Unprecedented Dinuclear CuII N,O-Donor Complex: Synthesis, Structural Characterization, Fluorescence Property, and Hirshfeld Analysis
Abstract
1. Introduction
2. Experimental
2.1. Materials and Instrumentation
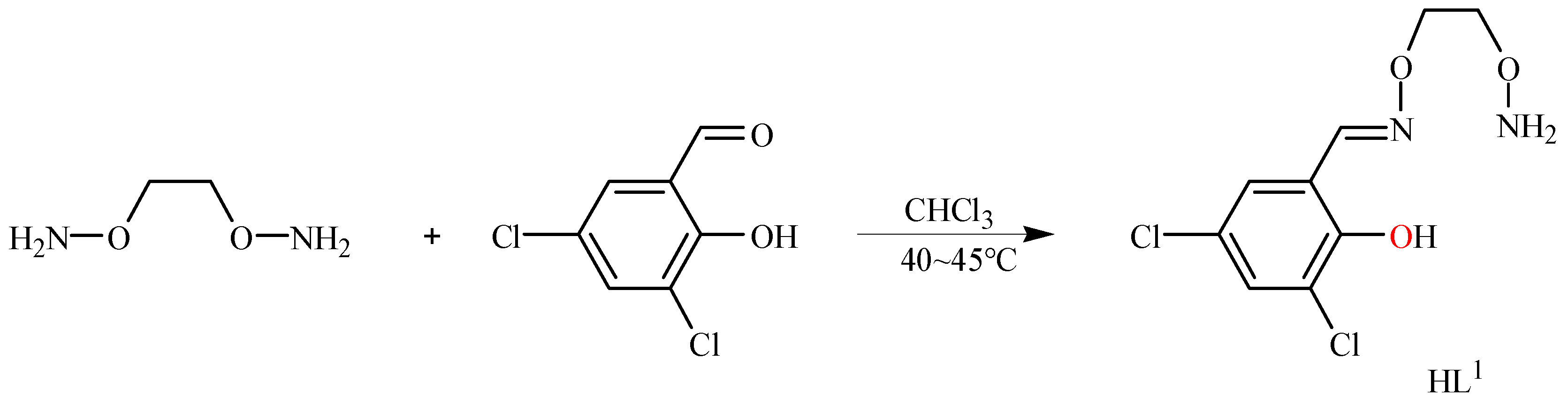
2.2. Preparation of the Ligand HL1
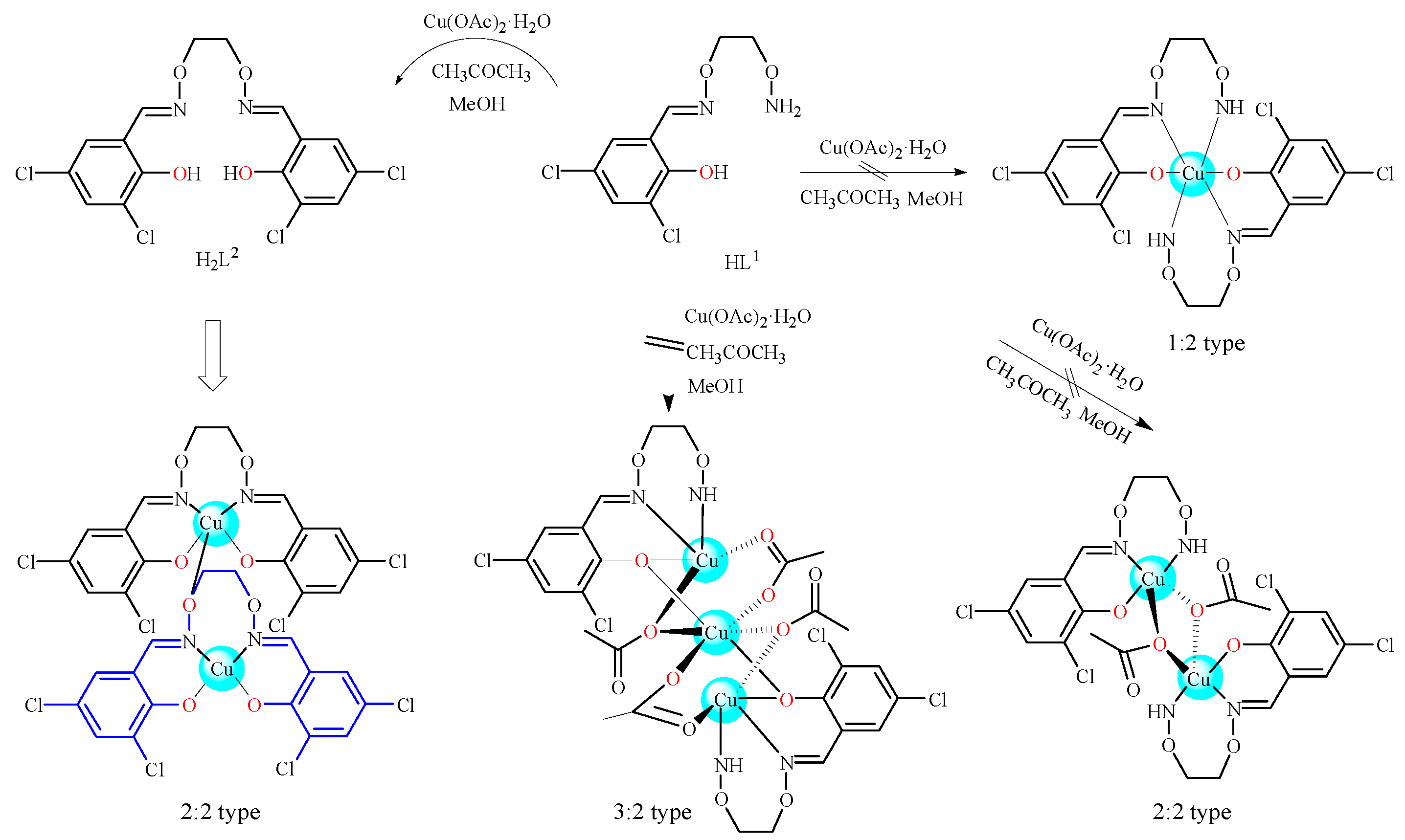
2.3. Preparation of the CuII Complex
2.4. Crystal Structure Determination of the CuII Complex
3. Results and Discussion
3.1. Infrared Spectra
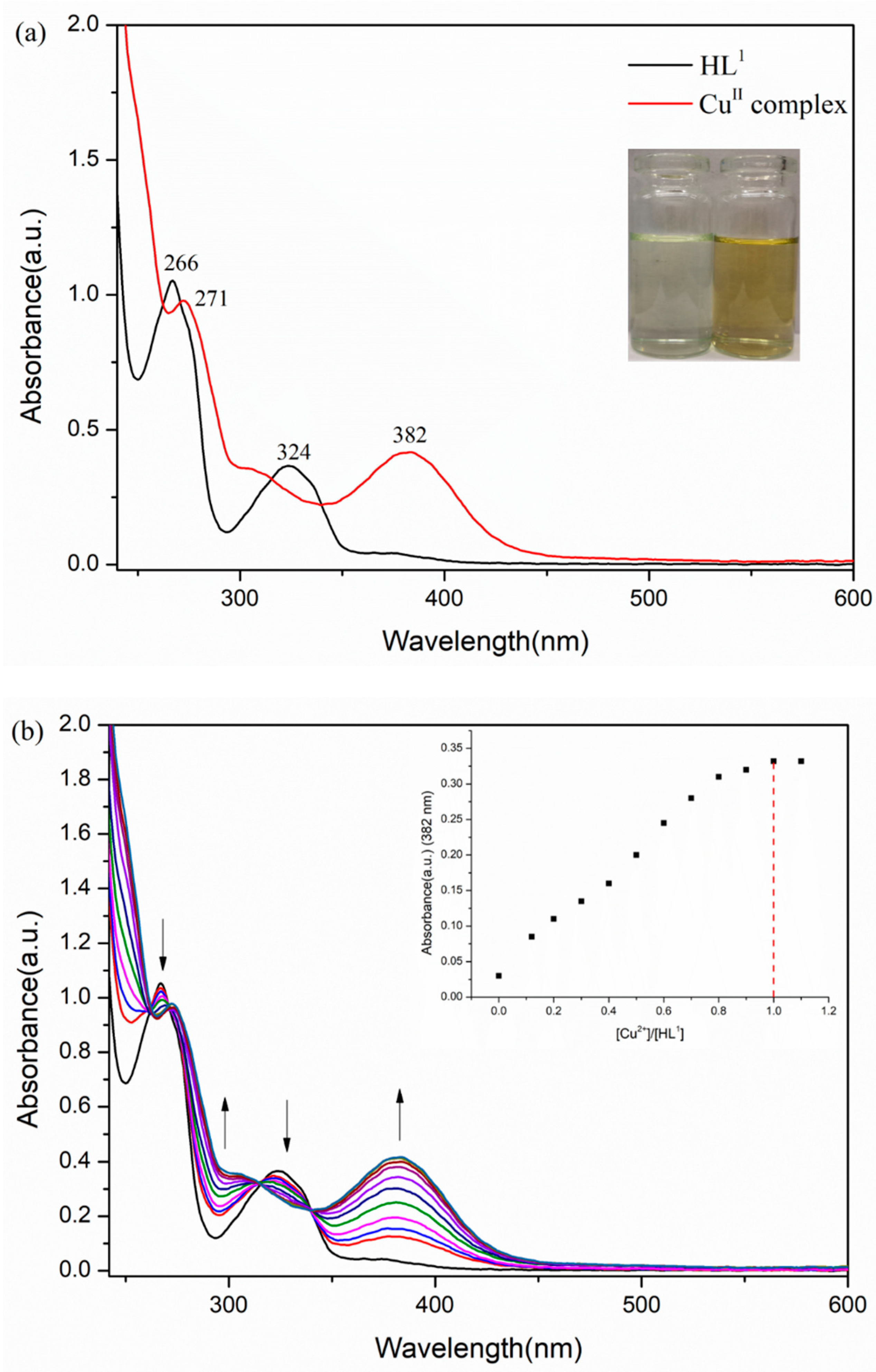
3.2. UV–Vis Spectra
3.3. Crystal Structure Description
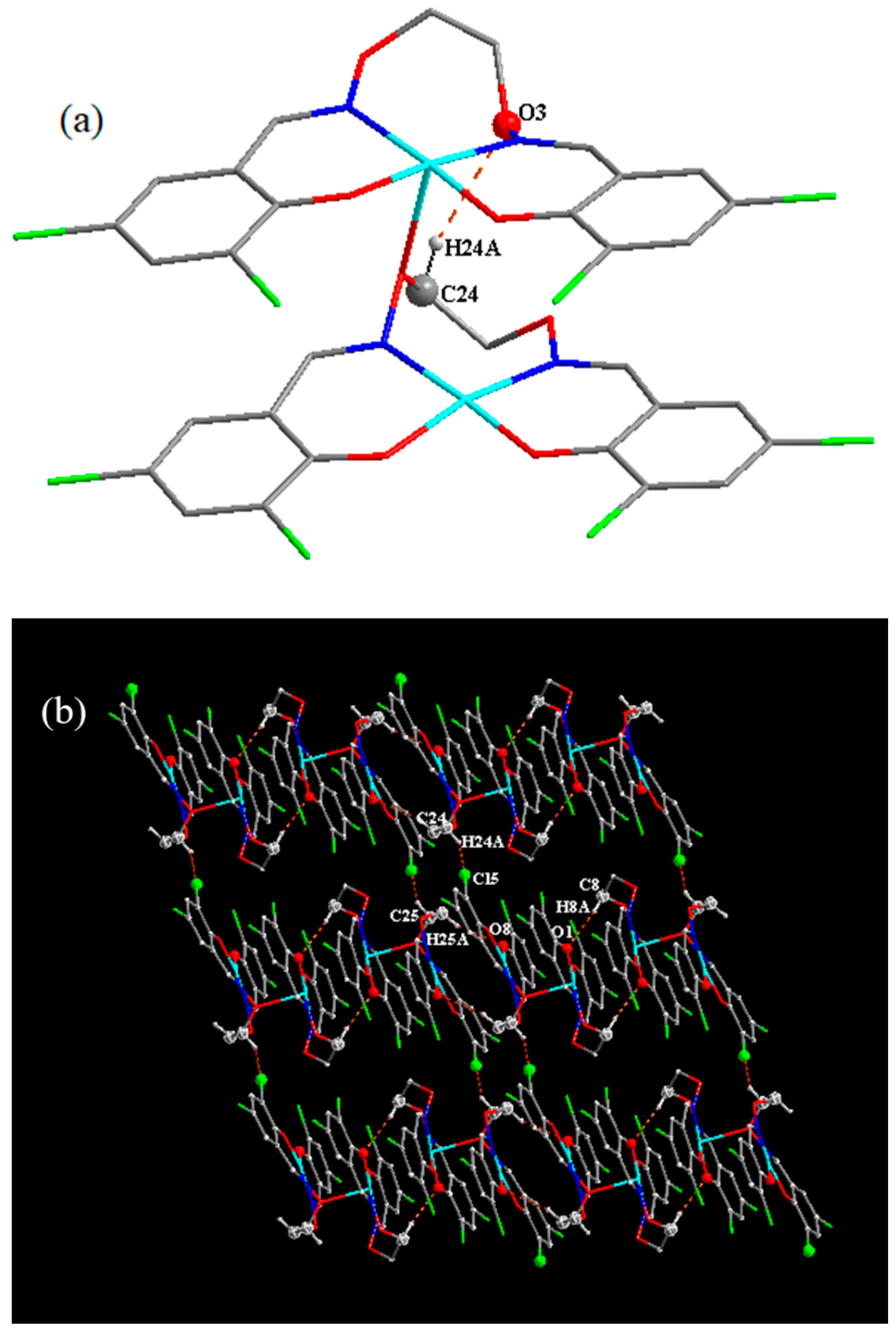
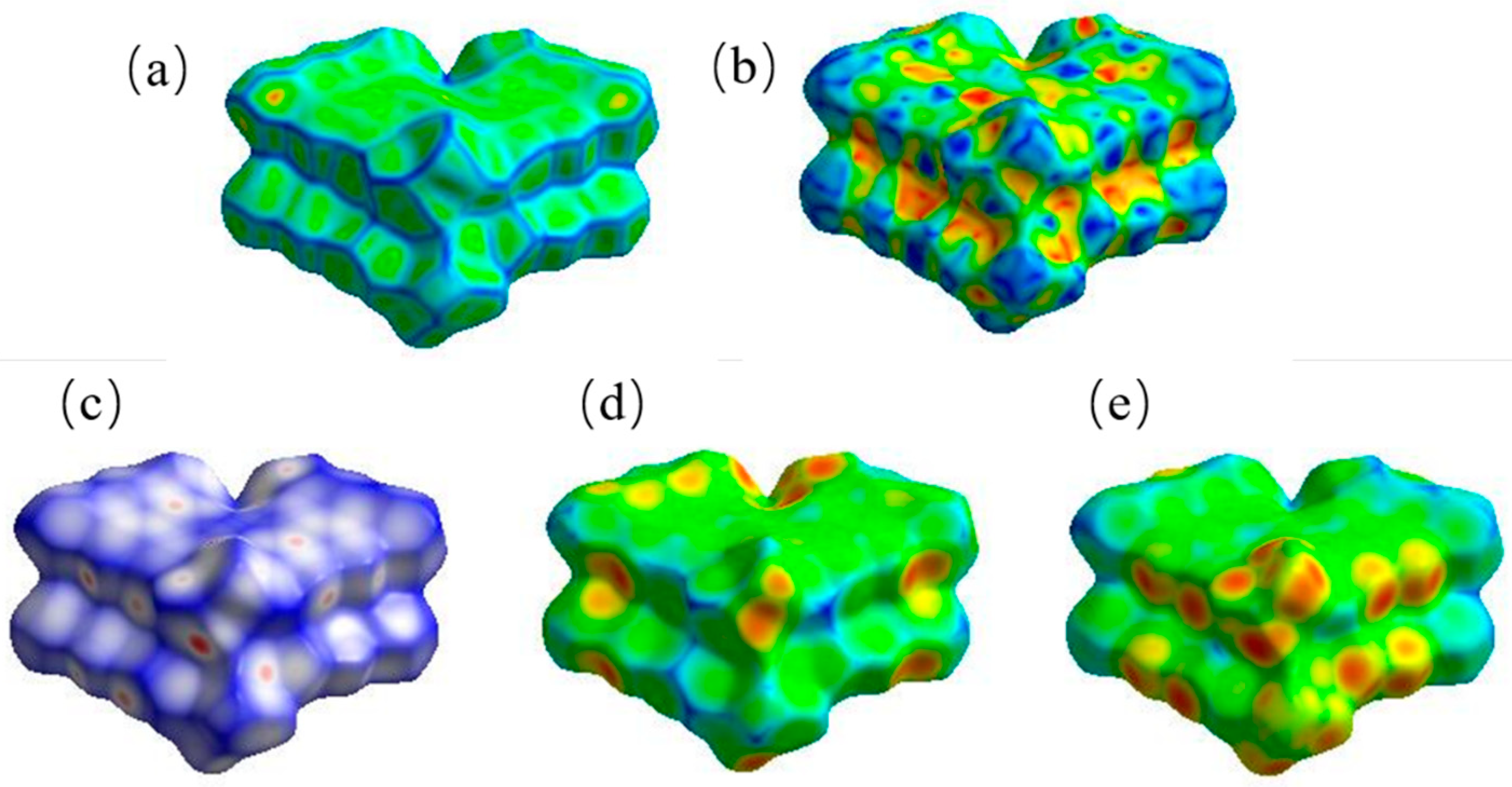
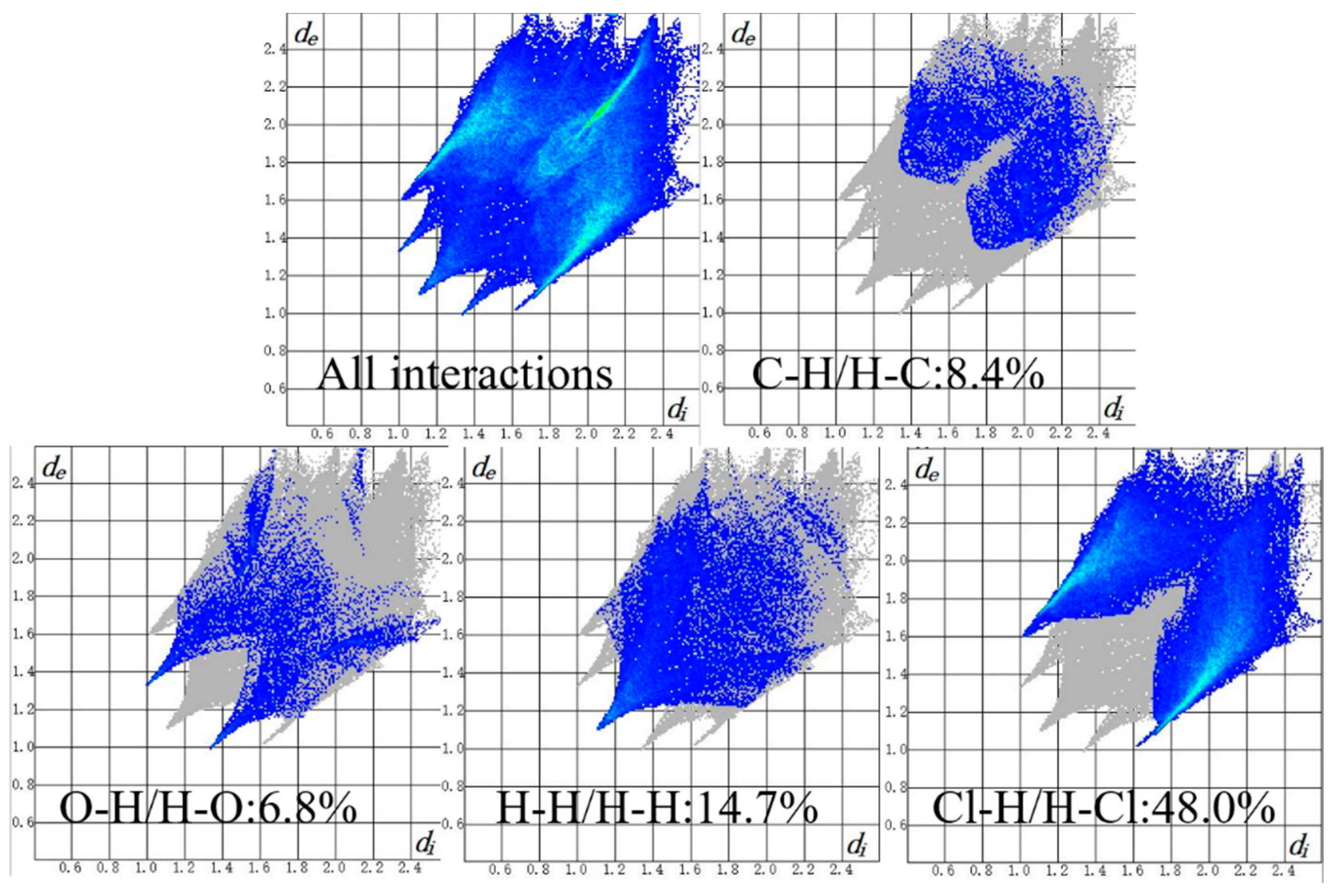
3.4. Supramolecular Interactions and Hirshfeld Surface Analysis
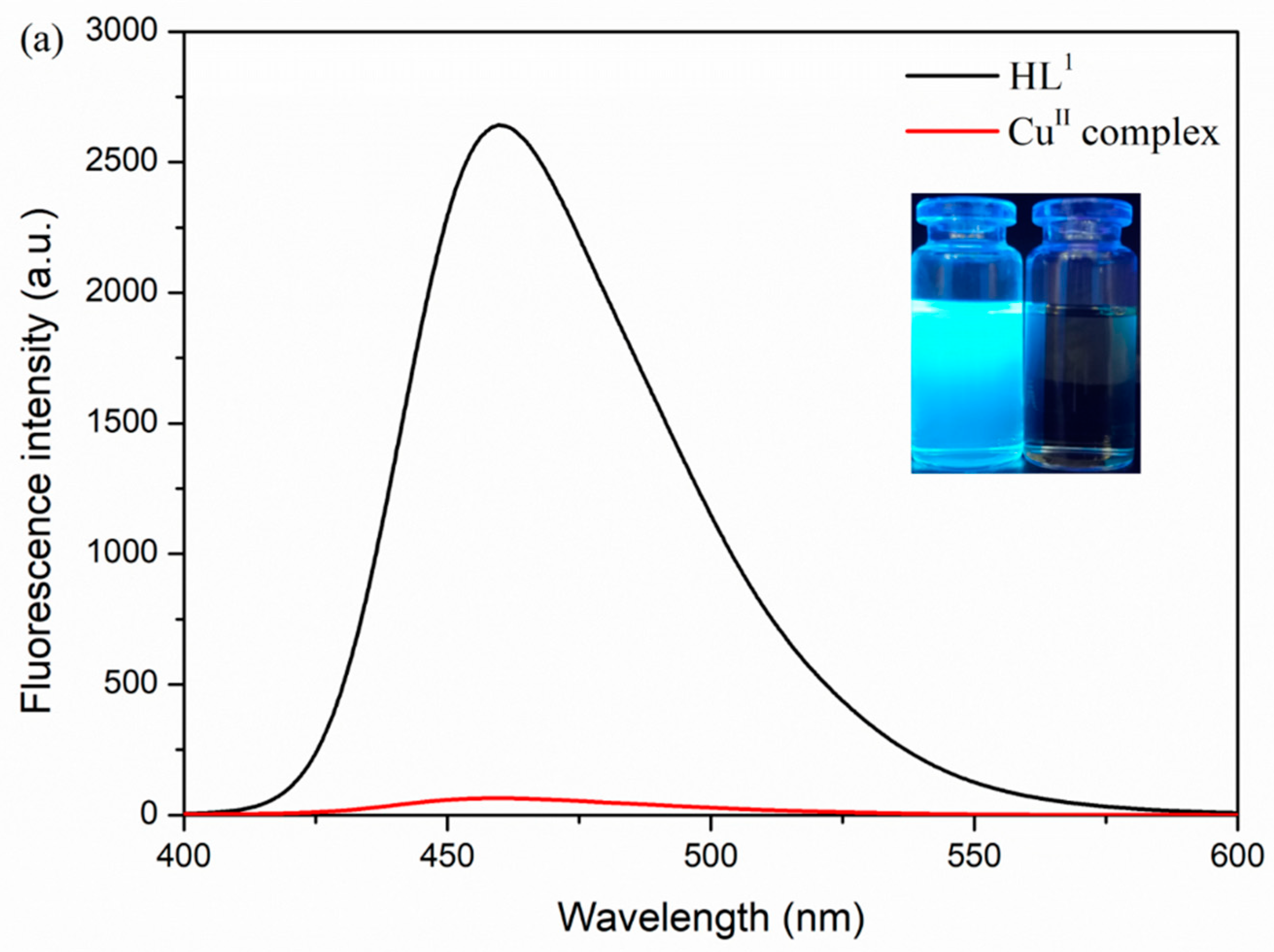
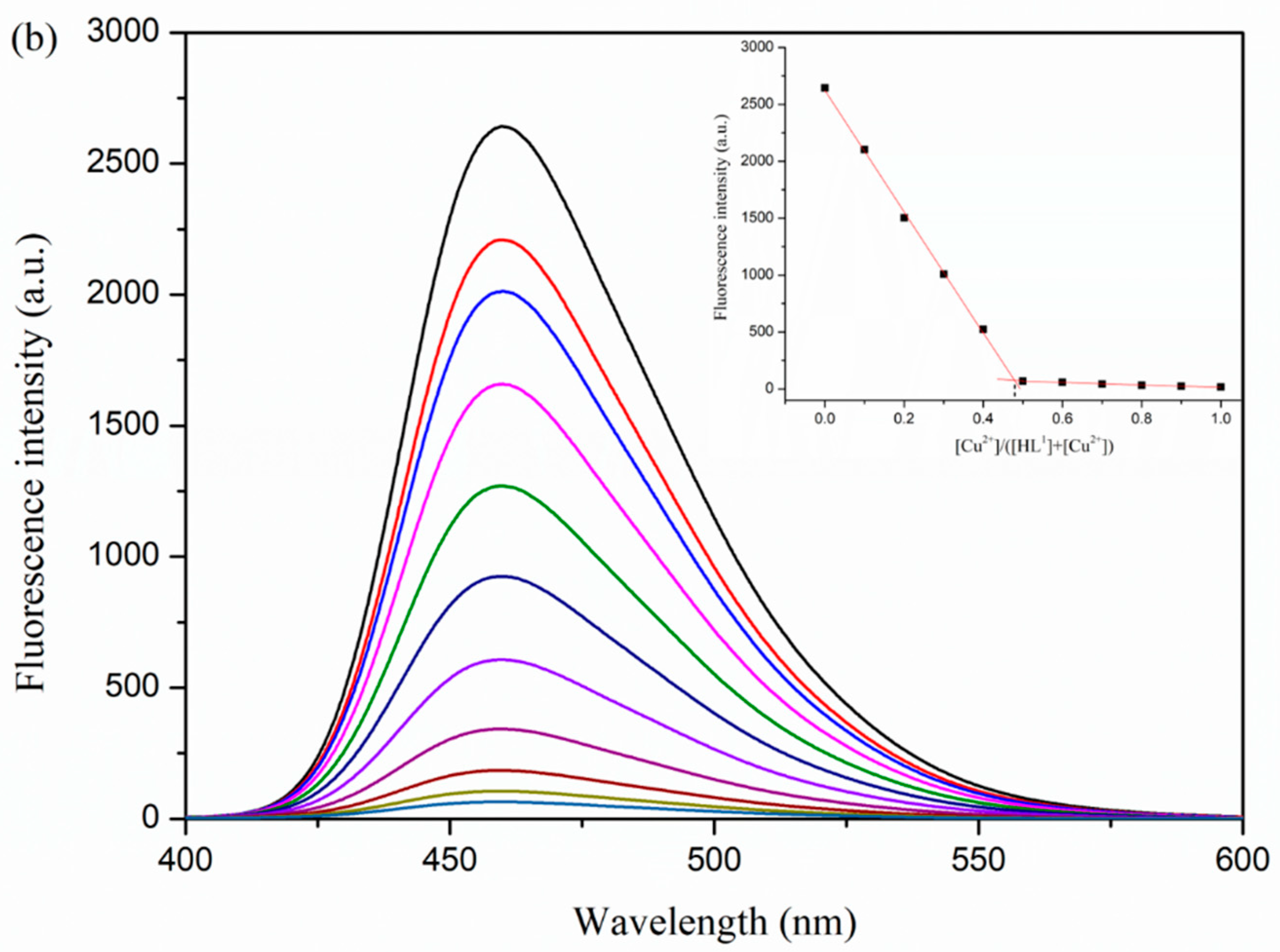
3.5. Fluorescence Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Akine, S.; Varadi, Z.; Nabeshima, T. Synthesis of planar metal complexes and the stacking abilities of naphthalenediol-based acyclic and macrocyclic salen-type ligands. Eur. J. Inorg. Chem. 2013, 35, 5987–5998. [Google Scholar] [CrossRef]
