Solvent Effects on the Spin Crossover Properties of Iron(II) Imidazolylimine Complexes
Abstract
1. Introduction
2. Materials and Methods
2.1. General Remarks
2.1.1. Synthesis of 4-ima-Bp
2.1.2. Synthesis of fac-[Fe(4-ima-Bp)3](ClO4)2⋅3EtOH 1
2.1.3. Synthesis of fac-[Fe(4-ima-Bp)3](ClO4)2⋅3MeOH 2
2.1.4. Synthesis of fac-[Fe(4-ima-Bp)3](BF4)2⋅EtOH⋅4H2O 3
2.1.5. Synthesis of fac-[Fe(4-ima-Bip)3](BF4)2⋅4H2O 4
2.1.6. Synthesis of fac-[Fe(4-ima-Bip)3](BF4)2⋅3.5MeCN 5
2.2. VSM and SQUID Magnetometry Studies
2.3. X-ray Crystallography
3. Results
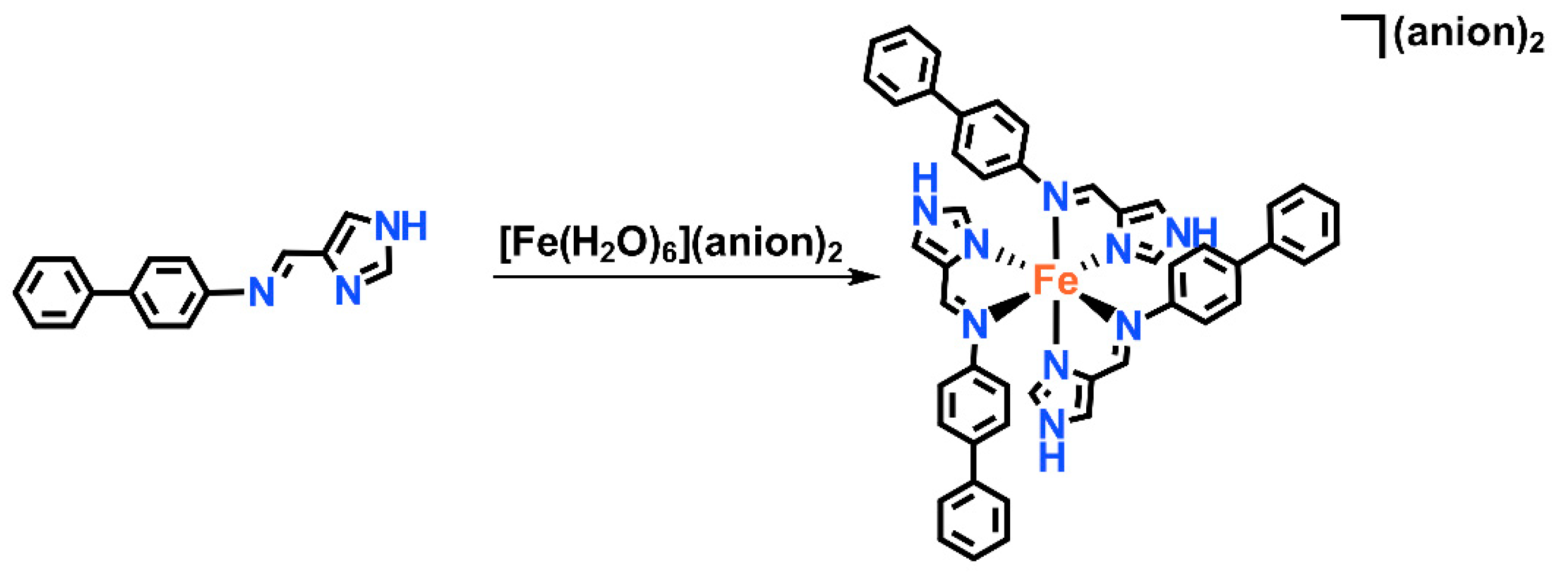
3.1. Synthesis and Characterization of fac-[Fe(4-ima-Bp)3](Y)2⋅sol Complexes
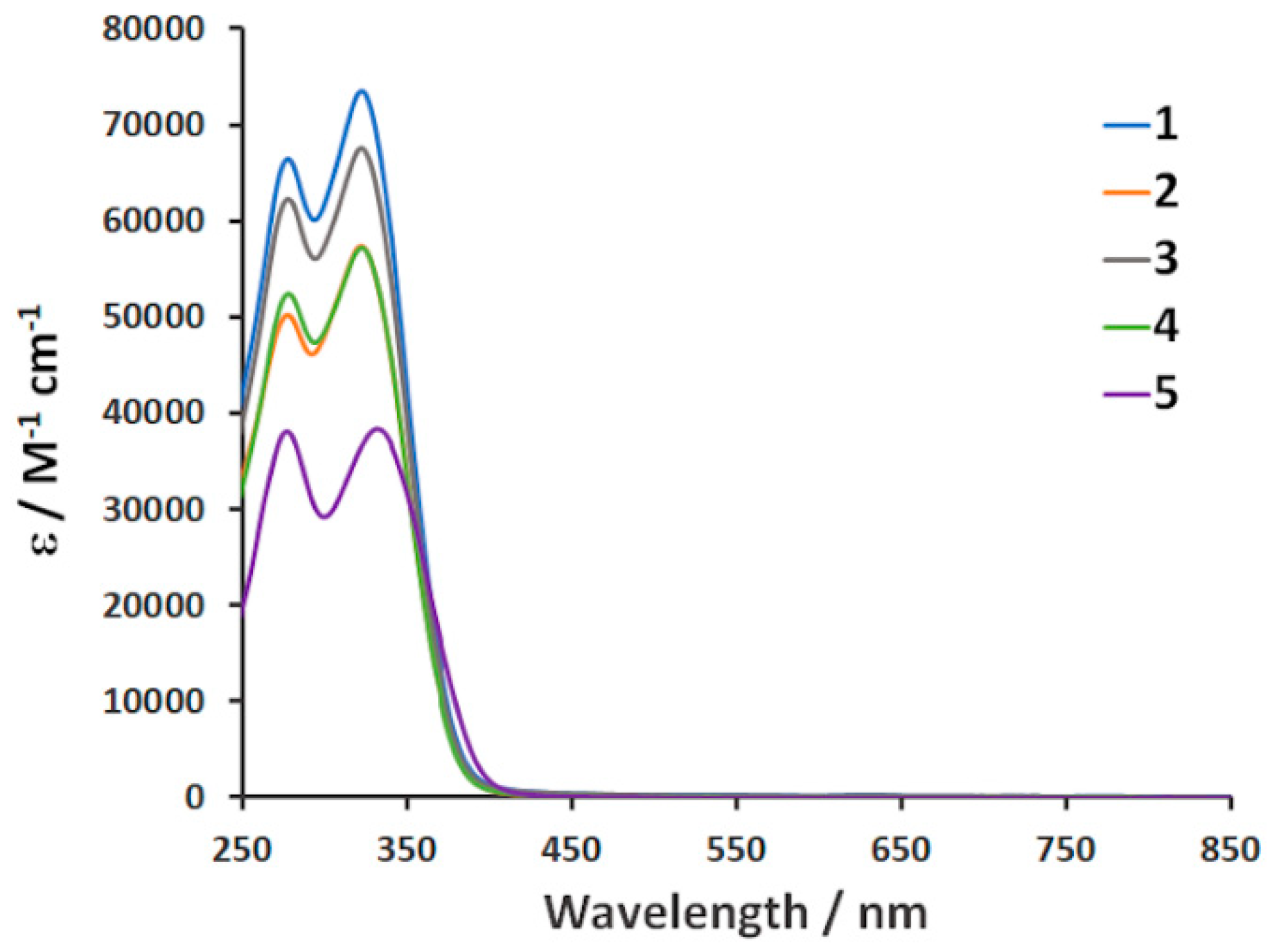
3.2. IR and UV–VIS Spectroscopy
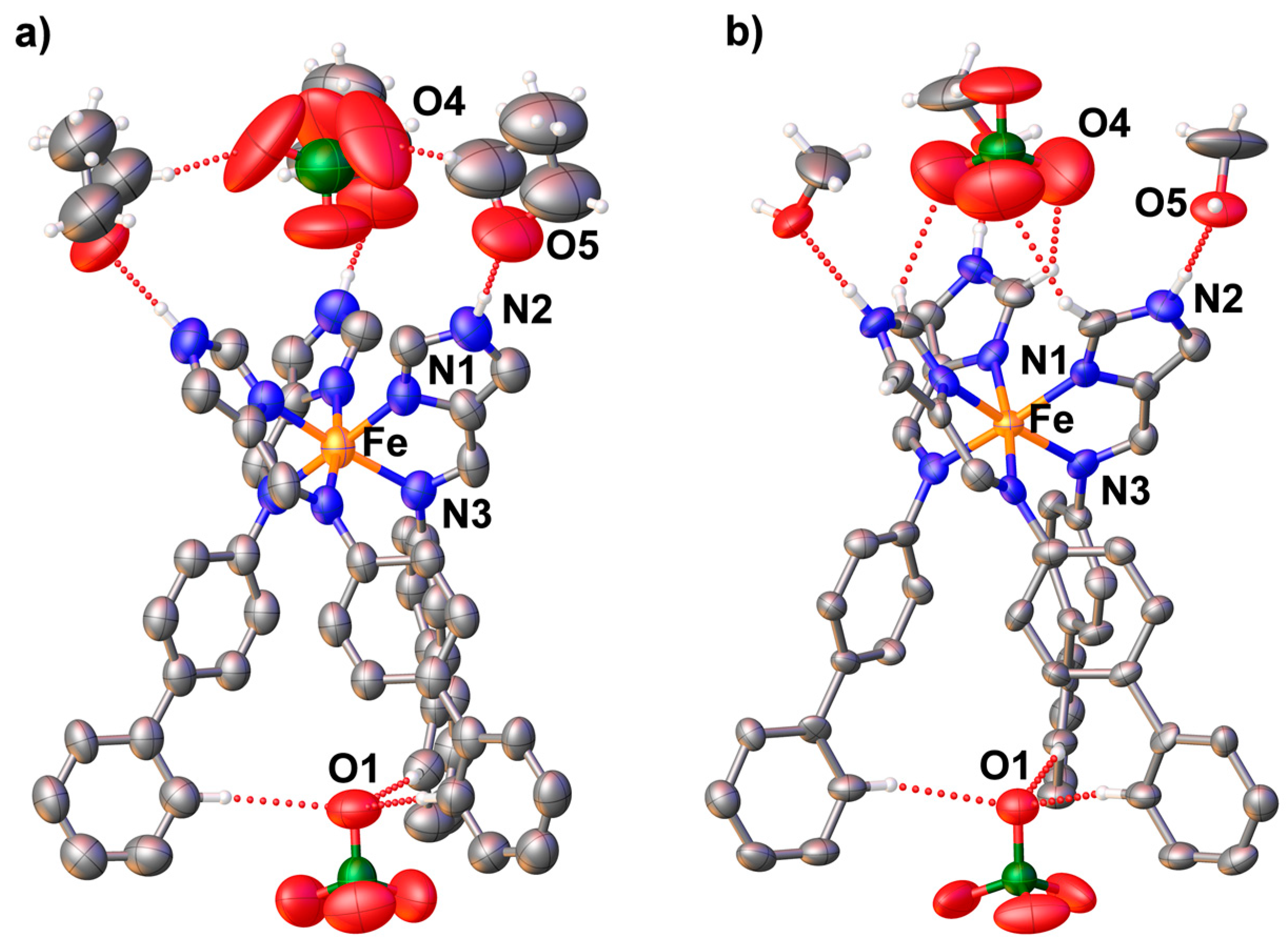
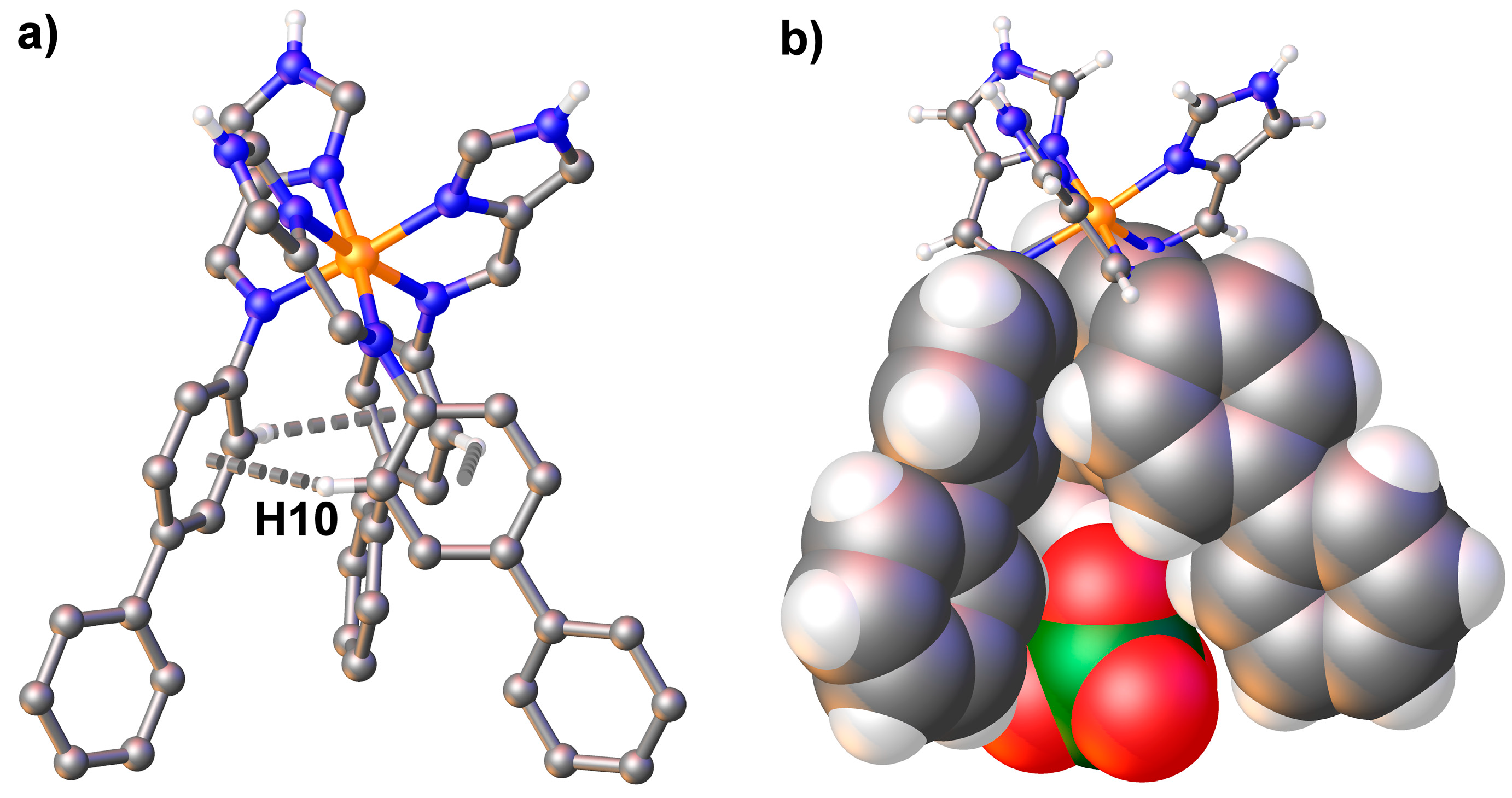
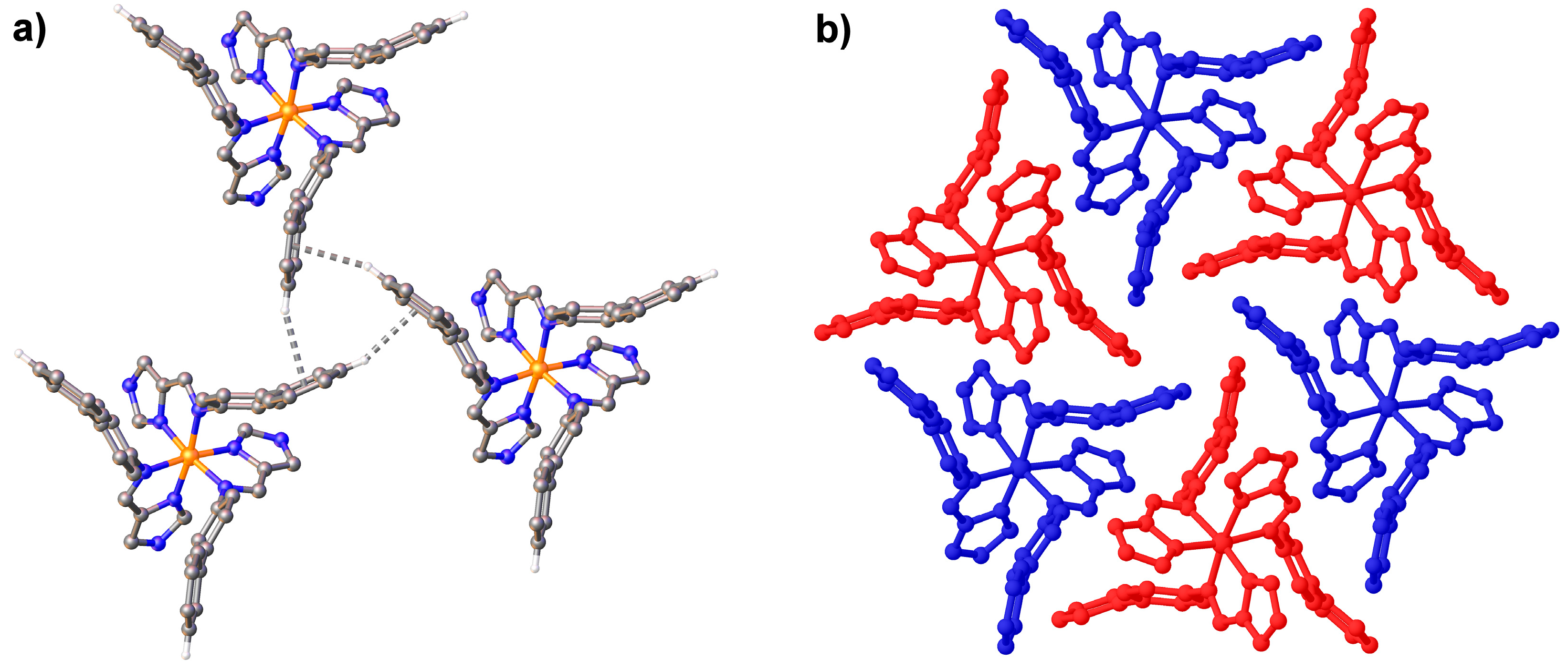
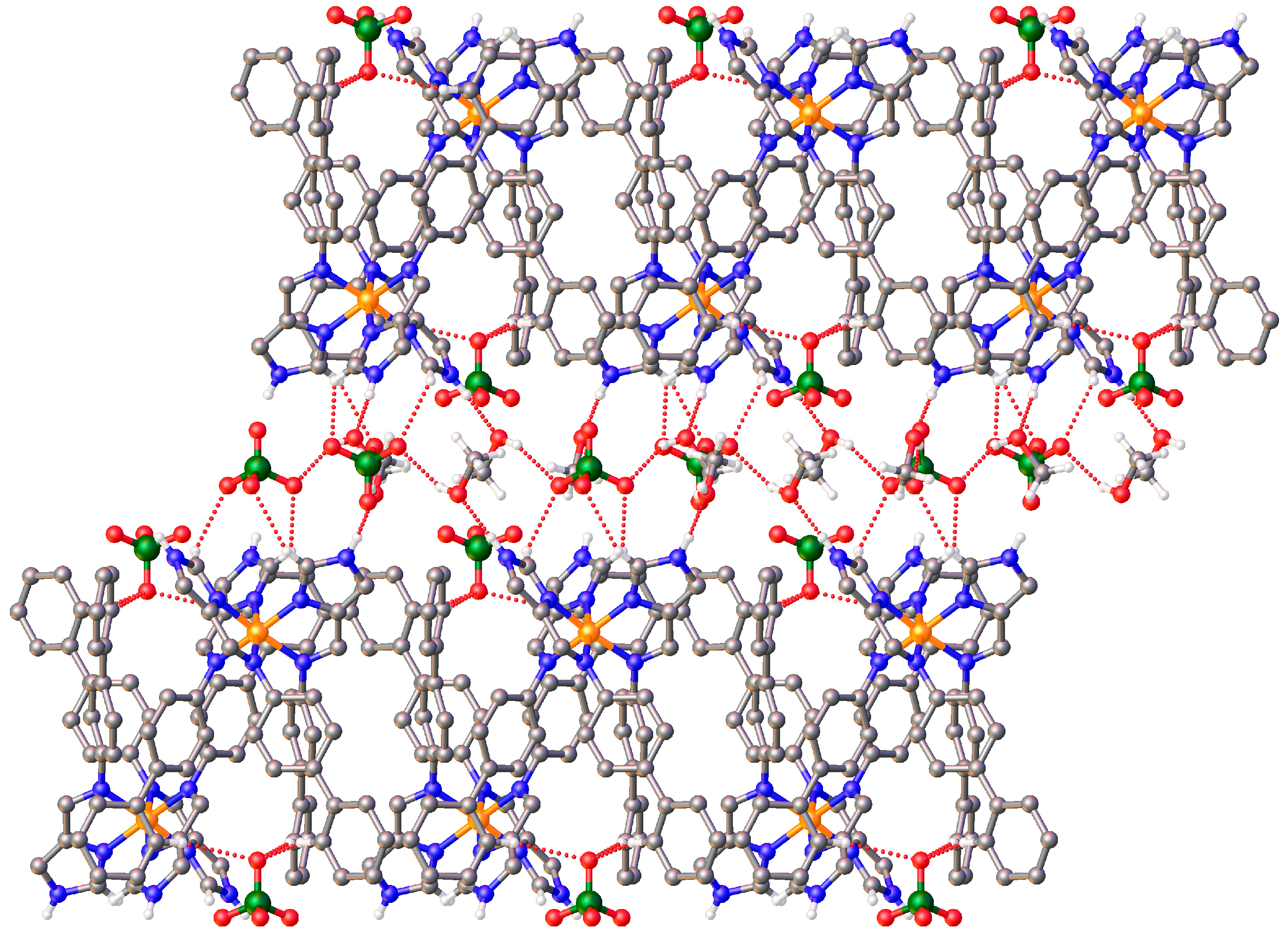
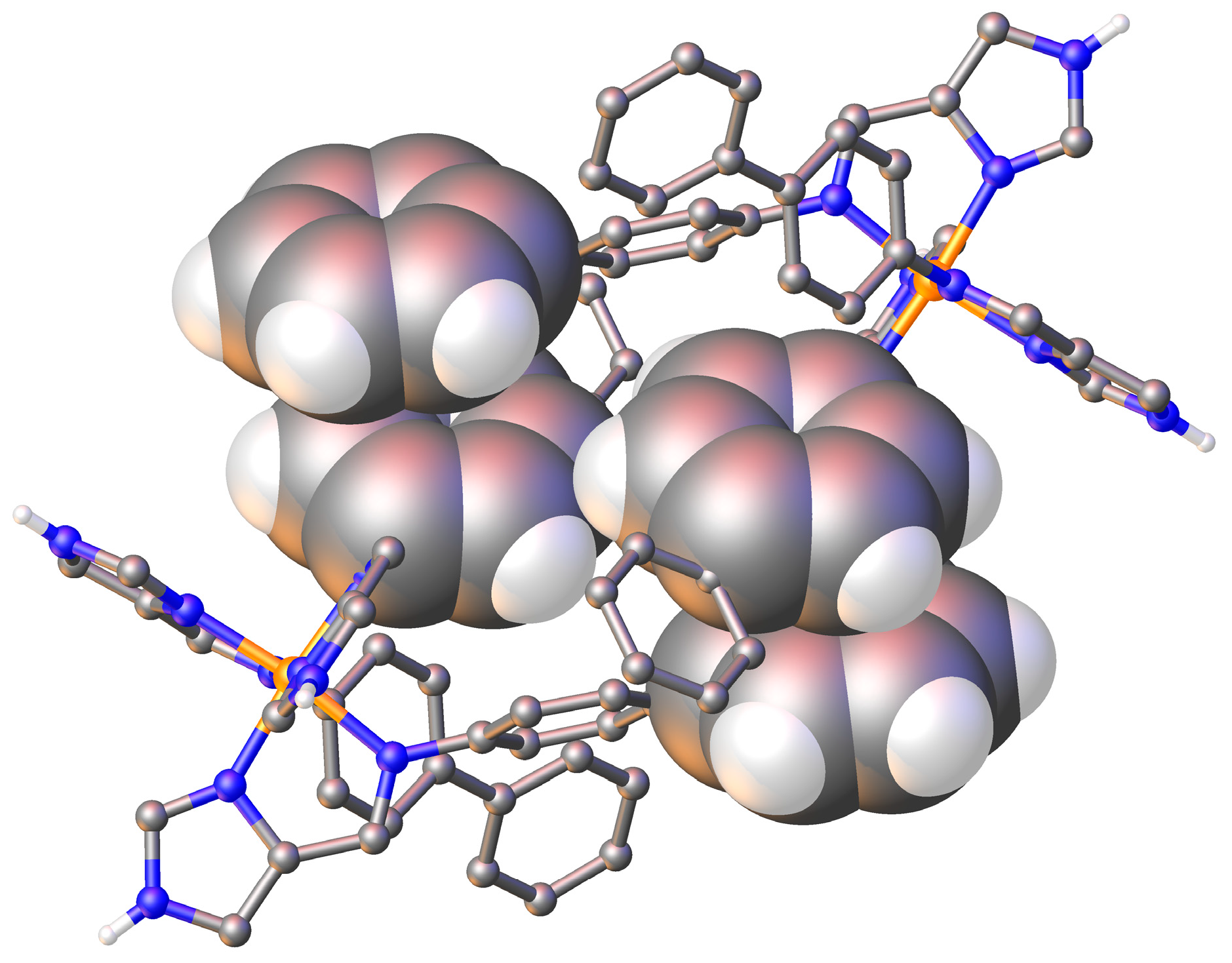
3.3. Structural Studies of fac-[Fe(4-ima-Bp)3](Y)2·sol Complexes
