Phase Behavior and DFT Calculations of Laterally Methyl Supramolecular Hydrogen-Bonding Complexes
Abstract
:1. Introduction
2. Experimental
2.1. Supramolecular Complexes Preparation
2.2. Characterizations
2.3. Computational Methods and Calculations
3. Results and Discussion
3.1. Phase Behavior of 1:1 Molar Supramolecular Complexes
3.2. Effect of Introduction Phenylazo Part to the 1:1 Supramolecular Complexes
3.3. DFT Calculations and Thermal Stability
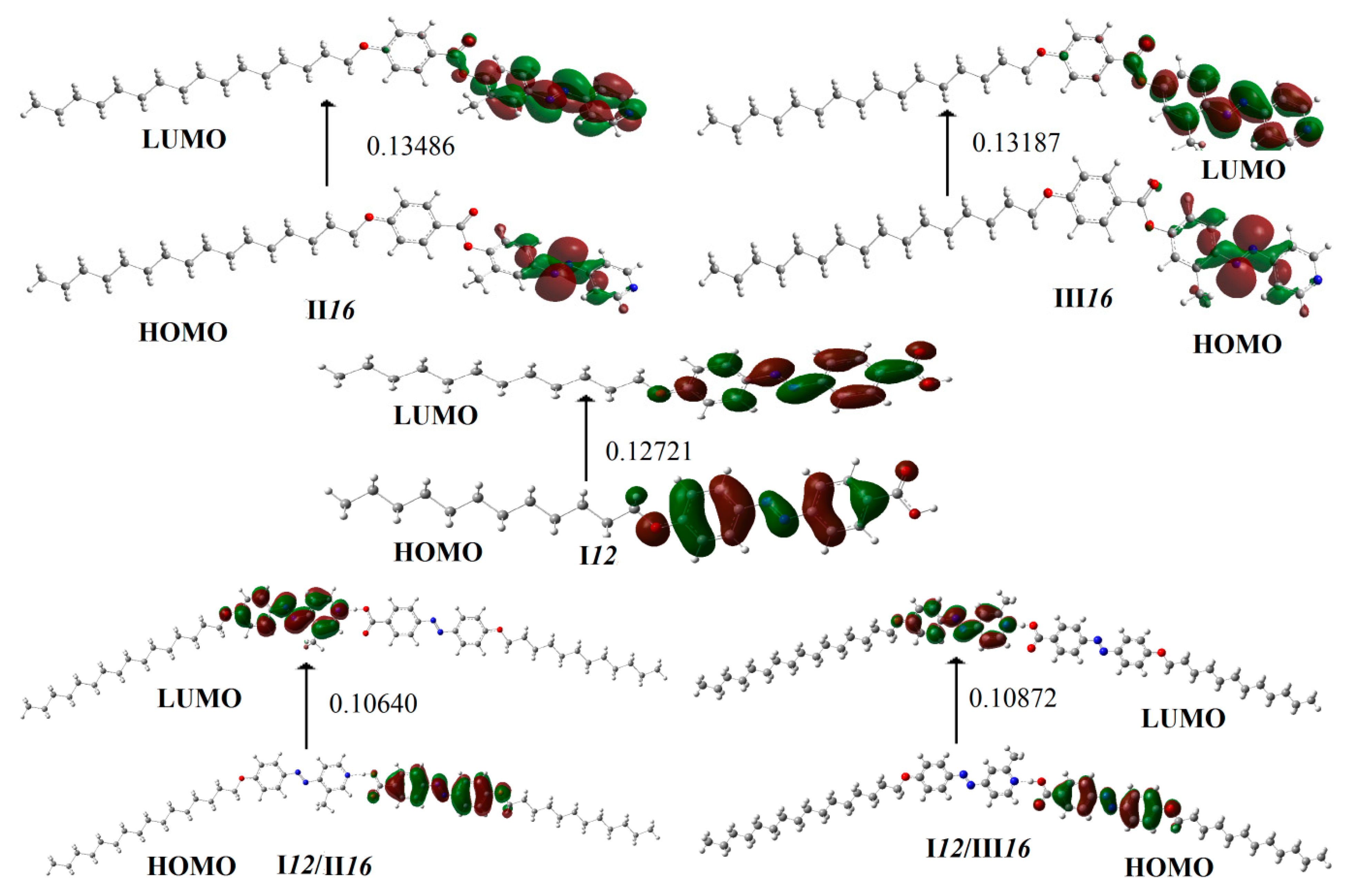
3.4. Frontier Molecular Orbitals
3.5. Molecular Electrostatic Potential (MEP)
3.6. Entropy Transition Changes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Imrie, C.T.; Lu, Z.; Picken, S.J.; Yildirim, Z. Oligomeric rod-disc nematic liquid crystals. Chem. Commun. 2007, 12, 1245–1247. [Google Scholar] [CrossRef] [PubMed]
- Fisch, M.R. Liquid Crystals, Laptops and Life; World Scientific Publishing Company: Jurong East, Singapore, 2004; Volume 23. [Google Scholar]
- Borshch, V.; Shiyanovskii, S.V.; Lavrentovich, O.D. Nanosecond electro-optic switching of a liquid crystal. Phys. Rev. Lett. 2013, 111, 107802. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, A.; Das, M.K.; Das, B.; Żurowska, M.; Dąbrowski, R. Electro-optical properties of a new series of fluorinated antiferroelectric orthoconic liquid crystalline esters. Liq. Cryst. 2015, 42, 412–421. [Google Scholar] [CrossRef]
- Bhattacharjee, D.; Alapati, P.R.; Bhattacharjee, A. Negative optical anisotropic behaviour of two higher homologues of 5O. m series of liquid crystals. J. Mol. Liq. 2016, 222, 55–60. [Google Scholar] [CrossRef]
- Vorländer, D. Über durchsichtig klare, krystallinische Flüssigkeiten. Ber. Der Dtsch. Chem. Ges. 1908, 41, 2033–2052. [Google Scholar] [CrossRef]
- Demus, D.; Diele, S.; Hauser, A.; Latif, I.; Selbmann, C.; Weissflog, W. Thermotropic liquid crystalline compounds with lateral long-chain substituents (IV). Physical properties of 1, 4-bis-[4-n-octyloxybenzoyloxy]-2-n-alkylbenzenes. Cryst. Res. Technol. 1985, 20, 1547–1558. [Google Scholar] [CrossRef]
