Intra-Cation versus Inter-Cation π-Contacts in [Cu(P^P)(N^N)][PF6] Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. [Cu(POP)(1)][PF6]
2.3. [Cu(xantphos)(1)][PF6]
2.4. [Cu(POP)(2)][PF6]
2.5. [Cu(xantphos)(2)][PF6]
2.6. [Cu(POP)(3)][PF6]
2.7. [Cu(xantphos)(3)][PF6]
2.8. Crystallography
2.9. [Cu(xantphos)(1)][PF6]·CH2Cl2
2.10. [Cu(xantphos)(2)][PF6]·CH2Cl2
2.11. [Cu(POP)(3)][PF6]·0.5H2O
3. Results and Discussion
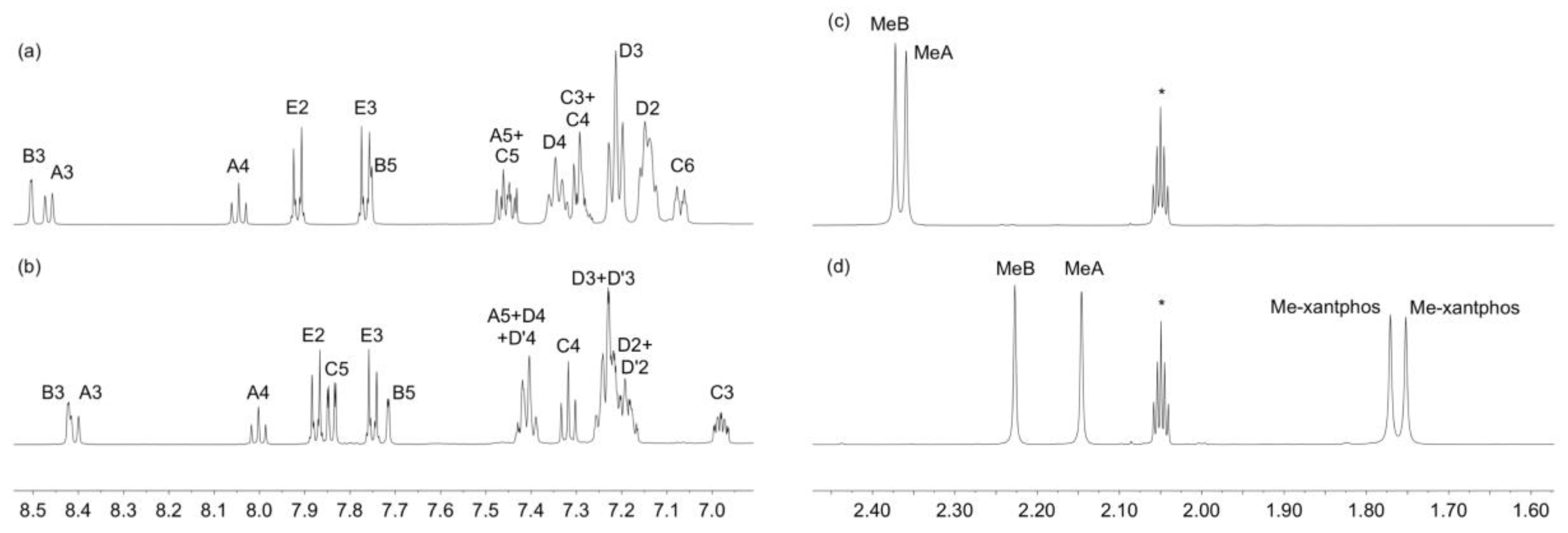
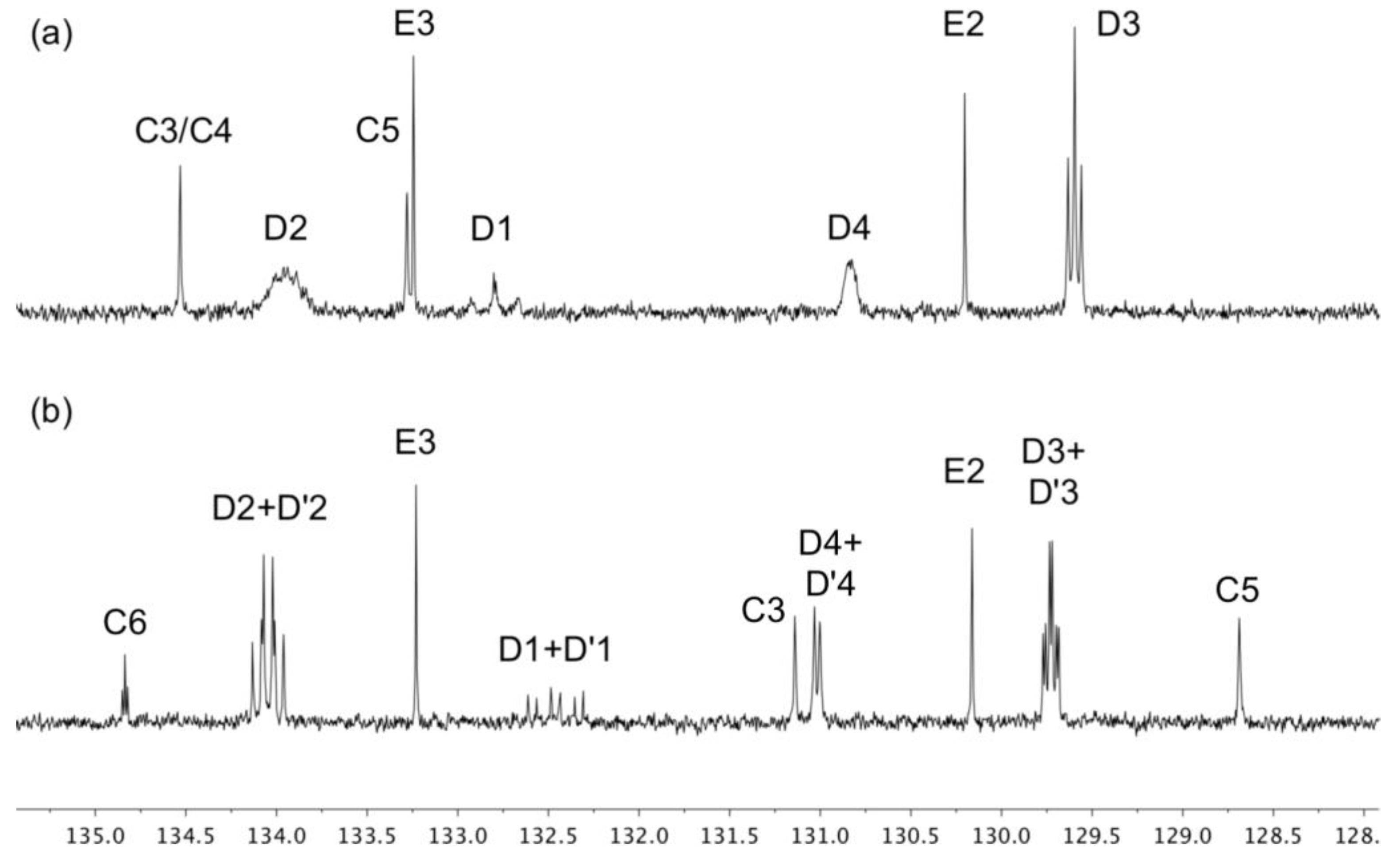
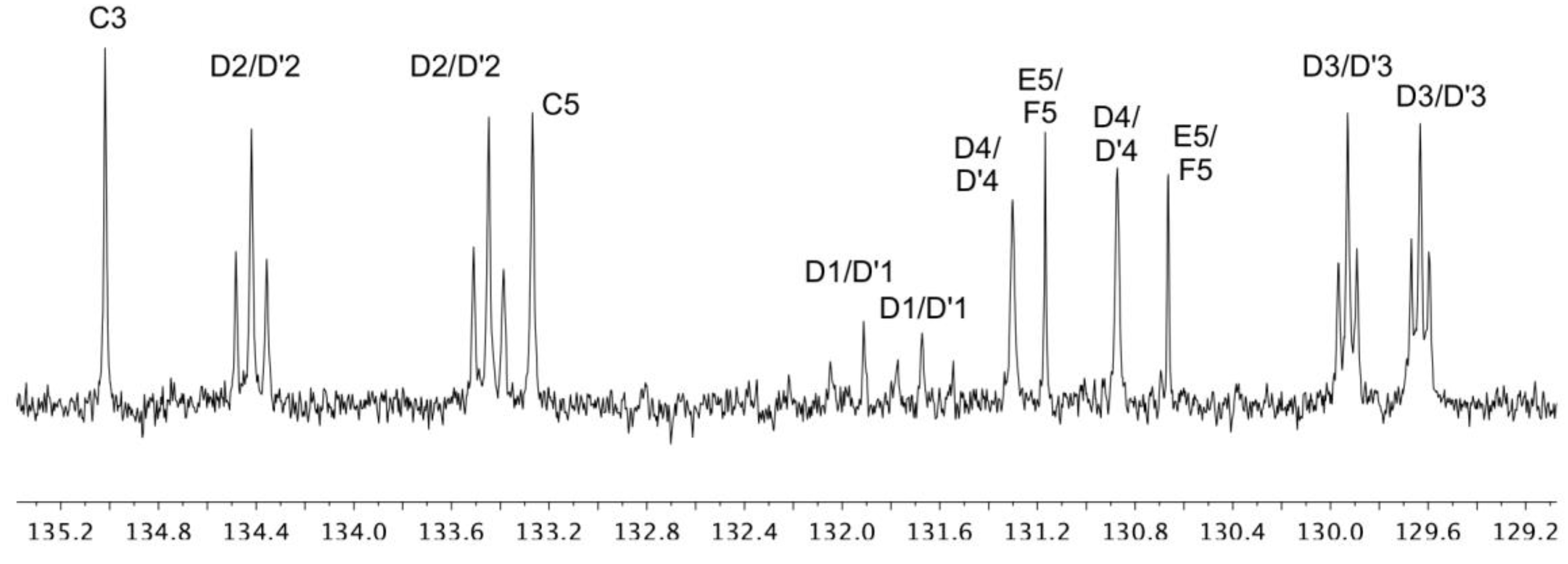
3.1. Synthesis and Mass Spectrometric and NMR Spectroscopic Characterization of the Copper(I) Compounds
3.2. Crystal Structures of [Cu(xantphos)(1)][PF6]·CH2Cl2, [Cu(xantphos)(2)][PF6]·CH2Cl2 and [Cu(POP)(3)][PF6]·0.5H2O
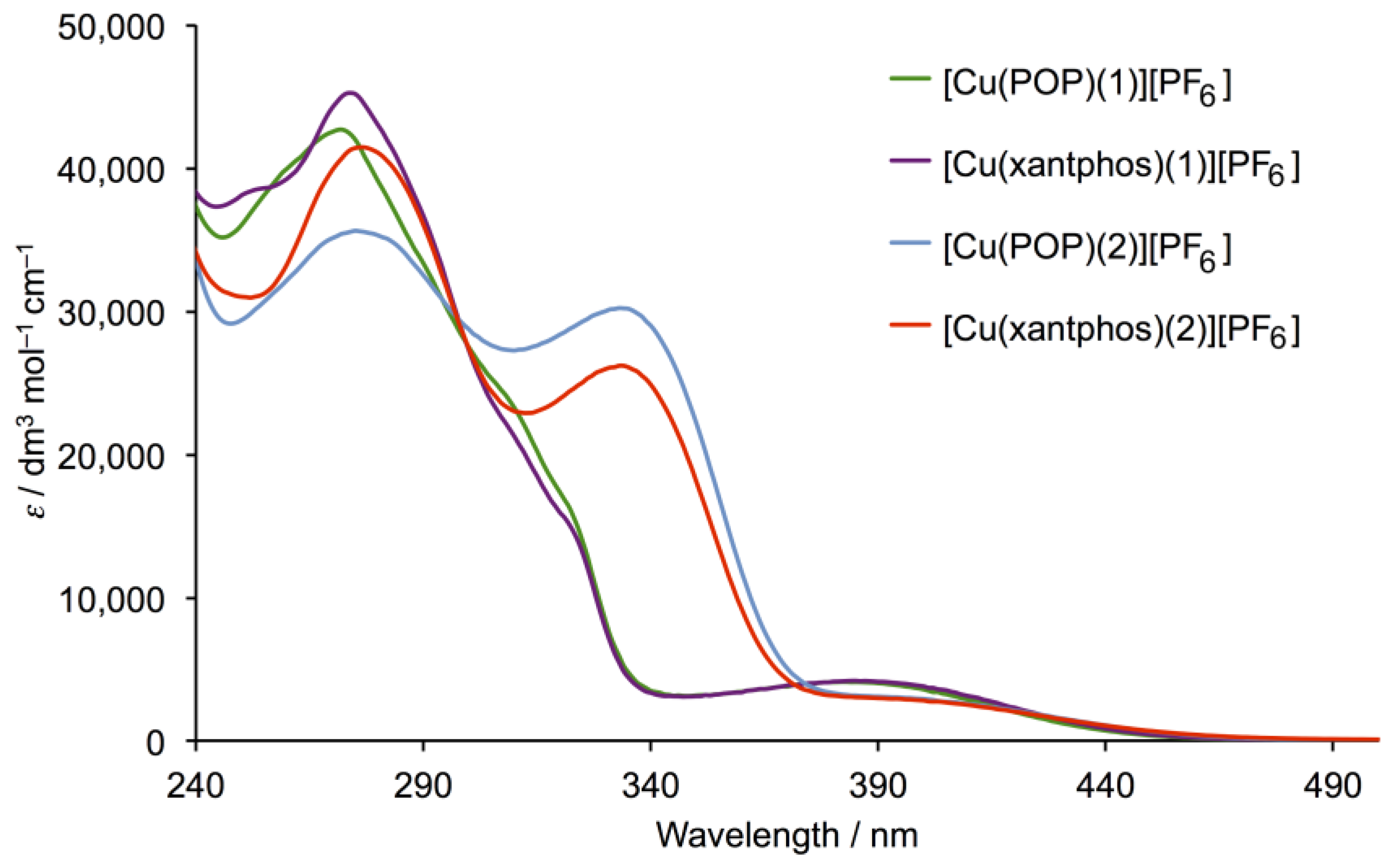
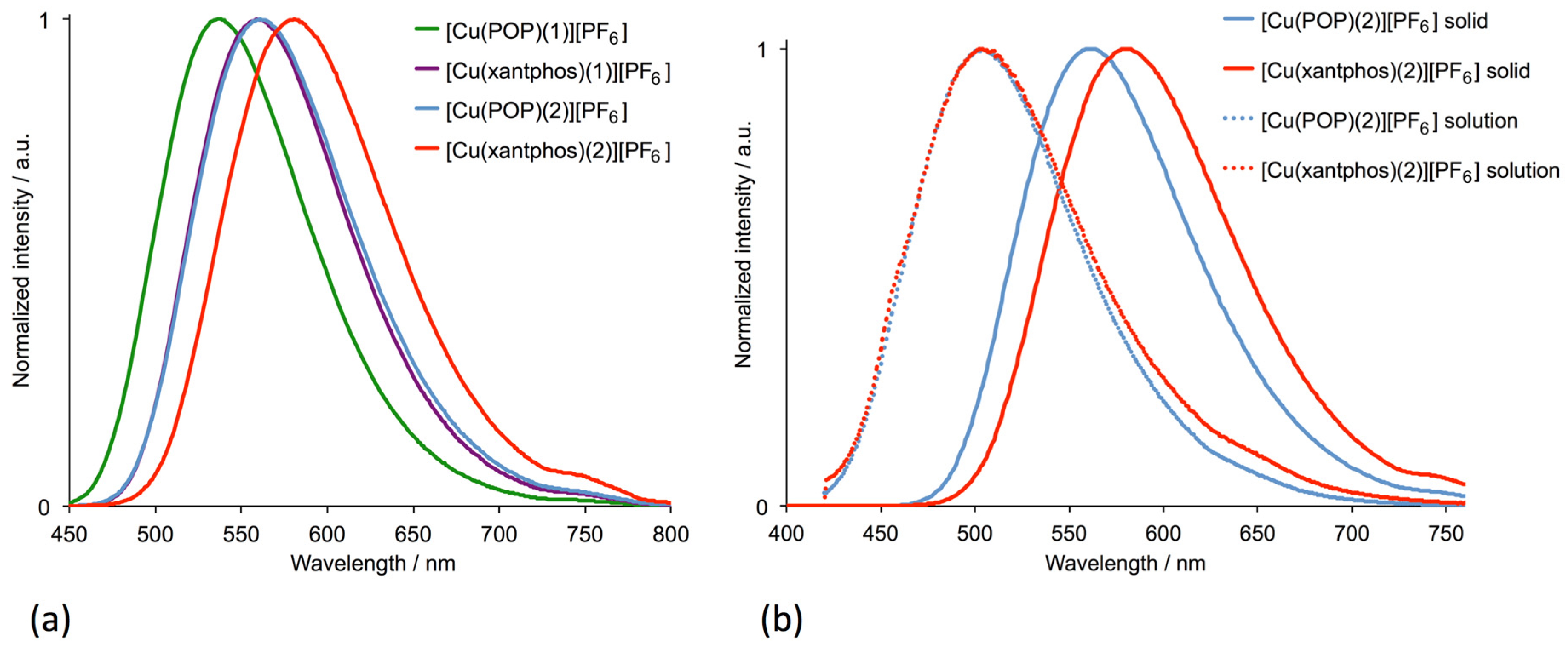
3.3. Electrochemical and Photophysical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Armaroli, N.; Accorsi, G.; Cardinali, F.; Listorti, A. Photochemistry and Photophysics of Coordination Compounds: Copper. Top. Curr. Chem. 2007, 280, 69–115. [Google Scholar] [CrossRef]
- Costa, R.D.; Ortí, E.; Bolink, H.J.; Monti, F.; Accorsi, G.; Armaroli, N. Luminescent ionic transition metal complexes for light-emitting electrochemical cells. Angew. Chem. Int. Ed. 2012, 51, 8178–8211. [Google Scholar] [CrossRef] [PubMed]