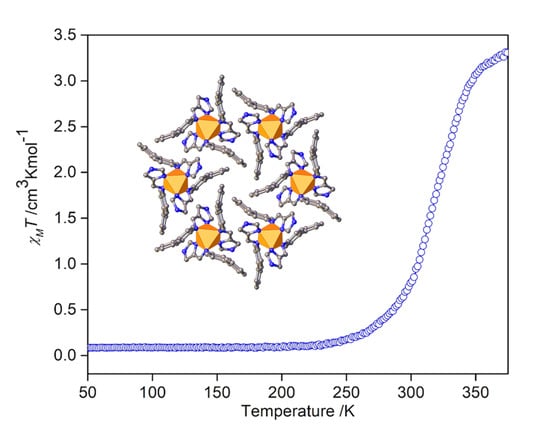
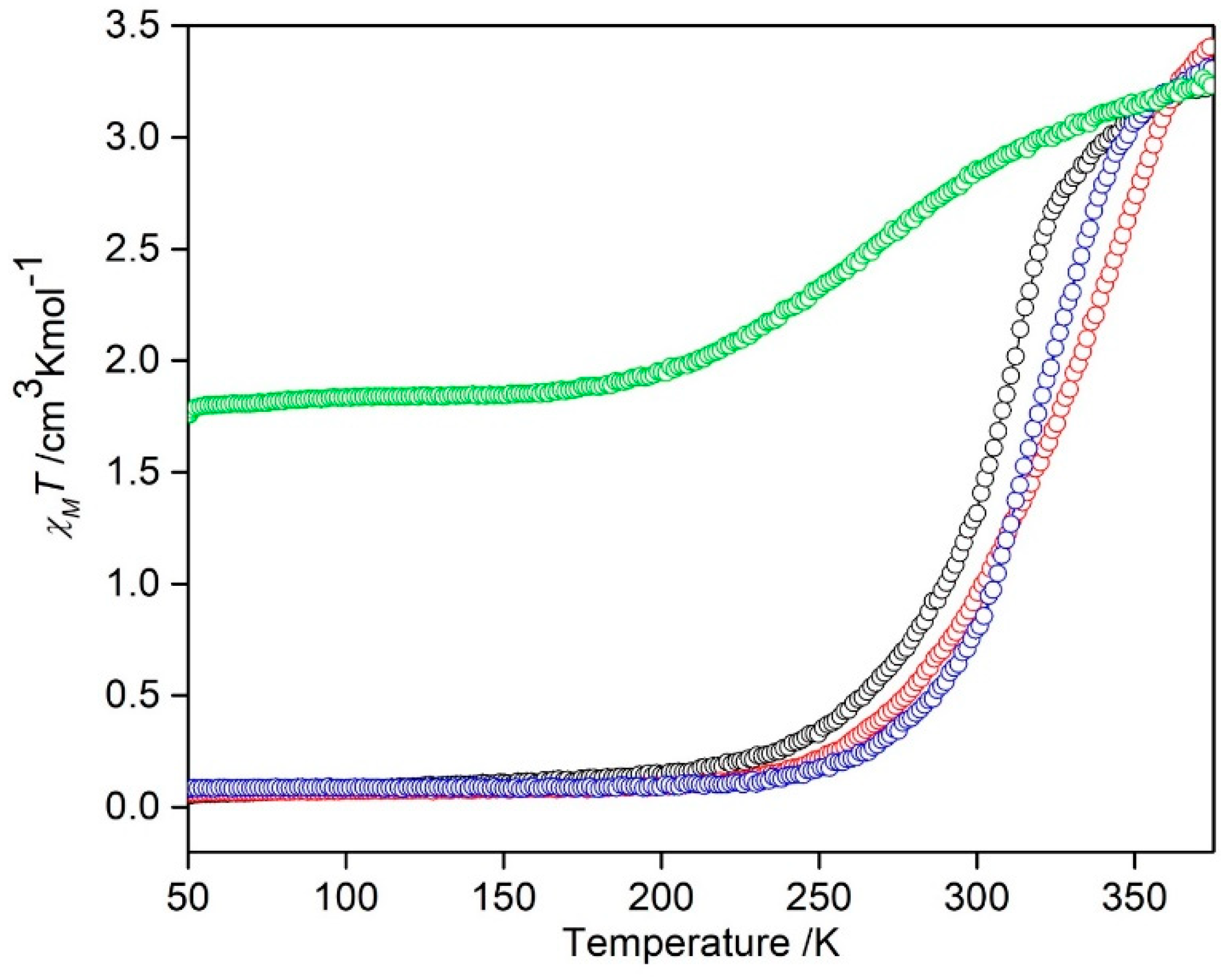
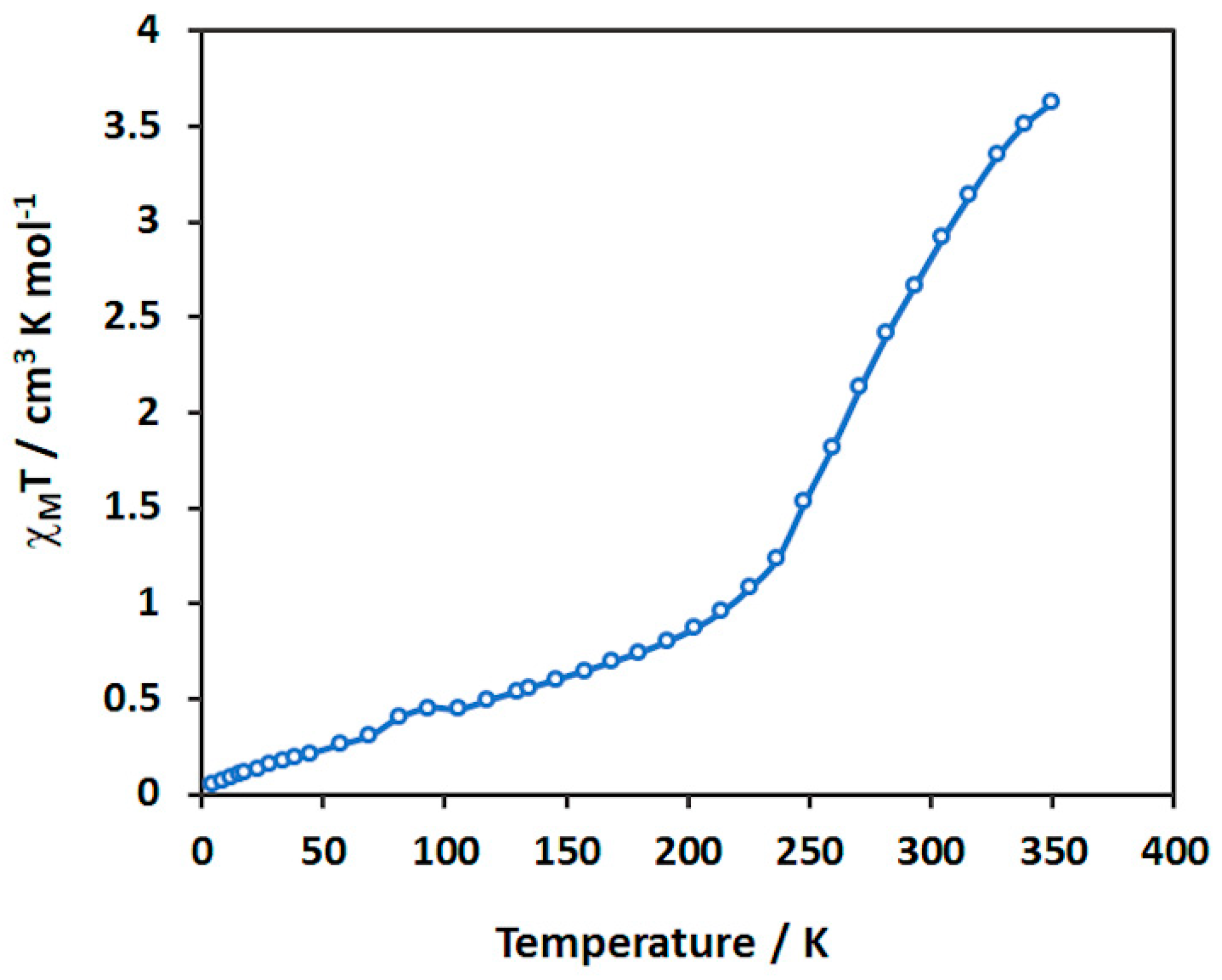
3.4. Magnetic studies of [Fe(4-ima-Bp)3](Y)2⋅sol Complexes
3.5. Thermochromism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Spin-Crossover Materials: Properties and Applications; Halcrow, M.A., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2013. [Google Scholar]
- Gütlich, P.; Goodwin, H.A. Spin Crossover—An Overall Perspective. Top. Curr. Chem. 2004, 233, 1–47. [Google Scholar]
- Boillot, M.L.; Weber, B. Mononuclear ferrous and ferric complexes. Comptes Rendus Chim. 2018, 21, 1196–1208. [Google Scholar] [CrossRef]
- Collet, E.; Guionneau, P. Structural analysis of spin-crossover materials: From molecules to materials. Comptes Rendus Chim. 2018, 21, 1133–1151. [Google Scholar] [CrossRef]
- Craig, G.A.; Roubeau, O.; Aromí, G. Spin state switching in 2,6-bis(pyrazol-3-yl)pyridine (3-bpp) based Fe(II) complexes. Coord. Chem. Rev. 2014, 269, 13–31. [Google Scholar] [CrossRef]