- Weissflog, W.; Demus, D. Compounds with lateral long-chain substituents—A new molecule structure concept for thermotropic liquid crystals. Cryst. Res. Technol. 1983, 18, K21–K24. [Google Scholar] [CrossRef]
- Demus, D.; Hauser, A. Selected Topics in Liquid Crystal Research; Akademie-Verlag: Berlin, Germany, 1990. [Google Scholar]
- Hey, M.; Gray, G.W. Molecular structure and the properties of liquid crystals. London and New York (Academic Press) 1962. vii+ 314 pp. Price 63s. Mineral. Mag. J. Mineral. Soc. 1963, 33, 440. [Google Scholar] [CrossRef]
- Osmiałowski, B.; Kolehmainen, E.; Dobosz, R.; Gawinecki, R.; Kauppinen, R.; Valkonen, A.; Koivukorpi, J.; Rissanen, K. Self-organization of 2-acylaminopyridines in the solid state and in solution. J. Phys. Chem. A 2010, 114, 10421–10426. [Google Scholar] [CrossRef] [PubMed]
- Osmiałowski, B.; Kolehmainen, E.; Gawinecki, R.; Dobosz, R.; Kauppinen, R. Complexation of 2, 6-Bis (acylamino) pyridines with dipyridin-2-ylamine and 4, 4-dimethylpiperidine-2, 6-dione. J. Phys. Chem. A 2010, 114, 12881–12887. [Google Scholar] [CrossRef] [PubMed]
- Ośmiałowski, B. Noncovalent interactions between classical supramolecular synthons in solution: Hydrogen bonding in hindered 2-acylaminopyridine/2-pyridone associates. J. Mol. Struct. 2012, 1018, 84–87. [Google Scholar] [CrossRef]
- Chen, K.-Y. Crystal Structure, Hydrogen-Bonding Properties, and DFT Studies of 2-((2-(2-Hydroxyphenyl) benzo [d] thiazol-6-yl) methylene) malononitrile. Mol. Cryst. Liq. Cryst. 2015, 623, 285–296. [Google Scholar] [CrossRef]
- Shoji, M.; Tanaka, F. Theoretical study of hydrogen-bonded supramolecular liquid crystals. Macromolecules 2002, 35, 7460–7472. [Google Scholar] [CrossRef]