- Celedón, S.; Dorcet, V.; Roisnel, T.; Singh, A.; Ledoux-Rak, I.; Hamon, J.R.; Carrillo, D.; Manzur, C. Main-chain oligomers from NiII- and CuII-centered unsymmetrical N2O2 Schiff-base complexes: Synthesis and spectral, structural, and second-order nonlinear optical properties. Eur. J. Inorg. Chem. 2014, 29, 4984–4993. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Drew, M.G.B.; Ghosh, A. Methylene spacer-regulated structural variation in cobalt(II/III) complexes with bridging acetate and salen- or salpn-type Schiff-base ligands. Eur. J. Inorg. Chem. 2008, 10, 1693–1701. [Google Scholar] [CrossRef]
- Akine, S. Novel ion recognition systems based on cyclic and acyclic oligo(salen)-type ligands. J. Incl. Phenom. Macrocycl. Chem. 2012, 72, 25–54. [Google Scholar] [CrossRef]
- Varga, Z.; Meana-Pañeda, R.; Song, G.L.; Paukku, Y.; Truhlar, D.G. Potential energy surface of triplet N2O2. J. Chem. Phys. 2016, 144, 024310. [Google Scholar] [CrossRef]
- Akine, S.; Utsuno, F.; Taniguchi, T.; Nabeshima, T. Dinuclear complexes of the N2O2 oxime chelate ligand with zinc(II)-lanthanide(III) as a selective sensitization system for Sm3+. Eur. J. Inorg. Chem. 2010, 20, 3143–3152. [Google Scholar] [CrossRef]
- Łępicka, K.; Pieta, P.; Francius, G.; Walcarius, A.; Kutner, W. Structure-reactivity requirements with respect to nickel-salen based polymers for enhanced electrochemical stability. Electrochim. Acta 2019, 315, 75–83. [Google Scholar] [CrossRef]
- Finelli, A.; Hérault, N.; Crochet, A.; Fromm, K.M. Threading salen-type Cu- and Ni-complexes into one-dimensional coordination polymers: Solution versus solid state, and the size effect of the alkali metal ion. Cryst. Growth Des. 2018, 18, 1215–1226. [Google Scholar] [CrossRef]
- Zhao, Q.; An, X.X.; Liu, L.Z.; Dong, W.K. Syntheses, luminescences and Hirshfeld surfaces analyses of structurally characterized homo-trinuclear ZnII and hetero-pentanuclear ZnII-LnIII (Ln = Eu, Nd) bis(salamo)-like complexes. Inorg. Chim. Acta 2019, 490, 6–15. [Google Scholar] [CrossRef]