- Brooker, S. Spin crossover with thermal hysteresis: practicalities and lessons learnt. Chem. Soc. Rev. 2015, 44, 2880–2892. [Google Scholar] [CrossRef] [PubMed]
- Halcrow, M.A. Structure:function relationships in molecular spin-crossover complexes. Chem. Soc. Rev. 2011, 40, 4119–4142. [Google Scholar] [CrossRef] [PubMed]
- Létard, J.-F.; Guionneau, P.; Goux-Capes, L. Towards Spin Crossover Applications. Top. Curr. Chem. 2004, 235, 221–249. [Google Scholar]
- Senthil, K.; Ruben, M. Emerging trends in spin crossover (SCO) based functional materials and devices. Coord. Chem. Rev. 2017, 346, 176–205. [Google Scholar] [CrossRef]
- Miller, R.G.; Brooker, S. Reversible quantitative guest sensing via spin crossover of an iron(ii) triazole. Chem. Sci. 2016, 7, 2501–2505. [Google Scholar] [CrossRef]
- Létard, J.-F. Photomagnetism of iron(II) spin crossover complexes the T(LIESST) approach. J. Mater. Chem. 2006, 16, 2550–2559. [Google Scholar] [CrossRef]
- Chastanet, G.; Desplanches, C.; Baldé, C.; Rosa, P.; Marchivie, M.; Guionneau, P. A critical review of the T(LIESST) temperature in spin crossover materials-What it is and what it is not. Chem. Sq. 2018, 2, 2. [Google Scholar] [CrossRef]
- Scott, H.S.; Staniland, R.W.; Kruger, P.E. Spin crossover in homoleptic Fe(II) imidazolylimine complexes. Coord. Chem. Rev. 2018, 362, 24–43. [Google Scholar] [CrossRef]
- Sunatsuki, Y.; Kawamoto, R.; Fujita, K.; Maruyama, H.; Suzuki, T.; Ishida, H.; Kojima, M.; Iijima, S.; Matsumoto, N. Structures and spin states of mono- and dinuclear iron(II) complexes of imidazole-4-carbaldehyde azine and its derivatives. Coord. Chem. Rev. 2010, 254, 1871–1881. [Google Scholar] [CrossRef]
- Yamada, M.; Ooidemizu, M.; Ikuta, Y.; Osa, S.; Matsumoto, N.; Iijima, S.; Kojima, M.; Dahan, F.; Tuchagues, J.P. Interlayer Interaction of Two-Dimensional Layered Spin Crossover Complexes [FeIIH3LMe][FeIILMe]X (X = ClO4–, BF4–). Inorg. Chem. 2003, 42, 8406–8416. [Google Scholar] [CrossRef] [PubMed]