- Fresta, E.; Costa, R.D. Beyond traditional light-emitting electrochemical cells—A review of new device designs and emitters. J. Mater. Chem. C 2017, 5, 5643–5675. [Google Scholar] [CrossRef]
- Costa, R.D. (Ed.) Light-Emitting Electrochemical Cells: Concepts, Advances and Challenges; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Costa, R.D.; Tordera, D.; Ortí, E.; Bolink, H.J.; Schönle, J.; Graber, S.; Housecroft, C.E.; Constable, E.C.; Zampese, J.A. Copper(I) complexes for sustainable light-emitting electrochemical cells. J. Mater. Chem. C 2011, 21, 16108–16118. [Google Scholar] [CrossRef]
- Buckner, M.T.; McMillin, D.R. Photoluminescence from copper(I) complexes with low-lying metal-to-ligand charge transfer excited states. J. Chem. Soc. Chem. Commun. 1978, 759–761. [Google Scholar] [CrossRef]
- Rader, R.A.; McMillin, D.R.; Buckner, M.T.; Matthews, T.G.; Casadonte, D.J.; Lengel, R.K.; Whittaker, S.B.; Darmon, L.M.; Lytle, F.E. Photostudies of 2,2′-bipyridine bis(triphenylphosphine)copper(1+), 1,10-phenanthroline bis(triphenylphosphine)copper(1+), and 2,9-dimethyl-1,10-phenanthroline bis(triphenylphosphine)copper(1+) in solution and in rigid, low-temperature glasses. Simultaneous multiple emissions from intraligand and charge-transfer states. J. Am. Chem. Soc. 1981, 103, 5906–5912. [Google Scholar] [CrossRef]
