A Cd(II) Coordination Polymer Based on Mixed Ligands: Synthesis, Crystal Structure, and Properties
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Instrumentation
2.2. Synthesis of [Cd(L)(frda)(H2O)]·0.5L·H2O (1)
2.3. Crystal Structure Determination
3. Results and Discussion
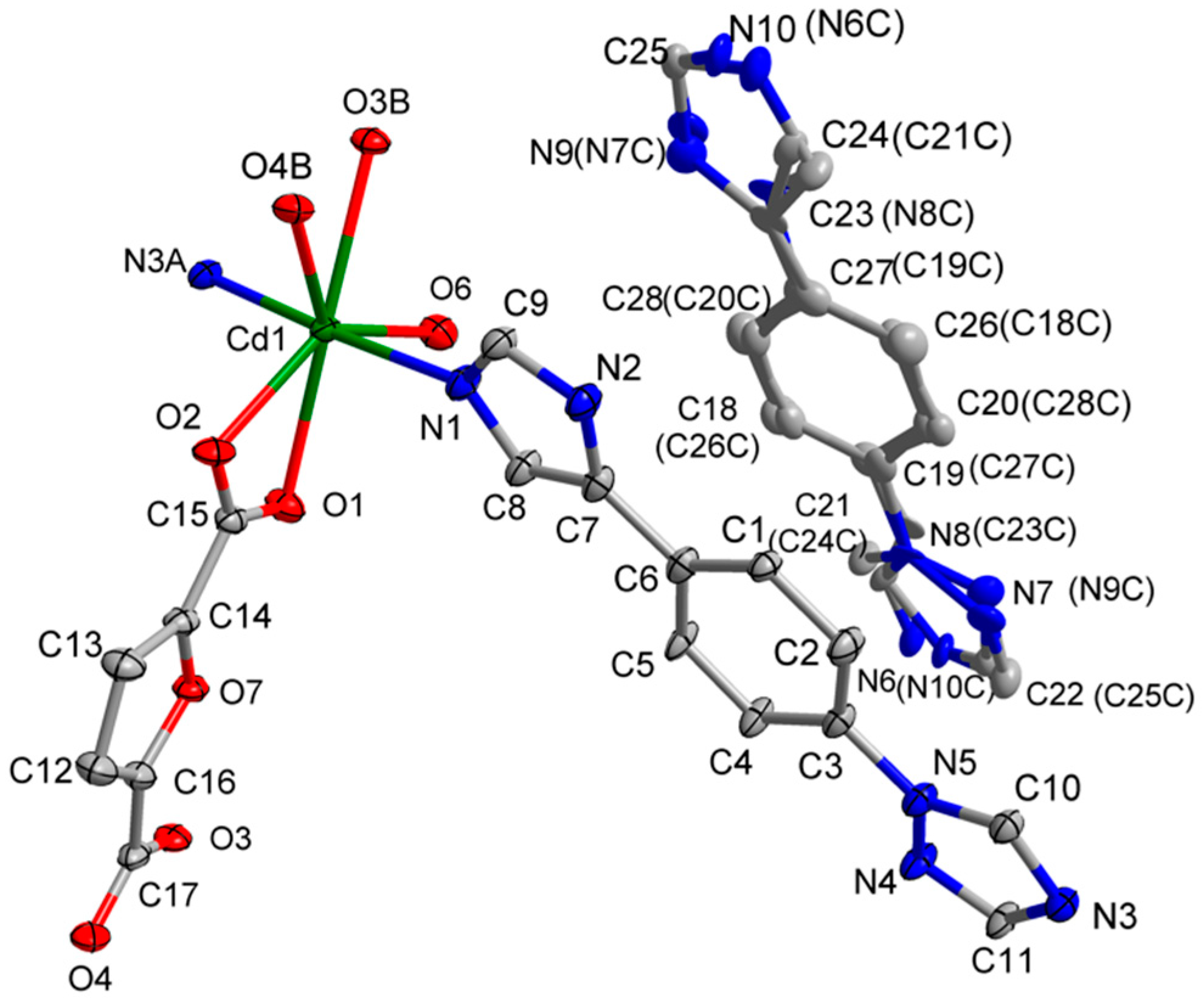
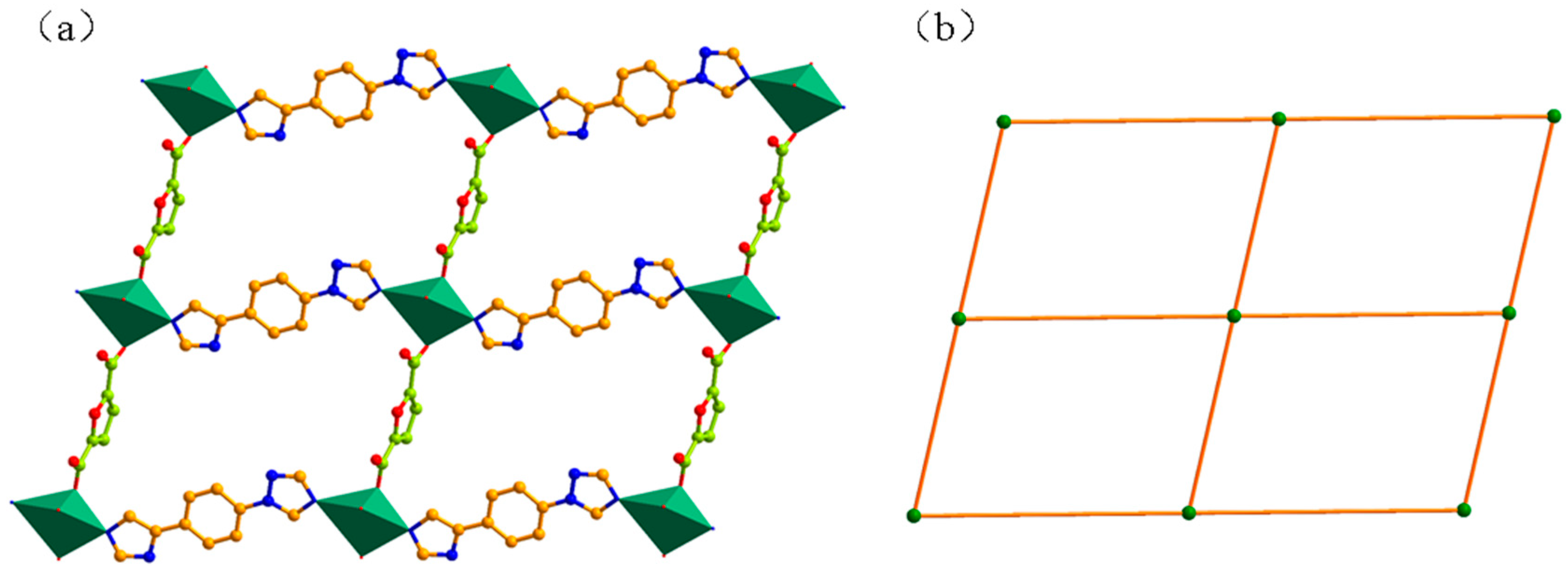
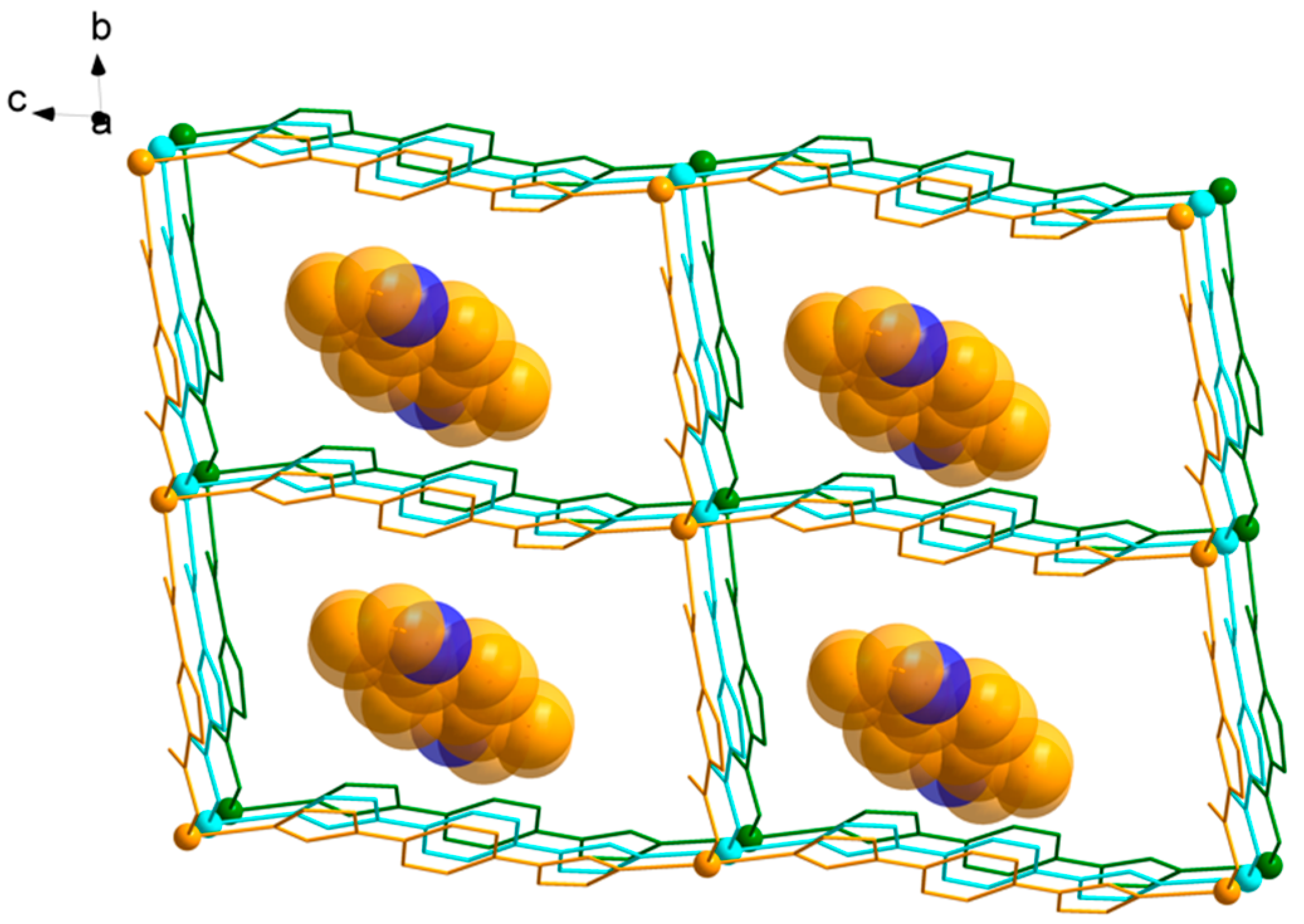
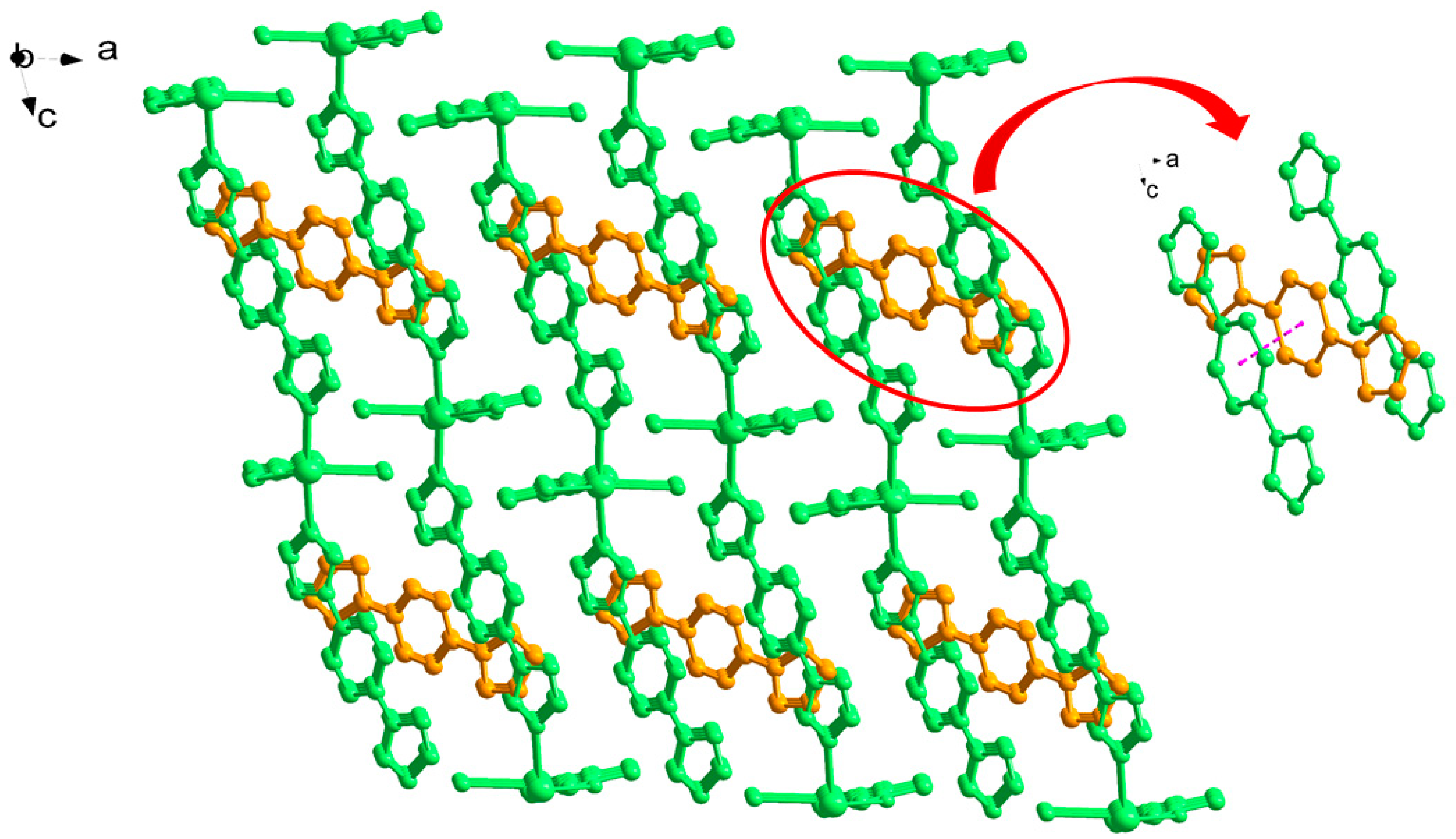
3.1. Structural of [Cd(L)(frda)(H2O)]·0.5L·H2O (1)
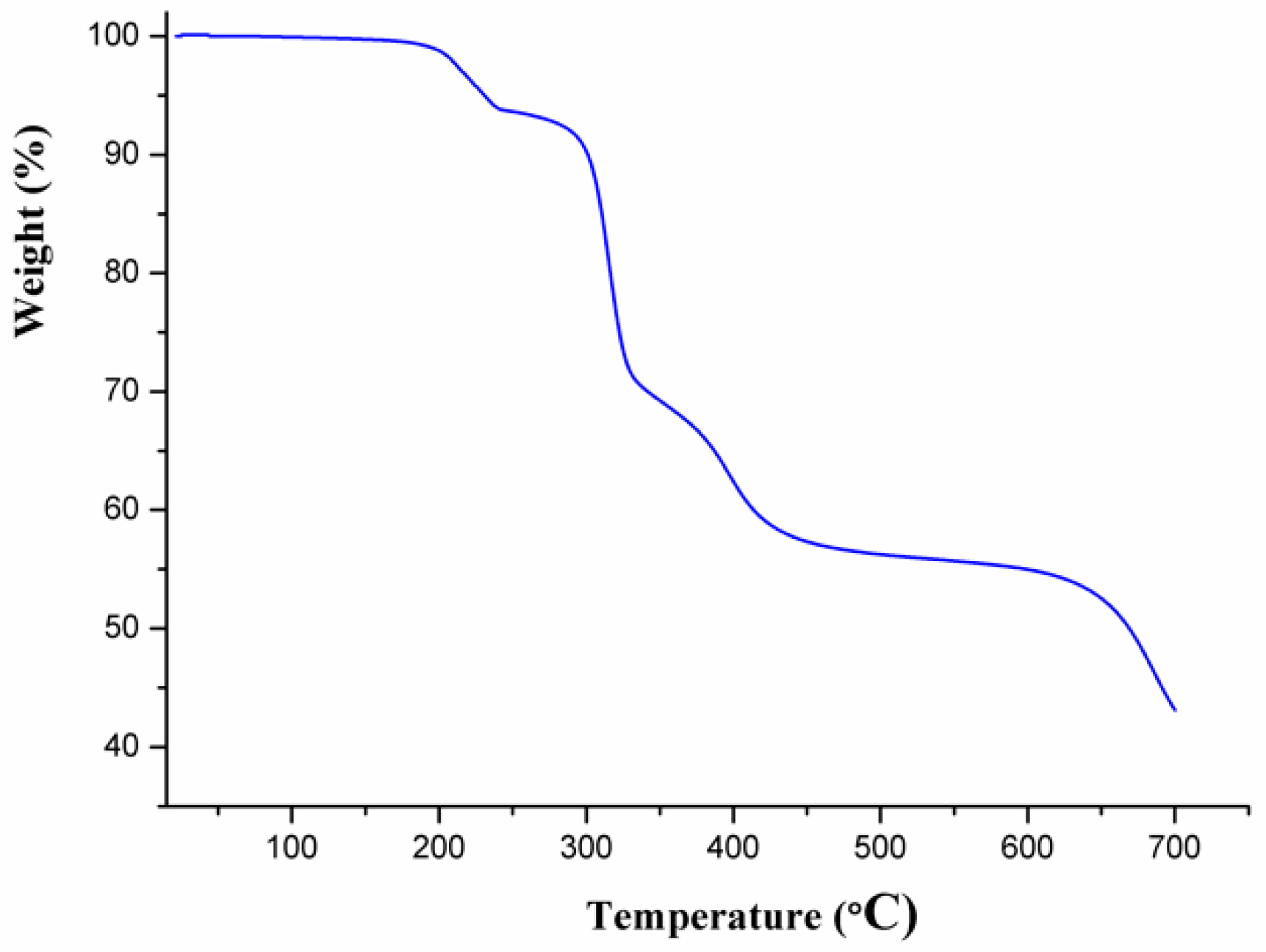
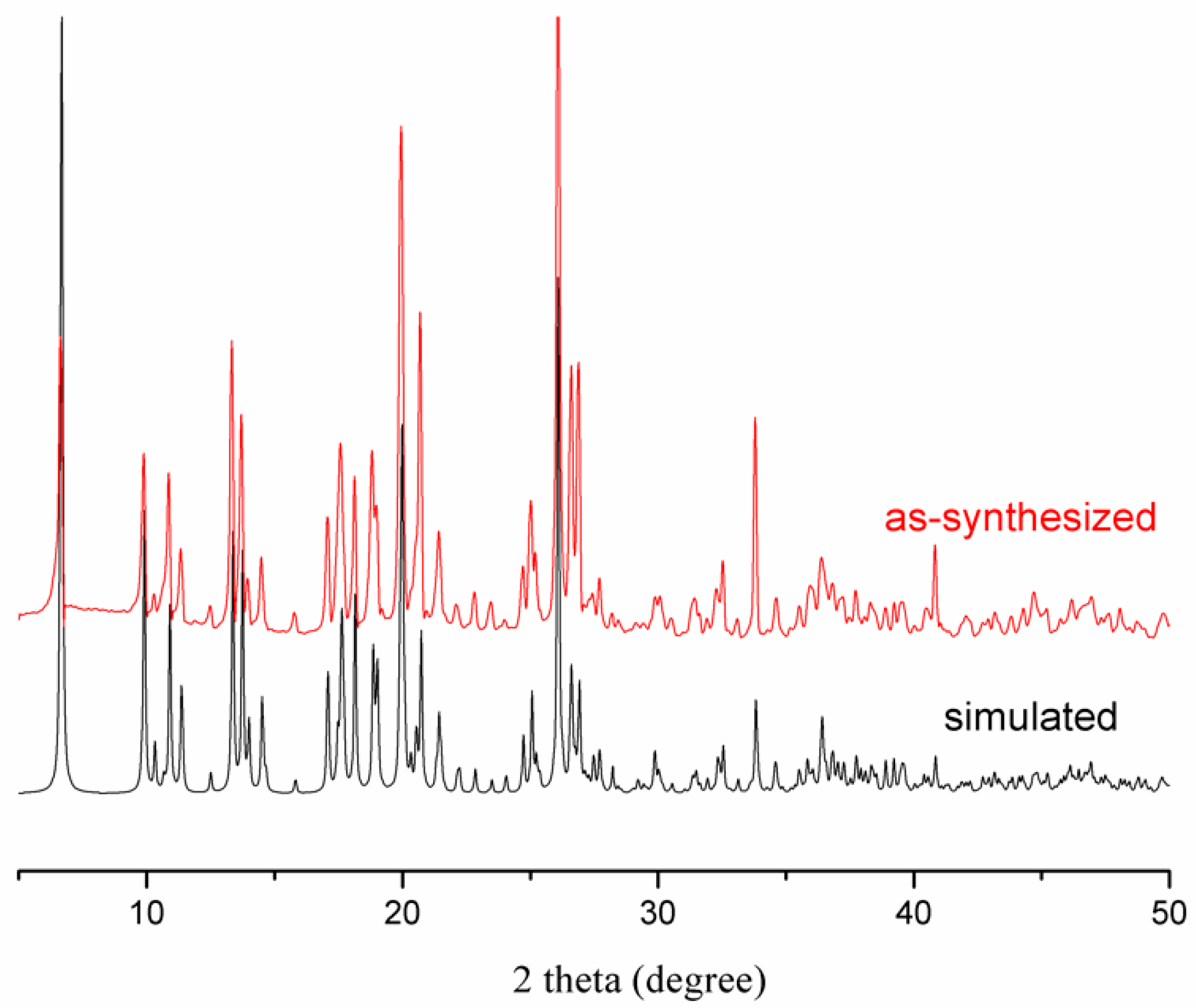
3.2. Thermal Analysis and Powder X-Ray Diffraction Analysis
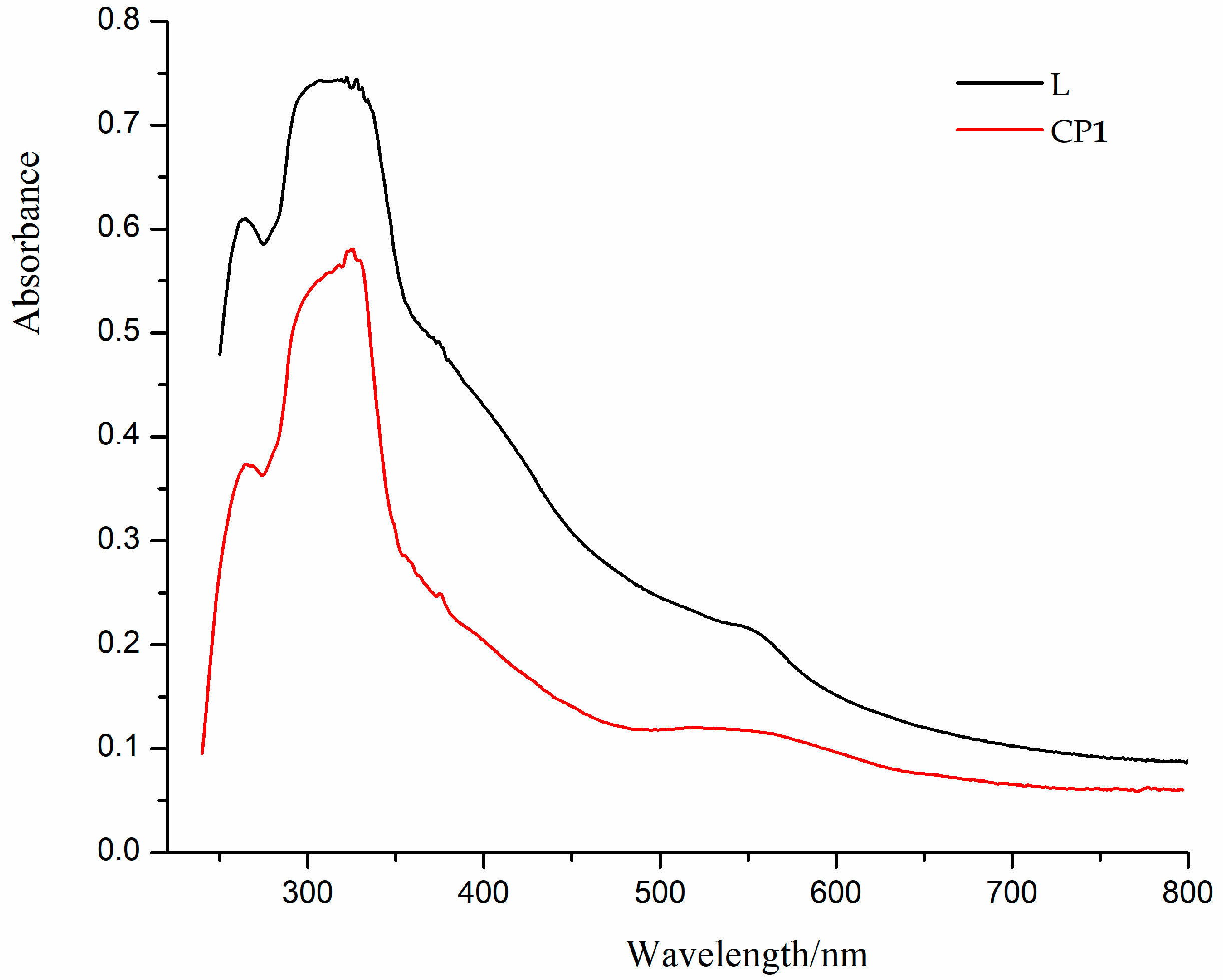
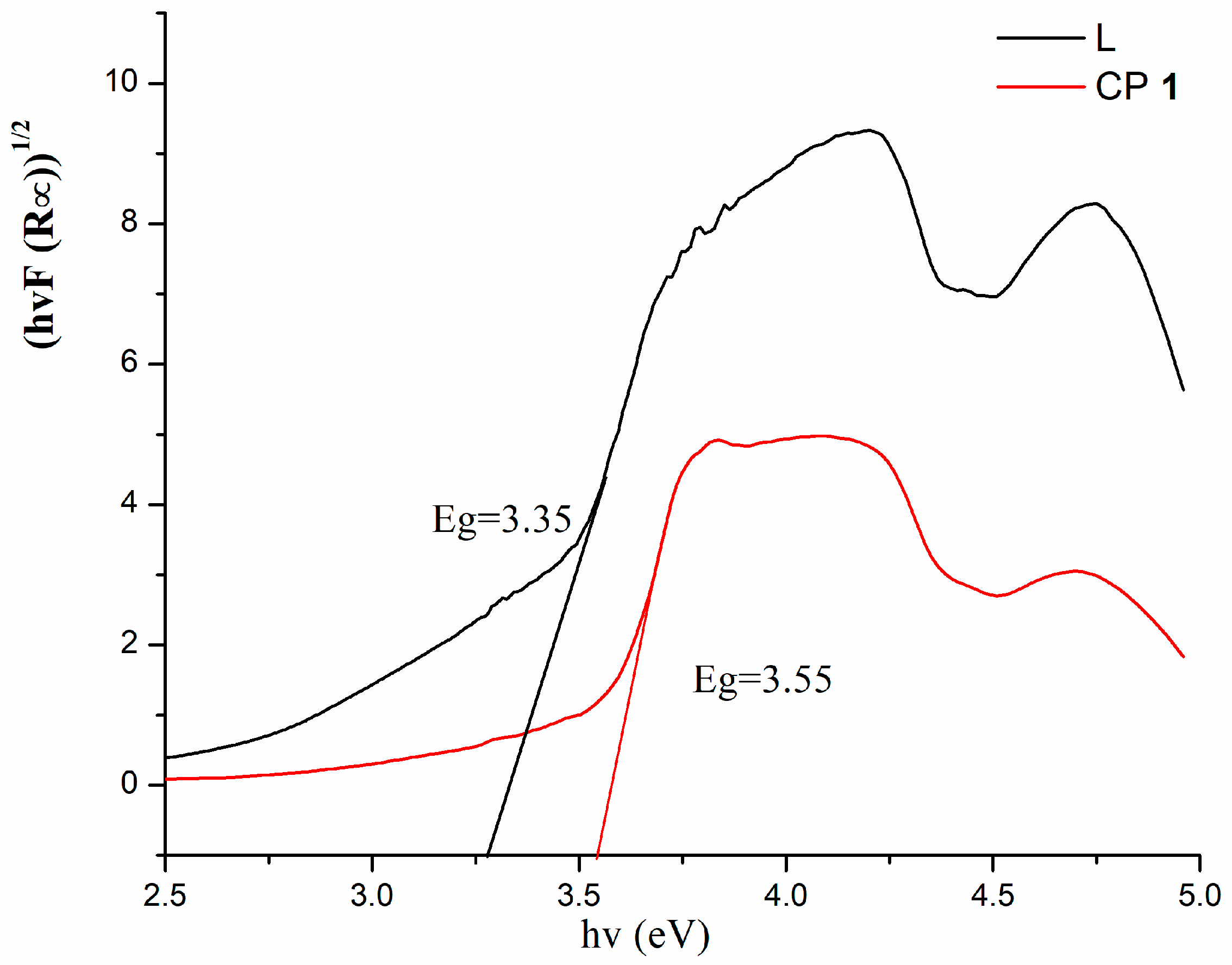
3.3. Diffuse Reflectance Spectra
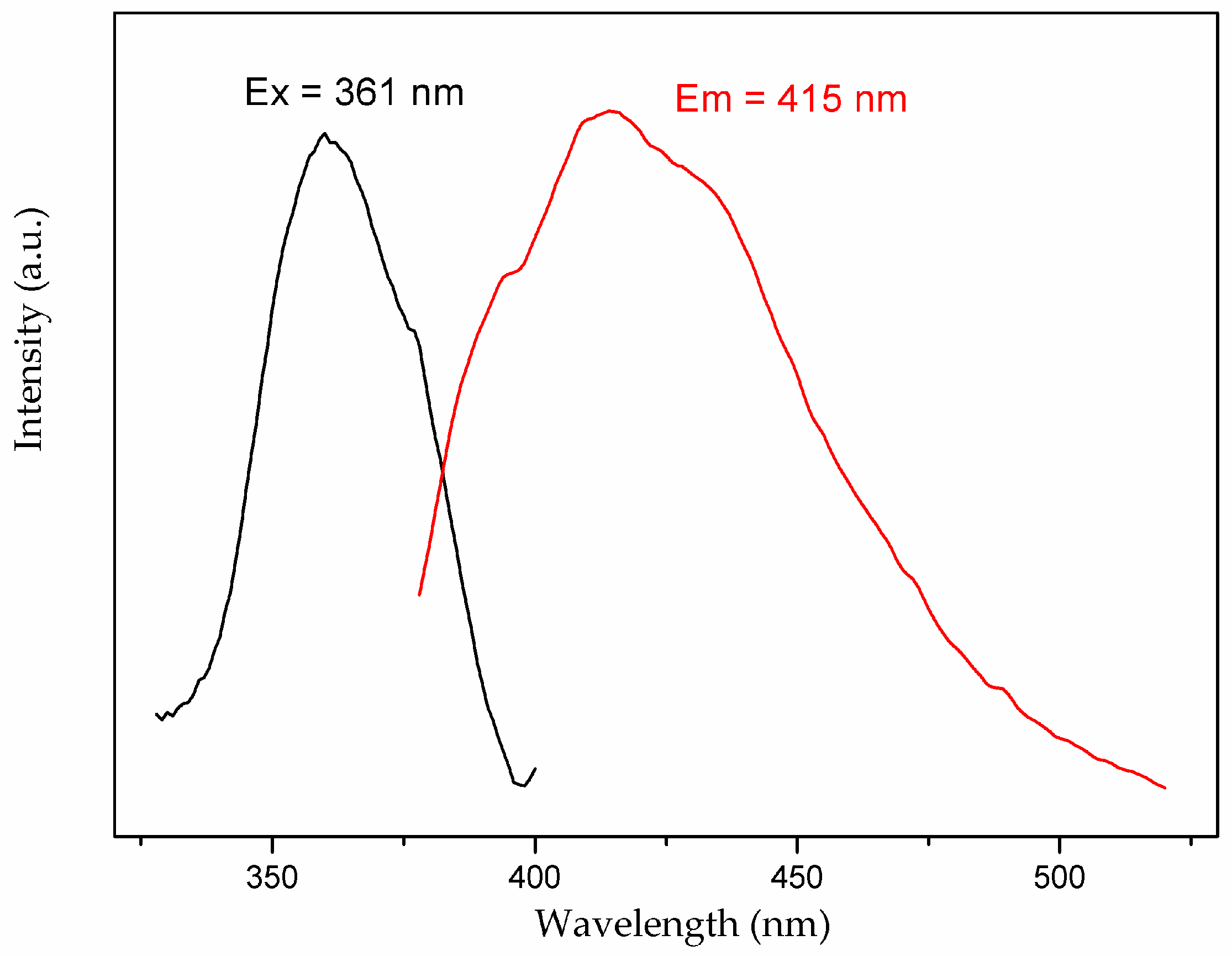
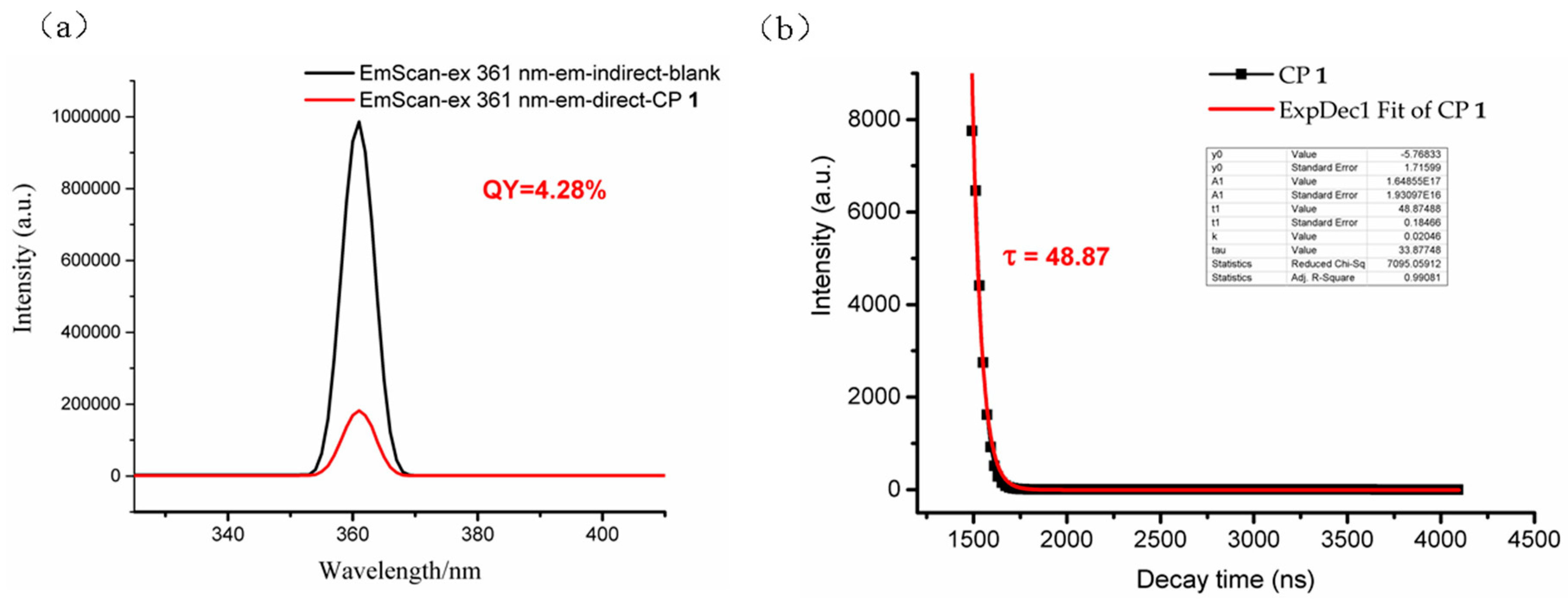
3.4. Photoluminescent Property
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, Z.C.; Deibert, B.J.; Li, J. Luminescent metal−organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, X.G.; Lu, X.M.; Yang, C.D.; Fan, N.N.; Yang, Z.T.; Wang, L.Y.; Ma, L.F. {Zn6} Cluster based metal–organic framework with enhanced room-temperature phosphorescence and optoelectronic performances. Inorg. Chem. 2019, 58, 6215–6221. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Zhao, Y.; Zhang, X.D.; Kang, Y.S.; Lu, Q.Y.; Azam, M.; Al-Resayes, S.I.; Sun, W.Y. Metal−organic frameworks with 1,4-di(1H-imidazol-4-yl)benzene and varied carboxylate ligands for selectively sensing Fe(III) ions and ketone molecules. Dalton Trans. 2017, 46, 13943–13951. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.G.; Ma, L.F.; Yan, D.P. Facile synthesis of 1D organic–inorganic perovskite micro-belts with high water stability for sensing and photonic applications. Chem. Sci. 2019, 10, 4567–4572. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Samanta, P.; Desai, A.V.; Ghosh, S.K. Guest responsive metal−organic frameworks as scaffolds for separation and sensing applications. Acc. Chem. Res. 2017, 50, 2457–2469. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Fan, N.N.; Han, M.L.; Yang, G.P.; Ma, L.F. Porous Zn(II)-based metal–organic frameworks decorated with carboxylate groups exhibiting high gas adsorption and separation of organic dyes. Cryst. Growth Des. 2018, 18, 7114–7121. [Google Scholar] [CrossRef]
- Zhang, K.; Xie, X.; Li, H.; Gao, J.; Nie, L.; Pan, Y.; Xie, J.; Tian, D.; Liu, W.; Fan, Q.; et al. Highly water-stable lanthanide–oxalate MOFs with remarkable proton conductivity and tunable luminescence. Adv. Mater. 2017, 29, 1701804. [Google Scholar] [CrossRef]
- Macreadie, L.K.; Mensforth, E.J.; Babarao, R.; Konstas, K.; Telfer, S.G.; Doherty, C.M.; Tsanaktsidis, J.; Batten, S.R.; Hill, M.R. CUB-5: A contoured aliphatic pore environment in a cubic framework with potential for benzene separation applications. J. Am. Chem. Soc. 2019, 141, 3828–3832. [Google Scholar] [CrossRef]
- Kang, Y.S.; Lu, Y.; Chen, K.; Zhao, Y.; Wang, P.; Sun, W.Y. Metal-organic frameworks with catalytic centers: From synthesis to catalytic application. Coord. Chem. Rev. 2019, 378, 262–280. [Google Scholar] [CrossRef]
- Cook, T.R.; Stang, P.J. Recent developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem. Rev. 2015, 115, 7001. [Google Scholar] [CrossRef]
