Influence of the Substituted Ethylenediamine Ligand on the Structure and Properties of [Cu(diamine)2Zn(NCS)4]∙Solv. Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of [Cu(diamine)2Zn(NCS)4]
2.3. Physical Measurements
2.4. Structural Analysis
3. Results and Discussion
3.1. General and Spectroscopic Characterization of 1 and 2
3.1.1. General Characterization
3.1.2. Electronic Spectra
3.1.3. Infrared Spectra
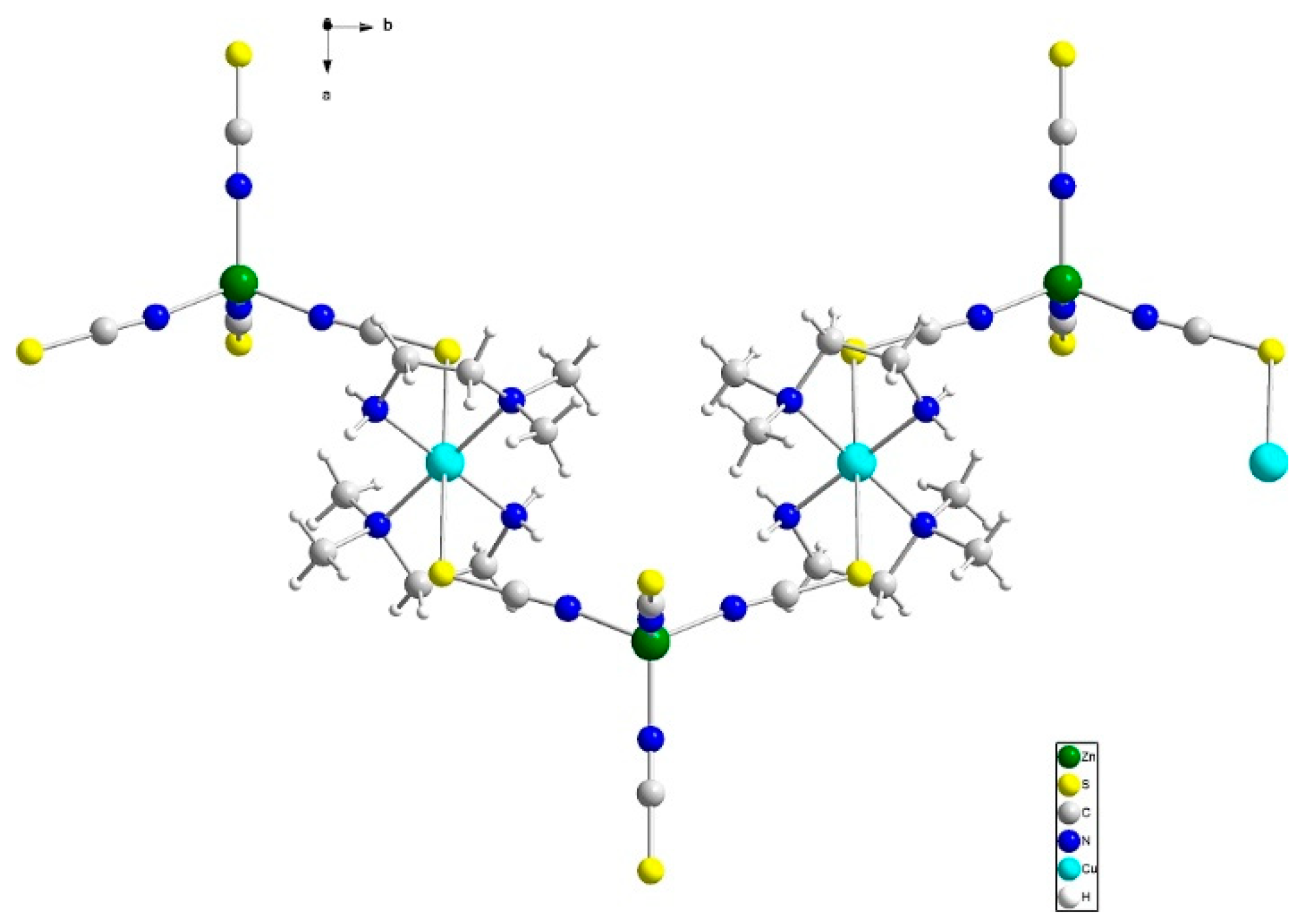
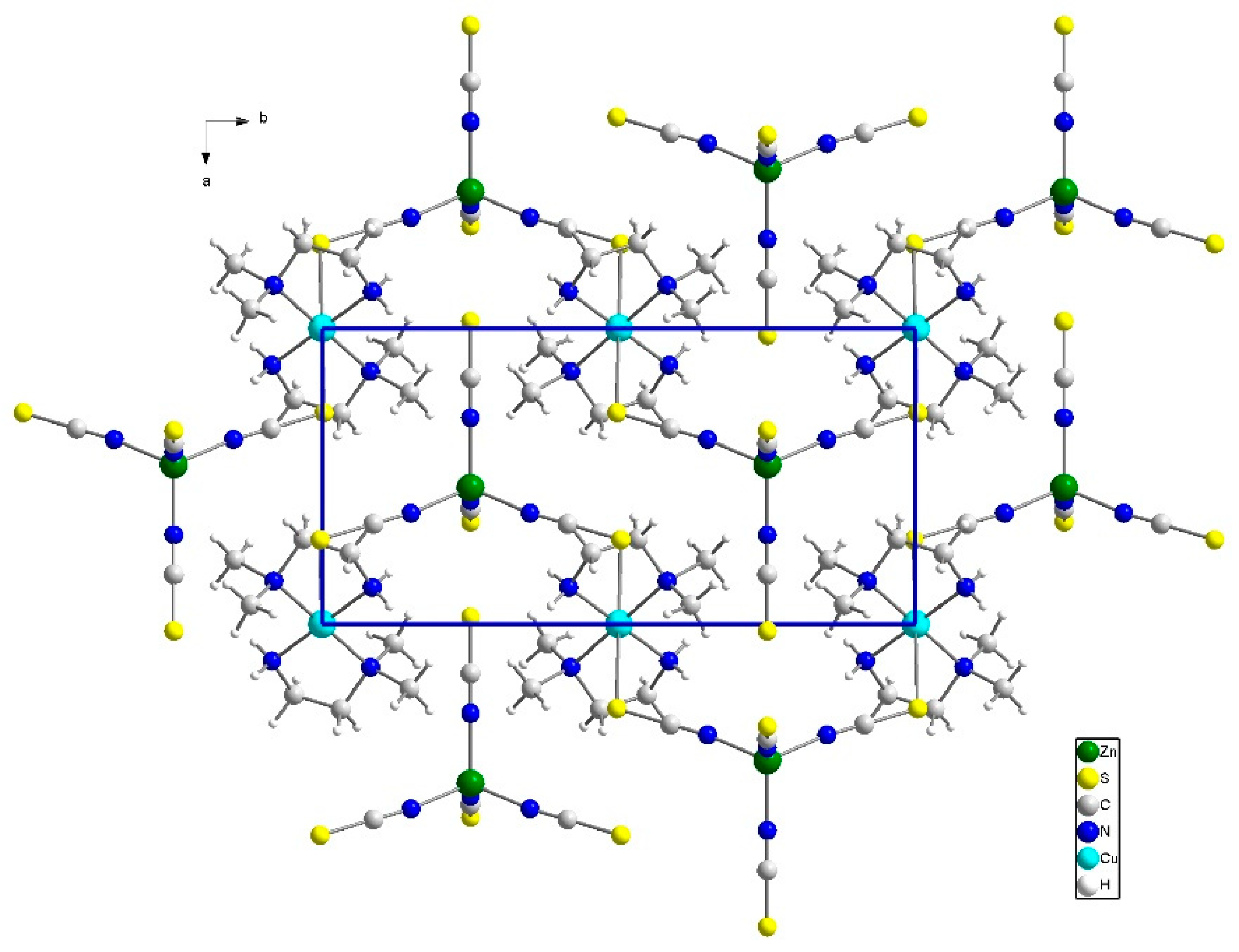
3.2. Description of the Crystal Structures
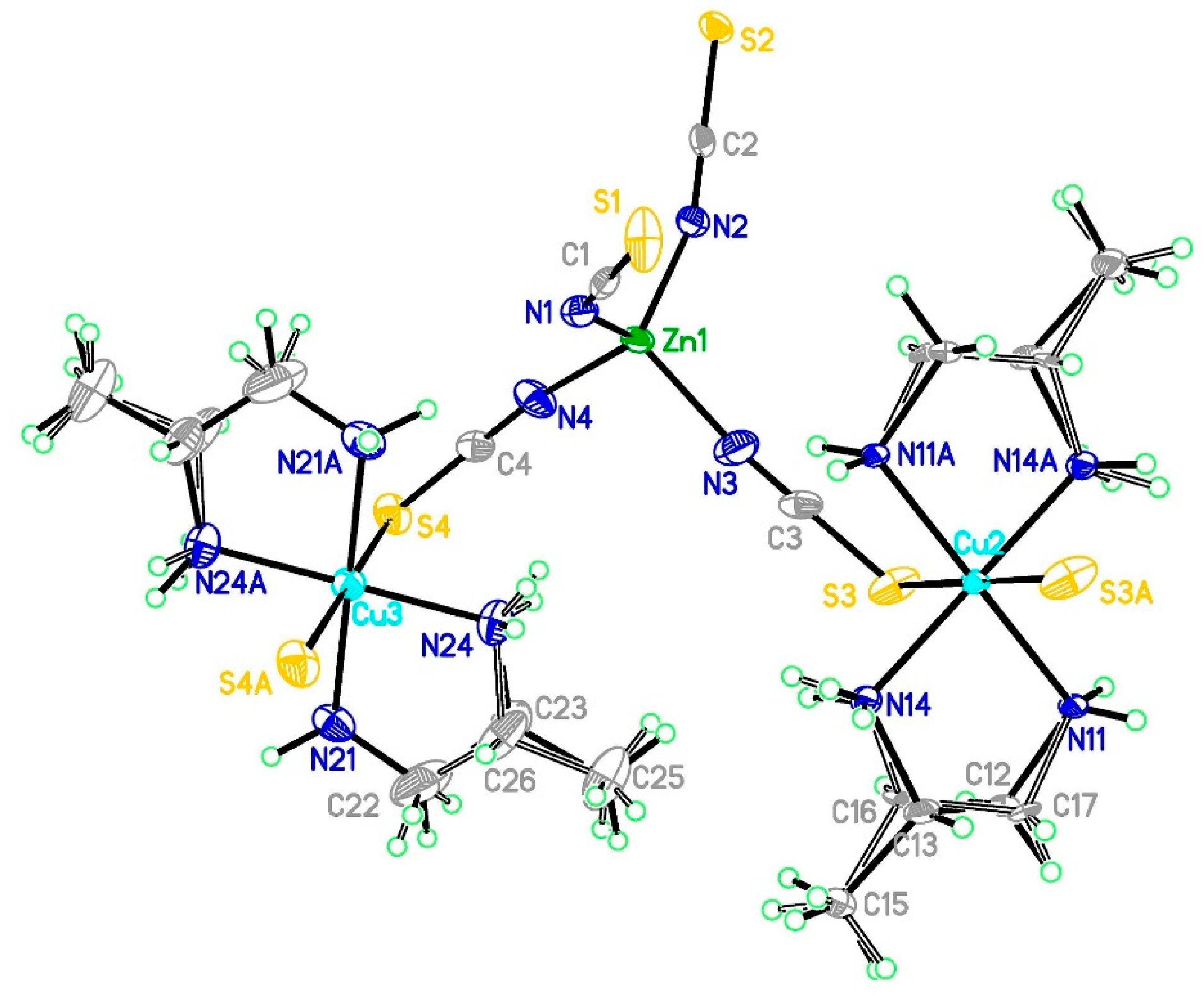
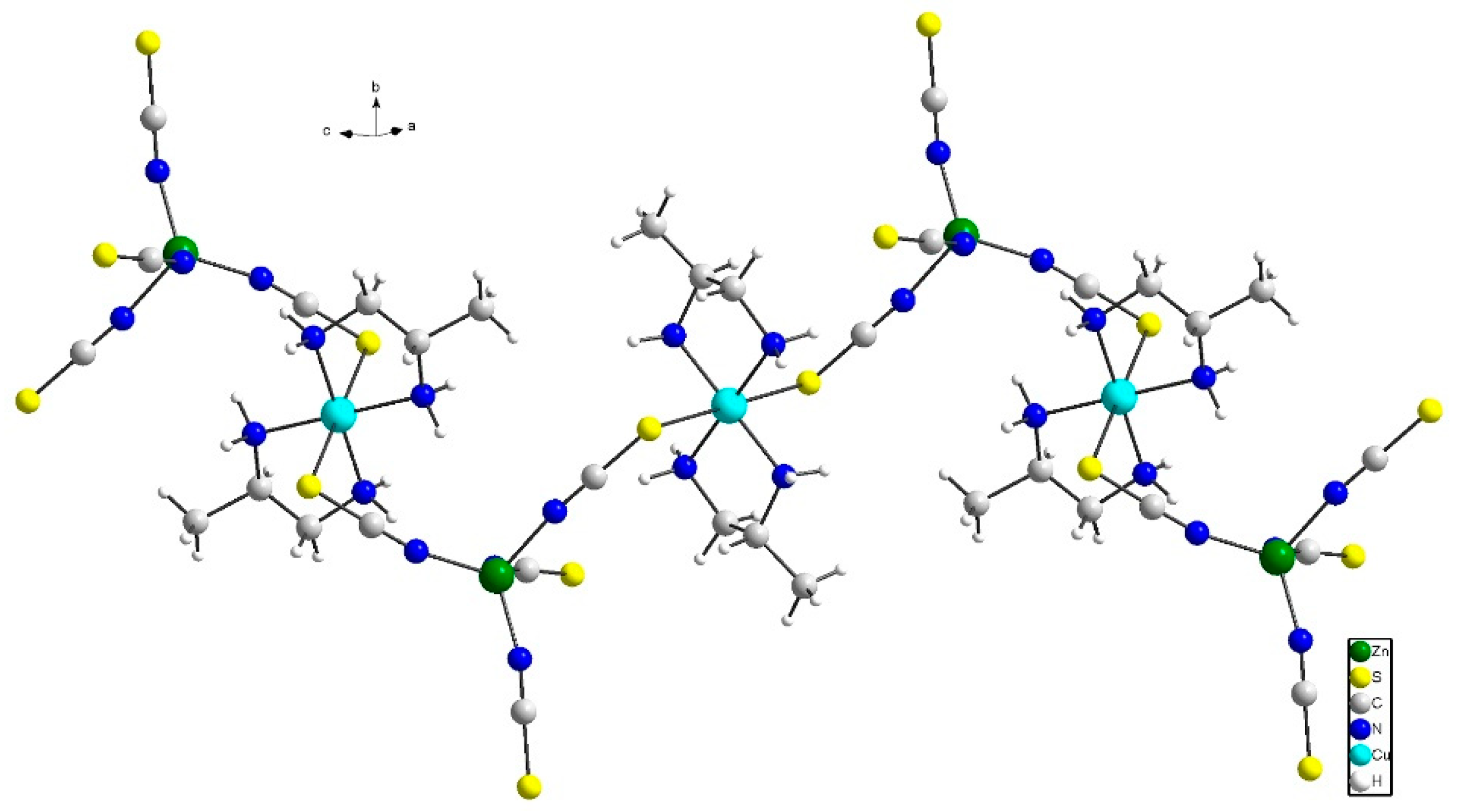
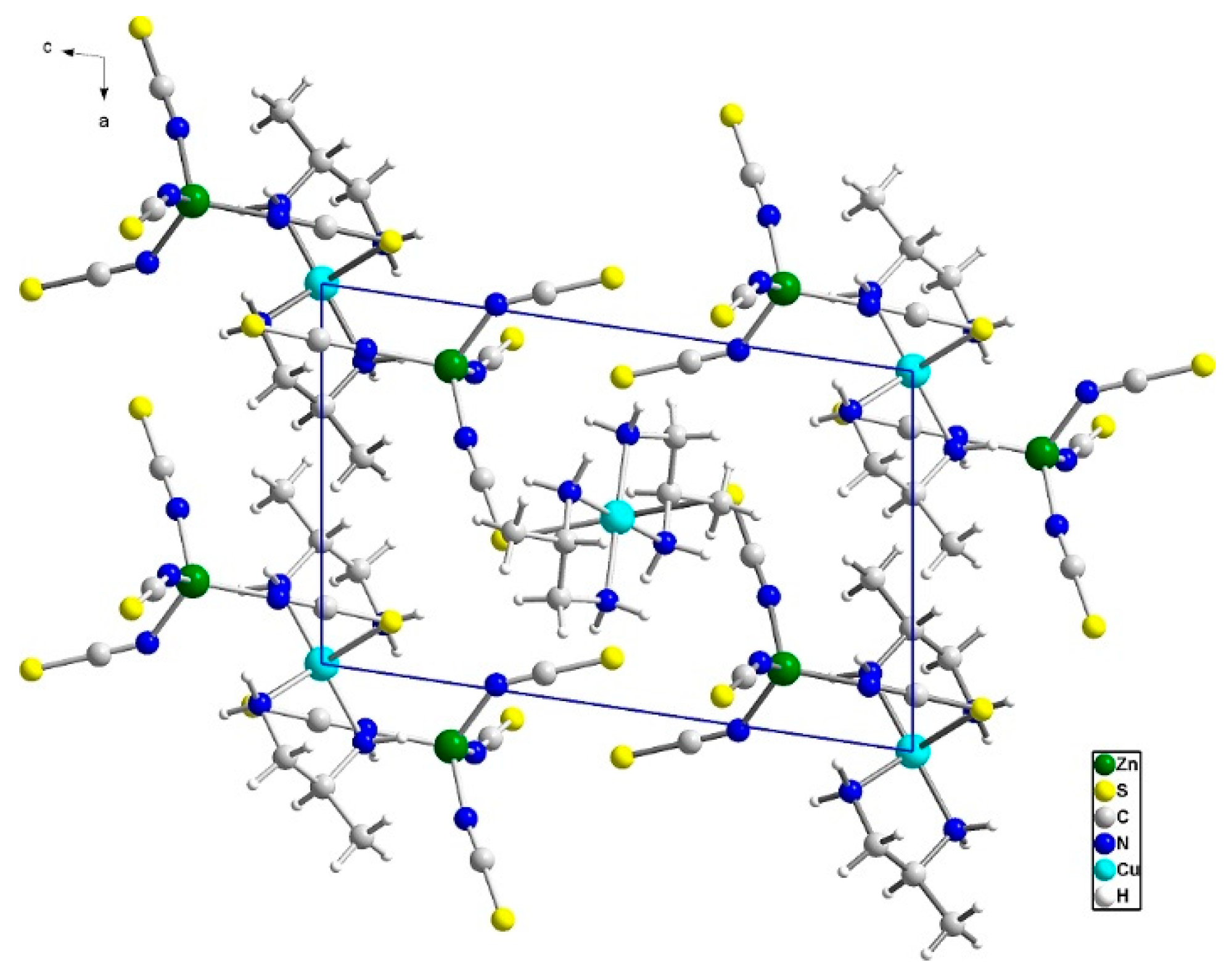
3.2.1. Structure of Compound (1)
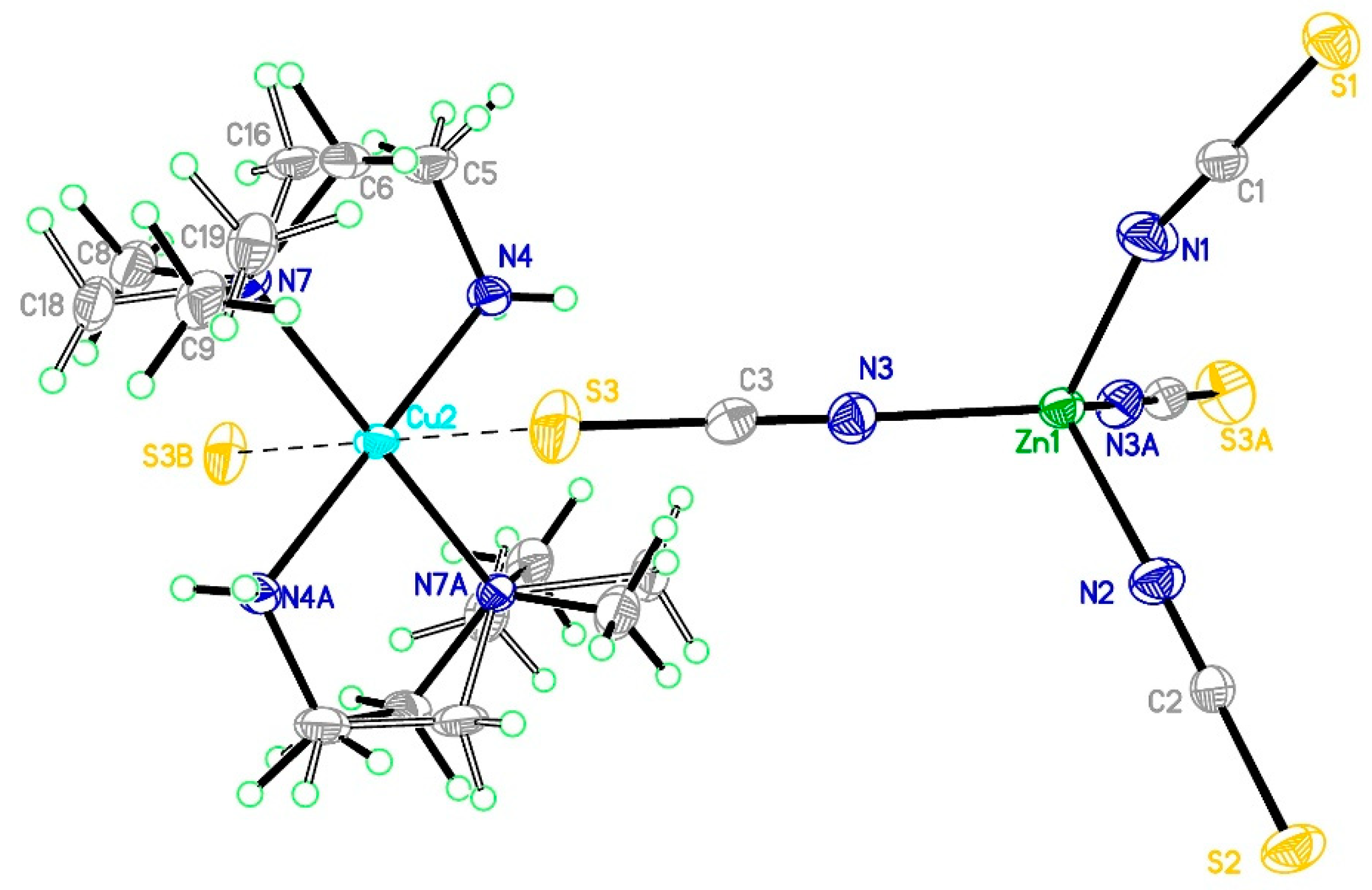
3.2.2. Structure of Compound 2
3.2.3. Structure of Compound 3
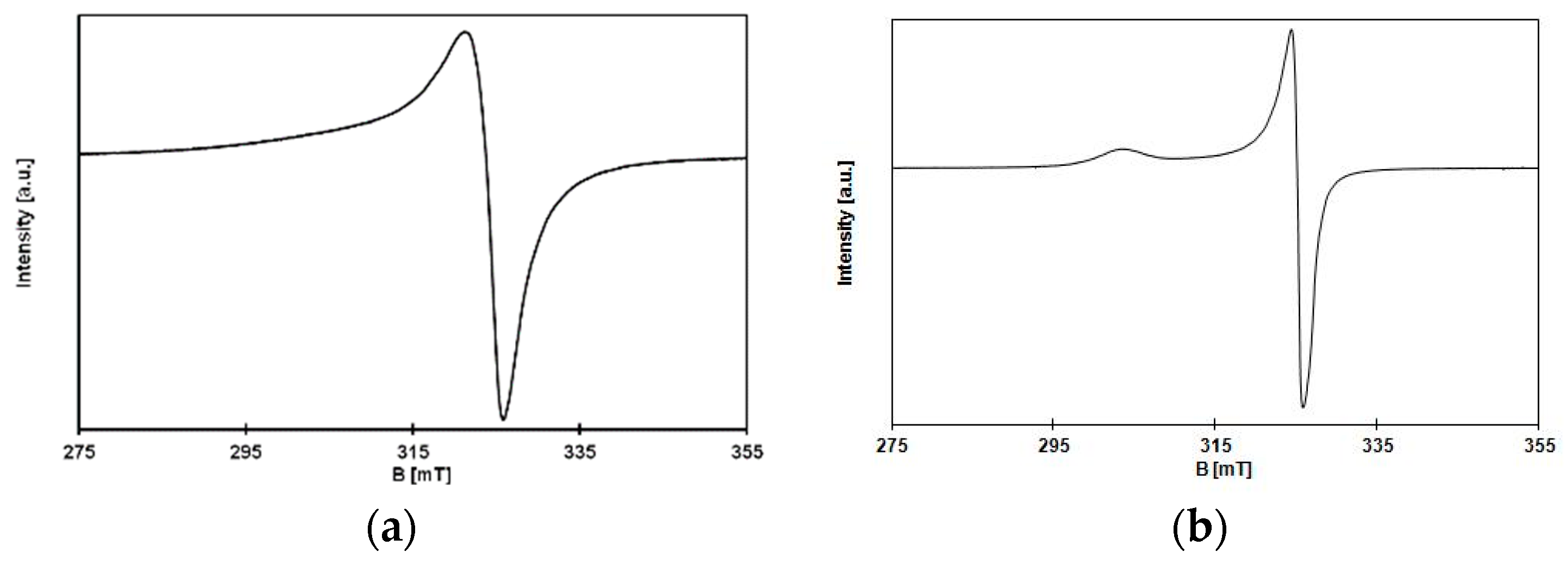
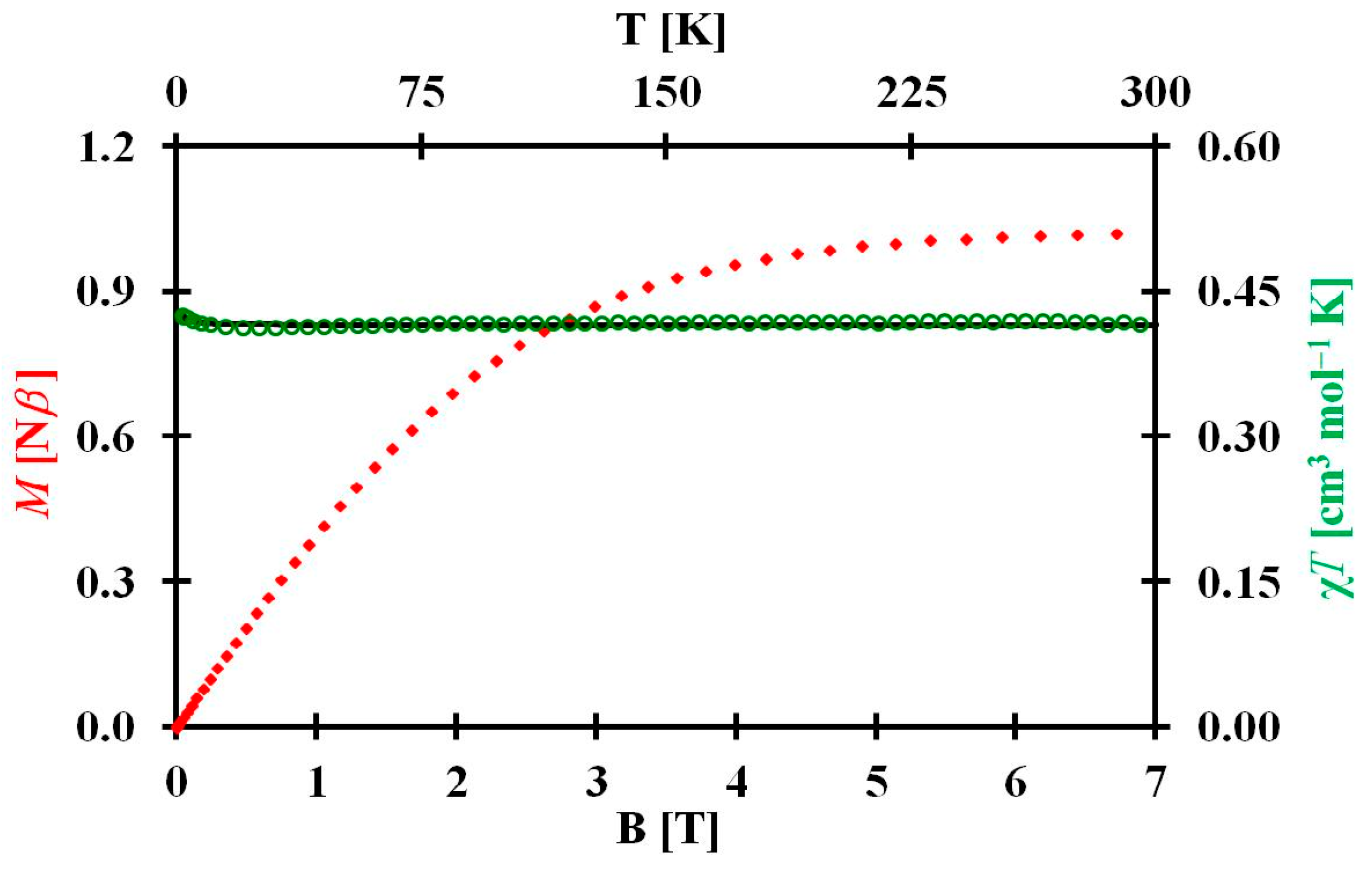
3.3. EPR and Magnetism of 1 and 2
3.3.1. EPR
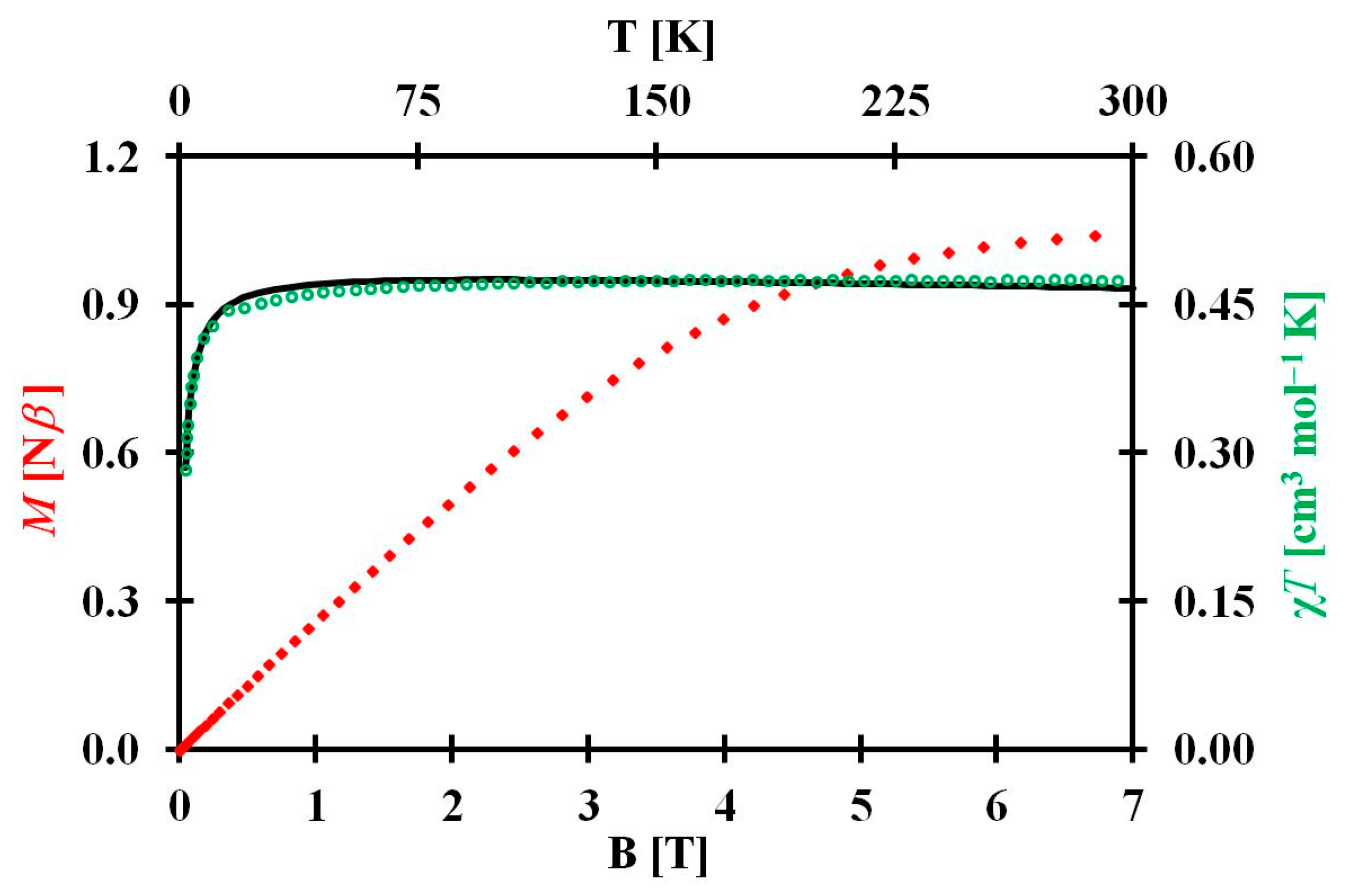
3.3.2. Magnetism
3.4. Modelling of Magnetic Interactions
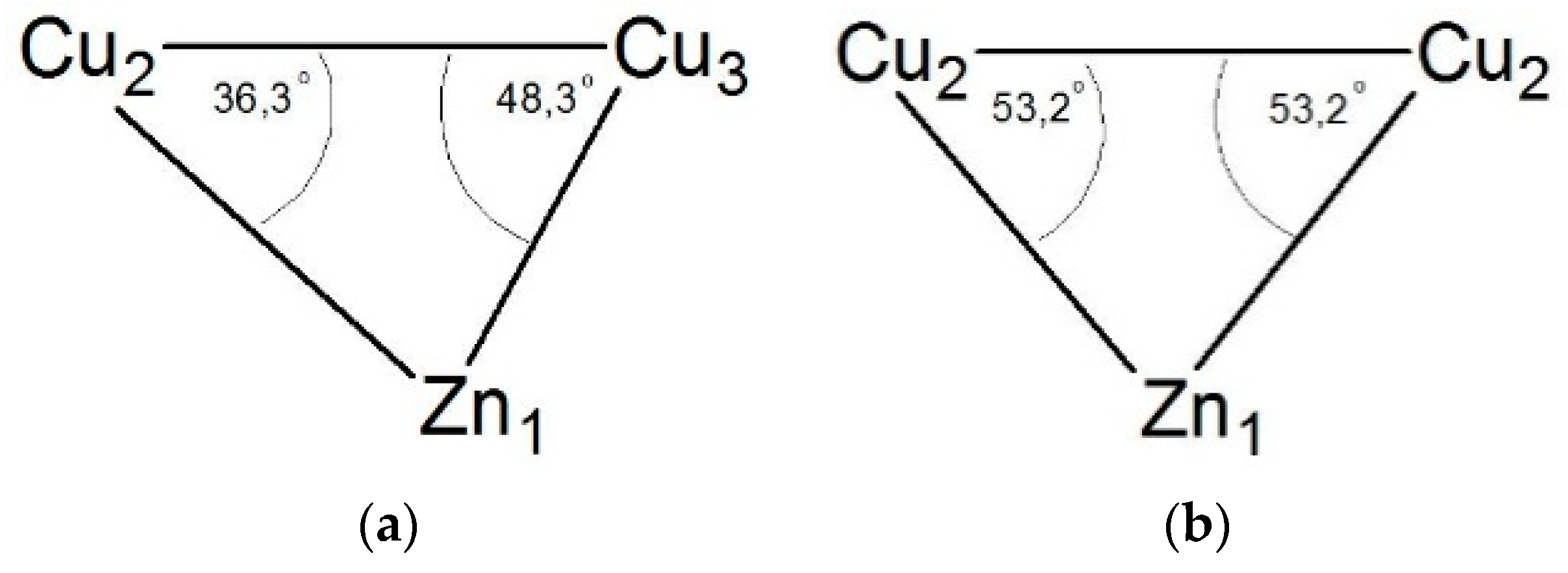
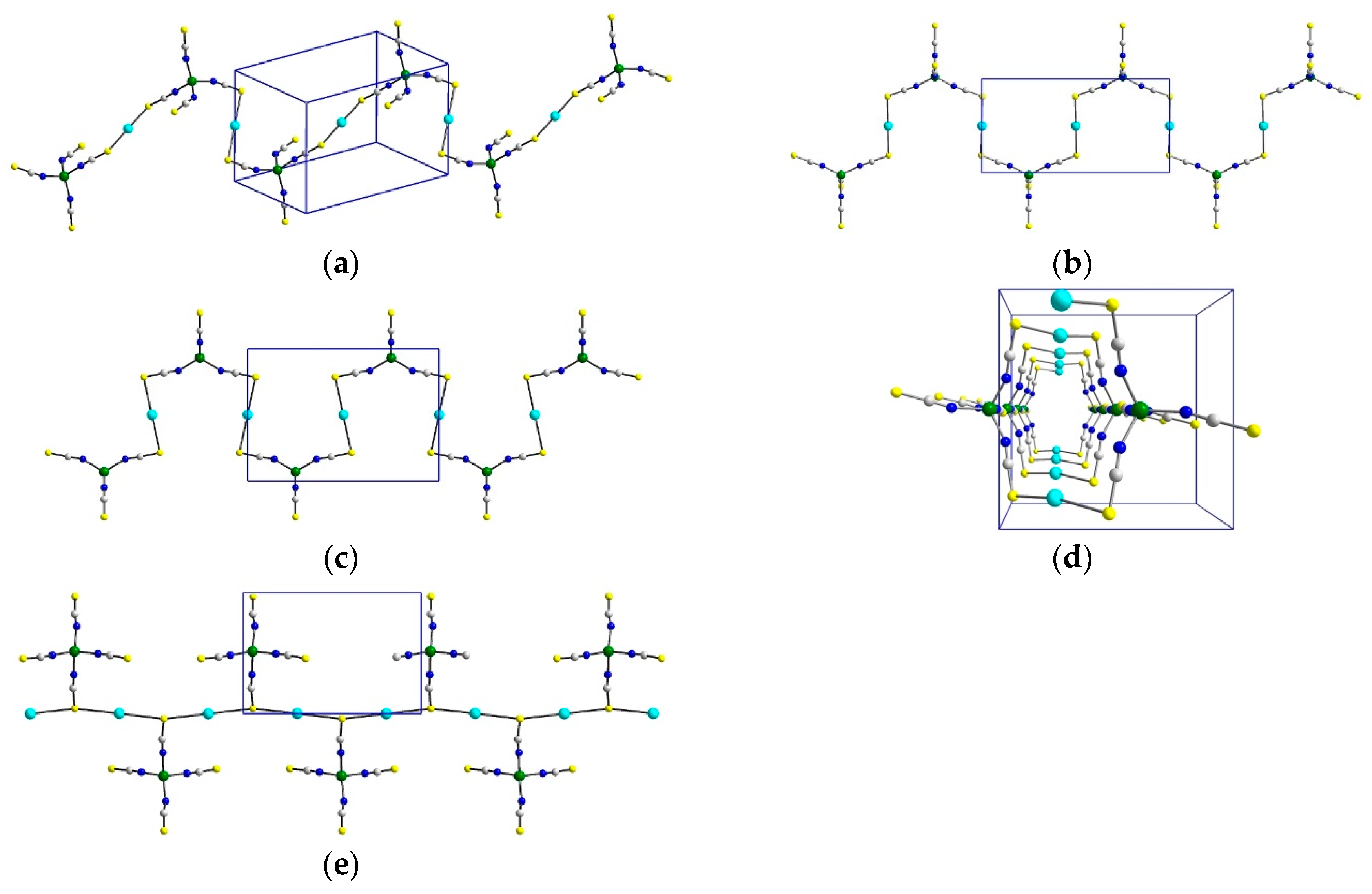
3.5. Comparison of Structures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Note
- Burmeister, J.L. Ambidentate ligands, the schizophrenics of coordination chemistry. Coord. Chem. Rev. 1990, 105, 77–133. [Google Scholar] [CrossRef]