- Bréfuel, N.; Watanabe, H.; Toupet, L.; Come, J.; Matsumoto, N.; Collet, E.; Tanaka, K.; Tuchagues, J.-P. Concerted spin crossover and symmetry breaking yield three thermally and one light-induced crystallographic phases of a molecular material. Angew. Chem. Int. Ed. 2009, 48, 9304–9307. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Hagiwara, H.; Torigoe, H.; Matsumoto, N.; Kojima, M.; Dahan, F.; Tuchagues, J.P.; Re, N.; Iijima, S. A variety of spin-crossover behaviors depending on the counter anion: Two-dimensional complexes constructed by NH⋯Cl-hydrogen bonds, [FeIIH3LMe]Cl·X (X = PF6–, AsF6–, SbF6–, CF3SO3–; H3LMe = tris[2-{[(2methylimidazol-4-yl)methyl idene]amino}ethyl]amine). Chem. Eur. J. 2006, 12, 4536–4549. [Google Scholar] [CrossRef] [PubMed]
- Seredyuk, M.; Muñoz, M.C.; Castro, M.; Romero-Morcillo, T.; Gaspar, A.B.; Real, J.A. Unprecedented multi-stable spin crossover molecular material with two thermal memory channels. Chem. Eur. J. 2013, 19, 6591–6596. [Google Scholar] [CrossRef] [PubMed]