- Keller, S.; Constable, E.C.; Housecroft, C.E.; Neuburger, M.; Prescimone, A.; Longo, G.; Pertegás, A.; Sessolo, M.; Bolink, H.J. [Cu(bpy)(P^P)]+ containing light-emitting electrochemical cells: Improving performance through simple substitution. Dalton Trans. 2014, 43, 16593–16596. [Google Scholar] [CrossRef] [Green Version]
- Keller, S.; Pertegás, A.; Longo, G.; Martínez, L.; Cerdá, J.; Junquera-Hernández, J.M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E.; Ortí, E.; et al. Shine bright or live long: Substituent effects in [Cu(N^N)(P^P)]+-based light-emitting electrochemical cells where N^N is a 6-substituted 2,2′-bipyridine. J. Mater. Chem. C 2016, 4, 3857–3871. [Google Scholar] [CrossRef] [Green Version]
- Alkan-Zambada, M.; Keller, S.; Martínez-Sarti, L.; Prescimone, A.; Junquera-Hernández, J.M.; Constable, E.C.; Bolink, H.J.; Sessolo, M.; Ortí, E.; Housecroft, C.E. [Cu(P^P)(N^N)][PF6] compounds with bis(phosphane) and 6-alkoxy, 6-alkylthio, 6-phenyloxy and 6-phenylthio-substituted 2,2′-bipyridine ligands for light-emitting electrochemical cells. J. Mater. Chem. C 2018, 6, 8460–8471. [Google Scholar] [CrossRef] [Green Version]