- Kabešová, M.; Boča, R.; Melník, M.; Valigura, D.; Dunaj-Jurčo, M. Bonding properties of thiocyanate groups in copper (II) and copper (I) complexes. Coord. Chem. Rev. 1995, 140, 115–135. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Sharpe, A.G. Inorganic Chemistry, 4th ed.; Pearson Education Limited: Harlow, UK, 2012; pp. 655, 824, 993. [Google Scholar]
- Shen, L.; Feng, X. Synthesis and Crystal Structure of a Novel Polymeric Thiocyanato-Bridged Heteronuclear Complex of Copper (II) and Cadmium (II). Struct. Chem. 2002, 13, 437–441. [Google Scholar] [CrossRef]
- Mroziński, J.; Kłak, J.; Kruszyński, R. Crystal structure and magnetic properties of the 1D bimetallic thiocyanate bridged compound: {(CuL1)[Co(NCS)4]}(L1 = N-rac-5,12-Me2-[14]-4,11-dieneN4). Polyhedron 2008, 27, 1401–1407. [Google Scholar] [CrossRef]
- Skorupa, A.; Korybut-Daszkiewicz, B.; Mroziński, J. Heteronuclear thiocyanate-bridged compounds of the type (NiL)3[M(NCS)6]2 (M = Fe(III), Cr(III); L = 5,6,12,13-Me4-[14]-4,11-dieneN4). Inorg. Chim. Acta 2002, 336, 65–70. [Google Scholar] [CrossRef]
- Skorupa, A.; Korybut-Daszkiewicz, B.; Mroziński, J. Crystal structure and magnetic properties of two heteronuclear thiocyanate bridged compounds: (CuL)[Co(NCS)4] (L = N-meso-(5,12-Me2-7,14-Et2-[14]-4,11-dieneN4) and N-rac-(5,12-Me2-7,14-Et2-[14]-4,11-dieneN4)). Inorg. Chim. Acta 2001, 324, 286–292. [Google Scholar] [CrossRef]