- Delgado, T.; Tissot, A.; Guénée, L.; Hauser, A.; Valverde-Muñoz, F.J.; Seredyuk, M.; Real, J.A.; Pillet, S.; Bendeif, E.-E.; Besnard, C. Very Long-Lived Photogenerated High-Spin Phase of a Multistable Spin-Crossover Molecular Material. J. Am. Chem. Soc. 2018, 140, 12870–12876. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Arata, S.; Matsumoto, N.; Iijima, S.; Sunatsuki, Y.; Ishida, H.; Kojima, M. One-dimensional Spin-crossover iron(II) complexes bridged by intermolecular imidazole-pyridine NH⋅⋅⋅N hydrogen bonds, [Fe(HLMe)3]X2 (HLMe = (2-Methylimidazol-4-yl-methylideneamino-2-ethylpyridine; X = PF6–, ClO4–, BF4–). Inorg. Chem. 2010, 49, 1517–1523. [Google Scholar] [CrossRef]
- Nishi, K.; Matsumoto, N.; Iijima, S.; Halcrow, M.A.; Sunatsuki, Y.; Kojima, M. A hydrogen bond motif giving a variety of supramolecular assembly structures and spin-crossover behaviors. Inorg. Chem. 2011, 50, 11303–11305. [Google Scholar] [CrossRef]
