Double Layer Conducting Salts: (CNB-EDT-TTF)4X, X = ClO4−, ReO4−, and SbF6−; Electrical Transport and Infrared Properties
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis
2.2. X-ray Structure
2.3. Electrical Transport Properties
2.4. Optical Properties
3. Results and Discussion
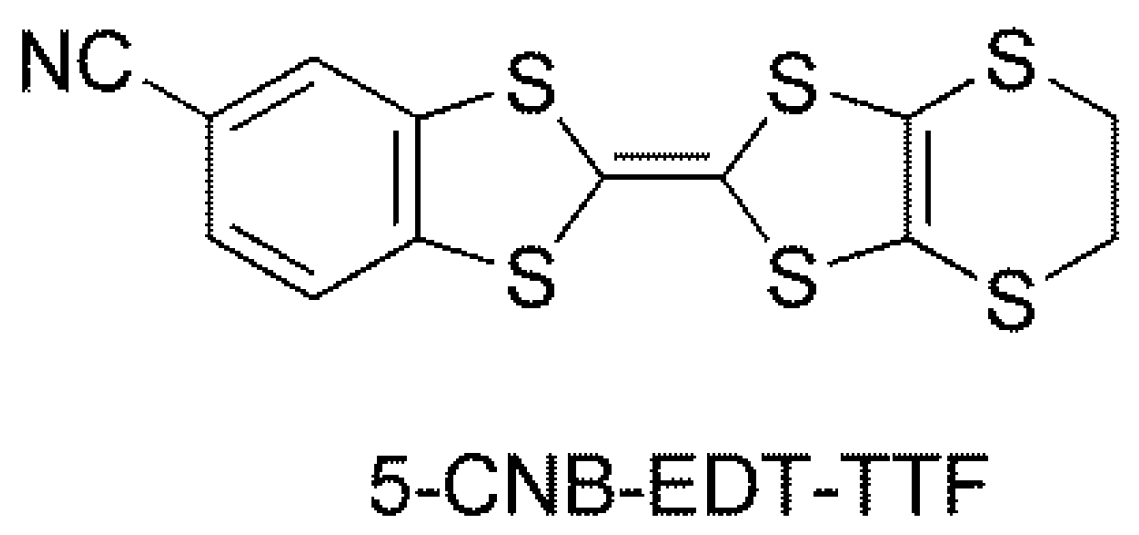
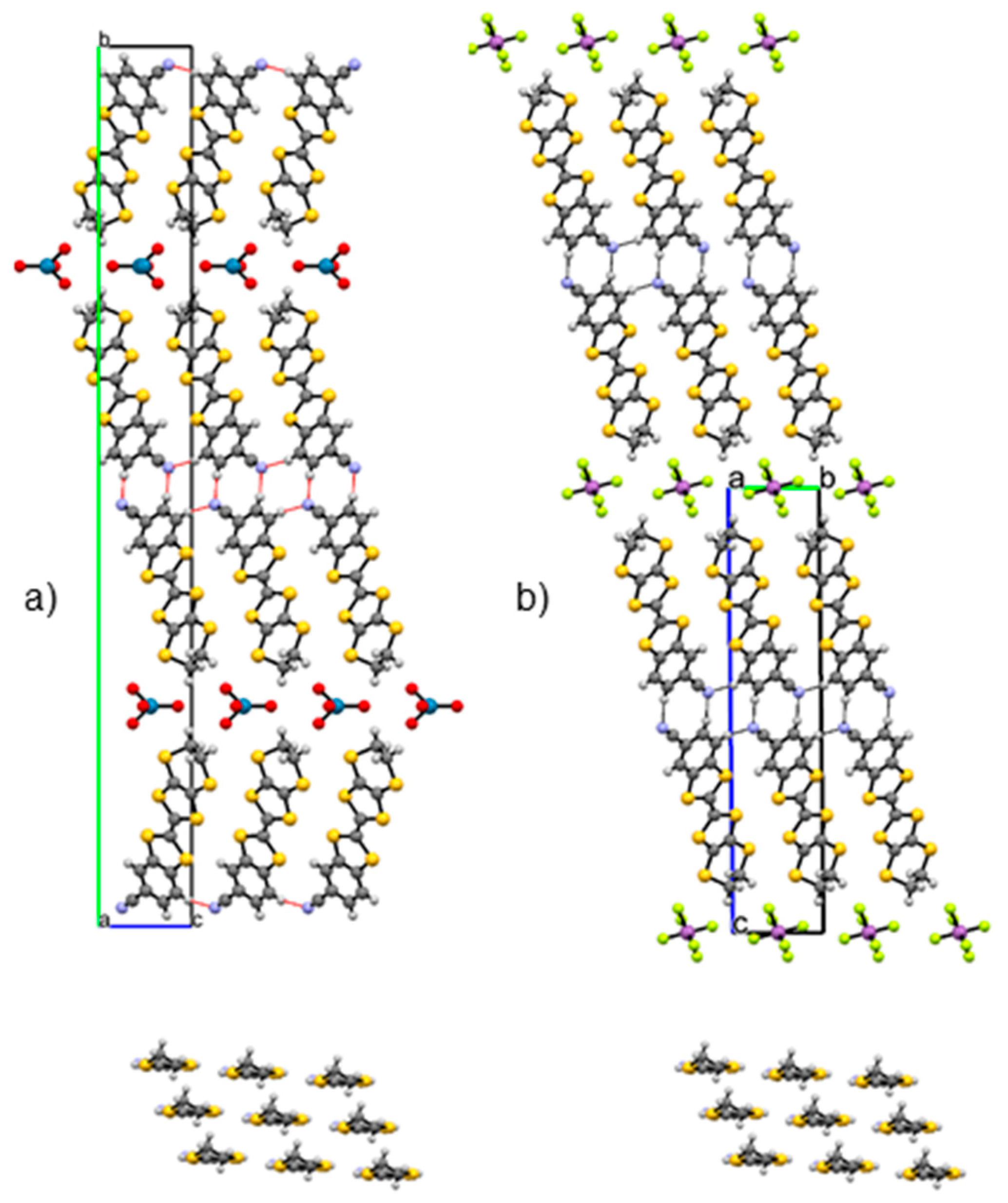
3.1. Crystal Structure
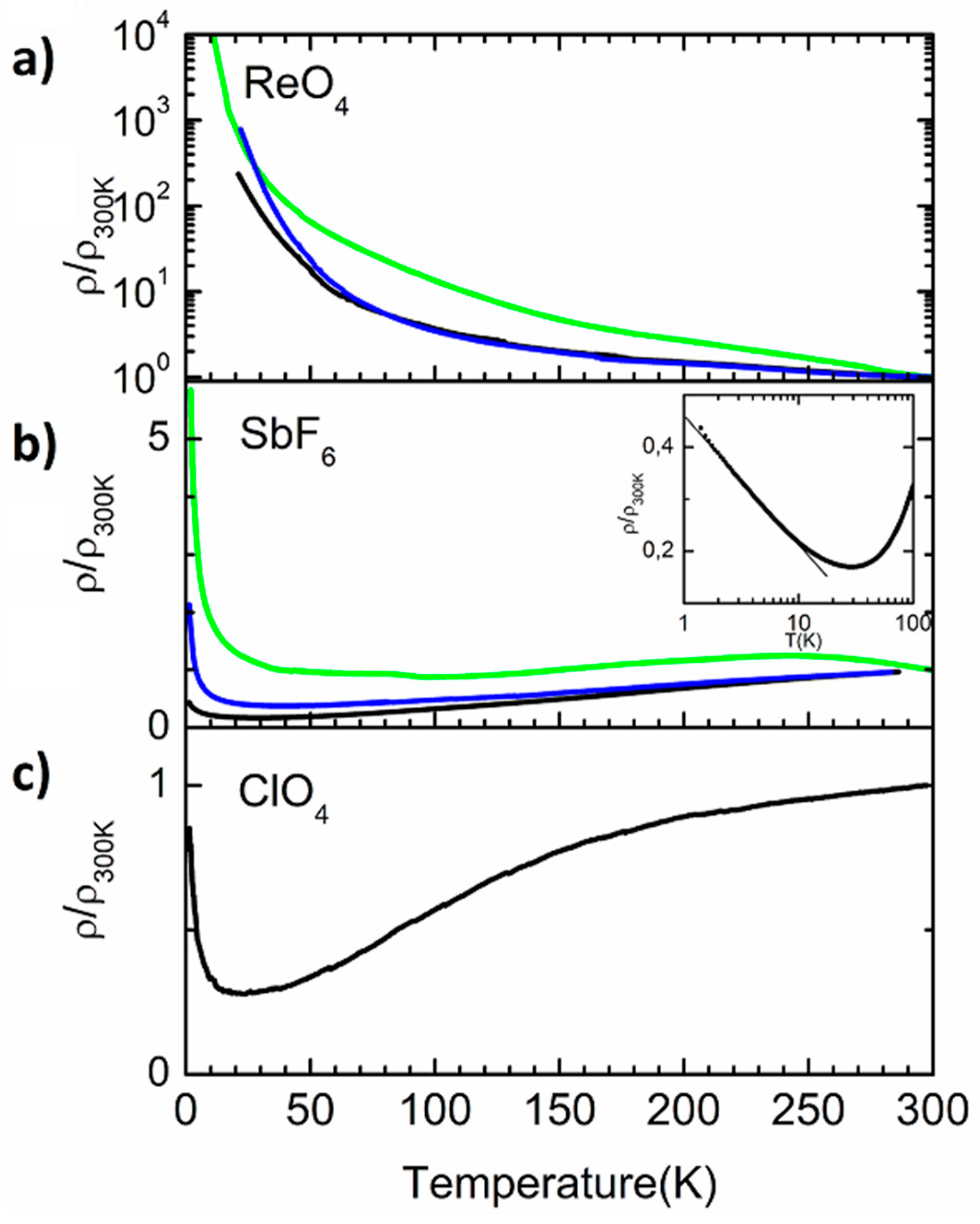
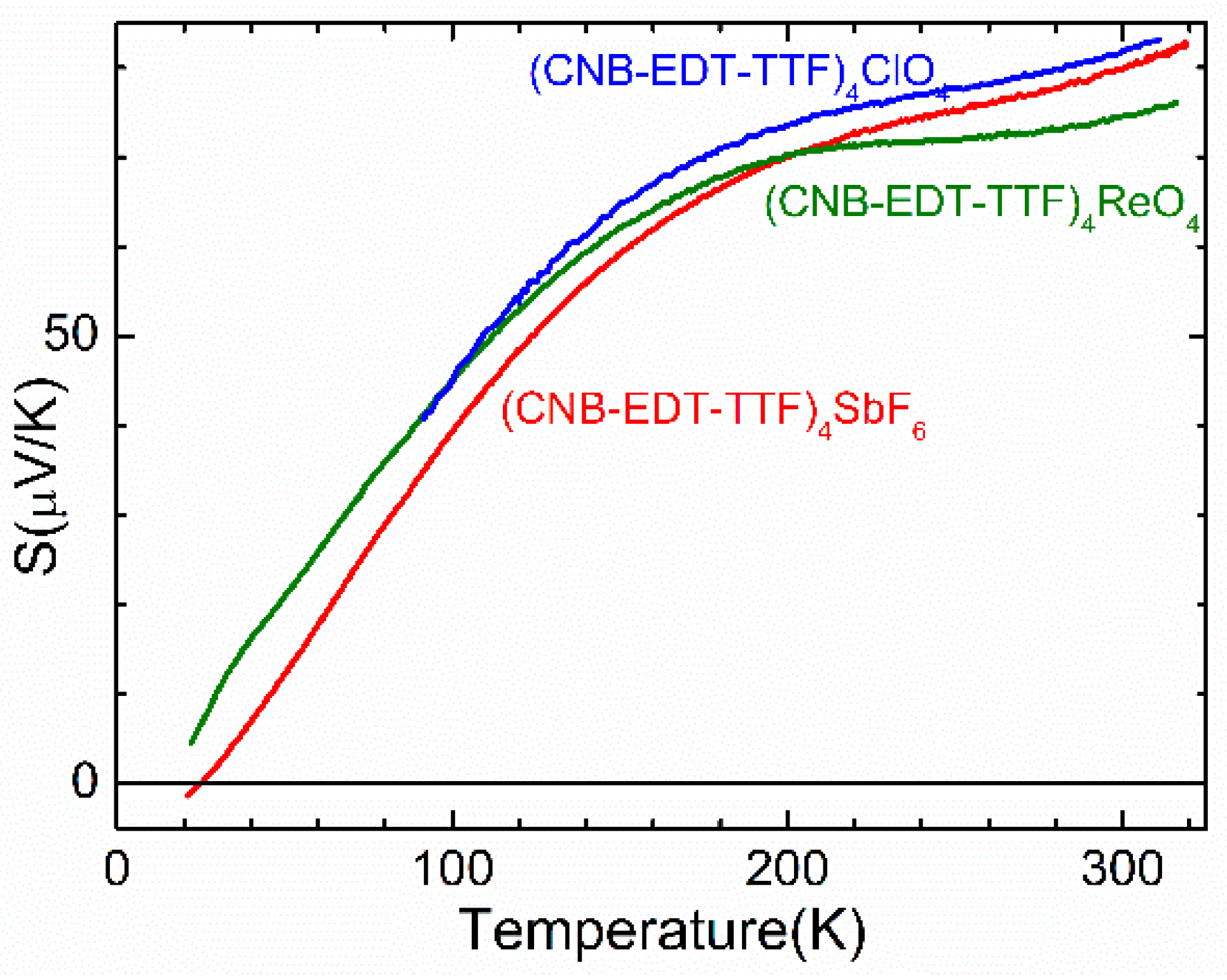
3.2. Electronic Transport Properties
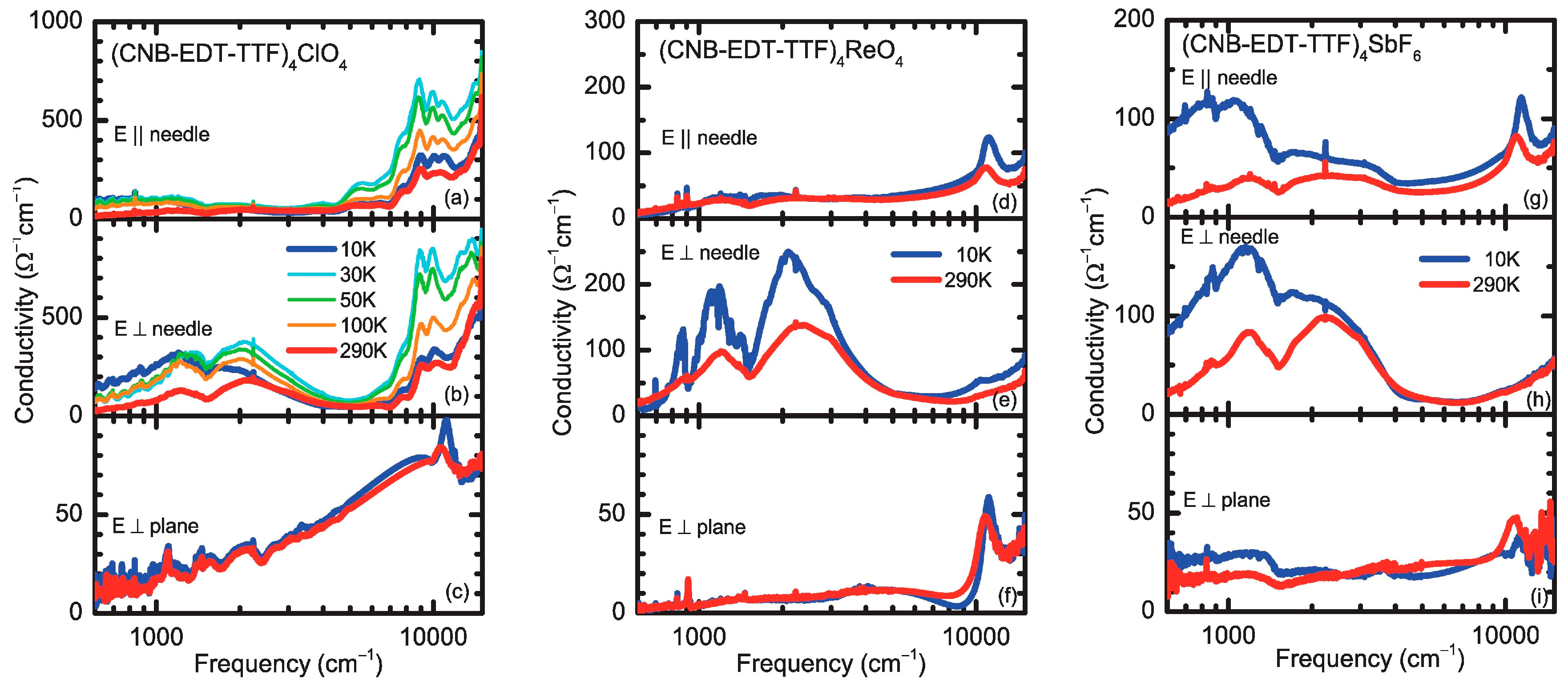
3.3. Optical Pproperties
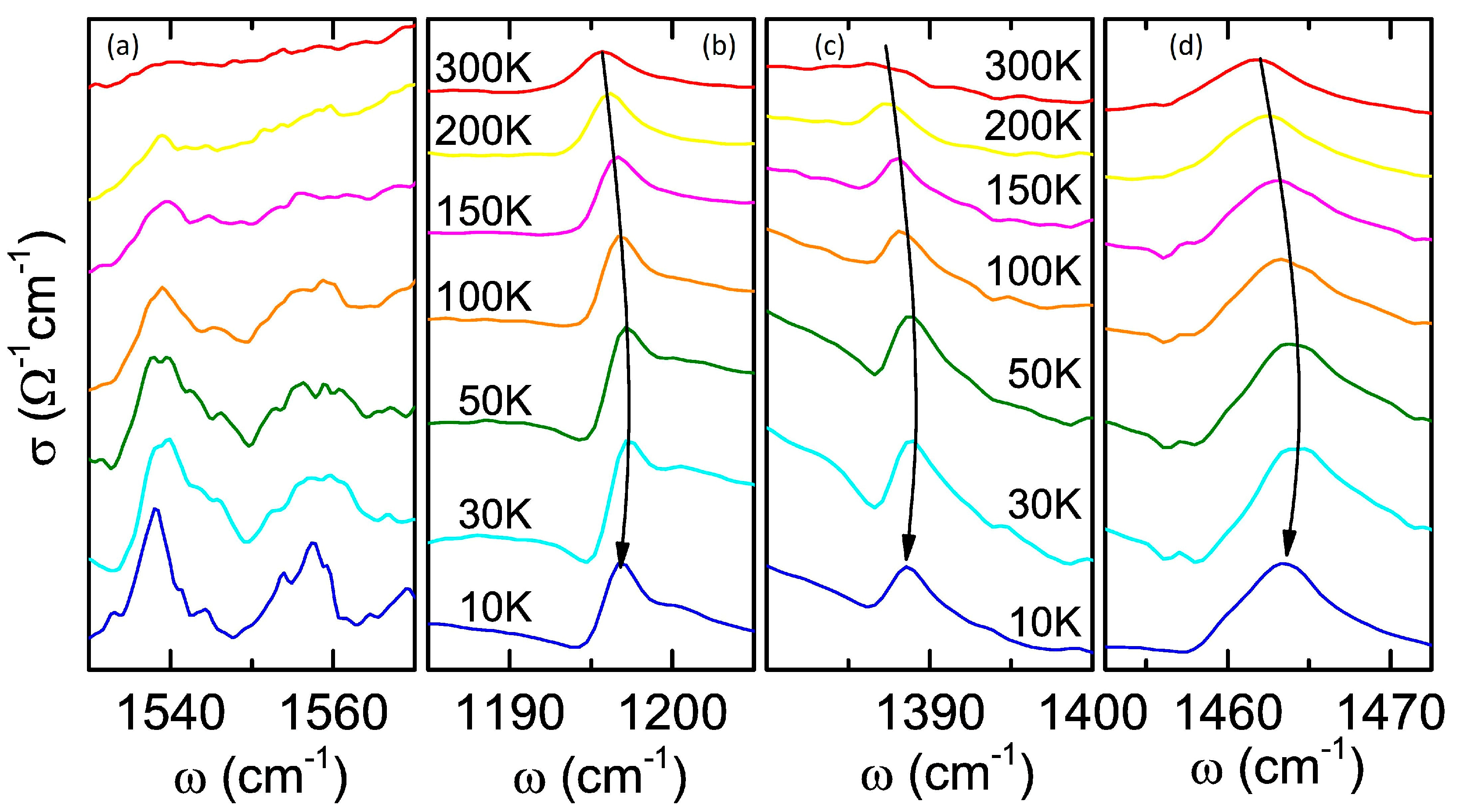
3.4. Vibrational Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Oliveira, S.; Belo, D.; Santos, I.C.; Rabaça, S.; Almeida, M. Synthesis and characterization of the cyanobenzene-ethylenedithio-TTF donor. Beilstein J. Org. Chem. 2015, 11, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.; Ministro, J.; Santos, I.C.; Belo, D.; Lopes, E.B.; Rabaça, S.; Canadell, E.; Almeida, M. Bilayer Molecular Metals Based on Dissymmetrical Electron Donors. Inorg. Chem. 2015, 