- Sundaram, S.; Jayaprakasam, R.; Dhandapani, M.; Senthil, T.; Vijayakumar, V. Theoretical (DFT) and experimental studies on multiple hydrogen bonded liquid crystals comprising between aliphatic and aromatic acids. J. Mol. Liq. 2017, 243, 14–21. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Alhaddadd, O. DFT Calculations and Mesophase Study of Coumarin Esters and Its Azoesters. Crystals 2018, 8, 359. [Google Scholar] [CrossRef]
- Hagar, M.; Soliman, S.M.; Ibid, F.; El Sayed, H. Quinazolin-4-yl-sulfanylacetyl-hydrazone derivatives; Synthesis, molecular structure and electronic properties. J. Mol. Struct. 2013, 1049, 177–188. [Google Scholar] [CrossRef]
- Soliman, S.M.; Hagar, M.; Ibid, F.; El Sayed, H. Experimental and theoretical spectroscopic studies, HOMO–LUMO, NBO analyses and thione–thiol tautomerism of a new hybrid of 1, 3, 4-oxadiazole-thione with quinazolin-4-one. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Hagar, M.; Soliman, S.M.; Ibid, F.; El Sayed, H. Synthesis, molecular structure and spectroscopic studies of some new quinazolin-4 (3H)-one derivatives; an account on the N-versus S-Alkylation. J. Mol. Struct. 2016, 1108, 667–679. [Google Scholar] [CrossRef]
- Aboelnaga, A.; Hagar, M.; Soliman, S.M. Ultrasonic Synthesis, Molecular Structure and Mechanistic Study of 1, 3-Dipolar Cycloaddition Reaction of 1-Alkynylpyridinium-3-olate and Acetylene Derivatives. Molecules 2016, 21, 848. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.A.; Gao, M.; Kim, Y.-K.; Jamali, A.; Finley, K.L.; Robles-Hernández, B.; Diez-Berart, S.; Salud, J.; de la Fuente, M.R.; Timimi, B.A. Understanding the twist-bend nematic phase: The characterisation of 1-(4-cyanobiphenyl-4′-yloxy)-6-(4-cyanobiphenyl-4′-yl) hexane (CB6OCB) and comparison with CB7CB. Soft Matter 2016, 12, 6827–6840. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Supramolecular binding thermodynamics by dispersion-corrected density functional theory. Chem. A Eur. J. 2012, 18, 9955–9964. [Google Scholar] [CrossRef] [PubMed]