- Zhang, H.J.; Chang, J.; Jia, H.R.; Sun, Y.X. Syntheses, supramolecular structures and spectroscopic properties of Cu(II) and Ni(II) complexes with Schiff base containing oxime group. Chin. J. Inorg. Chem. 2018, 34, 2261–2270. [Google Scholar]
- Kang, Q.P.; Li, X.Y.; Wang, L.; Zhang, Y.; Dong, W.K. Containing-PMBP N2O2-donors transition metal(II) complexes: Synthesis, crystal structure, Hirshfeld surface analyses and fluorescence properties. Appl. Organomet. Chem. 2019, 33, e5013. [Google Scholar] [CrossRef]
- Wang, F.; Liu, L.Z.; Gao, L.; Dong, W.K. Unusual constructions of two salamo-based copper(II) complexes. Spectrochim. Acta A 2018, 203, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.P.; Li, X.Y.; Wei, Z.L.; Zhang, Y.; Dong, W.K. Symmetric containing-PMBP N2O2-donors nickel(II) complexes: Syntheses, structures, Hirshfeld analyses and fluorescent properties. Polyhedron 2019, 165, 38–50. [Google Scholar] [CrossRef]
- An, X.X.; Zhao, Q.; Mu, H.R.; Dong, W.K. A new half-salamo-based homo-trinuclear nickel(II) complex: Crystal structure, Hirshfeld surface analysis, and fluorescence properties. Crystals 2019, 9, 101. [Google Scholar] [CrossRef]
- Peng, Y.D.; Li, X.Y.; Kang, Q.P.; An, G.X.; Zhang, Y.; Dong, W.K. Synthesis and fluorescence properties of asymmetrical salamo-type tetranuclear zinc(II) complex. Crystals 2018, 8, 107. [Google Scholar] [CrossRef]
- Li, X.Y.; Kang, Q.P.; Liu, L.Z.; Ma, J.C.; Dong, W.K. Trinuclear Co(II) and mononuclear Ni(II) salamo-type bisoxime coordination compounds. Crystals 2018, 8, 43. [Google Scholar] [CrossRef]
- Kang, Q.P.; Li, X.Y.; Zhao, Q.; Ma, J.C.; Dong, W.K. Structurally characterized homotrinuclear salamo-type nickel(II) complexes: Synthesis, solvent effect and fluorescence properties. Appl. Organomet. Chem. 2018, 32, e4379. [Google Scholar] [CrossRef]
- Song, X.Q.; Liu, P.P.; Liu, Y.A.; Zhou, J.J.; Wang, X.L. Two dodecanuclear heterometallic [Zn6Ln6] clusters constructed by a multidentate salicylamide salen-like ligand: Synthesis, structure, luminescence and magnetic properties. Dalton Trans. 2016, 45, 8154–8163. [Google Scholar] [CrossRef]