- Li, N.; Feng, R.; Zhu, J.; Chang, Z.; Bu, X.H. Conformation versatility of ligands in coordination polymers: From structural diversity to properties and applications. Coord. Chem. Rev. 2018, 375, 558–586. [Google Scholar] [CrossRef]
- Zhu, M.A.; Guo, X.Z.; Xiao, L.; Chen, S.S. A new Cd(II) coordination compound based on 4-(1,2,4-triazol-4-yl)phenylacetic acid: Synthesis, structure and photoluminescence property. Chin. J. Struct. Chem. 2018, 37, 437–444. [Google Scholar]
- Wu, Y.P.; Tian, J.W.; Liu, S.; Li, B.; Zhao, J.; Ma, L.F.; Li, D.S.; Lan, Y.Q.; Bu, X. Bi-microporous metal-organic-frameworks with cubane [M4(OH)4] (M = Ni, Co) clusters and pore space partition for electrocatalytic methanol oxidation reaction. Angew. Chem. Int. Ed. 2019, 58, 12185–12189. [Google Scholar] [CrossRef]
- Zhou, Z.; Han, M.L.; Fu, H.R.; Ma, L.F.; Luo, F.; Li, D.S. Engineering design toward exploring the functional group substitution in 1D channels of Zn-organic frameworks upon nitro explosives and antibiotics detection. Dalton Trans. 2018, 47, 5359–5365. [Google Scholar] [CrossRef]
- Zhao, X.; Bu, X.H.; Nguyen, E.T.; Zhai, Q.G.; Mao, C.Y.; Feng, P.Y. Multivariable modular design of pore space partition. J. Am. Chem. Soc. 2016, 138, 15102–15105. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Z.; Li, J.L.; Shi, S.S.; Zhou, H.; Han, S.S.; Chen, S.S. Synthesis, structure and luminescent property of a Zn(II) complex with mixed multi-N donor and 2,5-dihydroxy-terephthalic acid ligands. Chin. J. Struct. chem. 2018, 37, 1117–1124. [Google Scholar]
- Han, L.J.; Yan, W.; Chen, S.G.; Shi, Z.Z.; Zheng, H.G. Exploring the detection of metal ions by tailoring the coordination mode of V-shaped thienylpyridyl ligand in three MOFs. Inorg. Chem. 2017, 56, 2936–2940. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 974. [Google Scholar] [CrossRef]
