Abstract
The design and synthesis of magnets with low dimensionality is currently an attractive research topic. The reaction of a Schiff base metal complex with simple molybdate has led to the formation of a new {MnIII-Schiff-base}-decorated molybdate heterometallic cluster, [{Mn(salpn)(H2O)}3MoO4](CH3COO)·2H2O (1) (salpn = N,N’-(1,3-propylene)bis(salicylideneiminate)). The hybrid aggregate was characterized using a range of methods including elemental analysis, single crystal x-ray diffraction, Infrared spectra (IR) spectroscopy, and x-ray structural analysis. The crystals of 1 are hexagonal: P63/m, a = 14.027(5) Å, c = 17.487(5) Å, V = 1142.6(3) Å3. Structural analyses indicate that we report on the preparation of the first triple metallic-oligomer held by orthomolybdate. Density functional theoretical calculation (DFT) studies have been performed to calculate electronic structure and potential energy landscapes. Additionally, the magnetic property of 1 indicates an antiferromagnetic coupling between the metal centers in the complex.
1. Introduction
Organic-inorganic hybrids, a class of promising materials that simultaneously combine the remarkable features of both components into one material, presenting properties from either part of the distinctive building blocks, have become a focus of research interest in the past few decades [1,2,3,4]. In recent years, more and more attention has been paid to Schiff base hybrids, not only because of their variety of architectures and fascinating topologies, but also for their remarkable applications in magnetism, asymmetric catalysis, adsorption, and medicine [5,6,7,8]. Thanks to the higher thermal and chemical stability of inorganic carriers, the migration of metal Schiff base building blocks onto inorganic carriers conceives an alternative scientific method for the construction of new types of hybrid materials [9,10].
In recent years, various types of pure inorganic building blocks have been successfully introduced into Mn-salen molecular packing in crystals. This opens up a plethora of applications ranging from the synthesis of interesting magnetic compounds to the explorations of magnetic exchange [11,12,13,14,15]. As reported by our group previously, the introduction of diamagnetic B-type Anderson polyoxometalates (POMs) into Mn-dimer Schiff base precursors has led to the extraction of their intrinsic SMM behavior [16]. In the next couple of years, the continuous quest for large-size inorganic building units, especially the classical saturated polyoxoanion, incorporated with MnIII (Schiff base) systems will yield a sequence of new magnetic hybrids [17,18,19,20]. Although a vast number of saturated or monovacant polyoxoanions are known as a unique class of building units from which Mn-salen compounds are derived, tiny inorganic moieties largely remain unexplored. Meanwhile, the mechanism of the formation of crystalline materials has not been well clarified, especially for hybrid species compounds. Therefore, whether in theory or in practice, it is vital in the search for new reactions, which will in turn aid the investigation into the relationship between the hybrid structure and diverse precursors derived from the well-characterized Mn-Schiff based family and different inorganic building blocks.
Based on the above considerations, we introduce the molybdate (NH4)6Mo7O24·4H2O and [Mn(salpn)(H2O)]+ as starting materials for our design strategy resulting in the formation of a supramolecular aggregate [{Mn(salpn)(H2O)}3MoO4](CH3COO)·2H2O (1) (salpn = N,N’-(1,3-propylene)bis(salicylideneiminate)), see Scheme 1). As far as we know, compound 1 represents the first example of a triple metallic-oligomer linked by orthomolybdate.
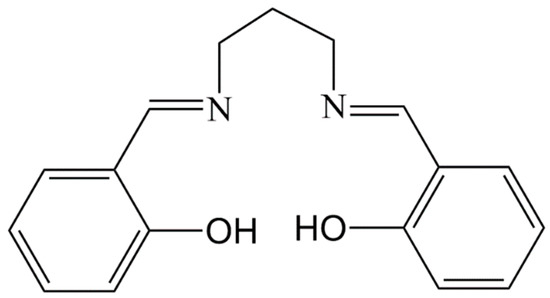
Scheme 1.
Molecular structure of title compound.