- Wrzeszcz, G.; Dobrzańska, L.; Grodzicki, A.; Wojtczak, A. Magnetostructural characterisation of the first bimetallic assemblies derived from the anionic building block [Cr(NCS)6]3−, [M(en)3]n[{M(en)2-µ-SCN-Cr(NCS)4-µ-NCS}2n] with M = Ni(II), Zn(II). J. Chem. Soc. Dalton Trans. 2002, 2862–2867. [Google Scholar] [CrossRef]
- Nesterova, O.V.; Petrusenko, S.R.; Kokozay, V.N.; Skelton, B.W.; Jezierska, J.; Linert, W.; Ozarowski, A. Structural, magnetic, high-frequency and high-field EPR investigation of double-stranded heterometallic [{Ni(en)2}2(µ-NCS)4Cd(NCS)2]n·nCH3CN polymer self-assembled from cadmium oxide, nickel thiocyanate and ethylenediamine. Dalton Trans. 2008, 1431–1436. [Google Scholar] [CrossRef]
- Shen, L.; Xu, Y.-Z. Structure and magnetic properties of a novel two-dimensional thiocyanato-bridged heterometallic polymer {Cu(en)2[Ni(en)(SCN)3]2}n. J. Chem. Soc. Dalton Trans. 2001, 3413–3414. [Google Scholar] [CrossRef]
- Krautscheid, H.; Emig, N.; Klaassen, N.; Seringer, P. Thiocyanato complexes of the coinage metals: Synthesis and crystal structures of the polymeric pyridine complexes [AgxCuy(SCN)x+y(py)z]. J. Chem. Soc. Dalton Trans. 1998, 3071–3077. [Google Scholar] [CrossRef]