- Mallik, S.; Nayak, A.; Daschakraborty, S.; Kumar, S.; Suresh, K.A. Supramolecular Self-Assembly of Ionic Discotic Liquid Crystalline Dimer with DNA at Interfaces. ChemistrySelect 2018, 3, 7318–7326. [Google Scholar] [CrossRef]
- Abdelkader, M.; Abdelmohsen, M. Hydrogen-bonded and supramolecular ferroelectricity in a new hybrid (C 12 H 25 NH 3) 2 CoCl 4. Mater. Res. Express 2018, 6, 025608. [Google Scholar] [CrossRef]
- Lehn, J.-M.; Sanders, J. Supramolecular chemistry. Concepts and perspectives. Angew. Chem. Engl. Ed. 1995, 34, 2563. [Google Scholar]
- Ahmed, H.; Naoum, M. Mesophase behavior of binary and ternary mixtures of benzoic acids bearing terminal substituents of different polarity and chain-lengths. Thermochim. Acta 2014, 575, 122–128. [Google Scholar] [CrossRef]
- Ahmed, H.; Naoum, M.; Saad, G. Mesophase behaviour of 1: 1 mixtures of 4-n-alkoxyphenylazo benzoic acids bearing terminal alkoxy groups of different chain lengths. Liq. Cryst. 2016, 43, 1259–1267. [Google Scholar] [CrossRef]
- Rahimi, M.; Roberts, T.F.; Armas-Pérez, J.C.; Wang, X.; Bukusoglu, E.; Abbott, N.L.; de Pablo, J.J. Nanoparticle self-assembly at the interface of liquid crystal droplets. Proc. Natl. Acad. Sci. USA 2015, 112, 5297–5302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohini, P.; Pongali Sathya Prabu, N.; Madhu Mohan, M. Comparison of mesomorphic properties exhibited by linear hydrogen bonded thermotropic liquid crystals. Mol. Cryst. Liq. Cryst. 2016, 631, 74–91. [Google Scholar] [CrossRef]
- Bhagavath, P.; Mahabaleshwara, S. Mesomorphism in binary mixtures of 4-((hexylimino) methyl) benzoic acid and 4-alkyloxybenzoic acids. J. Therm. Anal. Calorim. 2017, 129, 339–345. [Google Scholar] [CrossRef]
