Photodetectors. Appl. Phys. Lett. 2007, 90, 201118. [Google Scholar] [CrossRef]
- Xie, Y.; Wei, L.; Li, Q.; Chen, Y.; Yan, S.; Jiao, J.; Liu, G.; Mei, L. High-performance Self-powered UV Photodetectors Based on TiO2 Nano-branched Arrays. Nanotechnology 2014, 25, 075202. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wei, L.; Wei, G.; Li, Q.; Wang, D.; Chen, Y.; Yan, S.; Liu, G.; Mei, L.; Jiao, J. A Self-powered UV Photodetector Based on TiO2 Nanorod Arrays. Nanoscale Res. Lett. 2013, 8, 188. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Hu, C.; Wang, X.; Wang, S.; Tian, Y.; Zhang, H. UV Sensor Based on TiO2 Nanorod Arrays on FTO Thin Film. Sens. Actuators B Chem. 2011, 156, 114–119. [Google Scholar] [CrossRef]
- Wang, Y.; Han, W.; Zhao, B.; Chen, L.; Teng, F.; Li, X.; Gao, C.; Zhou, J.; Xie, E. Performance Optimization of Self-powered Ultraviolet Detectors Based on Photoelectrochemical Reaction by Utilizing Dendriform Titanium Dioxide Nanowires as Photoanode. Sol. Energy Mater. Sol. C 2015, 140, 376–381. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Song, W.; Li, G.; Yi, B.; Shao, Z. A Novel Photoelectrochemical Cell with Self-organized TiO2 Nanotubes as Photoanodes for Hydrogen Generation. Int. J. Hydrogen Energy 2011, 36, 14374–14380. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, L.; Wang, Y.; He, Y.; Pan, X.; Xie, E. An Overview on Emerging Photoelectrochemical Self-powered Ultraviolet Photodetectors. Nanoscale 2016, 8, 50–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cao, G. Nanostructured Photoelectrodes for Dye-sensitized Solar Cells. Nano Today 2011, 6, 91–109. [Google Scholar] [CrossRef]