- Brunner, F.; Graber, S.; Baumgartner, Y.; Häussinger, D.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. The effects of introducing sterically demanding aryl substituents in [Cu(N^N)(P^P)]+ complexes. Dalton Trans. 2017, 46, 6379–6391. [Google Scholar] [CrossRef] [Green Version]
- Keller, S.; Prescimone, A.; Bolink, H.J.; Sessolo, M.; Longo, G.; Martínez-Sarti, L.; Junquera-Hernández, J.-M.; Constable, E.C.; Ortí, E.; Housecroft, C.E. Luminescent copper(I) complexes with bisphosphane and halogen-substituted 2,2′-bipyridine ligands. Dalton Trans. 2018, 47, 14263–14276. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Brunner, F.; Junquera-Hernández, J.M.; Pertegás, A.; La-Placa, M.-G.; Prescimone, A.; Constable, E.C.; Bolink, H.J.; Ortí, E.; Housecroft, C.E. CF3 Substitution of [Cu(P^P)(bpy)][PF6] complexes: Effects on Photophysical Properties and Light-emitting Electrochemical Cell Performance. ChemPlusChem 2018, 83, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Leoni, E.; Mohanraj, J.; Holler, M.; Mohankumar, M.; Nierengarten, I.; Monti, F.; Sournia-Saquet, A.; Delavaux-Nicot, B.; Nierengarten, J.-F.; Armaroli, N. Heteroleptic Copper(I) Complexes Prepared from Phenanthroline and Bis-Phosphine Ligands: Rationalization of the Photophysical and Electrochemical Properties. Inorg. Chem. 2018, 57, 15537–15549. [Google Scholar] [CrossRef] [PubMed]