- Sundaram, S.; Subhasri, P.; Rajasekaran, T.; Jayaprakasam, R.; Senthil, T.; Vijayakumar, V. Observation of induced new smectic phase in supramolecular hydrogen bonded liquid crystals between mesogenic and non-mesogenic aliphatic compounds. Ferroelectrics 2017, 510, 103–120. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Alaasar, M.A.; Salem, R.A. Supramolecular liquid crystals in binary and ternary systems. Thermochim. Acta 2011, 517, 63–73. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Mohammady, S.Z.; Abaza, A.H. Effect of lateral substitution on supramolecular liquid crystal associates induced by hydrogen-bonding interactions between 4-(4′-pyridylazo-3-methylphenyl)-4″-alkoxy benzoates and 4-substituted benzoic acids. Liq. Cryst. 2010, 37, 475–486. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Alaasar, M.; Naoum, M. Wide nematic phases induced by hydrogen-bonding. Liq. Cryst. 2018, 1–10. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Aljuhani, A. Mesophase behavior of new linear supramolecular hydrogen-bonding complexes. RSC Adv. 2018, 8, 34937–34946. [Google Scholar] [CrossRef]
- Friot, B.; Boyd, D.; Willis, K.; Donnio, B.; Ungar, G.; Bruce, D.W. Hydrogen-bonded polycatenar mesogens. Liq. Cryst. 2000, 27, 605–611. [Google Scholar] [CrossRef]
- Paleos, C.M.; Tsiourvas, D. Supramolecular hydrogen-bonded liquid crystals. Liq. Cryst. 2001, 28, 1127–1161. [Google Scholar] [CrossRef]
- Kato, T.; Kamikawa, Y. Hydrogen-Bonded Systems: Discrete Defined Aggregates by Intermolecular H-Bonding, Amides, Carboxylic Acids, and Heterocycles. Handb. Liq. Cryst. 2014. [Google Scholar] [CrossRef]
- Jansze, S.M.; Martínez-Felipe, A.; Storey, J.; Marcelis, A.; Imrie, C.T. A Twist-Bend Nematic Phase Driven by Hydrogen Bonding. Angew. Chem. Int. Ed. 2015, 54, 643–646. [Google Scholar] [CrossRef]
- Alaasar, M.; Carsten, T. Nematic phases driven by hydrogen-bonding in liquid crystalline nonsymmetric dimers. Liq. Cryst. 2019, 46, 124–130. [Google Scholar] [CrossRef]