- Yamashita, A.; Watanabe, A.; Akine, S.; Nabeshima, T.; Nakano, M.; Yamamura, T.; Kajiwara, T. Wheel-shaped ErIIIZnII3 single-molecule magnet: A macrocyclic approach to designing magnetic anisotropy. Angew. Chem. Int. Ed. 2011, 50, 4016–4019. [Google Scholar] [CrossRef]
- Song, X.Q.; Liu, P.P.; Wang, C.Y.; Liu, Y.A.; Liu, W.S.; Zhang, M. Three sandwich-type zinc(II)-lanthanide(III) clusters: Structures, luminescence and magnetic properties. RSC Adv. 2017, 7, 22692–22698. [Google Scholar] [CrossRef]
- Song, X.Q.; Liu, P.P.; Xiao, Z.R.; Li, X.; Liu, Y.A. Four polynuclear complexes based on a versatile salicylamide salen-like ligand: Synthesis, structural variations and magnetic properties. Inorg. Chim. Acta 2015, 438, 232–244. [Google Scholar] [CrossRef]
- Ren, Z.L.; Hao, J.; Hao, P.; Dong, X.Y.; Bai, Y.; Dong, W.K. Synthesis, crystal structure, luminescence and electrochemical properties of a salamo-type trinuclear cobalt(II) complex. Z. Naturforsch. B 2018, 73, 203–210. [Google Scholar] [CrossRef]
