Crystal Growth and Physical Properties of Sr4Co3O7.5+xCl2 Single Crystals (x ∼ 0.14)
Abstract
1. Introduction
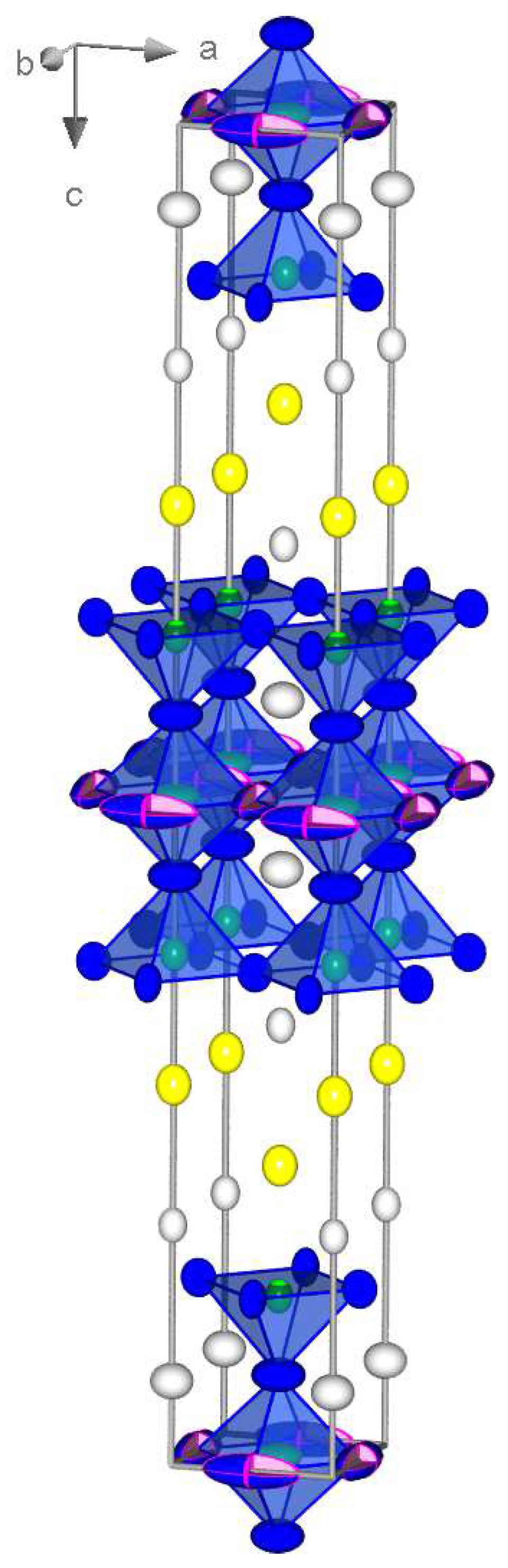
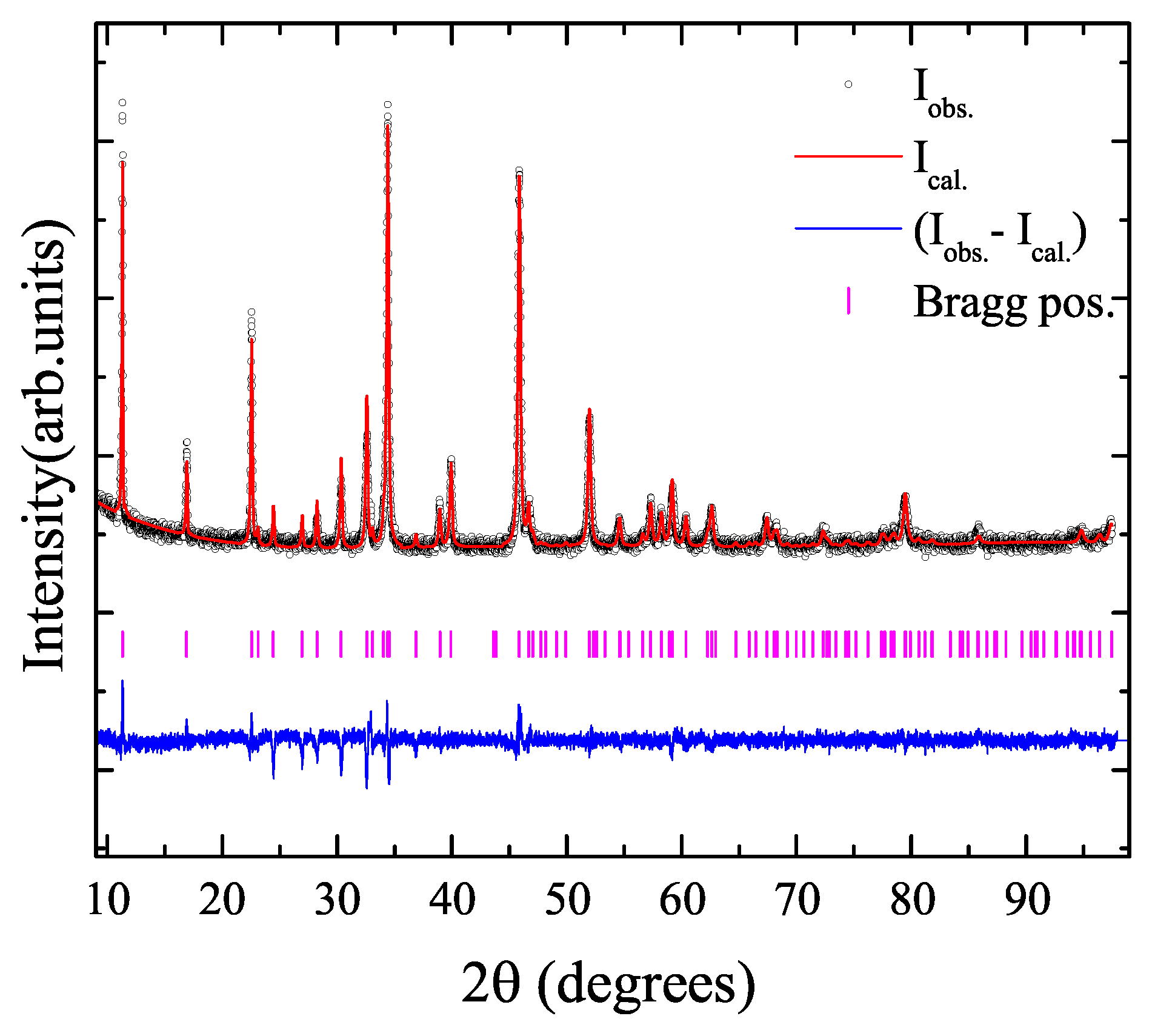
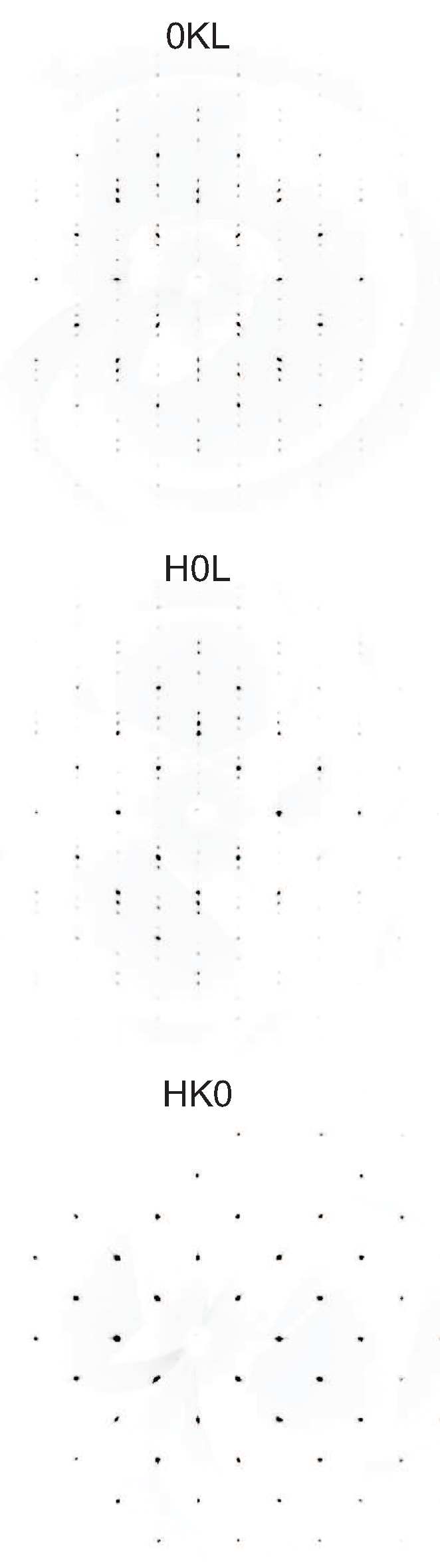
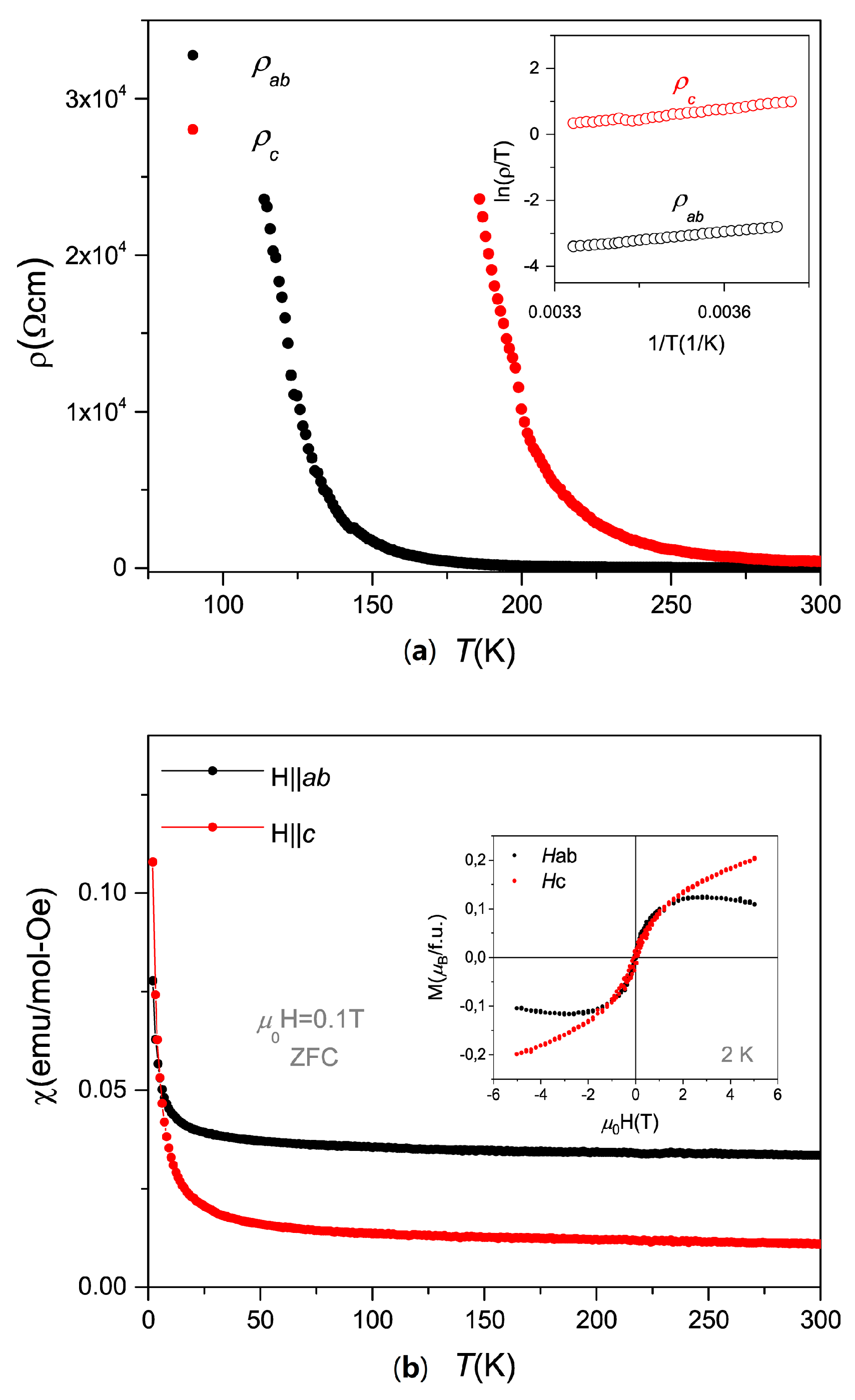
2. Results and Discussion
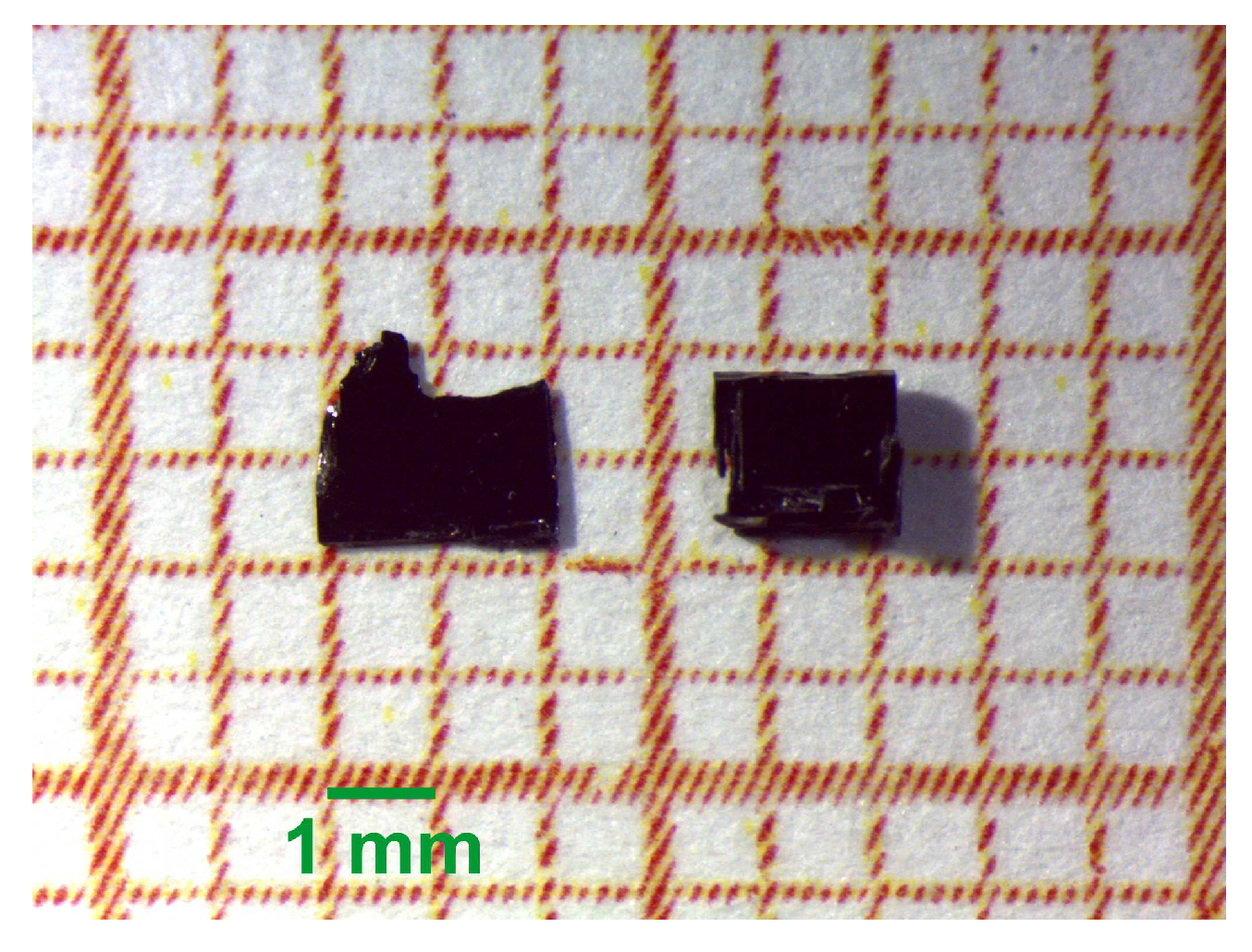
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takada, K.; Sakurai, H.; Takayama-Muromachi, E.; Izumi, F.; Dilanian, R.A.; Sasaki, T. Superconductivity in two-dimensional CoO2 layers. Nature 2003, 422, 53–55. [Google Scholar] [CrossRef]
- Ohtaki, M. Recent aspects of oxide thermoelectric materials for power generation from mid-to-high temperature heat source. J. Ceram. Soc. Jpn. 2011, 119, 770–775. [Google Scholar] [CrossRef]
- Boothroyd, A.T.; Babkevich, P.; Prabhakaran, D.; Freeman, P.G. Hour-glass magnetic spectrum in an insulating, hole-doped antiferromagnet. Nature 