54, 6677–6679. [Google Scholar] [CrossRef] [PubMed]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. Sect. B Struct. Sci. 1990, 46, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Etter, M.C. Hydrogen bonds as design elements in organic chemistry. J. Phys. Chem. 1991, 95, 4601–4610. [Google Scholar] [CrossRef]
- Rabaça, S.; Oliveira, S.; Gama, V.; Santos, I.C.; Belo, D.; Lopes, E.B.; Canadell, E.; Almeida, M. Polymorphism and Superconductivity in Bilayer Molecular Metals (CNB-EDT-TTF)4I3. Inorg. Chem. 2016, 55, 10343–10350. [Google Scholar] [CrossRef] [PubMed]
- Rabaça, S.; Oliveira, S.; Gonçalves, A.C.; Gama, V.; Santos, I.C.; Belo, D.; Almeida, M. Cyanobenzene–Ethylenedithio–Tetrathiafulvalene Salts with ClO4−: Bilayer Polymorphs and Different Stoichiometries. Cryst. Growth Des. 2017, 17, 2801–2808. [Google Scholar] [CrossRef]
- Rabaça, S.; Oliveira, S.; Gama, V.; Santos, I.C.; Oliveira, G.; Lopes, E.B.; Canadell, E.; Almeida, M. β″-(CNB-EDT-TTF)4BF4; Anion Disorder Effects in Bilayer Molecular Metals. Crystals 2018, 8, 142. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Bruker. SMART and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.; Giacovazzo, G.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R.J. SIR97: A new tool for crystal structure determination and refinement. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A.J. New Features for the Visualization and Investigation of Crystal Structures. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Chaikin, P.M.; Kwak, J.F. Apparatus for thermopower measurements on organic conductors. Rev. Sci. Instrum. 1975, 46, 218–220. [Google Scholar] [CrossRef]
- Almeida, M.; Alcácer, L.; Oostra, S. Anisotropy of Thermo-power in N-methyl-N-Ethylmorpholinium Bistetracyanoquinodime-thane, MEM(TCNQ)2, in the Region of the High-Temperature Phase Transitions. Phys. Rev. B Condens. Matter Mater. Phys. 1984, 30, 2839–2844. [Google Scholar] [CrossRef]
- Lopes, E.B. INETI-Sacavém; Internal Report; INETI Press: Sacavém, Portugal, 1991. [Google Scholar]
- Huebener, R.P. Thermoelectric Power of Lattice Vacancies in Gold. Phys. Rev. 1964, 135, A1281. [Google Scholar] [CrossRef]
- Altshuler, B.L.; Aronov, A.G. Electron-Electron Interaction in Disordered Conductors. In Electron-Electron Interactions in Disordered Systems; Efros, A.L., Pollak, M., Eds.; Elsevier: Amsterdam, The Netherlands, 1985; Volume 10, pp. 1–690. [Google Scholar]
- Prokhorova, T.G.; Yagubskii, E.B.; Zorina, L.V.; Simonov, S.V.; Zverev, V.N.; Shibaeva, R.P.; Buravov, L.I. Specific Structural Disorder in an Anion Layer and Its Influence on Conducting Properties of New Crystals of the (BEDT-TTF)4A+[M3+(ox)3]G Family, Where G Is 2-Halopyridine; M Is Cr, Ga; A+ Is [K0.8(H3O)0.2]+. Crystals 2018, 8, 92. [Google Scholar] [CrossRef]
- Dressel, M.; Drichko, N. Optical Properties of Two-Dimensional Organic Conductors: Signatures of Charge Ordering and Correlation Effects. Chem. Rev. 2004, 104, 5689–5716. [Google Scholar] [CrossRef] [PubMed]
- Dressel, M.; Dumm, M.; Knoblauch, T.; Masino, M. Comprehensive Optical Investigations of Charge Order in Organic Chain Compounds (TMTTF)2X. Crystals 2012, 2, 528–578. [Google Scholar] [CrossRef]