- Chai, L.Q.; Li, Y.X.; Chen, L.C.; Zhang, J.Y.; Huang, J.J. Synthesis, X-ray structure, spectroscopic, electrochemical properties and DFT calculation of a bridged dinuclear copper(II) complex. Inorg. Chim. Acta 2016, 444, 193–201. [Google Scholar] [CrossRef]
- Gerroll, B.H.R.; Bird, S.P.; Martin, E.T.; Mubarak, M.S.; Peters, D.G. Cyclohexyl bromide and iodide: Direct reduction at vitreous carbon cathodes together with nickel(I) salen- and cobalt(I) salen-catalyzed reductions in dimethylformamide. ChemElectroChem 2017, 5, 902–910. [Google Scholar] [CrossRef]
- Chai, L.Q.; Tang, L.J.; Chen, L.C.; Huang, J.J. Structural, spectral, electrochemical and DFT studies of two mononuclear manganese(II) and zinc(II) complexes. Polyhedron 2017, 122, 228–240. [Google Scholar] [CrossRef]
- Chin, T.K.; Endud, S.; Jamil, S.; Budagumpi, S.; Lintang, H.O. Oxidative dimerization of o-aminophenol by heterogeneous mesoporous material modified with biomimetic salen-type copper(II) complex. Catal. Lett. 2013, 143, 282–288. [Google Scholar] [CrossRef]