2011, 471, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Drees, Y.; Lamago, D.; Piovano, A.; Komarek, A.C. Hour-glass magnetic spectrum in a stripeless insulating transition metal oxide. Nat. Commun. 2013, 4, 2449. [Google Scholar] [CrossRef] [PubMed]
- Drees, Y.; Li, Z.W.; Ricci, A.; Rotter, M.; Schmidt, W.; Lamago, D.; Sobolev, O.; Rütt, U.; Gutowski, O.; Sprung, M.; et al. Hour-glass magnetic excitations induced by nanoscopic phase separation in cobalt oxides. Nat. Commun. 2014, 5, 5731. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Schmidt, W.; Tjeng, L.H.; Komarek, A.C. Charge correlations in cobaltates La2−xSrxCoO4. Phys. Status Solidi (RRL) 2015, 9, 580–582. [Google Scholar] [CrossRef]
- Li, Z.W.; Drees, Y.; Kuo, C.Y.; Guo, H.; Ricci, A.; Lamago, D.; Sobolev, O.; Rütt, U.; Gutowski, O.; Pi, T.W.; et al. Incommensurate spin correlations in highly oxidized cobaltates La2−xSrxCoO4. Sci. Rep. 2016, 6, 25117. [Google Scholar] [CrossRef]
- Guo, H.; Li, Z.W.; Sakong, S.; Ryu, G.; Zhao, L.; Piovano, A.; Schmidt, W.; Sprung, M.; Strempfer, J.; Francoual, S.; et al. Suppression of the outwards-dispersing branches in hour-glass magnetic spectra induced by nanoscale phase separation in La2−xSrxCoO4. Phys. Rev. B 2019, 100, 014411. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0 < x < −1): A new cathode material for batteries of high energy density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar]
- Hwang, J.; Rao, R.R.; Giordano, L.; Katayama, Y.; Yu, Y.; Shao-Horn, Y. Perovskites in catalysis and electrocatalysis. Science 2017, 358, 751–756. [Google Scholar] [CrossRef]
- Korotin, M.A.; Ezhov, S.Y.; Solovyev, I.V.; Anisimov, V.I.; Khomskii, D.I.; Sawatzky, G.A. Intermediate-spin state and properties of LaCoO3. Phys. Rev. B 1996, 54, 5309. [Google Scholar] [CrossRef] [PubMed]