- Liu, G.; Kong, L.; Yan, J.; Liu, Z.; Zhang, H.; Lei, P.; Xu, T.; Mao, H.; Chen, B. Nanocrystals in compression: Unexpected structural phase transition and amorphization due to surface impurities. Nanoscale 2016, 8, 11803–11809. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhou, J.; Zhang, Y.; Wang, Y.; Liu, X. Dominating Role of Aligned MoS2/Ni3S2 Nanoarrays Supported on Three-Dimensional Ni Foam with Hydrophilic Interface for Highly Enhanced Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2018, 10, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Guo, F.; Wang, D.; Liu, G.; Xu, Z.; Kong, L.; Wang, J.; Jiao, S.; Zhang, Y.; Yu, Q.; et al. Effect of MgO Surface Modification on the TiO2 Nanowires Electrode for Self-Powered UV Photodetectors. ACS Sustain. Chem. Eng. 2018, 6, 7265–7272. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y.; Sun, L.; Mao, Y.; Wang, M.; Lina, C. An Ultrasound-Assisted Deposition of NiO Nanoparticles on TiO2 Nanotube Arrays for Enhanced Photocatalytic Activity. J. Mater. Chem. A 2014, 2, 8223–8229. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, C.; Pu, W.; Tan, Y.; Yang, K.; Zhang, J. Liquid Phase Deposition of WO3/TiO2 Heterojunction Films with High Photoelectrocatalytic Activity under Visible Light Irradiation. Electrochim. Acta 2014, 148, 180–186. [Google Scholar] [CrossRef]
- Grönqvist, J.; Søndergaard, N.; Boxberg, F.; Guhr, T.; Åberg, S.; Xu, H. Strain in Semiconductor Core-shell Nanowires. J. Appl. Phys. 2009, 106, 53508. [Google Scholar] [CrossRef]
- Søndergaard, N.; He, Y.; Fan, C.; Han, R.; Guhr, T.; Xu, H. Strain Distributions in Lattice-mismatched Semiconductor Core-shell Nanowires. J. Vac. Sci. Technol. B 2009, 27, 827–830. [Google Scholar] [CrossRef]