- Rösslhuber, R.; Rose, E.; Ivek, T.; Pustogow, A.; Breier, T.; Geiger, M.; Schrem, K.; Untereiner, G.; Dressel, M. Structural and Electronic Properties of (TMTTF)2X Salts with Tetrahedral Anions. Crystals 2018, 8, 121. [Google Scholar] [CrossRef]
- Ivek, T.; Korin-Hamzić, B.; Milat, O.; Tomić, S.; Clauss, C.; Drichko, N.; Schweitzer, D.; Dressel, M. Electrodynamic response of the charge ordering phase: Dielectric and optical studies of α-(BEDT-TTF)2I3. Phys. Rev. B 2011, 83, 165128. [Google Scholar] [CrossRef]
| X | ReO4 | SbF6 |
|---|---|---|
| Empirical formula | C52H28N4O4ReS24 | C26H14F3N2S12Sb0.5 |
| Formula weight | 1728.42 | 856.99 |
| Temperature (K) | 150(2) | 150(2) |
| Crystal system, space group | Monoclinic, P21/m | Triclinic, P−1 |
| a (Å) | 4.8817(3) | 4.8532(4) |
| b (Å) | 54.581(3) | 5.8046(5) |
| c (Å) | 5.8117(4) | 28.368(2) |
| α (°) | 90.00 | 89.054(4) |
| β (°) | 95.376(4) | 86.377(4) |
| γ (°) | 90.00 | 84.651(4) |
| V (Å3) | 1541.70(17) | 794.03(11) |
| Z, DCal(mg/m3) | 1, 1.862 | 1, 1.792 |
| Absorption coeff. µ (mm−1) | 2.834 | 1.284 |
| Crystal size (mm) | 0.50 × 0.05 × 0.02 | 0.50 × 0.08 × 0.02 |