2. Experimental
2.1. General
All chemicals and solvents were commercially purchased and used as received (Aladdin, Shanghai, China). C, H, N, Mn, and Mo elemental analyses were performed on a Perkin-Elmer 240C atomic absorption spectrophotometer and a PLASMA-SPEC(1) ICP atomic emission spectrometer (PerkinElmer, Shanghai, China), respectively. IR spectra (Bruker, Karlsruhe, Germany) in 4000–400 cm−1 were measured with an Alpha-Centauri spectrometer from KBr pellets. [Mn(salpn)(H2O)]2(CH3COO)2 was prepared using a scaled-down version of Wei’s 1999 procedure [21].
Magnetic susceptibility measurements were carried out with powdered sample (16.52 mg) of the title compound using a Quantum Design SQUID-MPMS magnetometer (Quantum Design, San Diego, CA, USA). Diamagnetic corrections were made with Pascal’s constants for all the manganese atoms [22].
2.2. Synthesis
[Mn(salpn)(H2O)]2(CH3COO)2 (0.79 g) was dissolved in a methanol/water mixed solvent (15 mL). The solution was then stirred vigorously in a 100 mL Erlenmeyer flask to achieve a uniform mixture. Then, (NH4)6Mo7O24·4H2O (20 mL, 0.247 g) (Aladdin, Shanghai, China) was added to the mixture, and the pH was adjusted to 3.5. The solution was heated to 40 °C, stirred for another 3 h, and the resulting clear dark brown solution was filtered at room temperature, then the filtrate was left undisturbed in a flask sealed with pierced parafilm (Solarbio, Oshkosh, Wisconsin, USA). Brown crystals of 1 were isolated after one week. The hexagonal crystals were collected by filtration, washed with methanol, and dried under inert atmosphere. Yield: 31% based on Mo. Elemental analysis, calcd: C53H55Mn3MoN6O17 1: C, 48.63; H, 4.23; N, 6.42; Mn, 12.59; Mo, 7.33%; Found: C, 48.41; H, 4.20; N, 6.51; Mn, 12.61; Mo, 7.35 %. IR (KBr pellet, cm−1): 3376(m), 1612(s), 1542(s), 1451(s), 1395(m), 1271(s), 1119(m), 985(w), 904(s), 813(w), 771(w).
2.3. Crystal Structure Determination
Single crystal x-ray diffraction data were recorded on a Rigaku R-AXIS RAPID (Rigaku, Tokyo, Japan) image plate diffractometer (Mo KR radiation, λ = 0.71073 Å, room temperature). The total number of reflections measured was 21,315 (1822 unique, Rint = 0.0425). The heavy atoms were positioned with Patterson methods, while the remaining atoms were found using Fourier techniques. The structures were refined with the Olex2 refinement package using a full-matrix least-squares with respect to F2 [23]. Due to the presence of highly disordered solvent molecules and counter ions, the dataset was treated with the SQUEEZE [24] routine of the PLATON software [25] suite used to remove the contribution of the highly disordered lattice guests, and one counter ion acetate anion as well as two water molecules were estimated from the results of SQUEEZE and elemental analysis, where the assessment method and result are in accordance with a previously reported penta-nuclear analogue [13]. In the refinement process, the non-hydrogen atoms were refined anisotropically. The hydrogen atoms were geometrically located at calculated positions and refined in the riding model. As for the –CH2- structure, the refinement procedure obtained C–H = 0.97 Å, Uiso (H) = 1.5 Ueq of the attached C atoms for coordinated water molecules and 1.2 Ueq(C) for other H atoms, and aromatic C–H = 0.93Å. The crystallographic data and refinement parameters are given in Table 1, Tables S1 and S2 (Supplementary Materials).

Table 1.
Crystallographic characteristics of the compounds and parameters of the diffraction experiment.