- Haverkort, M.W.; Hu, Z.; Cezar, J.C.; Burnus, T.; Hartmann, H.; Reuther, M.; Zobel, C.; Lorenz, T.; Tanaka, A.; Brookes, N.B.; et al. Spin State Transition in LaCoO3 Studied Using Soft X-ray Absorption Spectroscopy and Magnetic Circular Dichroism. Phys. Rev. Lett. 2006, 97, 176405. [Google Scholar] [CrossRef] [PubMed]
- Moritomo, Y.; Higashi, K.; Matsuda, K.; Nakamura, A. Spin-state transition in layered perovskite cobalt oxides: La2−xSrxCoO4 (0.4 ≤ x ≤ 1.0). Phys. Rev. B 1997, 55, R14725. [Google Scholar] [CrossRef]
- Chang, C.F.; Hu, Z.; Wu, H.; Burnus, T.; Hollmann, N.; Benomar, M.; Lorenz, T.; Tanaka, A.; Lin, H.-J.; Hsieh, H.H.; et al. Spin Blockade, Orbital Occupation, and Charge Ordering in La1.5Sr0.5CoO4. Phys. Rev. Lett. 2009, 102, 116401. [Google Scholar] [CrossRef] [PubMed]
- Taskin, A.; Lavrov, A.N.; Ando, Y. Ising-Like Spin Anisotropy and Competing Antiferromagnetic- Ferromagnetic Orders in GdBaCo2O5.5 Single Crystals. Phys. Rev. Lett. 2003, 90, 227201. [Google Scholar] [CrossRef] [PubMed]