- Machura, B.; Świtlicka, A.; Zwoliński, P.; Mroziński, J.; Kalińska, B.; Kruszynski, R. Novel bimetallic thiocyanate-bridged Cu(II)–Hg(II) compounds—Synthesis, X-Ray studiem and magnetic properties. J. Solid State Chem. 2013, 197, 218–227. [Google Scholar] [CrossRef]
- Nikitina, V.M.; Nesterova, O.V.; Kokozay, V.N.; Zubatyuk, R.I.; Dyakonenko, V.V.; Shishkin, O.V.; Goreshnik, E.A.; Gómez-García, C.J.; Clemente-Juan, J.M.; Jezierska, J. Supramolecular diversity and magnetic properties of novel heterometallic Cu(II)/Cr(III) complexes prepared from copper powder, Reineckes salt and ethylenediamine. Inorg. Chim. Acta 2009, 362, 2237–2246. [Google Scholar] [CrossRef]
- Kobayashi, M.; Savard, D.; Geisheimer, A.R.; Sakai, K.; Leznoff, D.B. Heterobimetallic Coordination Polymers Based on the [Pt(SCN)4]2− and [Pt(SeCN)4]2− Building Blocks. Inorg. Chem. 2013, 52, 4842–4852. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, M.; Béreau, V.; Desplanches, C.; Duhayon, C.; Sutter, J.-P. Substantial exchange coupling for {Mo–NCS–M} combination: Illustration for 1-D [{Mo(NCS)6}{NiL}2(NCS)]n. Chem. Commun. 2010, 46, 7519–7521. [Google Scholar] [CrossRef] [PubMed]
