Influence of Pyruvic Acid and UV Radiation on the Morphology of Silica-carbonate Crystalline Biomorphs
Abstract
1. Introduction
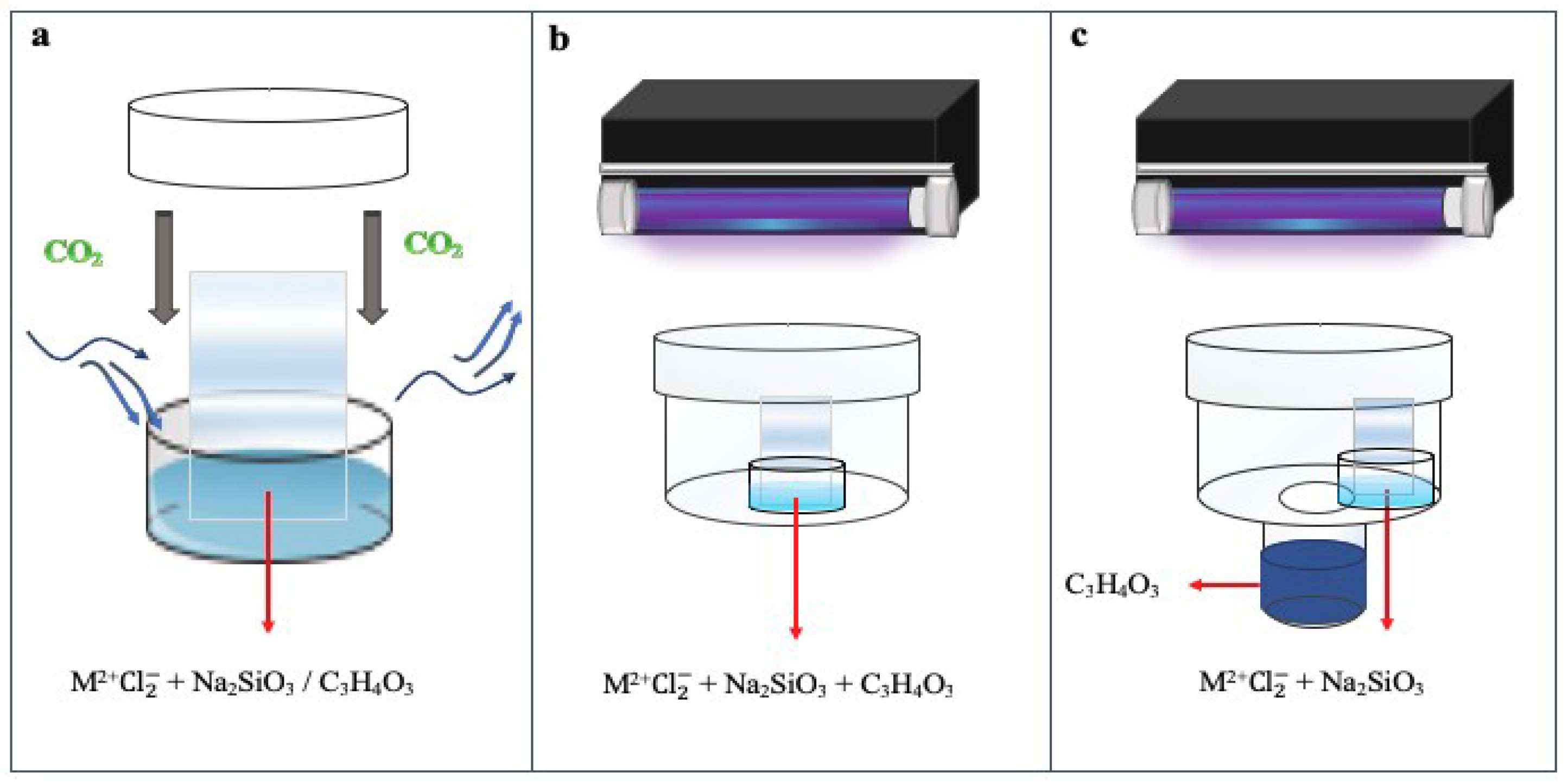
2. Materials and Methods
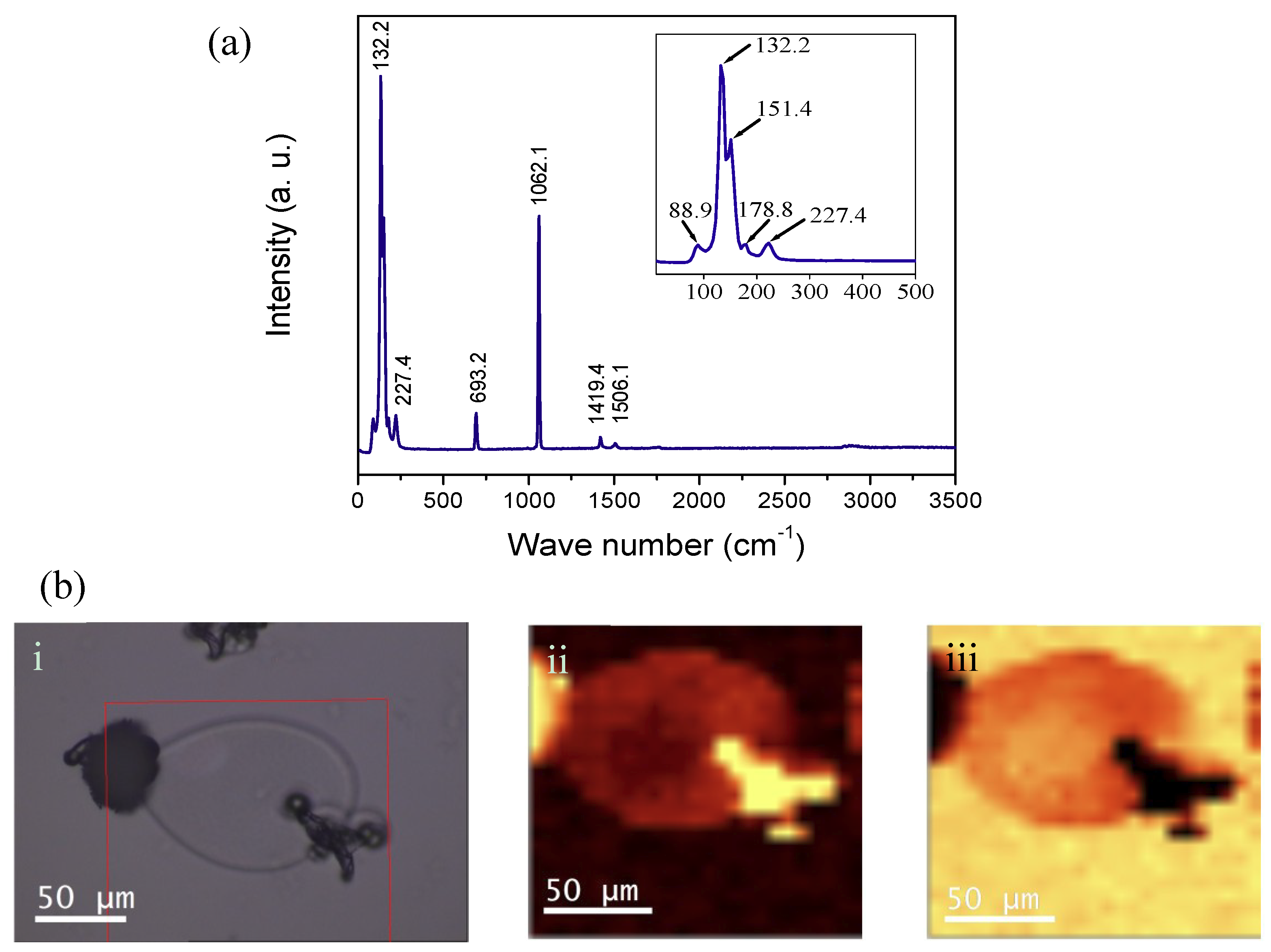
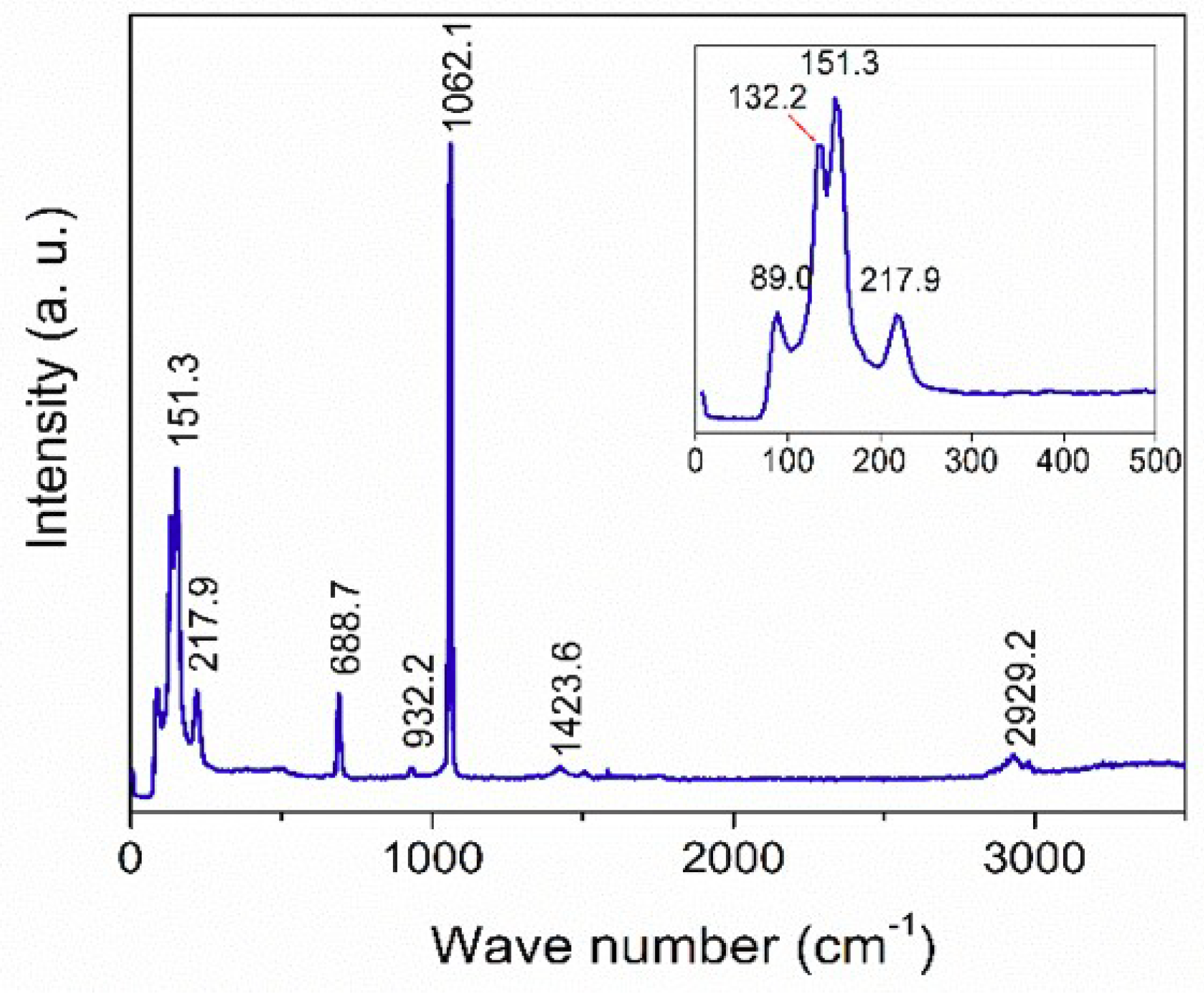
3. Characterization
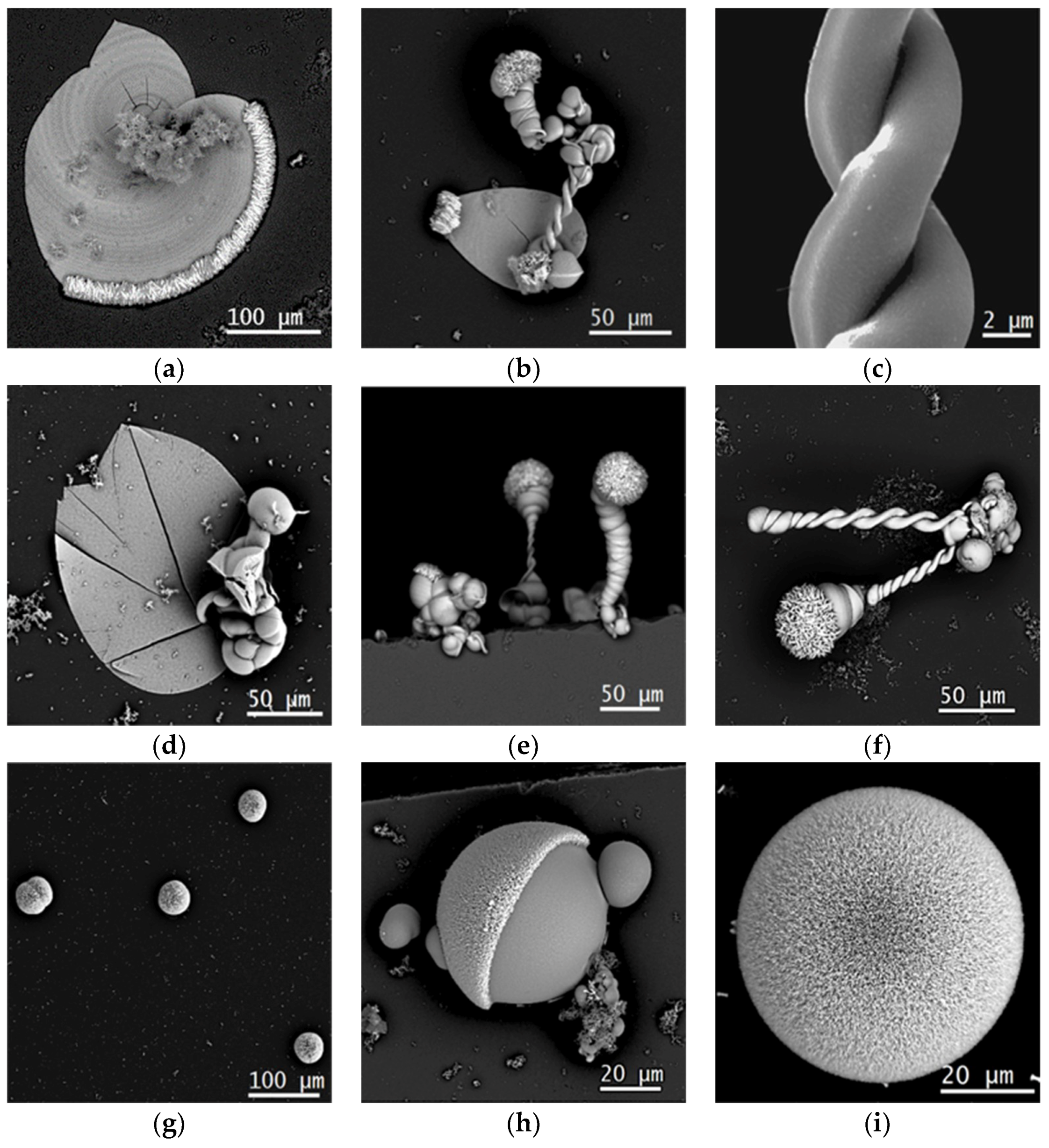
4. Results and discussion
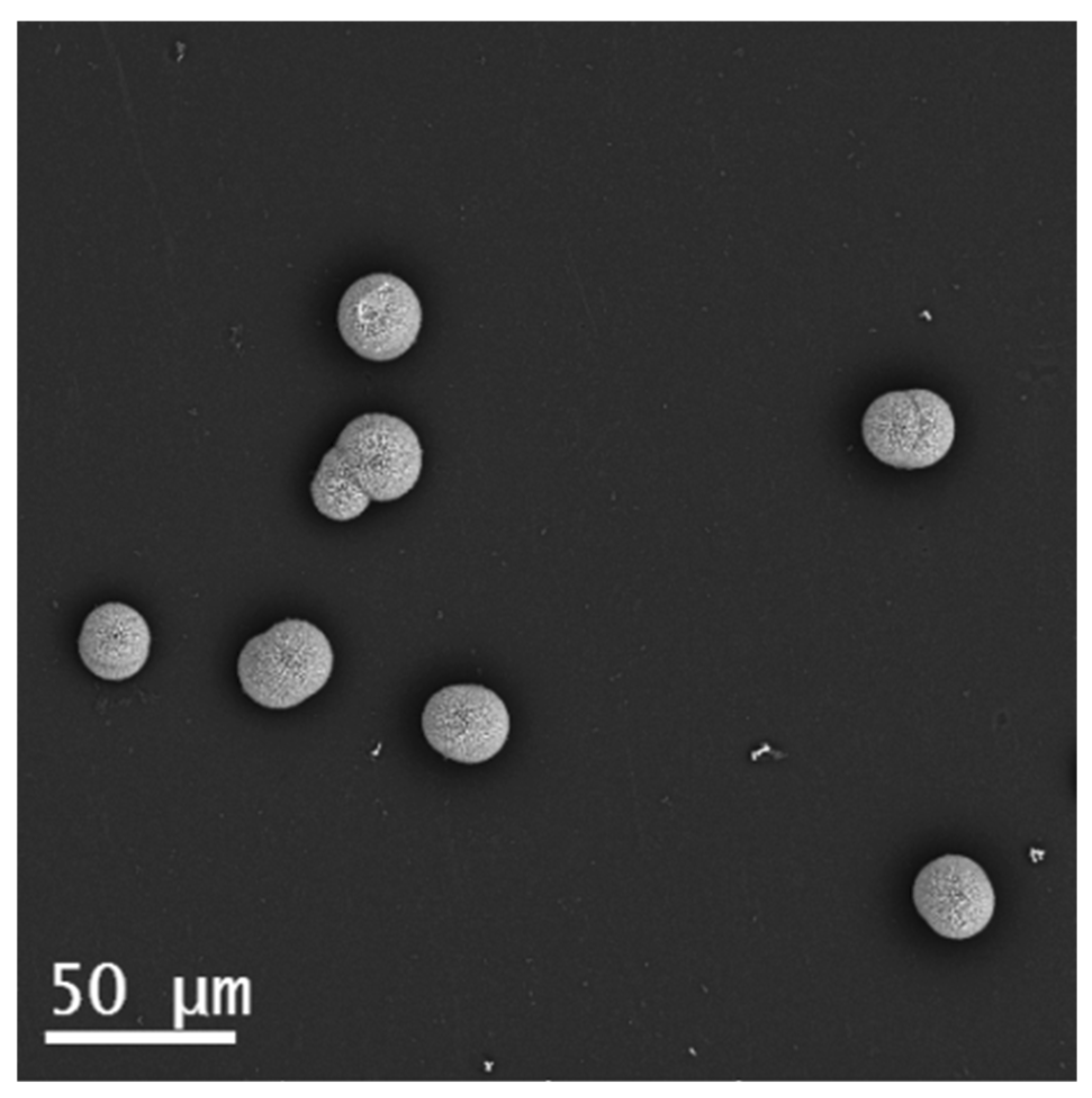
4.1. Gas Diffusion Experiment with PA
4.2. Single-phase Experiment under UV Radiation
4.3. Gas Diffusion Experiment under UV Radiation
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Schopf, J.W. Earth’s Earliest Biosphere; Princeton University Press: Princeton, NJ, USA, 1983. [Google Scholar]
- García-Ruiz, J.M. Inorganic self-organisation in precambrian cherts. Orig. Life Evol. B 1994, 24, 451–467. [Google Scholar] [CrossRef]
- García-Ruiz, J.M.; Carnerup, A.; Christy, A.G.; Welham, N.J.; Hyde, S.T. Morphology: An Ambiguous Indicator of Biogenicity. Astrobiology 2002, 2, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Hyde, S.T. Crystals: Animal, vegetable or mineral? Interface Focus 2015, 5, 20150027. [Google Scholar] [CrossRef] [PubMed]