- Boxberg, F.; Søndergaard, N.; Xu, H. Photovoltaics with Piezoelectric Core-shell Nanowires. Nano Lett. 2010, 10, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Progress in Piezotronics and Piezo-phototronics. Adv. Mater. 2012, 24, 4632–4646. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Kamat, P.V. Photosensitization of TiO2 Nanostructures with CdS Quantum Dots: Particulate Versus Tubular Support Architectures. Adv. Funct. Mater. 2009, 19, 805–811. [Google Scholar] [CrossRef]
- Zhang, J.; Jiao, S.; Wan, Y.; Gao, S.; Wang, D.; Wang, J. A Well-grown β-Ga2O3 Microrod Array Formed from GaOOH on a Si (100) Substrate and Growth Mechanism Study. CrystEngComm 2018, 20, 4329–4335. [Google Scholar] [CrossRef]
- Sanjines, R.; Tang, H.; Berger, H.; Gozzo, F.; Margaritondo, G.; Levy, F. Electronic Structure of Anatase TiO2 Oxide. J. Appl. Phys. 1994, 75, 2945–2951. [Google Scholar] [CrossRef]
- Aas, N.; Pringle, T.; Bowker, M. Adsorption and Decomposition of Methanol on TiO2, SrTiO3 and SrO. J. Chem. Soc. Faraday Trans. 1994, 90, 1015–1022. [Google Scholar] [CrossRef]
- Bai, H.; Liu, Z.; Sun, D. Hierarchical ZnO/Cu “Corn-like” Materials with High Photodegradation and Antibacterial Capability under Visible Light. Phys. Chem. Chem. Phys. 2011, 13, 6205–6210. [Google Scholar] [CrossRef] [PubMed]
- Yang, W. X-ray Photoelectron Spectroscopy and Electrical Properties Studies of La2O3-doped Strontium Titanate Ceramics Prepared by Sol-precipitation Method. J. Mater. Sci. 1999, 34, 3533–3544. [Google Scholar] [CrossRef]
- Cao, T.; Li, Y.; Wang, C.; Shao, C.; Liu, Y. A Facile in situ Hydrothermal Method to SrTiO3/TiO2 Nanofiber Heterostructures with High Photocatalytic Activity. Langmuir 2011, 27, 2946–2952. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Wu, H.; Wang, C.; Wang, B.; Zhang, L.; Cheng, Z.; Hu, P.; Pan, W.; Zhou, Z.; Yang, X.; et al. Nanowire-Based High-Performance “Micro Fuel Cells”: One Nanowire, One Fuel Cell. Adv. Mater. 2008, 20, 1644–1648. [Google Scholar] [CrossRef]