| θ range for data collection (°) | 3.52 to 25.35 | 2.158 to 26.451 |
| Index range (h, k, l) | −5/5, −64/65, −6/6 | −6/6, −5/7, −35/35 |
| Reflections collected / unique | 11339 / 2821 [R(int) = 0.0515] | 10330 / 2821 [R(int) = 0.0372] |
| Completeness to θ | 25.24 99.4% | 25.242 86.9% |
| Data / restraints / parameters | 2821 / 6 / 204 | 2821 / 0 / 208 |
| Goodness-of-fit on F2 | 1.049 | 1.197 |
| Final R indices [I > 2σ(I)] | R1 = 0.0774, wR2 = 0.2044 | R1 = 0.0996, wR2 = 0.2268 |
| Largest difference peak and hole (e Å3) | 3.520 and −1.576 | 0.759 and −1.101 |
| CCDC reference | 1,921,072 | 1,921,071 |
| Mode | (CNB-EDT-TTF)4ClO4 | (CNB-EDT-TTF)4ReO4 | (CNB-EDT-TTF)4SbF6 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| E ‖ Needle | E⊥ Needle | E ⊥ Plane | E ‖ Needle | E ⊥ Needle | E ⊥ Plane | E ‖ Needle | E ⊥ Needle | E ⊥ Plane | |
| ν1 | 697.2 | 697.2 | 695.7 | 695.5s | 696.2s | 696.1 | |||
| ν2 | 777.7 | 775.7 | 777.6 | ||||||
| ν3 | 835.0s | 835.0 | 834.7 | 833.1s | 833.6 | 834.1s | 835.4s | 835.5 | 835.6s |
| ν4 | 862.1 | 862.8 | 864.0 | 866.6 | 864.1 | 863.0 | |||