- Khandar, A.A.; Klein, A.; Bakhtiari, A.; Mahjoub, A.R.; Pohl, R.W.H. One-dimensional ladder like and two-dimensional polymorphs of heterometallic thiocyanate bridged copper (II) and mercury (II) coordination polymer: Syntheses, structural, vibration, luminescence and EPR studies. Inorg. Chim. Acta 2011, 366, 184–190. [Google Scholar] [CrossRef]
- Tercero, J.; Diaz, C.; Ribas, J.; Ruiz, E.; Mahía, J.; Maestro, M. New Oxamidato-Bridged CuII-NiII Complexes: Supramolecular Structures with Thiocyanate Ligands and Hydrogen Bonds. Magnetostructural Studies: DFT Calculations. Inorg. Chem. 2002, 41, 6780–6789. [Google Scholar] [CrossRef]
- Pryma, O.V.; Petrusenko, S.R.; Kokozay, V.N.; Skelton, B.W.; Shishkin, O.V.; Teplytska, T.S. A Facile Direct Synthesis of Bimetallic CuIIZnII Complexes with Ethylenediamine Revealing Different Types of Chain Crystal Structures. Eur. J. Inorg. Chem. 2003, 1426–1432. [Google Scholar] [CrossRef]
- Wrzeszcz, G.; Muzioł, T.M.; Tereba, N. Synthesis, characterization and crystal structure of a 1D thiocyanato bridged [Cu(en)2Zn(NCS)4]∙H2O. Comparison of the three structures with the same [Cu(en)2Zn(NCS)4] unit—Different in structural terms. J. Mol. Struct. 2015, 1083, 374–380. [Google Scholar] [CrossRef]