3. Results and Discussion
3.1. Crystal Structure
As is shown in Figure 1, there is a half Schiff-base ligand, one manganese cation, one molybdenum center with a 50% occupancy rate, and three oxygen atoms in the asymmetric unit, and the entire cationic [{Mn(salpn)(H2O)}3MoO4]+ unit is composed of a [MoO4]2− center and three MnIII salen-type moieties (Figure 2 and Figure S1 (Supplementary Materials)). The [MoO4]2− center exhibits a classic tetrahedron orthomolybdate structure in which the Mo6+ is tetrahedrally surrounded by four oxygen atoms. Three of these latter act as bridging ligands linked to {Mn(salpn)(H2O)} units (the bridging oxygen atom and the terminal one are O2 and O3, respectively). The center Mo atom is disordered and appears at two positions with identical occupancy in the [MoO4]2- unit. The Mo(1)–O(2) bond length is of 1.745(5) Å, with the O–Mo–O angle being of ca. 109.68(5)°. As shown by bond valence sum (BVS) calculations [24] and XPS spectroscopy data, the Mo is in the +6 oxidation state (Table 2). Additionally, MnIII ions exhibit the bipyramid coordination mode in the {Mn(salpn)(H2O)}+. [N2O2] atoms from the salpn ligand form the equatorial plane of MnIII. The distance of Mn–O(N) is 1.92(4) Å. The apical positions are occupied by two oxygen atoms from a [MoO4]2− group and coordination H2O molecule with Mn-O(H2O)/O([MoO4]2−) bond lengths ranging from 2.386(5) to 2.146(5) Å due to the presence of John-Teller distortion. As seen in the BVS calculations [24] and XPS spectra (ESI, Figure S2 (Supplementary Materials)), all Mn centers are in the +3 oxidation state (Table 2).
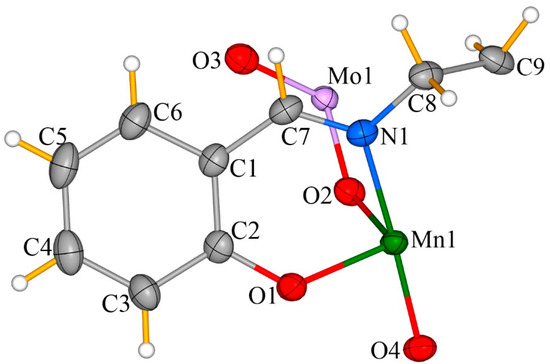
Figure 1.
Ball-and-stick and polyhedral illustration of the cationic hybrid cluster [{Mn(salpn)(H2O)}3MoO4]+ cluster of 1.
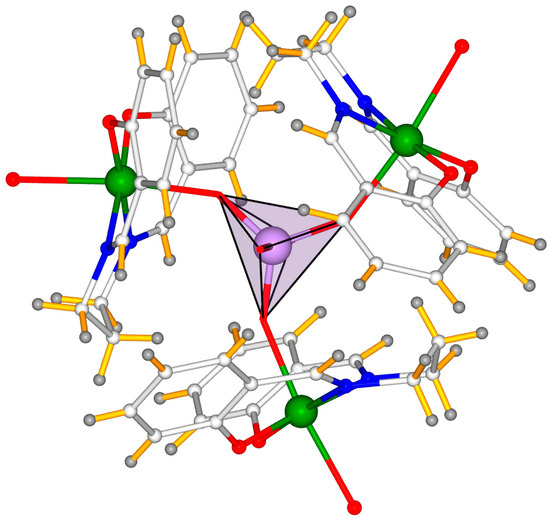
Figure 2.
Mixed polyhedral-ball & stick representation of [{Mn(salpn)(H2O)}3MoO4]+ cluster of 1.

Table 2.
Bond valence sum calculations of Mn and Mo centers in 1.
Three {Mn(salpn)(H2O)} moieties linked to the oxygen atoms of [MoO4]2- center are assembled in a staggered arrangement, resulting into the cationic cluster with a C3 symmetry (see Figure 1 and Figure 2). It is worth pointing out that, even though inorganic orthomolybdate/tungstate was proven to have the potential as versatile functional building blocks for the design of heterometallic clusters, up to now, only double and quadruple examples have been documented in the literature [13,14], and no example of triple-metal-organic fragments supported by orthomolybdate has been reported to date. Therefore, to the best of our knowledge, the structure of 1 exhibits the first tetranuclear heterometallic aggregate constructed with orthomolybdate and metal-Schiff base moieties. Moreover, according to the result of PLATON software [25], probability due to the present of counter cation and solvent molecules, the nearest distance between the benzene rings is longer than 4 Å, under this scenario, we deduce that electrostatic attraction and hydrogen bonding are the main driving forces in stabilizing the 3D structure of the title compound. The packing diagram of 1 along three different directions are shown in Figure 3.