- Lowenstam, H.A. Minerals formed by organisms. Science 1981, 211, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S. Biomineralization: A structural perspective. J. Struct. Biol. 2008, 163, 229–234. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, J.M. Teoría del crecimiento de cristales en geles. Precipitación polimórfica y agregados cristalinos de morfología inducida. Ph.D. Thesis, Universidad Complutense, Madrid, Spain, 1980. [Google Scholar]
- García-Ruiz, J.M.; Amorós, J.L. Morphological aspects of some symmetrical crystal aggregates grown by silica gel technique. J. Cryst. Growth 1981, 55, 379–383. [Google Scholar] [CrossRef]
- Baird, T.; Braterman, P.S.; Chen, P.; García-Ruiz, J.M.; Peacock, R.D.; Reid, A. Morphology of gel-grown barium carbonate aggregates—pH effect on control by silicate-carbonate membrane. Mater. Res. Bull. 1992, 27, 1031–1040. [Google Scholar] [CrossRef]
- Bittarello, E.; Aquilano, D. Self-assembled nanocrystals of barium carbonate in biomineral-like structures. Eur. J. Mineral. 2007, 19, 345–351. [Google Scholar] [CrossRef]
- Noorduin, W.L.; Grinthal, A.; Mahadevan, L.; Aiznberg, J. Rationally Designed Complex, Hierarchical Microarchitectures. Science 2013, 340, 832–837. [Google Scholar] [CrossRef]
- Nakouzi, E.; Knoll, P.; Hendrix, K.B.; Steinbock, O. Systematic characterization of polycrystalline silica-carbonate helices. Phys. Chem. Chem. Phys. 2016, 18, 23044–23052. [Google Scholar] [CrossRef]
- Nakouzi, E.; Steinbock, O. Self-organization in precipitation reactions far from the equilibrium. Sci. Adv. 2016, 2, e1601144. [Google Scholar] [CrossRef]
- Kellermeier, M.; Eiblmeier, J.; Melero-García, E.; Pretzl, M.; Fery, A.; Kunz, W. Evolution and Control of Complex Curved Form in Simple Inorganic Precipitation Systems. Cryst. Growth Des. 2012, 12, 3647–3655. [Google Scholar] [CrossRef]
- Sánchez-Puig, N.; Guerra-Flores, E.; López-Sánchez, F.; Juárez-Espinoza, P.A.; Ruíz-Arellano, R.; González-Muñoz, R.; Arreguín-Espinosa, R.; Moreno, A. Controlling the morphology of silica-carbonate biomorphs using proteins involved in biomineralization. J. Mater. Sci. 2012, 47, 2943–2950. [Google Scholar] [CrossRef]
- Opel, J.; Wimmer, F.P.; Kellermeier, M.; Cölfen, H. Functionalisation of silica-carbonate biomorphs. Nanoscale Horiz. 2016, 1, 144–149. [Google Scholar] [CrossRef]