- Harrison, R.; Koch, C. A Robust Analog VLSI Reichardt Motion Sensor. Analog Integr. Circ. Signal Proc. 2000, 24, 213–229. [Google Scholar] [CrossRef]
- Zhou, Z.; Fan, J.; Wang, X.; Zhou, W.; Du, Z.; Wu, S. Effect of Highly Ordered Single-crystalline TiO2 Nanowire Length on the Photovoltaic Performance of Dye-sensitized Solar Cells. ACS Appl. Mater. Interfaces 2011, 3, 4349–4353. [Google Scholar] [CrossRef] [PubMed]
- Hamedani, H.; Allam, N.; Garmestani, H.; ElSayed, M. Electrochemical Fabrication of Strontium-doped TiO2 Nanotube Array Electrodes and Investigation of Their Photoelectrochemical Properties. J. Phys. Chem. C 2011, 115, 13480–13486. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, S.; Mo, L.; Huang, Y.; Tian, H.; Hu, L.; Huo, Z.; Dai, S.; Kong, F.; Pan, X. Charge Recombination and Band-edge Shift in the Dye-sensitized Mg2+-doped TiO2 Solar Cells. J. Phys. Chem. C 2011, 115, 16418–16424. [Google Scholar] [CrossRef]
- Kim, C.; Suh, S.; Choi, M.; Kang, Y.; Kang, Y. Fabrication of SrTiO3-TiO2 Heterojunction Photoanode with Enlarged Pore Diameter for Dye-sensitized Solar Cells. J. Mater. Chem. A 2013, 1, 11820–11827. [Google Scholar] [CrossRef]
- Sun, H.; Pan, L.; Piao, X.; Sun, Z. Long Afterglow SrAl2O4:Eu,Dy Phosphors for CdS Quantum Dot-sensitized Solar Cells with Enhanced Photovoltaic Performance. J. Mater. Chem. A 2013, 1, 6388–6392. [Google Scholar] [CrossRef]