- Brunner, F.; Martínez-Sarti, L.; Keller, S.; Pertegás, A.; Prescimone, A.; Constable, E.C.; Bolink, H.J.; Housecroft, C.E. Peripheral halo-functionalization in [Cu(N^N)(P^P)]+ emitters: Influence on the performances of light-emitting electrochemical cells. Dalton Trans. 2016, 45, 15180–15192. [Google Scholar] [CrossRef] [Green Version]
- Norrby, T.; Börje, A.; Zhang, L.; Åkermark, B. Regioselective Functionalization of 2,2′-Bipyridine and Transformations into Unsymmetric Ligands for Coordination Chemistry. Acta Chem. Scand. 1998, 52, 77–85. [Google Scholar] [CrossRef]
- Schönhofer, E.; Bozic-Weber, B.; Martin, C.J.; Constable, E.C.; Housecroft, C.E.; Zampese, J.A. ‘Surfaces-as-ligands, surfaces-as-complexes’ strategies for copper(I) dye-sensitized solar cells. Dyes Pigment. 2015, 115, 154–165. [Google Scholar] [CrossRef] [Green Version]
- Constable, E.C.; Housecroft, C.E.; Kariuki, B.M.; Smith, C.B. Switching on Hydrogen Bonding in Oligopyridine Ligands. Supramol. Chem. 2006, 18, 305–309. [Google Scholar] [CrossRef]
- Carroll, J.; Woolard, H.G.; Mroz, R.; Nason, C.A.; Huo, S. Regiospecific acylation of cycloplatinated complexes. Scope, limitation, and mechanistic implications. Organometallics 2016, 35, 1313–1322. [Google Scholar] [CrossRef]
- Kubas, G.J. Tetrakis(acetonitrile)copper(I) hexafluorophosphate. Inorg. Synth. 1979, 19, 90–92. [Google Scholar] [CrossRef]