- Saladino, R.; Botta, G.; Bizarri, B.M.; Di Mauro, E.; García-Ruíz, J.M. A Global Scale Scenario for Prebiotic Chemistry: Silica-Based Self-Assembled Mineral Structures and Formamide. Biochemistry 2016, 55, 2806–2811. [Google Scholar] [CrossRef] [PubMed]
- Hazen, R.M.; Deamer, D.W. Hydrothermal Reactions of Pyruvic Acid: Synthesis, Selection, and Self-Assembly of Amphiphilic Molecules. Orig. Life Evol. Biosph. 2007, 37, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M.I.; Colussi, A.J.; Hoffmann, M.R. Photoinduced Oligomerization of Aqueous Pyruvic Acid. J. Phys. Chem. A 2006, 110, 3619–3626. [Google Scholar] [CrossRef]
- Lim, H.-J.; Carlton, A.G.; Turpin, B.J. Isoprene Forms Secondary Organic Aerosol through Cloud Processing: Model Simulations. Environ. Sci. Technol. 2005, 39, 4441–4446. [Google Scholar] [CrossRef]
- Carlton, A.G.; Turpin, B.J.; Lim, H.J.; Altieri, K.E.; Seitzinger, S. Link between isoprene and secondary organic aerosol (SOA): Pyruvic acid oxidation yields low volatility organic acid in clouds. Geophys. Res. Lett. 2006, 33, L06822. [Google Scholar] [CrossRef]