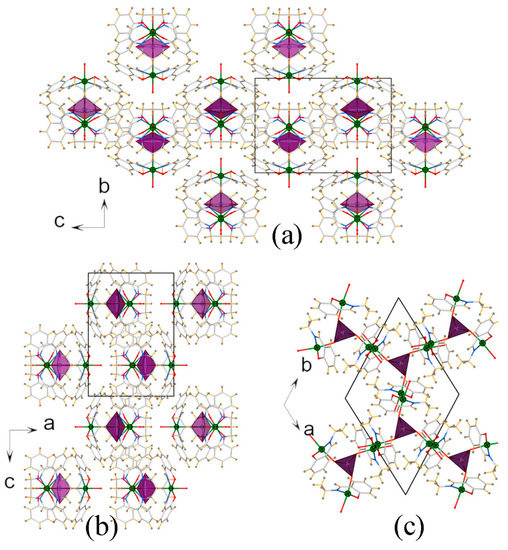
Figure 3.
The packing diagrams viewed along the a-axis (a), b-axis (b), and c-axis (c). The compound crystallizes in the hexagonal sp. gr. P63/m.
3.2. Porous Analysis
Furthermore, the heterometallic [{Mn(salpn)(H2O)}3MoO4]+ clusters are linked via hydrogen bonds and intermolecular interactions to assemble into larger structures and the calculated inner surface of the porous framework is shown along with them (Figure 4) [27,28]. The porous between the cationic heterometallic clusters are isolated from each other, and the program PLATON [25] indicates that the potential solvent area has a diameter of about 7 Å and possesses a 474.5 Å3 void of a total 15.9% space in the unit cell. It is noteworthy that because the SQUEEZE routine [24] was used to remove the highly disordered reflections of the dataset, the calculated empty spaces are probably filled with acetate counter ions and solvent water molecules.
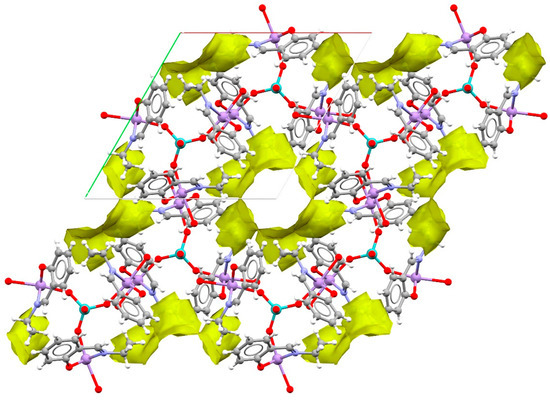
Figure 4.
The empty spaces in the packing crystal structure of 1.
3.3. Frontier Molecular Orbitals
The frontier molecular orbitals (FMO) play a significant role in revealing the electronic properties, the kinetical stability, and chemical reactivity of the complex [29]. DFT calculations were carried out at the B3LYP/6-311+G(d,p) level of theory. The Frontier molecular orbitals 3D plots of 1 are displayed in Figure 5.
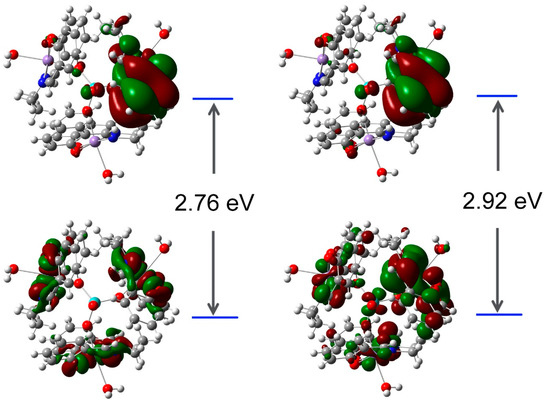
Figure 5.