| ν5 | 877.5 | 876.5 | 875.5 | 875.6 | 874.8 | 875.4 | |||
| ν6 | 897.0 | 902.7s | 902.9 | 899.1 | 894.2 | ||||
| ν7 | 1196.9 | 1196.1 | 1196.0 | 1198.3 | 1195.8 | 1196.7 | 1196.0 | ||
| ν8 | 1286.7 | 1285.3 | 1286.6 | 1288.0 | 1285.0 | 1286.3 | 1285.0 | 1286.7 | |
| ν9 | 1388.6 | 1387.6 | 1387.7 | 1388.8 | 1387.7 | ||||
| ν10 | 1463.4 | 1464.1 | 1460.2 | 1463.5 | 1461.4 | 1464.3 | 1457.0 | 1460.0 | |
| ν11 | 1498.5a | 1505.7a | 1505.7a | 1522.6a | |||||
| ν12 | 1538.1 | 1537.0 | 1538.0 | 1537.7 | 1538.0 | ||||
| ν13 | 1557.3 | 1553.0 | 1546.1 | 1553.4 | 1556.1 | ||||
| ν14 | 2234.7 | 2235.7 | 2235.5 | 2232.2 | 2234.0 | 2233.6 | 2236.2s | 2234.8s | 2236.6 |
| ν15 | 3017.9 | 3017.9 | |||||||
| ν16 | 3076.2 | 3085.0 | 3071.0 | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, S.; Gonçalves, A.C.; Oliveira, G.; Günther, D.; Rohwer, A.; Gama, V.; Lopes, E.B.; Santos, I.C.; Rabaça, S.; Almeida, M.; et al. Double Layer Conducting Salts: (CNB-EDT-TTF)4X, X = ClO4−, ReO4−, and SbF6−; Electrical Transport and Infrared Properties. Crystals 2019, 9, 608. https://doi.org/10.3390/cryst9120608