- Wu, H. Spin state and phase competition in TbBaCo2O5.5 and the lanthanide series LBaCo2O5+δ (0 < ∼δ<∼1). Phys. Rev. B 2001, 64, 092413. [Google Scholar]
- Hu, Z.; Wu, H.; Haverkort, M.W.; Hsieh, H.H.; Lin, H.J.; Lorenz, T.; Baier, J.; Reichl, A.; Bonn, I.; Felser, C.; et al. Different Look at the Spin State of Co3+ Ions in a CoO5 Pyramidal Coordination. Phys. Rev. Lett. 2004, 92, 207402. [Google Scholar] [CrossRef]
- Loureiro, S.M.; Felser, C.; Huang, Q.; Cava, R.J. Refinement of the Crystal Structures of Strontium Cobalt Oxychlorides by Neutron Powder Diffraction. Chem. Mater. 2000, 12, 3181–3185. [Google Scholar] [CrossRef]
- McGlothlin, N.; Ho, D.; Cava, R.J. Sr3Co2O5Cl2 and Sr2CoO3Cl: two layered cobalt oxychlorides. Mater. Res. Bull. 2000, 35, 1035. [Google Scholar] [CrossRef]
- Mueller Buschbaum, H.; Boje, J. Zur Kenntnis eines Halogenooxo-Cobaltats(III): Sr8Co6O15Cl4. Z. Anorg. Allg. Chem. 1991, 592, 73–78. [Google Scholar] [CrossRef]
- De Groot, F.M.F. X-ray absorption and dichroism of transition metals and their compounds. J. Electron Spectrosc. Relat. Phenom. 1994, 67, 529. [Google Scholar] [CrossRef]
- Tanaka, A.; Jo, T. Resonant 3d, 3p and 3s Photoemission in Transition Metal Oxides Predicted at 2p Threshold. J. Phys. Soc. Jpn. 1994, 63, 2788. [Google Scholar] [CrossRef]
- Petricek, V.; Dusek, M.; Palatinus, L. Structure Determination Software Programs; Institute of Physics, University of Prague: Prague, Czech Republic, 2000. [Google Scholar]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A Computer Program for the Solution of Crystal Structures by Charge Flipping in Arbitrary Dimensions. J. Appl. Crystallogr. 2007, 40, 786. [Google Scholar] [CrossRef]
- Bruker-AXS TOPAS, version 3; Bruker: Karlsruhe, Germany, 2005.