Frontier molecular orbital plots of the title compound obtained by the B3LYP/6-311G(d,p) method.
It was revealed that the HOMO (highest occupied molecular orbital) surfaces are localized mostly over the {Mn(salpn)(H2O)}+cations, whilst the LUMO (lowest unoccupied molecular orbital) surfaces are over the molecule. This suggests that electronic communication exists between [MoO4]2− and metal–organic units. The value of the energy separation between the HOMO and LUMO was also calculated as 3.35 eV for the α orbitals and 3.51 eV for β orbitals, respectively, which indicates a high kinetic stability of 1.
3.4. Magnetic Properties
Magnetic susceptibility measurements were carried out on the powdered sample of the title compound. Temperature-dependent magnetism susceptibility data were collected over the temperature range of 2 to 300 K with χmT vs T displayed in Figure 6.
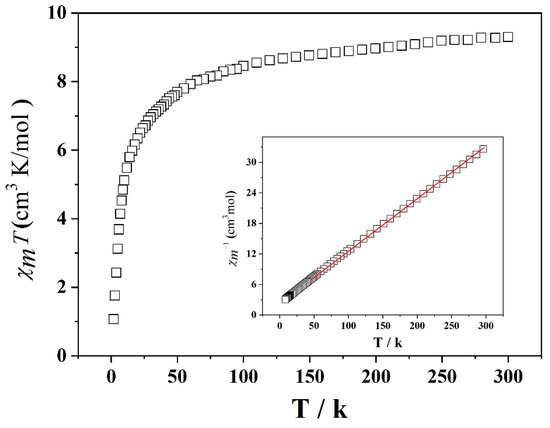
Figure 6.
Temperature dependence of χmT vs. T of 1 from 2 to 300 K.
The generation of magnetic behavior of 1 from the MnIII ions can be ascribed to the diamagnetic [MoO4]2−. The χmT value of 1 decreases slowly with decreasing temperature from 9.1 cm3∙mol−1∙K at 300 K to 8.2 cm3∙mol−1∙K at 50 K. Upon further cooling, the χmT value drops rapidly to a minimum value of 0.51 cm3∙mol−1∙K at 2.0 K.
The χmT value at 300 K is slightly higher than the spin-only value of 9 cm3∙mol−1∙K for three uncoupled Mn(III) with S = 4 and g = 2. The χ−1 versus T obeys the Curie–Weiss law over the whole temperature range, providing parameters of C = 9.37 cm 3·K∙mol−1 and θ = −7.41 K. The negative θ values indicate that there exists overall antiferromagnetic behavior of the title compound.
4. Conclusions
In conclusion, we have synthesized and structurally characterized a new organic-inorganic hybrid [{Mn(salpn)(H2O)}3MoO4](CH3COO)·2H2O (1) (salpn = N,N’-(1,3-propylene)bis(salicylideneiminate)). Structural analyses show that compound 1 represents the first example of tri-metallic-polymer decorated orthomolybdate characterized by single crystal x-ray diffraction. The DFT calculation results not only suggest that compound 1 possesses good stability, but also indicate that there is effective communication between inorganic [MoO4]2− unit and metal-organic {Mn(salpn)(H2O)}+ moieties. Magnetic analysis studies disclosed that the [MoO4]2− unit transmits very weak antiferromagnetic coupling within three [Mn(salpn)(H2O)]+ moieties. Our research, to some extent, provides a new path for the design and synthesis of hybrid materials between metal-Schiff base complexes and simple inorganic salts. Further research will focus on the reactions between different types of inorganic salts and metal-Schiff base complexes to obtain new classes of metal-Schiff base hybrids.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4352/9/12/657/s1, Figure S1: ORTEP drawing of the synthetic heterometallic cationic cluster, Figure S2: XPS spectra of 1, Table S1 and S2: Selected bond lengths and angles of 1.