- Dong, X.Y.; Si, C.D.; Fan, Y.; Hu, D.C.; Yao, X.Q.; Yang, Y.X.; Liu, J.C. Effect of N-donor ligands and metal ions on the coordination polymers based on a semirigid carboxylic acid ligand: Structures analysis, magnetic properties, and photoluminescence. Cryst. Growth Des. 2016, 16, 2062–2073. [Google Scholar] [CrossRef]
- Roztocki, K.; Jedrzejowski, D.; Hodorowicz, M.; Senkovska, I.; Kaskel, S.; Matoga, D. Effect of linker substituent on layers arrangement, stability, and sorption of Zn-isophthalate/acylhydrazone frameworks. Cryst. Growth Des. 2018, 18, 488–497. [Google Scholar] [CrossRef]
- Yang, G.P.; Hou, L.; Ma, L.F.; Wang, Y.Y. Investigation on the prime factors influencing the formation of entangled metal−organic frameworks. CrystEngComm 2013, 15, 2561–2578. [Google Scholar] [CrossRef]
- Singh, R.; Bharadwaj, P.K. Coordination polymers built with a linear bis-imidazole and different dicarboxylates: Unusual entanglement and emission properties. Cryst. Growth Des. 2013, 13, 3722–3733. [Google Scholar] [CrossRef]
- Chen, S.S.; Chen, M.; Takamizawa, S.; Wang, P.; Lv, G.C.; Sun, W.Y. Temperature dependent selective gas sorption of the microporous metal-imidazolate framework [Cu(L)][H2L = 1, 4-di (1H-imidazol-4-yl) benzene]. Chem. Commun. 2011, 47, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Chen, M.; Takamizawa, S.; Wang, P.; Lv, G.C.; Sun, W.Y. Porous cobalt(II)-imidazolate supramolecular isomeric frameworks with selective gas sorption property. Chem. Commun. 2011, 47, 4902–4904. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Wang, P.; Takamizawa, S.; Okamura, T.A.; Chen, M.; Sun, W.Y. Zinc (II) and cadmium(II) metal−organic frameworks with 4-imidazole containing tripodal ligand: Sorption and anion exchange properties. Dalton Trans. 2014, 43, 6012–6020. [Google Scholar] [CrossRef]
- Chen, S.S.; Sheng, L.Q.; Zhao, Y.; Liu, Z.D.; Qiao, R.; Yang, S. Syntheses, structures, and properties of a series of polyazaheteroaromatic core-based Zn (II) coordination polymers together with carboxylate auxiliary ligands. Cryst. Growth Des. 2016, 16, 229–241. [Google Scholar] [CrossRef]
- Chen, S.S. The roles of imidazole ligands in coordination supramolecular systems. CrystEngComm 2016, 18, 6543–6565. [Google Scholar] [CrossRef]
- Chen, S.S.; Qiao, R.; Sheng, L.Q.; Zhao, Y.; Yang, S.; Chen, M.M.; Liu, Z.D.; Wang, D.H. Cadmium(II) and zinc(II) complexes with rigid 1-(1H-imidazol-4-yl)-3-(4H-tetrazol-5-yl)benzene and varied carboxylate ligands. CrystEngComm 2013, 15, 5713–5725. [Google Scholar] [CrossRef]
- Ten Have, R.; Huisman, M.; Meetsma, A.; van Leusen, A.M. Novel Synthesis of 4(5)-Monosubstituted Imidazoles via Cycloaddition of Tosylmethyl Isocyanide to Aldimines. Tetrahedron 1997, 53, 11355–11368. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Blatov, V.A. TOPOS, A Multipurpose Crystallochemical Analysis with the Program Package; Samara State University: Samara, Russia, 2009. [Google Scholar]
- Chen, S.S.; Liu, Q.; Zhao, Y.; Qiao, R.; Sheng, L.Q.; Liu, Z.D.; Yang, S.; Song, C.F. New metal−organic frameworks constructed from the 4-imidazole-carboxylate ligand: Structural diversities, luminescence, and gas adsorption properties. Cryst. Growth Des. 2014, 14, 3727–3741. [Google Scholar] [CrossRef]