Oliveira S, Gonçalves AC, Oliveira G, Günther D, Rohwer A, Gama V, Lopes EB, Santos IC, Rabaça S, Almeida M, et al. Double Layer Conducting Salts: (CNB-EDT-TTF)4X, X = ClO4−, ReO4−, and SbF6−; Electrical Transport and Infrared Properties. Crystals. 2019; 9(12):608. https://doi.org/10.3390/cryst9120608
Chicago/Turabian StyleOliveira, Sandrina, Ana Cristina Gonçalves, Gonçalo Oliveira, Dominik Günther, Andrea Rohwer, Vasco Gama, Elsa Branco Lopes, Isabel Cordeiro Santos, Sandra Rabaça, Manuel Almeida, and et al. 2019. "Double Layer Conducting Salts: (CNB-EDT-TTF)4X, X = ClO4−, ReO4−, and SbF6−; Electrical Transport and Infrared Properties" Crystals 9, no. 12: 608. https://doi.org/10.3390/cryst9120608
APA StyleOliveira, S., Gonçalves, A. C., Oliveira, G., Günther, D., Rohwer, A., Gama, V., Lopes, E. B., Santos, I. C., Rabaça, S., Almeida, M., & Dressel, M. (2019). Double Layer Conducting Salts: (CNB-EDT-TTF)4X, X = ClO4−, ReO4−, and SbF6−; Electrical Transport and Infrared Properties. Crystals, 9(12), 608. https://doi.org/10.3390/cryst9120608