Author Contributions
Methodology, Q.W. and Y.X.; Investigation, Q.W., F.J., X.-D.X., T.L., and Y.L.; Resources J.-R.S.; Software, Conceptualization Q.W. and J.-C.X.; Formal analysis, Y.L. and J.-R.S.; Validation, J.-C.X. and T.L.; Supervision, Q.W.; Writing-original draft preparation, J.-C.X.; Project administration, Q.W.; Visualization, Q.W. and J.-C.X.; Writing-review and editing, J.-C.X.; Funding acquisition, Q.W.
Acknowledgments
The authors thank the National Nature Science Foundation of China (No. 31760257) as well as the Joint Basic Research Program (partial) of Yunnan Provincial Undergraduate Universities (2017FH001-002) and the Recruitment Program of Yunnan Province Experts Provincial Young Talents (2019HB098) for financial support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Mirzaei, M.; Eshtiagh-Hosseini, H.; Alipour, M.; Frontera, A. Recent developments in the crystal engineering of diverse coordination modes (0–12) for Keggin-type polyoxometalates in hybrid inorganic–organic architectures. Coordin. Chem. Rev. 2014, 275, 1–18. [Google Scholar] [CrossRef]
- Miras, H.N.; Yan, J.; Long, D.-L.; Cronin, L. Engineering polyoxometalates with emergent properties. Chem. Soc. Rev. 2012, 41, 7403–7430. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Luo, T.; Zhang, J.; Li, X.-X.; Lv, S.-F.; Peng, J.-J.; Li, M.; Li, W.; Nakamura, T. Two Supramolecular Inorganic—Organic Hybrid Crystals Based on Keggin Polyoxometalates and Crown Ethers. Crystals 2018, 8, 17. [Google Scholar] [CrossRef]
- Sosa, J.D.; Bennett, T.F.; Nelms, K.J.; Liu, B.M.; Tovar, R.C.; Liu, Y. Metal–Organic Framework Hybrid Materials and Their Applications. Crystals 2018, 8, 325. [Google Scholar] [CrossRef]
- Gupta, K.C.; Sutar, A.K. Catalytic activities of Schiff base transition metal complexes. Coordin. Chem. Rev. 2008, 252, 1420–1450. [Google Scholar] [CrossRef]
- Miyasaka, H.; Saitoh, A.; Abe, S. Magnetic assemblies based on Mn(III) salen analogues. Coord. Chem. Rev. 2007, 251, 2622–2664. [Google Scholar] [CrossRef]
- Fu, Z.; Liao, H.; Xiong, D.; Zhang, Z.; Yan, J.; Yin, D. A highly-efficient and environmental-friendly method for the preparation of Mn(III)–Salen complexes encapsulated HMS by using microwave irradiation. Microporous Mesoporous Mater. 2007, 106, 298–303. [Google Scholar] [CrossRef]
- Watanabe, T.; Owada, S.H.; Kawakami, H.; Nagaoka, S.; Murakami, E.; Ishiuchi, A.; Enomoto, T.; Jinnouchi, Y.; Sakurai, J.; Tobe, N. Protective effects of MnM2Py4P and Mn-salen against small bowel ischemia/reperfusion injury in rats using an in vivo and an ex vivo electron paramagnetic resonance technique with a spin probe. Transplant. Proc. 2007, 39, 3002–3006. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Day, C.S.; Lachgar, A. Self-assembly and solvent-mediated structural transformation of one-dimensional cluster-based coordination polymer. CrystEngComm 2011, 13, 133–137. [Google Scholar] [CrossRef]
- Donmez, A.; Coban, M.B.; Kara, H. Cyan-Blue Luminescence and Antiferromagnetic Coupling of CN-Bridged Tetranuclear Complex Based on Manganese(III) Schiff Base and Hexacyanoferrate(III). J. Clust. Sci. 2018, 29, 951–958. [Google Scholar] [CrossRef]