- Software for the Integration of CCD Detector System; Bruker Analytical X-ray Systems, Bruker axs: Madison, WI, USA, 2013.
- Sheldrick, G.M. ShelXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with ShelXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and Analysis of Crystal Structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 955–964. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Brunner, F.; Babaei, A.; Pertegás, A.; Junquera-Hernández, J.M.; Prescimone, A.; Constable, E.C.; Bolink, H.J.; Sessolo, M.; Ortí, E.; Housecroft, C.E. Phosphane tuning in heteroleptic [Cu(N^N)(P^P)]+ complexes for light-emitting electrochemical cells. Dalton Trans. 2019, 48, 446–460. [Google Scholar] [CrossRef] [Green Version]
- Weber, M.D.; Viciano-Chumillas, M.; Armentano, D.; Cano, J.; Costa, R.D. σ-Hammett parameter: A strategy to enhance both photo- and electro-luminescence features of heteroleptic copper(II) complexes. Dalton Trans. 2017, 46, 6312–6323. [Google Scholar] [CrossRef]
- Dance, I.; Scudder, M. Supramolecular motifs: Sextuple aryl embraces in crystalline [M(2,2′-bipy)3] and related complexes. J. Chem. Soc. Dalton Trans. 1998, 1341–1350. [Google Scholar] [CrossRef]

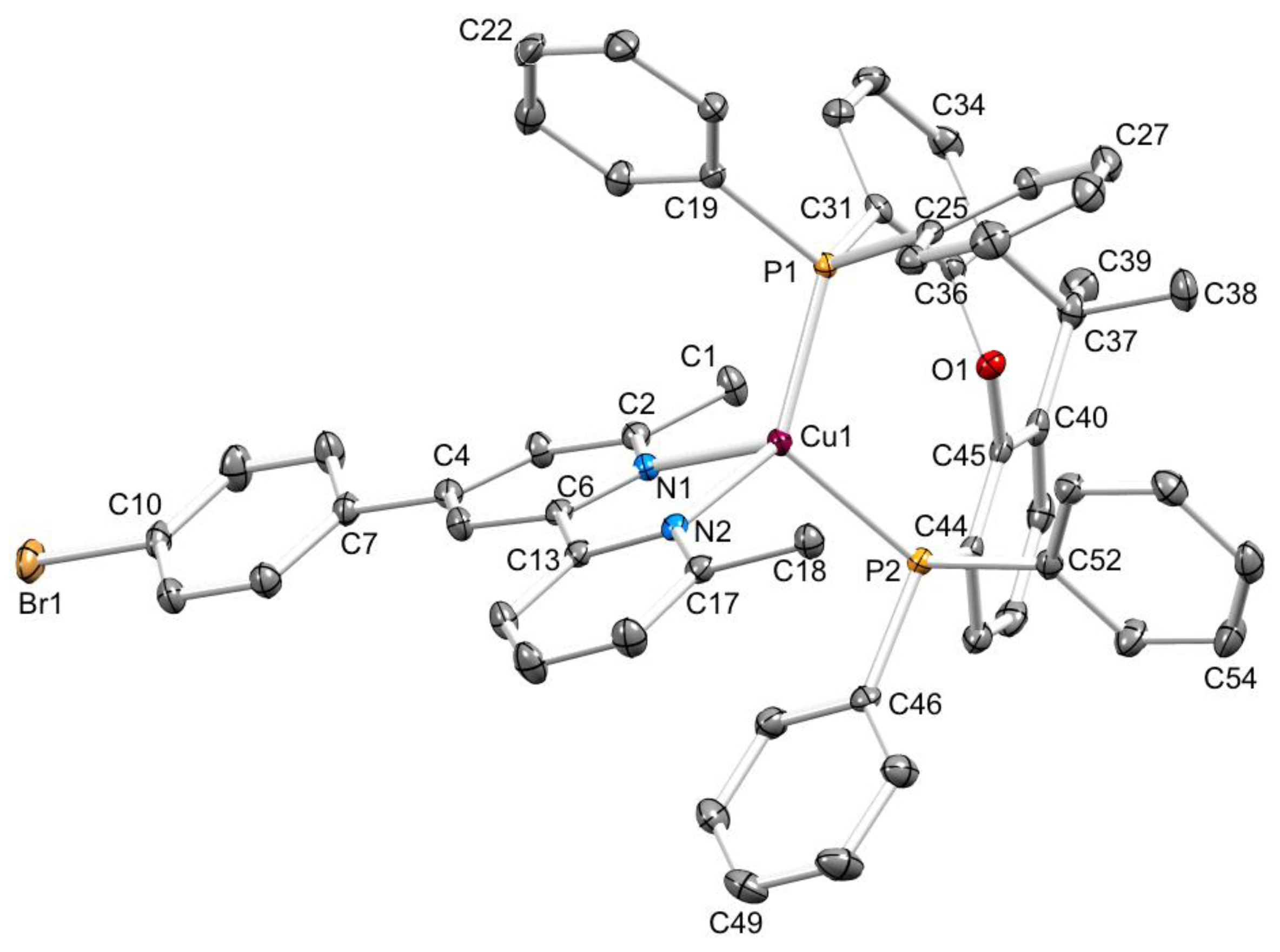

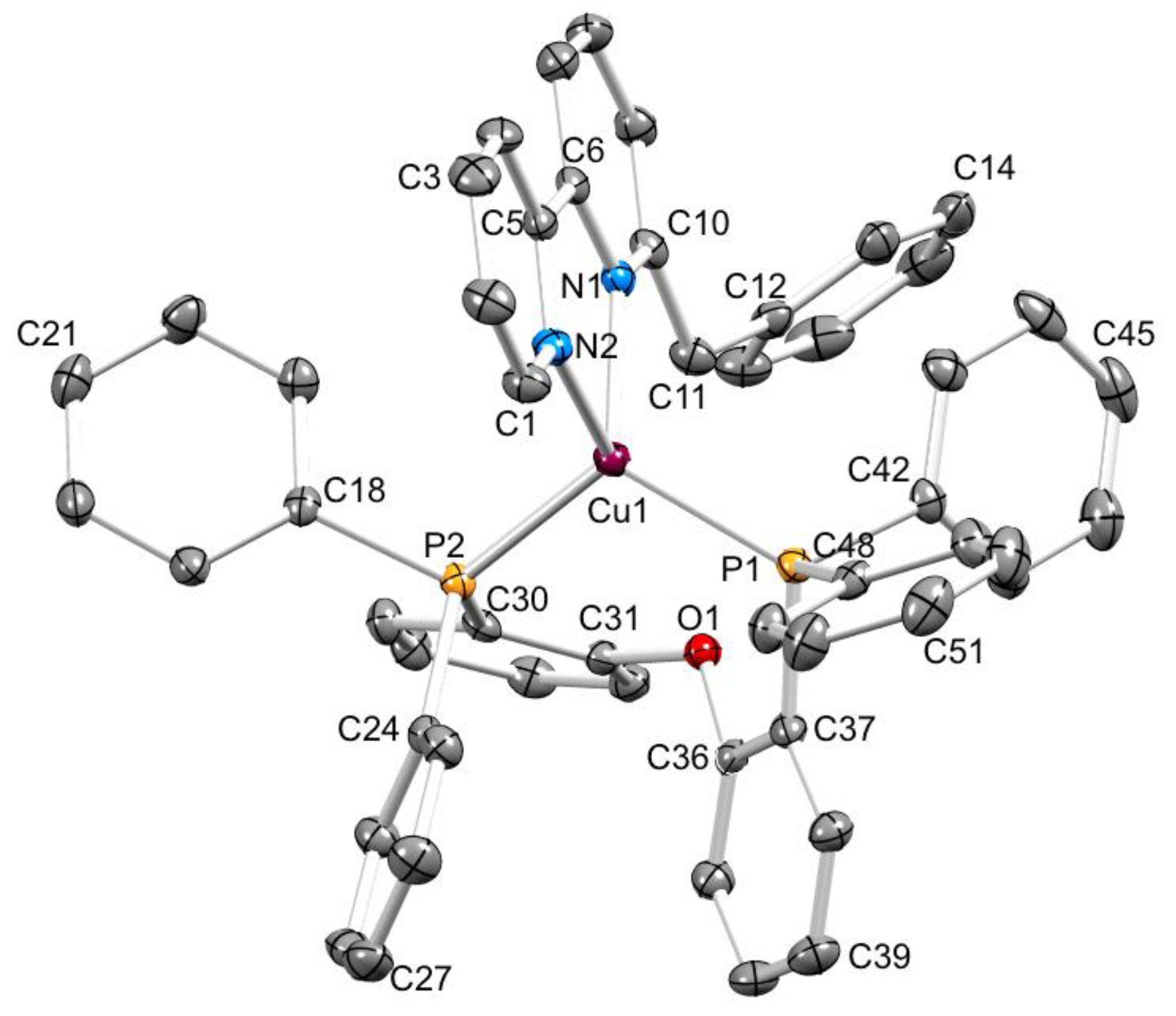
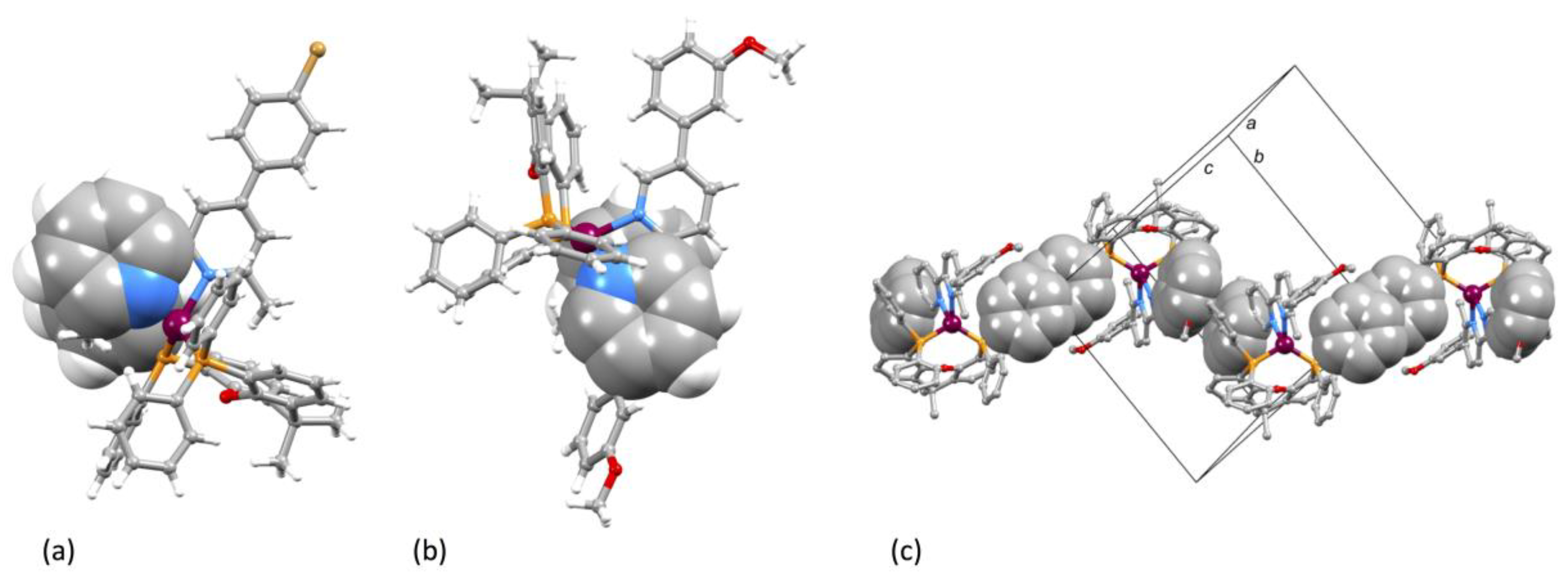
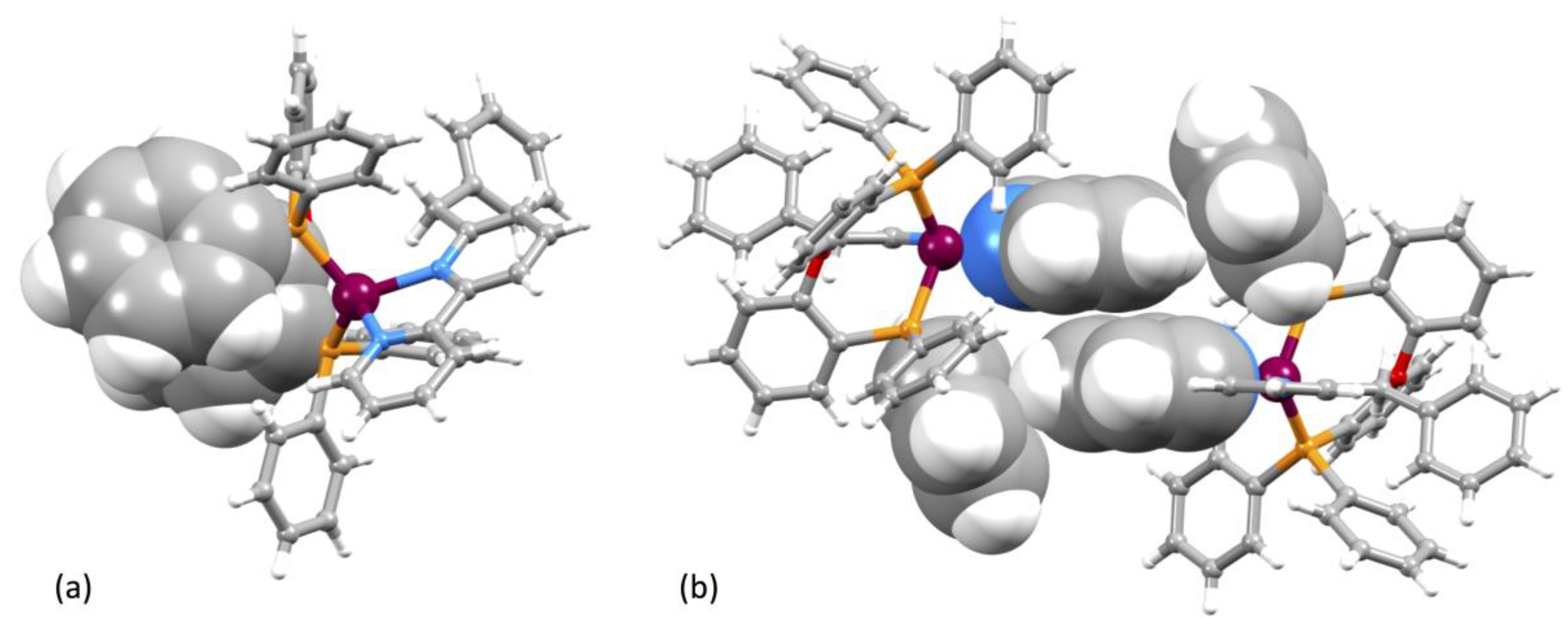