- Zaban, A.; Greenshtein, M.; Bisquert, J. Determination of the Electron Lifetime in Nanocrystalline Dye Solar Cells by Open-Circuit Voltage Decay Measurements. ChemPhysChem 2003, 4, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Bisquert, J.; Zaban, A.; Greenshtein, M.; Mora-Sero, I. Determination of Rate Constants for Charge Transfer and the Distribution of Semiconductor and Electrolyte Electronic Energy Levels in Dye-sensitized Solar Cells by Open-circuit Photovoltage Decay Method. J. Am. Chem. Soc. 2004, 126, 13550–13559. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, X.; Tai, Q.; Hu, H.; Chen, B.; Huang, N.; Sebo, B.; Zhao, X. Efficiency Enhancement in Dye-sensitized Solar Cells by Interfacial Modification of Conducting Glass/Mesoporous TiO2 Using a Novel ZnO Compact Blocking Film. J. Power Sources 2011, 196, 475–481. [Google Scholar] [CrossRef]
- Liu, G.; Kong, L.; Gong, J.; Yang, W.; Mao, H.; Hu, Q.; Liu, Z.; Schaller, R.D.; Zhang, D.; Xu, T. Pressure-Induced Bandgap Optimization in Lead-Based Perovskites with Prolonged Carrier Lifetime and Ambient Retainability. Adv. Func. Mater. 2017, 27, 1604208. [Google Scholar] [CrossRef]
- Kong, L.; Liu, G.; Gong, J.; Hu, Q.; Schaller, R.D.; Dera, P.; Zhang, D.; Liu, Z.; Yang, W.; Zhu, K.; et al. Simultaneous band-gap narrowing and carrier-lifetime prolongation of organic–inorganic trihalide perovskites. Proc. Natl. Acad. Sci. USA 2016, 113, 8910–8915. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Yin, L. Enhanced Photovoltaic Performance of Dye-sensitized Solar Cells Based on Sr-doped TiO2/SrTiO3 Nanorod Array Heterostructures. J. Mater. Chem. A 2015, 3, 17417–17425. [Google Scholar] [CrossRef]

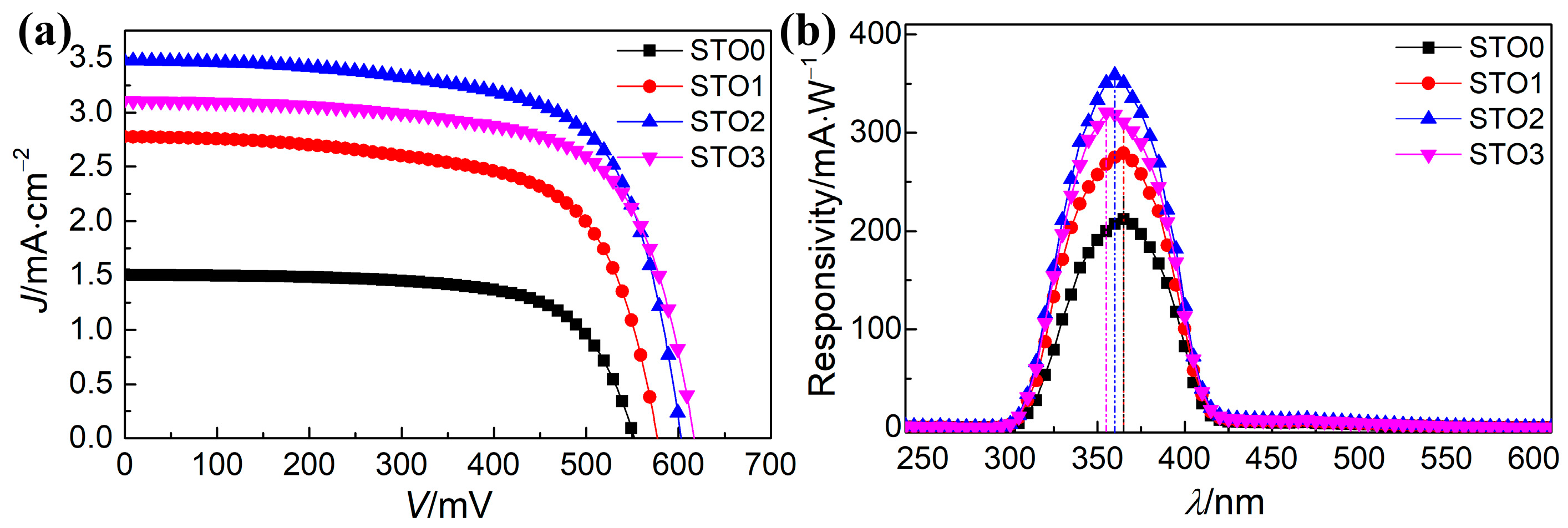
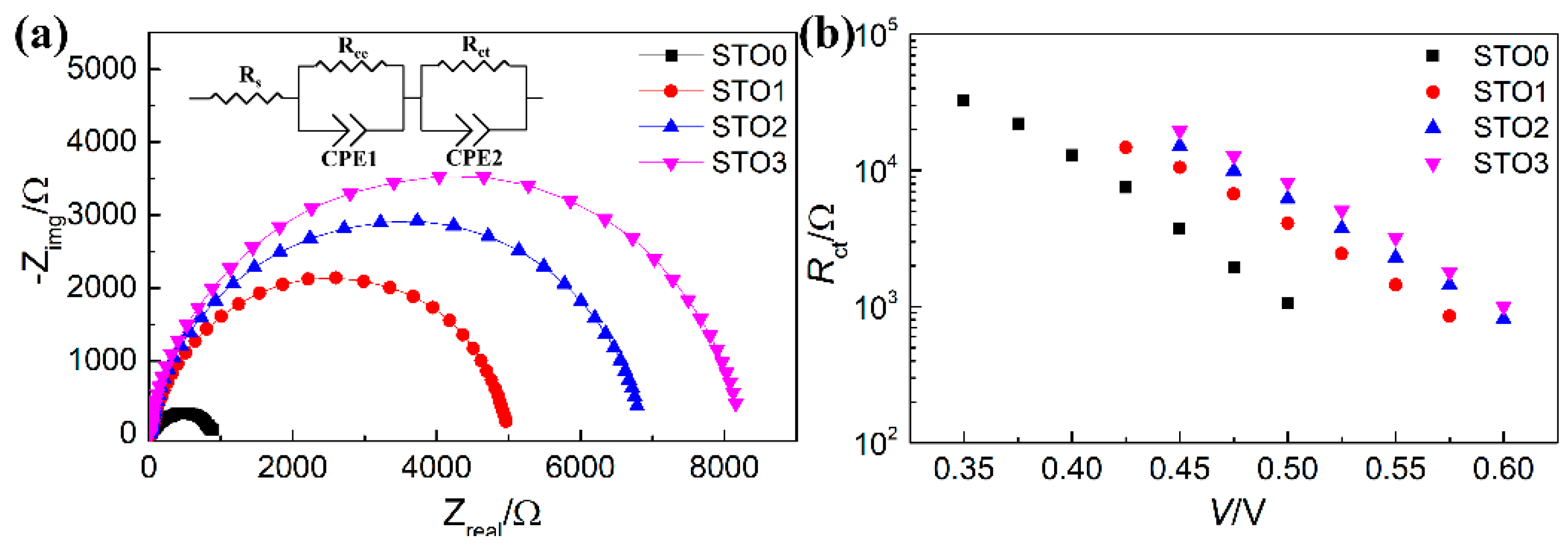
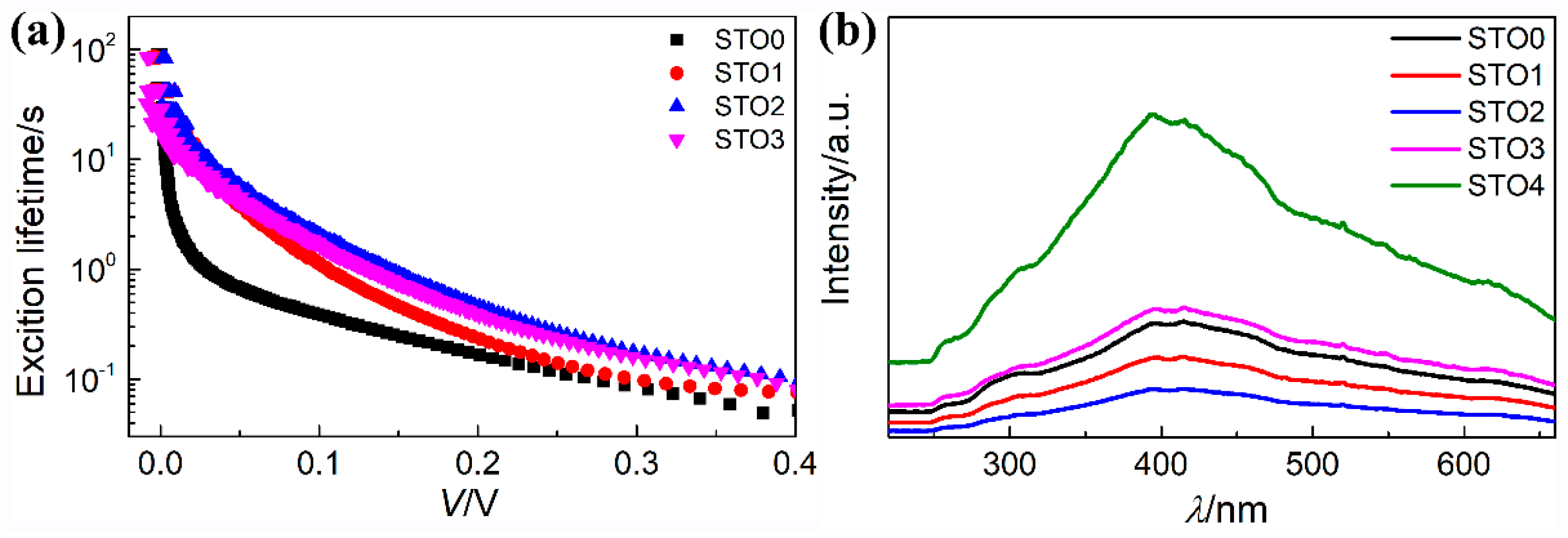
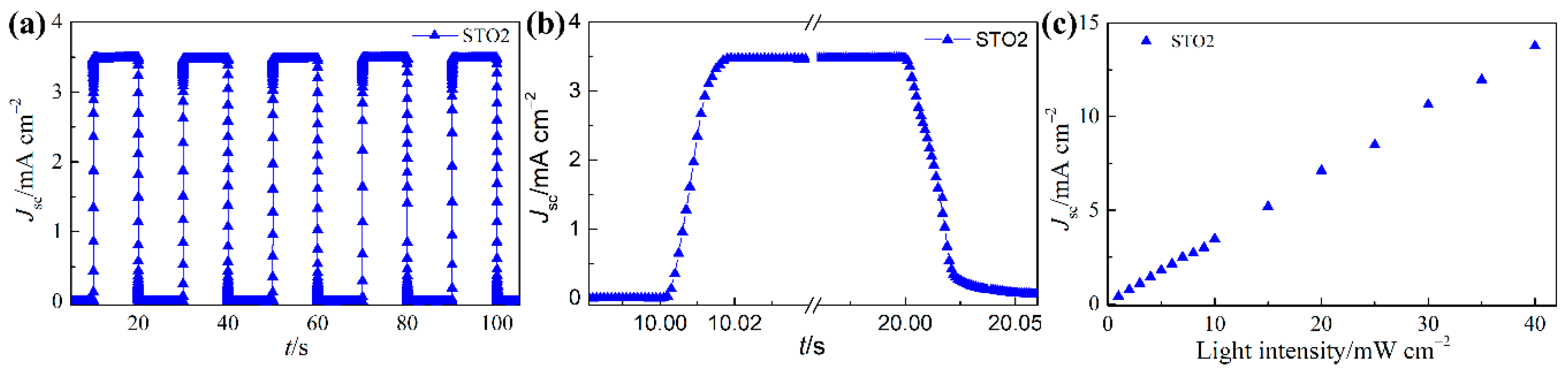
| Sample | Jsc (mA·cm−2) | Voc (mV) | FF (%) | η (%) | Photoresponsivity (mA·W−1) |