- Wang, T.T.; Bao, S.S.; Ren, M.; Cai, Z.S.; Zheng, Z.H.; Xu, Z.L.; Zheng, L.M. Assembly of {Mn2(salen)2}2+ Dimers by Cyclic V 4 O 12 4− Clusters: A 3 D Compound with Open-Framework Structure Exhibiting Slow Magnetization Relaxation. Chem. Asian J. 2013, 8, 1772–1775. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chen, W.L.; Liu, D.; Liang, C.; Li, Y.G.; Lin, S.W.; Wang, E. New class of organic-inorganic hybrid aggregates based on polyoxometalates and Metal-Schiff-base. Dalton Trans. 2011, 40, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.-X.; Tan, H.-Q.; Liu, D.; Zhang, Z.-M.; Wang, E.-B. Two organic–inorganic hybrid materials based on the penta-nuclear {MMn4} (M = W and Mo) heterometallic clusters and Schiff-base ligands. Inorg. Chem. Commun. 2013, 29, 49–52. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, H.; Hu, X.; Lu, J.; Wang, H. A New 1-D Organic–Inorganic Hybrid Dimer Chain Complex Constructed by Manganese(III)-(Schiff-base) and [WO4]2− Unit. J. Clust. Sci. 2015, 26, 1203–1213. [Google Scholar] [CrossRef]
- Suzuki, K.; Sato, R.; Mizuno, N. Reversible switching of single-molecule magnet behaviors by transformation of dinuclear dysprosium cores in polyoxometalates. Chem. Sci. 2013, 4, 596–600. [Google Scholar] [CrossRef]
- Wu, Q.; Li, Y.-G.; Wang, Y.-H.; Clérac, R.; Lu, Y.; Wang, E.-B. Polyoxometalate-based {MnIII2}–Schiff base composite materials exhibiting single-molecule magnet behaviour. Chem. Commun. 2009, 5743–5745. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Kosaka, W.; Hayashi, Y.; Miyasaka, H. Coulombic aggregations of Mn III salen-type complexes and keggin-type polyoxometalates: Isolation of Mn 2 single-molecule magnets. Inorg. Chem. 2012, 51, 4824–4832. [Google Scholar] [CrossRef]
- Meng, X.; Wang, H.-N.; Wang, X.-L.; Yang, G.-S.; Wang, S.; Shao, K.-Z.; Su, Z.-M. Construction and property investigation of inorganic–organic hybrid materials based on metal–salens and Keggin polyoxometalates. Inorg. Chim. Acta 2012, 390, 135–142. [Google Scholar] [CrossRef]
- Meng, X.; Qin, C.; Wang, X.L.; Su, Z.M.; Li, B.; Yang, Q.H. Chiral salen-metal derivatives of polyoxometalates with asymmetric catalytic and photocatalytic activities. Dalton Trans. 2011, 40, 9964–9966. [Google Scholar] [CrossRef]
- Lin, S.; Wu, Q.; Tan, H.; Wang, E. A new organic–inorganic hybrid based on Mn–salen and decavanadate. J. Coord. Chem. 2011, 64, 3661–3669. [Google Scholar] [CrossRef]
- Shyu, H.L.; Wei, H.H.; Wang, Y. Structure and magnetic properties of dinuclear [Mn(III)(salen)(H2O)]2(ClO4)2 and polynuclear [Mn(III)(salen)(NO3)]n. Inorg. Chim. Acta 1999, 290, 8–13. [Google Scholar] [CrossRef]
- Boudreaux, E.A.L.; Mulay, L.N. Theory and Applications of Molecular Paramagnetism; Boudreaux, E.A., Mulay, L.N., Eds.; Wiley-Interscience: New York, NY, USA, 1976; 510p. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2010, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. 2015, 71, 9–18. [Google Scholar]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Cambridge Crystallographic Data Centre. Available online: https://www.ccdc.cam.ac.uk/ (accessed on 2 December 2019).
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the Inorganic Crystal Structure Database. Acta Crystallogr. Section B 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Barbour, L.J. Crystal porosity and the burden of proof. Chem Commun. 2006, 11, 1163–1168. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).