- Collings, P.J.; Hird, M. Introduction to Liquid Crystals: Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Frisch, M.; Trucks, G.; Schlegel, H.B.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision a. 02; Gaussian. Inc.: Wallingford, CT, USA, 2009; p. 200. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.; Millam, J. GaussView, Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Muhammad, S.; Al-Sehemi, A.G.; Irfan, A.; Algarni, H.; Qiu, Y.; Xu, H.; Su, Z.; Iqbal, J. The substitution effect of heterocyclic rings to tune the optical and nonlinear optical properties of hybrid chalcones: A comparative study. J. Mol. Graph. Model. 2018, 81, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Hagar, M.; Ahmed, H.A.; Alhaddad, O.A. Experimental and theoretical approaches of molecular geometry and mesophase behaviour relationship of laterally substituted azopyridines. Liq. Cryst. 2019. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; El-Sayed, T.H.; Alnoman, R.B. Schiff base/ester liquid crystals with different lateral substituents: mesophase behaviour and DFT calculations. Liq. Cryst. 2019. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.A.; Saad, G. Synthesis and mesophase behaviour of Schiff base/ester 4-(arylideneamino) phenyl-4 ″-alkoxy benzoates and their binary mixtures. J. Mol. Liq. 2019, 273, 266–273. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; Saad, G. Impact of The Proportionation of Dialkoxy Chain Length on The Mesophase Behaviour of Schiff base/ester Liquid Crystals; Experimental and Theoritical Study. Liq. Cryst. 2019. [Google Scholar] [CrossRef]
- Imrie, C. Laterally substituted dimeric liquid crystals. Liq. Cryst. 1989, 6, 391–396. [Google Scholar] [CrossRef]
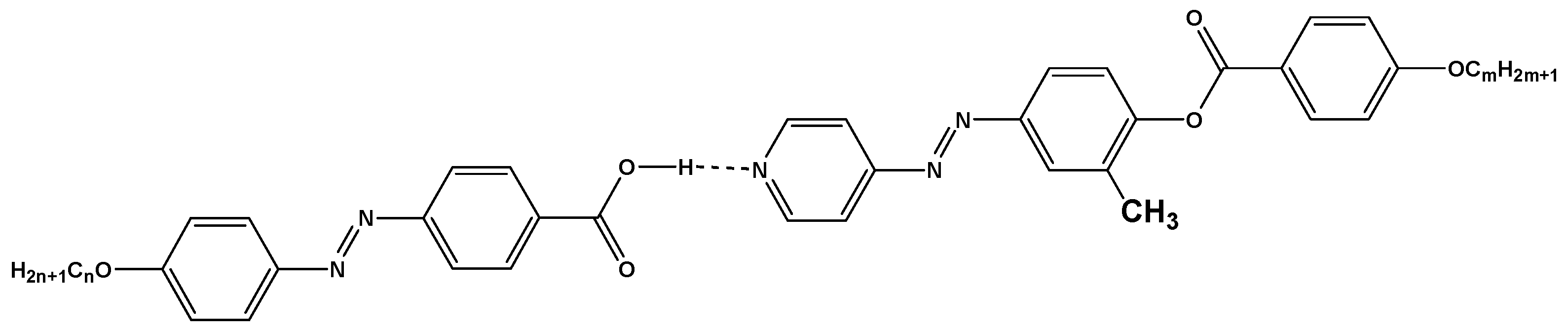
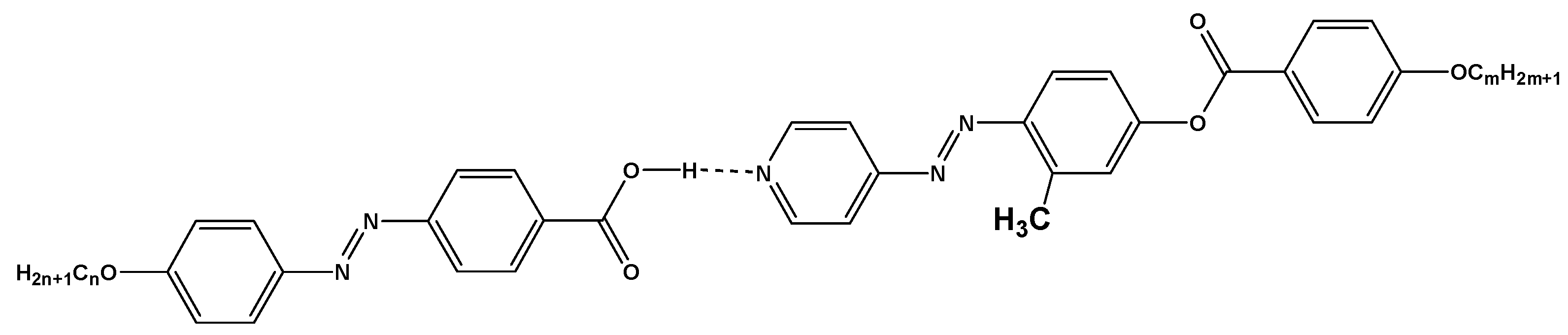
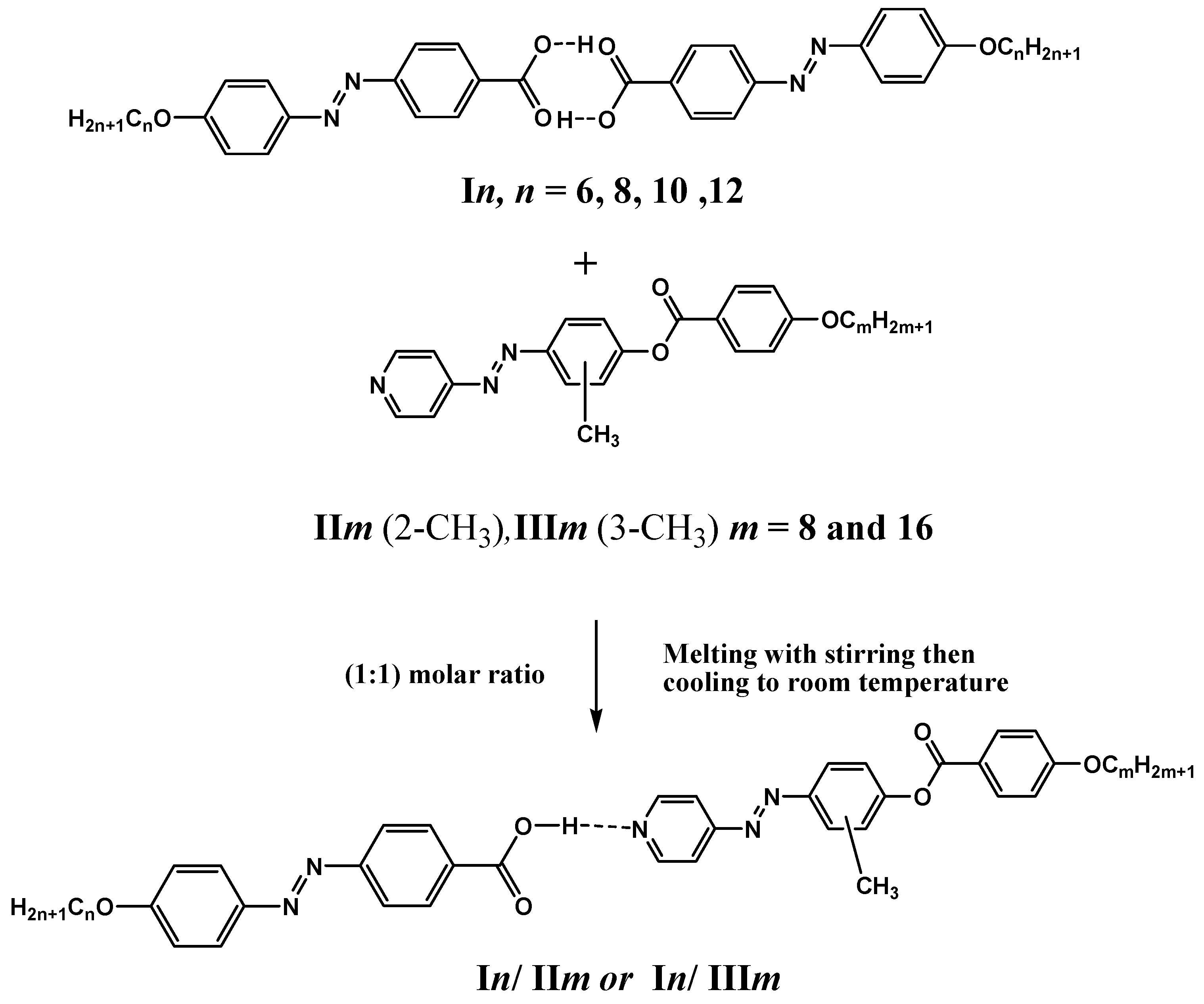
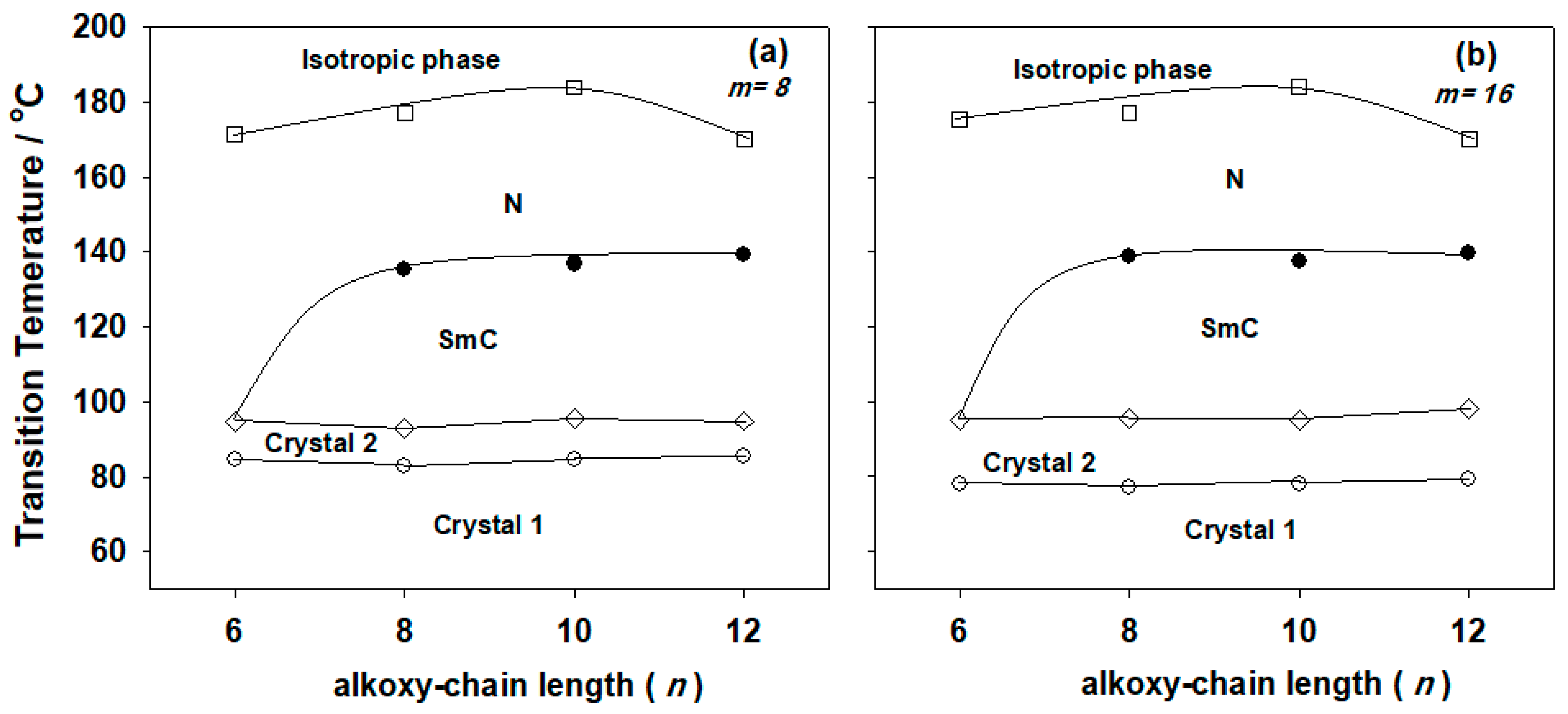
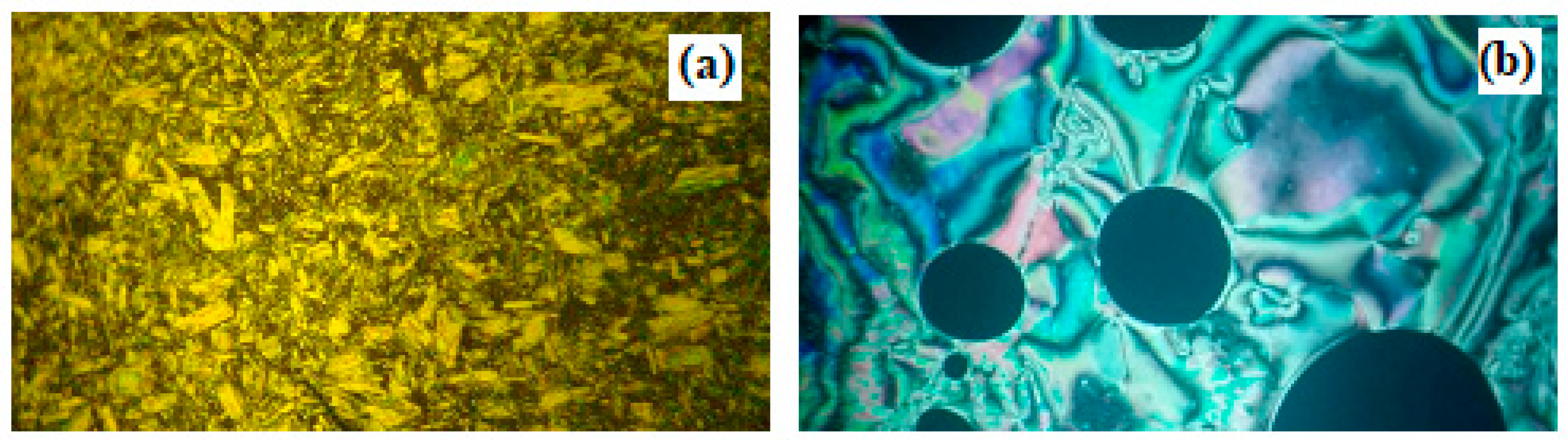
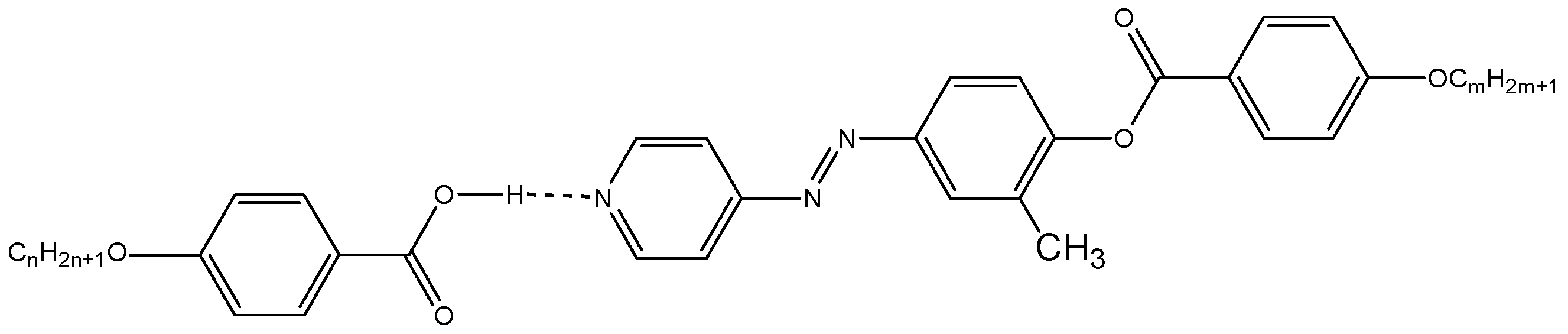


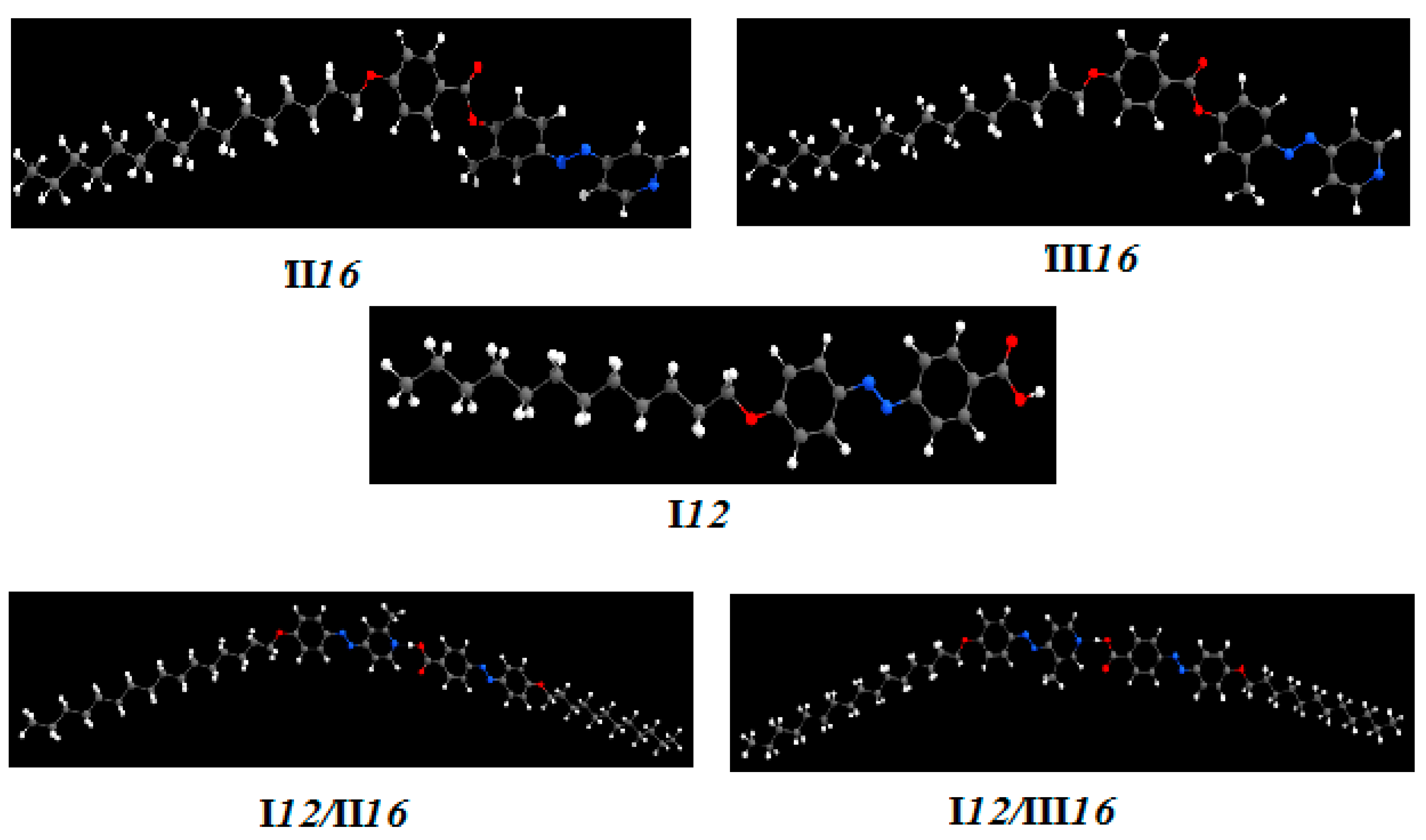

 ).