| [Cu(P^P)(N^N)]+ Cation | Cu–N/Å | Cu–P/Å | P–Cu–P/o | N–Cu–N/o | τ4 1 |
|---|---|---|---|---|---|
| [Cu(xantphos)(1)]+ | 2.1142(13), 2.0796(13) | 2.2589(5), 2.3038(5) | 116.050(17) | 79.05(5) | 0.83 |
| [Cu(xantphos)(2)]+ | 2.0654(18), 2.0693(18) | 2.2241(6), 2.2729(6) | 120.05(2) | 79.45(7) | 0.82 |
| [Cu(POP)(3)]+ | 2.060(2), 2.127(2) | 2.2789(7), 2.2662(7) | 112.83(3) | 79.68(8) | 0.89 |
| Cation in [Cu(P^P)(N^N)][PF6] | E1/2ox/V | Epc − Epa/mV | E1/2red/V | Epc − Epa/mV |
|---|---|---|---|---|
| [Cu(POP)(1)]+ | +0.92 a | - | −2.21, −2.11 b | - |
| [Cu(xantphos)(1)]+ | +0.87 | 80 | −2.18, −2.10 b | - |
| [Cu(POP)(2)]+ | +0.81 | 100 | −2.04 | 130 |
| [Cu(xantphos)(2)]+ | +0.84 | 110 | −2.00 | 130 |
| Cation in [Cu(P^P)(N^N)][PF6] | λmax/nm (ε/dm3 mol−1 cm−1) | |
|---|---|---|
| Ligand-Based Absorptions | MLCT | |
| [Cu(POP)(1)]+ | 260 sh (40,000), 272 (42,600), 308 sh (24,100), 322 sh (16,300) | 390 (4100) |
| [Cu(xantphos)(1)]+ | 253 sh (38,500), 274 (45,200), 321 sh (15,100) | 390 (4100) |
| [Cu(POP)(2)]+ | 276 (35,500), 333 (30,200) | 400 (2700) |
| [Cu(xantphos)(2)]+ | 277 (41,450), 333 (26,200) | 400 (2700) |
| Cation in [Cu(P^P)(N^N)][PF6] | λexc/nm | λem/nm | PLQY a (Non-Deaerated)/% | PLQY a (Deaerated)/% |
|---|---|---|---|---|
| [Cu(POP)(1)]+ | 365 | 575 | 2 | 20 |
| [Cu(xantphos)(1)]+ | 365 | 570 | 1 | 12 |