- Fujinami, T.; Nishi, K.; Hamada, D.; Murakami, K.; Matsumoto, N.; Iijima, S.; Kojima, M.; Sunatsuki, Y. Scan Rate Dependent Spin Crossover Iron(II) Complex with Two Different Relaxations and Thermal Hysteresis fac-[FeII(HLn-Pr)3]Cl·PF6 (HLn-Pr = 2-Methylimidazol-4-yl-methylideneamino-n-propyl). Inorg. Chem. 2015, 54, 7291–7300. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.R.; Archer, R.J.; Hawes, C.S.; Ferguson, A.; Wattiaux, A.; Mathonière, C.; Clérac, R.; Kruger, P.E. Thermally and photo-induced spin crossover behaviour in an Fe(II) imidazolylimine complex: [FeL3](ClO4)2. Dalton Trans. 2012, 41, 12720–12725. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.-G.; Pang, C.-Y.; Qiu, D.; Zhang, J.; Huang, J.-L.; Qin, L.-F.; Sun, A.-Q.; Li, Z. Homochiral iron(II) complexes based on imidazole Schiff-base ligands: Syntheses, structures, and spin-crossover properties. Inorg. Chem. Commun. 2013, 35, 164–168. [Google Scholar] [CrossRef]
- Rigaku. Rigaku XRD; Rigaku Corporation: Tokyo, Japan, 1996. [Google Scholar]
- Bruker APEXII; Bruker AXS Inc.: Madison, WI, USA, 2005.
- SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2003.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–342. [Google Scholar] [CrossRef]
- Fujinami, T.; Nishi, K.; Matsumoto, N.; Iijima, S.; Halcrow, M.A.; Sunatsukid, Y.; Kojima, M. 1D and 2D assembly structures by imidazole···chloride hydrogen bonds of iron(II) complexes [FeII(HLn-Pr)3]Cl·Y(HLn-Pr = 2-methylimidazol-4-yl-methylideneamino-n-propyl; Y = AsF6–, BF4–) and their spin states. Dalton Trans. 2011, 40, 12301–12309. [Google Scholar] [CrossRef]
- Sunatsuki, Y.; Kawamoto, R.; Fujita, K.; Maruyama, H.; Suzuki, T.; Ishida, H.; Kojima, M.; Iijima, S.; Matsumoto, N. Structures and spin states of bis(tridentate)-type mononuclear and triple helicate dinuclear iron(II) complexes of imidazole-4-carbaldehyde azine. Inorg. Chem. 2009, 48, 8784–8795. [Google Scholar] [CrossRef]
- McCusker, J.K.; Rheingold, A.L.; Hendrickson, D.N. Variable-Temperature Studies of Laser-Initiated 5T2 to 1A1 Intersystem Crossing in Spin-Crossover Complexes: Empirical Correlations between Activation Parameters and Ligand Structure in a Series of Polypyridyl Ferrous Complexes. Inorg. Chem. 1996, 35, 2100–2112. [Google Scholar] [CrossRef]
- Marchivie, M.; Guionneau, P.; Létard, J.F.; Chasseau, D. Photo-induced spin-transition: the role of the iron(II) environment distortion. Acta Crystallogr. Sect. B Struct. Sci. 2005, 61, 25–28. [Google Scholar] [CrossRef]
- Furushou, D.; Hashibe, T.; Fujinami, T.; Nishi, K.; Hagiwara, H.; Matsumoto, N.; Sunatsuki, Y.; Kojima, M.; Iijima, S. Reprint of "facial and meridional geometrical isomers of tris(2-methylimidazol-4-yl-methylideneaminobenzyl)iron(II) with delta- and lambda-configurations and their enantio-discriminative assembly via imidazole···chloride hydrogen bonding and spin crossover. Polyhedron 2012, 44, 194–203. [Google Scholar] [CrossRef]

| Compound | 1 | 2 | 5 |
|---|---|---|---|
| Formula | C54H57Cl2FeN9O11 | C51H51Cl2FeN9O11 | C55.5H49.5B2FeN12.5F8 |
| Molecular weight/gmol−1 | 1134.83 | 1092.76 | 1080.04 |
| Crystal system | Trigonal | Trigonal | Triclinic |
| Space group | R | R | P |
| a/Å | 13.1242(9) | 12.9080(15) | 13.0204(7) |
| b/Å | 13.1242(9) | 12.9080(15) | 13.1932(8) |
| c/Å | 53.258(4) | 52.5240(4) | 18.4021(11) |
| α/° | 90 | 90 | 73.540(4) |
| β/° | 90 | 90 | 86.375(4) |
| γ/° | 120 | 120 | 61.569(3) |
| T/K | 143(2) | 153(2) | 123(2) |
| Cell volume/Å3 | 7944.4(12) | 7578.9(18) | 2656.3(3) |
| Z | 6 | 6 | 4 |
| Absorption coefficient/mm−1 | 3.797 | 3.958 | 0.363 |
| Reflections collected | 23981 | 19730 | 39419 |
| Independent reflections, Rint | 3413, 0.094 | 2875, 0.092 | 9354, 0.0825 |
| Max. and min. transmission | 0.561, 1.000 | 1.000 and 0.772 | - |
| Restraints/parameters | 2/206 | 0/224 | 0/878 |
| Final R indices [I>2σ(I)]: R1, wR2 | 0.1383, 0.3738 | 0.122, 0.392 | 0.1142, 0.2301 |
| CCDC no. | 1892568 | 1892567 | 1892569 |
| Compound | %yield | Colour | IR (cm−1) | ||||
|---|---|---|---|---|---|---|---|
| νC=N | νC=C | νOH | νArH | νanion | |||
| 1 (ClO4⋅3EtOH) | 53 | Dark red | 1620 | 1484 | 3362 | 3128 | 1087 |
| 2 (ClO4⋅3EtOH) | 67 | Dark red | 1621 | 1484 | 3380 | 3135 | 1089 |
| 3 (BF4⋅EtOH⋅4H2O) | 77 | Dark red | 1620 | 1484 | 3377 | 3137 | 1083 |
| 4 (BF4⋅4H2O) | 57 | Dark red | 1620 | 1484 | 3377 | 3137 | 1083 |
| 5 (BF4⋅3.5MeCN) | 63 | Red Orange | 1620 | 1484 | - | 3144 | 1051 |
| Compound | λmax/nm (εmax/M−1cm−1) |
|---|---|
| 1 | 328 (65,000), 283 (72,000) |
| 2 | 326 (57,000), 283 (50,000) |
| 3 | 327 (66,200), 283 (61,000) |
| 4 | 328 (56,000), 283 (51,000) |
| Bond lengths | 1-143 K | 2-153 K | 5-123 K | |
|---|---|---|---|---|
| Fe1–N1 | 1.962(6) | 1.964(8) | Fe1–N1 | 1.971(4) |
| - | - | Fe1–N3 | 1.995(4) | |
| - | - | Fe1–N4 | 1.962(4) | |
| Fe1–N3 | 2.005(5) | 1.983(8) | Fe1–N6 | 1.992(4) |
| - | - | Fe1–N7 | 1.963(4) | |
| - | - | Fe1–N9 | 2.001(4) | |
| Σ [33] | 68.7 | 55.7 | 57.9 | |
| Θ [34] | 196.4 | 127.3 | 125.0 | |
| N2-H2⋅⋅⋅O5 | 1.829(9) | 1.832(11) | N8-H8⋅⋅⋅F3 | 2.109(4) |
| O5-H5⋅⋅⋅O4 | - | 2.09(2) | N8-H8⋅⋅⋅F2 | 2.426(4) |
| N5-H5⋅⋅⋅F1 | 1.939(4) | |||
| N2-H2⋅⋅⋅N10 | 2.041(5) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sertphon, D.; Harding, P.; Murray, K.S.; Moubaraki, B.; Neville, S.M.; Liu, L.; Telfer, S.G.; Harding, D.J. Solvent Effects on the Spin Crossover Properties of Iron(II) Imidazolylimine Complexes. Crystals 2019, 9, 116. https://doi.org/10.3390/cryst9020116
Sertphon D, Harding P, Murray KS, Moubaraki B, Neville SM, Liu L, Telfer SG, Harding DJ. Solvent Effects on the Spin Crossover Properties of Iron(II) Imidazolylimine Complexes. Crystals. 2019; 9(2):116. https://doi.org/10.3390/cryst9020116
Chicago/Turabian StyleSertphon, Darunee, Phimphaka Harding, Keith S. Murray, Boujemaa Moubaraki, Suzanne M. Neville, Lujia Liu, Shane G. Telfer, and David J. Harding. 2019. "Solvent Effects on the Spin Crossover Properties of Iron(II) Imidazolylimine Complexes" Crystals 9, no. 2: 116. https://doi.org/10.3390/cryst9020116
APA StyleSertphon, D., Harding, P., Murray, K. S., Moubaraki, B., Neville, S. M., Liu, L., Telfer, S. G., & Harding, D. J. (2019). Solvent Effects on the Spin Crossover Properties of Iron(II) Imidazolylimine Complexes. Crystals, 9(2), 116. https://doi.org/10.3390/cryst9020116