- Li, J.L.; Li, W.D.; He, Z.W.; Han, S.S.; Chen, S.S. Synthesis, crystal structure, and properties of a Zn(II) coordination polymer based on a difunctional ligand containing triazolyl and carboxyl groups. Crystals 2018, 8, 424. [Google Scholar] [CrossRef]
- Yang, Y.J.; Wang, M.J.; Zhang, K.L. A novel photoluminescent Cd(II)–organic framework exhibiting rapid and efficient multi-responsive fluorescence sensing for trace amounts of Fe3+ ions and some NACs, especially for 4-nitroaniline and 2-methyl-4-nitroaniline. J. Mater. Chem. C 2016, 4, 11404–11418. [Google Scholar] [CrossRef]
- Su, J.; Yao, L.; Zhao, M.; Wang, H.; Zhang, Q.; Cheng, L.; Tian, Y. Structural induction effect of a zwitterion pyridiniumolate for metal–organic frameworks. Inorg. Chem. 2015, 54, 6169–6175. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Sun, W.Y. Zinc(II)– and cadmium(II)–organic frameworks with 1-imidazole-containing and 1-imidazolecarboxylate ligands. CrystEngComm 2015, 17, 4045–4063. [Google Scholar] [CrossRef]
- Zou, J.Y.; Gao, H.L.; Shi, W.; Cui, J.Z.; Cheng, P. Auxiliary ligand-assisted structural diversities of three metal-organic frameworks with potassium 1H-1,2,3-triazole-4,5-dicarboxylic acid: Syntheses, crystal structures and luminescence properties. CrystEngComm 2013, 15, 2682–2687. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhang, J.P.; Lin, Y.Y.; Chen, X.M. Syntheses, structures, and photoluminescence of three coordination polymers of cadmium dicarboxylates. Cryst. Growth Des. 2006, 6, 1684. [Google Scholar] [CrossRef]