).
 ).
).
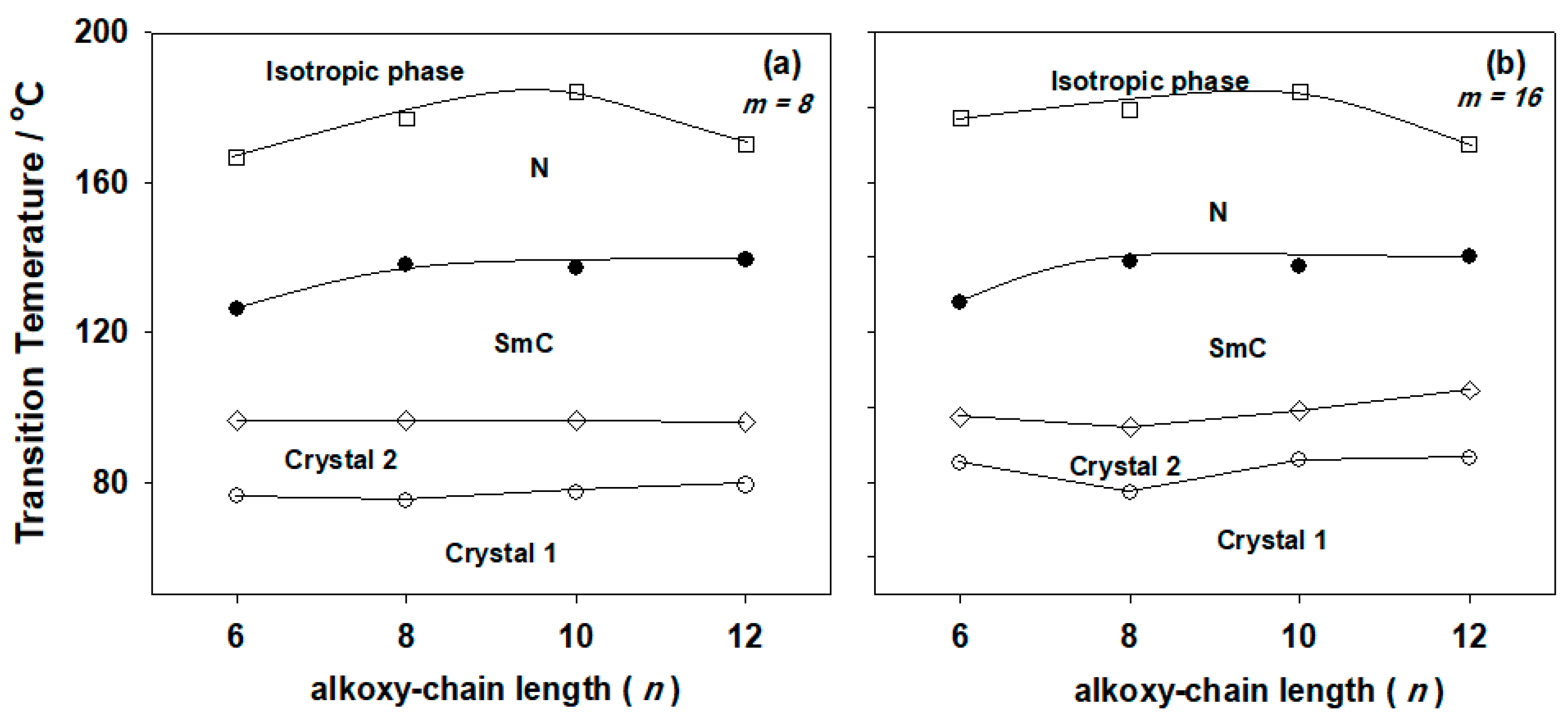
| System | TCr1-Cr2 | TCr2-C | ∆HCr2-C | TC-N | ∆HC-N | TN-I | ∆HN-I |
|---|---|---|---|---|---|---|---|
| I6/II8 | 76.3 | 96.4 | 34.5 | 126.2 | 3.1 | 166.6 | 1.2 |
| I8/II8 | 74.8 | 96.3 | 25.8 | 137.8 | 6.5 | 177.0 | 1.7 |
| I10/II8 | 77.3 | 96.3 | 32.7 | 137.0 | 5.6 | 184.1 | 1.9 |
| I12/II8 | 79.1 | 95.9 | 31.9 | 139.3 | 6.9 | 170.2 | 2.8 |
| I6/II16 | 85.0 | 97.4 | 68.6 | 128.0 | 4.6 | 177.2 | 1.6 |
| I8/II16 | 77.1 | 94.5 | 54.5 | 138.8 | 3.9 | 179.4 | 1.1 |
| I10/II16 | 85.7 | 99.0 | 66.4 | 137.7 | 4.1 | 184.3 | 1.4 |
| I12/II16 | 86.3 | 104.5 | 51.9 | 140.0 | 3.7 | 170.2 | 1.2 |
| I6/III8 | 84.5 | 94.7 | 42.6 | - | - | 171.5 | 2.1 |
| I8/III8 | 82.8 | 93.1 | 37.4 | 135.3 | 3.1 | 177.3 | 1.2 |
| I10/III8 | 84.6 | 95.5 | 35.4 | 136.9 | 4.6 | 184.0 | 2.6 |
| I12/III8 | 85.4 | 94.9 | 36.5 | 139.3 | 6.6 | 170.3 | 3.2 |
| I6/III16 | 78.0 | 95.2 | 59.3 | - | - | 175.4 | 1.8 |
| I8/III16 | 77.1 | 95.4 | 53.7 | 139.0 | 4.7 | 176.9 | 1.3 |
| I10/III16 | 78.1 | 95.0 | 51.7 | 137.5 | 5.2 | 184.2 | 2.6 |
| I12/III16 | 79.2 | 98.2 | 51.3 | 139.8 | 5.3 | 170.2 | 2.8 |
| Parameter | I12 | II16 | III16 | I12/II16 | I12/III16 | |
|---|---|---|---|---|---|---|
| Ecorr | 0.552074 | 0.762269 | 0.762263 | 1.220213 | 1.219892 | |
| ZPVE | −1307.701790 | −1751.112125 | −1751.111524 | −2639.339419 | −2639.343300 | |
| Etot | −1307.670531 | −1751.069093 | −1751.068433 | −2639.270880 | −2639.274657 | |
| H | −1307.669587 | −1751.068149 | −1751.067489 | −2639.269936 | −2639.273713 | |
| G | −1307.770916 | −1751.199293 | −1751.199207 | −2639.465599 | −2639.470155 | |
| ΔH | 000000 | 0.003777 | ||||
| ΔG | 000000 | 0.004556 | ||||
| Dipole Moment | X | 1.8494776 | 3.2045878 | 2.9735829 | 2.4718444 | −2.1457531 |
| Y | −0.2220247 | −0.546798 | 0.6756452 | −0.0119251 | −0.4343364 | |
| Z | 0.025892 | −0.0004635 | 0.0391378 | 0.8166558 | −0.0123549 | |
| Total | 4.7351 | 8.2629 | 7.7514 | 6.6169 | 7.7514 | |
| Compound | OHCOOH | C=OCOOH | C–OCOOH | C=NPyr | C=CPyr | N=Nacid | N=Nbase | H-bond |
|---|---|---|---|---|---|---|---|---|
| I12 | 0.97588 | 1.23711 | 1.38408 | 1.28188 | ||||
| II16 | 1.35599 | 1.39091 | 1.27854 | |||||
| III16 | 1.35597 | 1.39088 | 1.27988 | |||||
| I12/II16 | 1.04420 | 1.25123 | 1.34683 | 1.34989 | 1.38555 | 1.28110 | 1.28236 | 1.56777 |
| I12/III16 | 1.04760 | 1.25104 | 1.34753 | 1.35153 | 1.39348 | 1.28112 | 1.28166 | 1.57783 |