- Harris, A.E.R.; Cazaunau, M.; Gratien, A.; Pangui, E.; Doussin, J.-F.; Vaida, V. Multiphase Photochemistry of Pyruvic Acid under Atmospheric Conditions. J. Phys Chem. A 2017, 121, 3327–3339. [Google Scholar] [CrossRef]
- Lin, C.-C.; Liu, L.-G. Post-Aragonite Phase-Transitions in Strontianite and Cerussite—A High-Pressure Raman Spectroscopy Study. J. Phys. Chem. Solids 1997, 58, 977–987. [Google Scholar] [CrossRef]
- Kuo, S.-C.; Tsai, Y.I.; Tsai, C.-H.; Hsieh, L.-Y. Carboxylic acids in PM2.5 over Pinus morrisonicola forest and related photoreaction mechanisms identified via Raman spectroscopy. Atmos. Environ. 2011, 45, 6741–6750. [Google Scholar] [CrossRef]
- Opel, J.; Hecht, M.; Rurack, K.; Eiblmeier, J.; Kunz, W.; Cölfen, H.; Kellermeier, M. Probng local pH-based precipitation processes in self-assembled silica-carbonate hybrid materials. Nanoscale 2015, 7, 17434–17440. [Google Scholar] [CrossRef] [PubMed]
- Eiblmeier, J.; Kellermeier, M.; Deng, M.; Kienle, L.; García-Ruiz, J.M.; Kunz, W. Bottom-Up Self-Assembly of Amorphous Core-Shell-Shell Nanoparticles and Biomimetic Crystal Forms in Inorganic Silica-Carbonate Systems. Chem. Mater. 2013, 25, 1842–1851. [Google Scholar] [CrossRef]