- Ye, R.P.; Zhang, X.; Zhai, J.Q.; Qin, Y.Y.; Zhang, L.; Yao, Y.G.; Zhang, J. N-donor ligands enhancing luminescence properties of seven Zn/CdIJII) MOFs based on a large rigid π-conjugated carboxylate ligand. CrystEngComm 2015, 17, 9155–9166. [Google Scholar] [CrossRef]
- Wang, D.Z.; Fan, J.Z.; Jia, D.Z.; Du, C.C. Zinc and cadmium complexes based on bis-(1Htetrazol-5-ylmethyl/ylethyl)-amine ligands: Structures and photoluminescence properties. CrystEngComm 2016, 18, 6708–6723. [Google Scholar] [CrossRef]
| Empirical Formula | C45H39N15O14Cd2 |
|---|---|
| Formula weight | 1238.73 |
| Temperature/K | 293(2) |
| Crystal system | Triclinic |
| Space group | P-1 |
| a/Å | 10.0552(2) |
| b/Å | 10.1437(2) |
| c/Å | 14.1937(2) |
| α/° | 75.690(10) |
| β/° | 69.664(2) |
| γ/° | 62.167(2) |
| Volume/Å3 | 1194.13(4) |
| Z | 1 |
| ρcalcmg/mm3 | 1.723 |
| μ/mm−1 | 0.976 |
| F(000) | 622 |
| Index ranges | −12 ≤ h ≤ 13, −12 ≤ k ≤ 12, −13 ≤ l ≤ 18 |
| Reflections collected | 14832 |
| Independent reflections | 4980 |
| Data/restraints/parameters | 5560/0/396 |
| Goodness-of-fit on F2 | 1.030 |
| Final R indexes [I ≥ 2σ(I)] | R1 = 0.0310, wR2 = 0.0709 |
| Final R indexes [all data] | R1 = 0.0361, wR2 = 0.0742 |
| Largest diff. peak/hole / e Å−3 | 0.470/−0.460 |
| Bond | d | Bond | d |
|---|---|---|---|
| Cd(1)-N(1) | 2.288(2) | Cd(1)-N(3) i | 2.281(2) |
| Cd(1)-O(6) | 2.346(2) | Cd(1)-O(2) | 2.3470(16) |
| Cd(1)-O(4) ii | 2.3871(19) | Cd(1)-O(3) ii | 2.5427(18) |
| Cd(1)-O(1) | 2.5866(18) | ||
| Angle | ω | Angle | ω |
| N(3) i-Cd(1)-N(1) | 175.41(7) | N(3) i-Cd(1)-O(6) | 90.86(8) |
| N(1)-Cd(1)-O(6) | 84.94(8) | N(3) i-Cd(1)-O(2) | 93.45(6) |
| N(1)-Cd(1)-O(2) | 90.84(7) | O(6)-Cd(1)-O(2) | 135.86(7) |