| Compound | OHCOOH | C=OCOOH | C-OCOOH | C=NPyr | C=CPyr | N=Nacid | N=Nbase |
|---|---|---|---|---|---|---|---|
| I12 | 3360 | 1666 (1679) | 1374 (1295) | 1601 (1589) | 1424 (1417) | ||
| II16 | 1591 (1600) | 1616 (1615) | 1434 (1469) | ||||
| III16 | 1590 (1595) | 1617 (1615) | 1434 (1472) | ||||
| I12/II16 | 2362 (2353) | 1668 (1681) | 1319 (1298) | 1593 (1597) | 1625 | 1426 (1420) | 1428 (1469) |
| I12/III16 | 2304 (2353) | 1672 (1681) | 1319 (1296) | 1592 (1592) | 1635 | 1426 (1420) | 1421 (1469) |
| Compound | EHOMO (a.u) | ELUMO (a.u) | ΔE(ELUMO – EHOMO) (a.u) | η = ΔE(ELUMO − EHOMO)/2 | S = 1/2η |
|---|---|---|---|---|---|
| I12 | −0.22959 | −0.10238 | 0.12721 | 0.063605 | 0.031803 |
| II16 | −0.23916 | −0.10430 | 0.13486 | 0.06743 | 0.033715 |
| III16 | −0.23827 | −0.10640 | 0.13187 | 0.065935 | 0.032968 |
| I12/II16 | −0.21766 | −0.11126 | 0.10640 | 0.0532 | 0.0266 |
| I12/III16 | −0.21814 | −0.10942 | 0.10872 | 0.05436 | 0.02718 |
| System | ∆SSmC-N/R | ∆SN-I/R | System | ∆SSmC-N/R |
|---|---|---|---|---|
| I6/II8 | 0.9 | 0.3 | IV6/II8 | 1.1 |
| I8/II8 | 1.9 | 0.5 | IV8/II8 | 1.3 |
| I10/II8 | 1.6 | 0.5 | IV10/II8 | 1.1 |
| I12/II8 | 2.0 | 0.8 | IV12/II8 | 1.1 |
| I6/II16 | 1.4 | 0.4 | IV6/II16 | 1.2 |
| I8/II16 | 1.1 | 0.3 | IV8/II16 | 1.4 |
| I10/II16 | 1.2 | 0.4 | IV10/II16 | 2.3 |
| I12/II16 | 1.1 | 0.3 | IV12/II16 | 2.4 |
| I6/III8 | - | 0.6 | IV6/III8 | - |
| I8/III8 | 0.9 | 0.3 | IV8/III8 | 0.3 |
| I10/III8 | 1.3 | 0.7 | IV10/III8 | 0.5 |
| I12/III8 | 1.9 | 0.9 | IV12/III8 | 0.4 |
| I6/III16 | - | 0.5 | IV6/III16 | - |
| I8/III16 | 1.4 | 0.3 | IV8/III16 | 0.5 |
| I10/III16 | 1.5 | 0.7 | IV10/III16 | 0.4 |
| I12/III16 | 1.5 | 0.8 | IV12/III16 | 2.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, H.A.; Hagar, M.; Alhaddad, O.A. Phase Behavior and DFT Calculations of Laterally Methyl Supramolecular Hydrogen-Bonding Complexes. Crystals 2019, 9, 133. https://doi.org/10.3390/cryst9030133
Ahmed HA, Hagar M, Alhaddad OA. Phase Behavior and DFT Calculations of Laterally Methyl Supramolecular Hydrogen-Bonding Complexes. Crystals. 2019; 9(3):133. https://doi.org/10.3390/cryst9030133
Chicago/Turabian StyleAhmed, Hoda A., Mohamed Hagar, and Omaima A. Alhaddad. 2019. "Phase Behavior and DFT Calculations of Laterally Methyl Supramolecular Hydrogen-Bonding Complexes" Crystals 9, no. 3: 133. https://doi.org/10.3390/cryst9030133