- Wrzeszcz, G.; Muzioł, T.M.; Tereba, N. Corrigendum to Synthesis, characterization and crystal structure of a 1D thiocyanato bridged [Cu(en)2Zn(NCS)4]∙H2O. Comparison of the three structures with the same [Cu(en)2Zn(NCS)4] unit—Different in structural terms [J. Mol. Struct. 1083 (2015) 374–380]. J. Mol. Struct. 2015, 1091, 236. [Google Scholar] [CrossRef]
- Legendre, A.O.; Mauro, A.E.; Ferreira, J.G.; Ananias, S.R.; Santos, R.H.A.; Netto, A.V.G. A 2D coordination polymer with brick-wall network topology based on the [Cu(NCS)2(pn)] monomer. Inorg. Chem. Commun. 2007, 10, 815–820. [Google Scholar] [CrossRef]
- You, Z.-L.; Ng, S.W. (1,3-Propanediamine-κ2N,N′)bis(thiocyanato-κN)copper(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63, m2347. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Boča, R. Magnetic Parameters and Magnetic Functions in Mononuclear Complexes Beyond the Spin-Hamiltonian Formalism. In Structure and Bonding; Mingos, D.M.P., Ed.; Springer: Berlin, Germany, 2006; Volume 117, pp. 1–264. [Google Scholar] [CrossRef]
- CrysAlis RED and CrysAlis CCD; Oxford Diffraction Ltd.: Abingdon, UK, 2000.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Adv. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K. DIAMOND, Release 2.1e; Crystal Impact GbR: Bonn, Germany, 2001. [Google Scholar]
- Farrugia, L.J. ORTEP-3 for Windows-a version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- www.ccdc.cam.ac.uk.data_request/cif.