|---|---|---|---|---|---|
| STO0 | 1.50 | 552 | 68.1 | 0.566 | 212 at 365 nm |
| STO1 | 2.77 | 577 | 67.9 | 1.045 | 279 at 365 nm |
| STO2 | 3.48 | 602 | 67.7 | 1.418 | 358 at 360 nm |
| STO3 | 3.11 | 616 | 67.6 | 1.294 | 321 at 355 nm |
| Sample | Dark Current (mA·cm−2) | On/Off Ratio | τr (ms) | τd (ms) |
|---|---|---|---|---|
| STO0 | 2.39 × 10−4 | 6276 | 10 | 12 |
| STO1 | 1.53 × 10−4 | 18104 | 11 | 13 |
| STO2 | 1.24 × 10−4 | 28064 | 11 | 15 |
| STO3 | 1.36 × 10−4 | 22867 | 11 | 14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, S.; Guo, F.; Wang, D.; Jiao, S.; Wang, J.; Zhang, Y.; Wang, B.; Zhao, L. Optimal Sr-Doped Free TiO2@SrTiO3 Heterostructured Nanowire Arrays for High-Efficiency Self-Powered Photoelectrochemical UV Photodetector Applications. Crystals 2019, 9, 134. https://doi.org/10.3390/cryst9030134
Ni S, Guo F, Wang D, Jiao S, Wang J, Zhang Y, Wang B, Zhao L. Optimal Sr-Doped Free TiO2@SrTiO3 Heterostructured Nanowire Arrays for High-Efficiency Self-Powered Photoelectrochemical UV Photodetector Applications. Crystals. 2019; 9(3):134. https://doi.org/10.3390/cryst9030134
Chicago/Turabian StyleNi, Shiming, Fengyun Guo, Dongbo Wang, Shujie Jiao, Jinzhong Wang, Yong Zhang, Bao Wang, and Liancheng Zhao. 2019. "Optimal Sr-Doped Free TiO2@SrTiO3 Heterostructured Nanowire Arrays for High-Efficiency Self-Powered Photoelectrochemical UV Photodetector Applications" Crystals 9, no. 3: 134. https://doi.org/10.3390/cryst9030134
APA StyleNi, S., Guo, F., Wang, D., Jiao, S., Wang, J., Zhang, Y., Wang, B., & Zhao, L. (2019). Optimal Sr-Doped Free TiO2@SrTiO3 Heterostructured Nanowire Arrays for High-Efficiency Self-Powered Photoelectrochemical UV Photodetector Applications. Crystals, 9(3), 134. https://doi.org/10.3390/cryst9030134