| N(3) i-Cd(1)-O(4) ii | 99.08(7) | N(1)-Cd(1)-O(4) ii | 82.57(7) |
| O(6)-Cd(1)-O(4) ii | 133.33(7) | O(2)-Cd(1)-O(4) ii | 89.19(6) |
| N(3) i-Cd(1)-O(3) ii | 83.11(7) | N(1)-Cd(1)-O(3) ii | 94.58(7) |
| O(6)-Cd(1)-O(3) ii | 83.62(7) | O(2)-Cd(1)-O(3) ii | 140.50(6) |
| O(4) ii-Cd(1)-O(3) ii | 53.03(6) | N(3) i-Cd(1)-O(1) | 87.07(7) |
| N(1)-Cd(1)-O(1) | 94.26(7) | O(6)-Cd(1)-O(1) | 83.57(7) |
| O(2)-Cd(1)-O(1) | 52.90(6) | O(4) ii-Cd(1)-O(1) | 142.01(6) |
| O(3) ii-Cd(1)-O(1) | 163.71(6) |
| D–H···A | d(D–H) | d(H···A) | d(D···A) | ∠DHA |
|---|---|---|---|---|
| O(6)–H(6A)···O(5) a | 0.70(4) | 2.18(4) | 2.872(4) | 167(5) |
| C(6)–H(6B)···O(6) b | 0.79(4) | 1.96(4) | 2.738(3) | 169(5) |
| C(2)–H(2)···O(2) c | 0.9300 | 2.3200 | 3.142(3) | 148.00 |
| C(4) –H(4)···N(4) | 0.9300 | 2.5100 | 2.826(4) | 100.00 |
| C(9)–H(9)···O(4) a | 0.9300 | 2.5400 | 3.086(3) | 118.00 |
| C(10)–H(10)···O(2) c | 0.9300 | 2.2300 | 3.101(4) | 155.00 |
| C(11)–H(11)···O(6) d | 0.9300 | 2.5700 | 3.410(4) | 151.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.-S.; He, Z.-W.; Li, L.; Chen, S.-S. A Cd(II) Coordination Polymer Based on Mixed Ligands: Synthesis, Crystal Structure, and Properties. Crystals 2019, 9, 625. https://doi.org/10.3390/cryst9120625
Han S-S, He Z-W, Li L, Chen S-S. A Cd(II) Coordination Polymer Based on Mixed Ligands: Synthesis, Crystal Structure, and Properties. Crystals. 2019; 9(12):625. https://doi.org/10.3390/cryst9120625
Chicago/Turabian StyleHan, Shuai-Shuai, Zi-Wei He, Lei Li, and Shui-Sheng Chen. 2019. "A Cd(II) Coordination Polymer Based on Mixed Ligands: Synthesis, Crystal Structure, and Properties" Crystals 9, no. 12: 625. https://doi.org/10.3390/cryst9120625