| Atom | x | y | z |
| Sr1 | 0 | 0 | 0.179355 (8) |
| Sr2 | 0 | 0 | 0.063130 (9) |
| Co1 | 0.5 | 0.5 | 0.118567 (12) |
| Co2 | 0.5 | 0.5 | 0 |
| Cl1 | 0.5 | 0.5 | 0.21597 (2) |
| O1 | 0.5 | 0 | 0.12824 (5) |
| O2 | 0.5 | 0.5 | 0.06001 (7) |
| O3 | 0 | 0.5 | 0 |
| Atom | U (Å) | U (Å) | U (Å) |
| Sr1 | 0.00691 (8) | 0.00691 (8) | 0.01062 (10) |
| Sr2 | 0.01534 (10) | 0.01534 (10) | 0.01105 (11) |
| Co1 | 0.00652 (10) | 0.00652 (10) | 0.00978 (13) |
| Co2 | 0.0200 (2) | 0.0200 (2) | 0.00476 (18) |
| Cl1 | 0.01032 (18) | 0.01032 (18) | 0.0144 (3) |
| O1 | 0.0086 (7) | 0.0157 (8) | 0.0187 (7) |
| O2 | 0.0351 (12) | 0.0351 (12) | 0.0136 (10) |
| O3 | 0.0179 (19) | 0.118 (5) | 0.0141 (13) |
| Atoms | Bond Distances (Å) | ||
| Co1-Cl1 | 4× | 3.0843 (9) | |
| Co1-O1 | 4× | 1.9761 (3) | |
| Co1-O2 | 1× | 1.854 (2) | |
| Co2-O2 | 2× | 1.900 (2) | |
| Co2-O3 | 4× | 1.95220 (10) | |
| Co1 | BVS: | 2.556 (4) | |
| Co2 | BVS: | 2.758 (5) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, G.; Hu, Z.; Chang, C.-F.; Kuo, C.-Y.; Shin, K.; Lin, H.-J.; Chen, C.-T.; Tjeng, L.H.; Komarek, A.C. Crystal Growth and Physical Properties of Sr4Co3O7.5+xCl2 Single Crystals (x ∼ 0.14). Crystals 2019, 9, 623. https://doi.org/10.3390/cryst9120623
Ryu G, Hu Z, Chang C-F, Kuo C-Y, Shin K, Lin H-J, Chen C-T, Tjeng LH, Komarek AC. Crystal Growth and Physical Properties of Sr4Co3O7.5+xCl2 Single Crystals (x ∼ 0.14). Crystals. 2019; 9(12):623. https://doi.org/10.3390/cryst9120623
Chicago/Turabian StyleRyu, Gihun, Zhiwei Hu, Chun-Fu Chang, Chang-Yang Kuo, K. Shin, Hong-Ji Lin, Chien-Te Chen, Liu Hao Tjeng, and Alexander C. Komarek. 2019. "Crystal Growth and Physical Properties of Sr4Co3O7.5+xCl2 Single Crystals (x ∼ 0.14)" Crystals 9, no. 12: 623. https://doi.org/10.3390/cryst9120623
APA StyleRyu, G., Hu, Z., Chang, C.-F., Kuo, C.-Y., Shin, K., Lin, H.-J., Chen, C.-T., Tjeng, L. H., & Komarek, A. C. (2019). Crystal Growth and Physical Properties of Sr4Co3O7.5+xCl2 Single Crystals (x ∼ 0.14). Crystals, 9(12), 623. https://doi.org/10.3390/cryst9120623