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1984; pp. 554–572. [Google Scholar]
- Procter, I.M.; Hathaway, B.J.; Nicholls, P. The Electronic Properties and Stereochemistry of the Copper (II) Ion. Part I. Bis(ethylenediamine)copper(II) Complexes. J. Chem. Soc. A 1968, 1678–1684. [Google Scholar] [CrossRef]
- Hathaway, B.J.; Billing, D.E. The electronic properties and stereochemistry of mono-nuclear complexes of the copper (II) ion. Coord. Chem. Rev. 1970, 5, 143–207. [Google Scholar] [CrossRef]
- Dobrzańska, L.; Wrzeszcz, G.; Grodzicki, A.; Rozpłoch, F. Synthesis and Characterization of Thiocyanato-Bridged Heteropolynuclear Chromium (III)–Copper (II) Complexes. Polish J. Chem. 2000, 74, 199–206. [Google Scholar]
- Dobrzańska, L.; Wrzeszcz, G.; Grodzicki, A.; Rozpłoch, F. Synthesis, Spectroscopy and Magnetism of New µ-Thiocyanato Polynuclear Copper (II)–Chromium (III) Complexes. Polish J. Chem. 2001, 75, 1689–1694. [Google Scholar]
- Wrzeszcz, G.; Dobrzańska, L.; Grodzicki, A.; Rozpłoch, F. Synthesis and Characterization of New Thiocyanato Bridged Complexes with the General Formula [MLn]3[Cr(NCS)6]2∙mH2O, where M = Cu(II), Ni(II), Co(II); L = Various Substituted Imidazoles. Polish J. Chem. 2003, 77, 147–156. [Google Scholar]
- Wrzeszcz, G. Synthesis and Characterization of New Thiocyanato Bridged Heterobimetallic Complexes with the General Formula: [Cu(diamine)2]3[Cr(NCS)6]2∙nH2O. Polish J. Chem. 2003, 77, 845–854. [Google Scholar]
- Wrzeszcz, G.; Dobrzańska, L. Magnetic and Thermal Properties of New Thiocyanato Bridged Complexes of the Type [M(diamine)2]3[Cr(NCS)6]2∙nH2O, where M = Cu(II), Ni(II). Polish J. Chem. 2003, 77, 1245–1254. [Google Scholar]
- Wrzeszcz, G.; Grzebielucha, T. Synthesis and Properties of New Bimetallic Complexes of General Formula: [Cu(diamine)2][Cr(NCS)4(NH3)2]2. Polish J. Chem. 2009, 83, 1575–1582. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 120–126. [Google Scholar]
- Lever, A.B.P.; Mantovani, E. The Far-Infrared and Electronic Spectra of Some Bis-Ethylenediamine and Related Complexes of Copper (II) and the Relevance of These Data to Tetragonal Distortion and Bond Strengths. Inorg. Chem. 1971, 10, 817–826. [Google Scholar] [CrossRef]
- Forster, D.; Horrocks, W.D., Jr. Vibrational spectra and force constants of Zn(NCO)42−, Zn(NCS)42−, and Zn(NCSe)42−. Inorg. Chem. 1967, 6, 339–343. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Bhar, K.; Das, S.; Chantrapromma, S.; Fun, H.-K.; Ghosh, B.K. Syntheses, structures and properties of homo- and heterobimetallic complexes of the type [Zn(tren)NCS]2[M(NCS)4] [tren = tris(2-aminoethyl)amine; M = Zn, Cu]. J. Mol. Struct. 2010, 967, 112–118. [Google Scholar] [CrossRef]
- Brown, B.W.; Lingafelter, E.C. The Crystal Structure of Bis(ethylenediamine)copper(II) Thiocyanate. Acta Crystallogr. 1964, 17, 254–259. [Google Scholar] [CrossRef]
- Nesterova (Pryma), O.V.; Petrusenko, S.R.; Kokozay, V.N.; Skelton, B.W.; Linert, W. A new 2D heterometallic Cu/Cd mixed-anion polymer with dicyanamide and thiocyanate bridges formed via the reaction of elemental copper, cadmium dicyanamide and ethylenediamine. Inorg. Chem. Comm. 2004, 7, 450–454. [Google Scholar] [CrossRef]
- Hathaway, B.J. The Correlation of the Electronic Properties and Stereochemistry of Mononuclear {CuN4-6} Chromophores. J. Chem. Soc. Dalton Trans. 1972, 1196–1199. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH Publishers Inc.: New York, NY, USA, 1993; pp. 10–12. [Google Scholar]
- Fisher, M.E. Magnetism in One-Dimensional Systems—The Hesisenberg Model for Infinite Spin. Am. J. Phys. 1964, 32, 343–346. [Google Scholar] [CrossRef]
- Bonner, J.C.; Fisher, M.E. Linear Magnetic Chains with Anisotropic Coupling. Phys. Rev. 1964, 135, A640–A658. [Google Scholar] [CrossRef]
- Hiller, W.; Strähle, J.; Datz, A.; Hanack, M.; Hatfield, W.E.; Ter Haar, L.W.; Gütlich, P. Synthesis, Structure, and Magnetic Properties of catena-(µ-Oxo)(hemiporphyrazinato)iron(IV), the First Polymeric µ-Oxo-Bridged Complex of Iron. J. Am. Chem. Soc. 1984, 106, 329–335. [Google Scholar] [CrossRef]
- Francese, G.; Ferlay, S.; Schmalle, H.W.; Decurtins, S. A New 1D bimetal lic thiocyanate-bridged copper (II)-cobalt(II) compound. New J. Chem. 1999, 23, 267–269. [Google Scholar] [CrossRef]
- Tomkiewicz, A.; Kłak, J.; Mroziński, J. Bimetallic complexes with macrocyclic ligands. Variation of magnetic exchange interactions in some heteronuclear thiocyanato-bridged compounds. Mater. Sci. Pol. 2004, 22, 253–263. [Google Scholar]
- Quan, Y.-P.; Yin, P.; Han, N.-N.; Yang, A.-H.; Gao, H.-L.; Cui, J.-Z.; Shi, W.; Cheng, P. Novel hetero-polynuclear metal complexes (CuL)3[Mn(NCS)5]2 and (NiL)3[Mn(NCS)5]2 containing trigonal bipyramidal geometric [Mn(NCS)5]3− as bridging ligand. Inorg. Chem. Commun. 2009, 12, 469–472. [Google Scholar] [CrossRef]
- Kou, H.-Z.; Liao, D.-Z.; Cheng, P.; Jiang, Z.-H.; Yan, S.-P.; Wang, G.-L.; Yao, X.-K.; Wang, H.-G. A new one-dimensional thiocyanato-bridged bimetallic compound [Cu(en)2Mn(NCS)4(H2O)2]n. Synthesis, crystal structure, and magnetic properties. Can. J. Chem. 1998, 76, 1102–1107. [Google Scholar] [CrossRef]
- Zhang, K.-L.; Chen, W.; Xu, Y.; Wang, Z.; Zhong, Z.J.; You, X.-Z. Crystal structure and magnetic properties of a thiocyanato-bridged dinuclear Cr (III)-Cu (II) complex [(HLCu(SCN)Cr(NCS)3(NH3)2]·DMF. Polyhedron 2001, 20, 2033–2036. [Google Scholar] [CrossRef]
- Nikitina, V.M.; Nesterova, O.V.; Kokozay, V.N.; Goreshnik, E.A.; Jezierska, J. The first heterometallic Cu(II)/Cr(III) complex with an open-chain Schiff-base ligand self-assembled from copper powder, Reineckes salt, ethylenediamine and acetone. Polyhedron 2008, 27, 2426–2430. [Google Scholar] [CrossRef]
- Smékal, Z.; Březina, F.; Šindelář, Z.; Klička, R.; Nádvornik, M. Polynuclear complexes of chromium (III), copper (II) or nickel (II) with thiocyanato as a bridging ligand. Transition Met. Chem. 1997, 22, 299–301. [Google Scholar] [CrossRef]
- Vasková, Z.; Moncol, J.; Korabik, M.; Valigura, D.; Švorec, J.; Lis, T.; Valko, M.; Melník, M. Supramolecular dimer formation through hydrogen bond extensions of carboxylate ligands—Path for magnetic exchange. Polyhedron 2010, 29, 154–163. [Google Scholar] [CrossRef]
- O’Connor, C.J. Magnetochemistry—Advances in Theory and Experimentation. In Progress in Inorganic Chemistry; Lippard, S.J., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1982; Volume 29, pp. 203–283. [Google Scholar] [CrossRef]