| [Cu(POP)(2)]+ | 365 | 500 | 3 | 10 |
| [Cu(xantphos)(2)]+ | 365 | 500 | 3 | 10 |
| Cation in [Cu(P^P)(N^N)][PF6] | λexc/nm | λem/nm | PLQY/% | τ1/2/μs |
|---|---|---|---|---|
| [Cu(POP)(1)]+ | 365 | 535 | 41 | 9.4 |
| [Cu(xantphos)(1)]+ | 365 | 560 | 35 | 11.1 |
| [Cu(POP)(2)]+ | 365 | 560 | 27 | 9.2 |
| [Cu(xantphos)(2)]+ | 365 | 577 | 19 | 5.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzeo, F.; Brunner, F.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Intra-Cation versus Inter-Cation π-Contacts in [Cu(P^P)(N^N)][PF6] Complexes. Crystals 2020, 10, 1. https://doi.org/10.3390/cryst10010001
Mazzeo F, Brunner F, Prescimone A, Constable EC, Housecroft CE. Intra-Cation versus Inter-Cation π-Contacts in [Cu(P^P)(N^N)][PF6] Complexes. Crystals. 2020; 10(1):1. https://doi.org/10.3390/cryst10010001
Chicago/Turabian StyleMazzeo, Francesca, Fabian Brunner, Alessandro Prescimone, Edwin C. Constable, and Catherine E. Housecroft. 2020. "Intra-Cation versus Inter-Cation π-Contacts in [Cu(P^P)(N^N)][PF6] Complexes" Crystals 10, no. 1: 1. https://doi.org/10.3390/cryst10010001
APA StyleMazzeo, F., Brunner, F., Prescimone, A., Constable, E. C., & Housecroft, C. E. (2020). Intra-Cation versus Inter-Cation π-Contacts in [Cu(P^P)(N^N)][PF6] Complexes. Crystals, 10(1), 1. https://doi.org/10.3390/cryst10010001