- García-Ruiz, J.M.; Nakouzi, E.; Kotopoulou, E.; Tamborrino, L.; Steinbock, O. Biomimetic mineral self-organization from silica-rich spring waters. Sci. Adv. 2017, 3, e1602285. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Arellano, R.R.; Moreno, A. Obtainment of Spherical-Shaped Calcite Crystals Induced by Intramineral Proteins from Eggshells of Ostrich and Emu. Cryst. Growth Des. 2014, 14, 5137–5143. [Google Scholar] [CrossRef]
- Nakouzi, E.; Knoll, P.; Steinbock, O. Biomorph growth in single-phase systems: Expanding the spectrum and pH range. Chem. Commun. 2016, 52, 2107–2110. [Google Scholar] [CrossRef] [PubMed]
- Grgić, I.; Nieto-Gligorovski, L.I.; Net, S.; Temime-Roussel, B.; Gligorovski, S.; Wortham, H. Light induced multiphase chemistry of gas-phase ozone on aqueous pyruvic and oxalic acids. Phys. Chem. Chem. Phys. 2010, 12, 698–707. [Google Scholar] [CrossRef]
- Altieri, K.E.; Carlton, A.G.; Lim, H.-J.; Turpin, B.J.; Seitzinger, S.P. Evidence for oligomer formation in clouds: Reactions of isoprene oxidation products. Environ. Sci. Technol. 2006, 40, 4956–4960. [Google Scholar] [CrossRef]
- Rouillard, J.; García-Ruiz, J.M.; Gong, J.; van Zuilen, M.A. A morphogram for silica-witherite biomorphs and its application to microfossil identification in the early earth rock record. Geobiology 2018, 16, 279–296. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, K.S.; Moreno, A. Influence of Pyruvic Acid and UV Radiation on the Morphology of Silica-carbonate Crystalline Biomorphs. Crystals 2019, 9, 67. https://doi.org/10.3390/cryst9020067
Pérez KS, Moreno A. Influence of Pyruvic Acid and UV Radiation on the Morphology of Silica-carbonate Crystalline Biomorphs. Crystals. 2019; 9(2):67. https://doi.org/10.3390/cryst9020067
Chicago/Turabian StylePérez, Karina S., and Abel Moreno. 2019. "Influence of Pyruvic Acid and UV Radiation on the Morphology of Silica-carbonate Crystalline Biomorphs" Crystals 9, no. 2: 67. https://doi.org/10.3390/cryst9020067
APA StylePérez, K. S., & Moreno, A. (2019). Influence of Pyruvic Acid and UV Radiation on the Morphology of Silica-carbonate Crystalline Biomorphs. Crystals, 9(2), 67. https://doi.org/10.3390/cryst9020067