- Mueller, U.; Förster, R.; Hellmig, M.; Huschmann, F.U.; Kastner, A.; Malecki, P.; Pühringer, S.; Röwer, M.; Sparta, K.; Steffien, M.; et al. The macromolecular crystallography beamlines at BESSY II of the Helmholtz-Zentrum Berlin: Current status and perspectives. Eur. Phys. J. Plus 2015, 130, 141. [Google Scholar] [CrossRef]
| Parameters | 1 | 2 |
|---|---|---|
| Empirical formula | C10H20CuN8S4Zn | C12H24CuN8S4Zn |
| Formula weight | 509.49 | 537.54 |
| Temperature [K] | 100(2) | 293(2) |
| Wavelength [Å] | 0.7999 | 0.71073 |
| Space group | Triclinic, P –1 | Monoclinic, P 21/m |
| Unit cell dimensions [Å] and [°] | a = 8.6630(6) α = 97.190(7) b = 8.9219(7) β = 97.164(6) c = 13.5420(11) γ = 98.772(6) | a = 8.2626(3) α = 90 b = 16.4982(6) β = 95.317(4) c = 8.2895(3) γ = 90 |
| Volume [Å3] | 1015.19(14) | 1125.14(7) |
| Z, Calculated density [Mg/m3] | 2, 1.667 | 2, 1.587 |
| Absorption coefficient [mm−1] | 2.650 | 2.396 |
| F(000) | 518 | 550 |
| Crystal size [mm] | 0.205 × 0.180 × 0.080 | 0.500 × 0.360 × 0.090 |
| Theta range for data collection [°] | 2.924–32.209 | 2.468−26.370 |
| Limiting indices | −11 ≤ h ≤ 11 −11 ≤ k ≤ 11 −18 ≤ l ≤18 | −10 ≤ h ≤ 9 −20 ≤ k ≤ 20 −10 ≤ l ≤ 10 |
| Reflections collected / unique | 16727/4431 [R(int) = 0.0298] | 6640 / 2383 [R(int) = 0.0291] |
| Completeness to theta | 28.681° 89.5% | 25.242° 99.9% |
| Absorption correction | Numerical | Numerical |
| Max. and min. transmission | 0.816 and 0.613 | 0.813 and 0.380 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 4431 / 0 / 247 | 2383 / 2 / 164 |
| Goodness-of-fit on F2 | 1.056 | 1.068 |
| Final R indices [I > 2sigma(I)] | R1a = 0.0381, wR2b = 0.1000 | R1a = 0.0345, wR2b = 0.0917 |
| R indices (all data) | R1a = 0.0413, wR2b = 0.1018 | R1a = 0.0417, wR2b = 0.0946 |
| Extinction coefficient | N/a | 0.0264(19) |
| Largest diff. peak and hole [eÅ−3] | 1.039 and −0.871 | 0.631 and −0.546 |
| Bond | Length | Bond | Length | Bond | Length |
|---|---|---|---|---|---|
| Zn1−N2 | 1.950(3) | Cu2−N14 i | 1.993(2) | Cu3−N24 | 2.006(3) |
| Zn1−N1 | 1.963(3) | Cu2−N14 | 1.993(2) | Cu3−N24 ii | 2.006(3) |
| Zn1−N3 | 1.969(3) | Cu2−N11 i | 2.031(3) | Cu3−N21 | 2.017(3) |
| Zn1−N4 | 1.969(3) | Cu2−N11 | 2.031(3) | Cu3−N21 ii | 2.017(3) |
| Cu2−S3 i | 2.9718(8) | Cu3−S4 ii | 3.0432(9) | ||
| Cu2−S3 | 2.9718(8) | Cu3−S4 | 3.0432(9) |
| D-H | A | d(D-H) [Å] | d(H∙∙∙A) [Å] | d(D∙∙∙A) [Å] | <DHA [°] |
|---|---|---|---|---|---|
| N11-H11A | S2 [1+x,1+y,z] | 0.990 | 2.85 | 3.696(3) | 144 |
| N14-H14A | S1 [–x,1–y,1–z] | 0.990 | 2.46 | 3.431(3) | 167 |
| N21-H21A | N1 [–x,1–y,2–z] | 0.990 | 2.51 | 3.283(4) | 134 |
| N21-H21A | N4 [–x,1–y,2–z] | 0.990 | 2.50 | 3.365(5) | 146 |
| N21-H21B | S3 [1–x,1–y,2–z] | 0.990 | 2.62 | 3.514(3) | 151 |
| N24-N24B | S2 [x,1+y,z] | 0.990 | 2.54 | 3.446(3) | 152 |
| Bond | Length | Bond | Length |
|---|---|---|---|
| Zn1−N3 | 1.950(3) | Cu2−N4 | 1.980(2) |
| Zn1−N3 i | 1.950(3) | Cu2−N4 ii | 1.980(2) |
| Zn1−N1 | 1.955(4) | Cu2−N7 ii | 2.085(2) |
| Zn1−N2 | 1.967(4) | Cu2−N7 | 2.085(2) |
| Cu2−S3 ii | 3.3477(14) | ||
| Cu2−S3 | 3.3477(14) |
| D-H | A | d(D-H) [Å] | d(H∙∙∙A) [Å] | d(D∙∙∙A) [Å] | <DHA [°] |
|---|---|---|---|---|---|
| N4-H4A | S2 [x,y,–1+z] | 0.822(15) | 2.778(13) | 3.532(3) | 153.2(13) |
| N4-H4B | S1 [–1+x,y,–1+z] | 0.821(14) | 2.767(14) | 3.485(2) | 147.0(13) |
| C9-H9A | S3 [x,y,z] | 0.96 | 2.66 | 3.505(8) | 147 |
| Model | HDVV | Curie–Weiss and Molecular Field | ||||||
|---|---|---|---|---|---|---|---|---|
| Compound | 2J | g | R·106 | C | θ [K] | G | zJ’ | R·106 |
| [cm–1] | [cm3 mol–1 K] | [cm–1] | ||||||
| 1 | 0.040 | 2.103 | 8.02 | 0.415 | 0.05 | 2.104 | 0.063 | 7.97 |
| 2 | −1.054 | 2.261 | 2.99 | 0.478 b | −1.40 b | 2.259 | −2.011 | 1.92 |
| a | −0.222 | 2.164 | 1.70 | 0.439 | −0.24 | 2.163 | −0.331 | 1.58 |
| Diamine (Solv.) | pn (1) (None) | N,N-Me2-en (2) (None) | en (H2O) | en (½H2O) | en (MeCN) |
|---|---|---|---|---|---|
| Crystal system | Triclinic | Monoclinic | Orthorhombic | Orthorhombic | Monoclinic |
| Space group | P ‒1 | P 21/m | C mcm | P 21 21 21 | P 21 |
| Chain type | “Irregular” zigzaga | Zigzag | Zigzag | Helical | Linear |
| No. of NCS‒ bridging ions | 2 | 2 | 2 | 2 | 1 |
| Cu−S [Å] | Cu2 2.9718 Cu3 3.0432 | 3.3477 | 3.0336 | 2.921 3.218 | 3.202 3.232 |
| Cu−N [Å] | Cu2 1.993 | 1.980 | 2.022 | 1.99 | 1.918 |
| Cu2 2.031 | 2.085 | 1.99 | 1.990 | ||
| Cu3 2.006 | 2.01 | 2.085 | |||
| Cu3 2.017 | 2.04 | 2.085 | |||
| Comment to Cu−N distances | Four pairs of identical (two for each centers) | Two pairs of identical | Four identical | Pair + single + single | Single + single + pair |
| Copper environment | 4 + 2 | 4 + 2 | 4 + 2 | 4 + 1 + 1 | 4 + 1 + 1 ≈ 4 + 2 |
| Δ in Zn−N [Å] | 0.019 | 0.017 | 0.280 | 0.080 | 0.157 |
| Δ in N−Zn−N [°] | 10.45 | 12.60 | 14.89 | 5.30 | 23.1 |
| Calculated density [Mg/m3] | 1.667 | 1.587 | 1.729 | 1.649 | 1.627 |
| Cu···Cu [Å] intrachain | 8.481 | 8.249 | 7.484 | 9.001b | 6.308 |
| Cu···Cu [Å] interchain | 7.569 8.663 | 8.263 | 8.074 12.414 | 7.947b 8.651b | 9.496b 11.409b |
| Zn···Zn [Å] intrachain | 10.078 12.726 | 13.758 | 11.659 | 8.261b | 11.574b |
| Zn···Zn [Å] interchain | 8.663 | 8.263 11.149 | 8.074 | 7.807b 9.120b | 6.314b 9.496b |
| Cu···Zn [Å] intrachain | 5.039 6.363 | 6.872 | 5.829 | 5.491b 6.242b | 5.783b 5.791b |
| Cu···Zn [Å] interchain | 7.156 7.675 | 7.011 7.701 | 7.281 | 5.780b 6.233b | 5.602b 5.629b |
| Magnetic interaction | Ferro | Antiferro | Antiferro | No data | No data |
| Reference | This work | This work | [19] | [18] | [18] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tereba, N.; Muzioł, T.M.; Podgajny, R.; Wrzeszcz, G. Influence of the Substituted Ethylenediamine Ligand on the Structure and Properties of [Cu(diamine)2Zn(NCS)4]∙Solv. Compounds. Crystals 2019, 9, 637. https://doi.org/10.3390/cryst9120637
Tereba N, Muzioł TM, Podgajny R, Wrzeszcz G. Influence of the Substituted Ethylenediamine Ligand on the Structure and Properties of [Cu(diamine)2Zn(NCS)4]∙Solv. Compounds. Crystals. 2019; 9(12):637. https://doi.org/10.3390/cryst9120637
Chicago/Turabian StyleTereba, Natalia, Tadeusz M. Muzioł, Robert Podgajny, and Grzegorz Wrzeszcz. 2019. "Influence of the Substituted Ethylenediamine Ligand on the Structure and Properties of [Cu(diamine)2Zn(NCS)4]∙Solv. Compounds" Crystals 9, no. 12: 637. https://doi.org/10.3390/cryst9120637
APA StyleTereba, N., Muzioł, T. M., Podgajny, R., & Wrzeszcz, G. (2019). Influence of the Substituted Ethylenediamine Ligand on the Structure and Properties of [Cu(diamine)2Zn(NCS)4]∙Solv. Compounds. Crystals, 9(12), 637. https://doi.org/10.3390/cryst9120637