- Haak, R.M.; Decortes, A.; Escudero-Adán, E.C.; Belmonte, M.M.; Martin, E.; Benet-Buchholz, J.; Kleij, A.W. Shape-persistent octanuclear zinc salen clusters: Synthesis, characterization, and catalysis. Inorg. Chem. 2011, 50, 7934–7936. [Google Scholar] [CrossRef]
- Stößer, T.; Williams, C.K. Selective polymerization catalysis from monomer mixtures: Using a commercial Cr-salen catalyst to access ABA block polyesters. Angew. Chem. Int. Ed. 2018, 57, 6337–6341. [Google Scholar] [CrossRef]
- Abd-El-Sater, M.; Jaber, N.; Schulz, E. Chiral salen complexes for asymmetric heterogeneous catalysis: Recent examples for recycling and cooperativity. ChemCatChem. 2019, 11, 1–27. [Google Scholar] [CrossRef]
- Li, X.Y.; Kang, Q.P.; Liu, C.; Zhang, Y.; Dong, W.K. Structurally characterized homo-trinuclear ZnII and hetero-pentanuclear [ZnII4LnIII] complexes constructed from an octadentate bis(salamo)-based ligand: Hirshfeld surfaces, fluorescence and catalytic properties. New J. Chem. 2019, 43, 4605–4619. [Google Scholar] [CrossRef]
- Yamamura, M.; Takizawa, H.; Sakamoto, N.; Nabeshima, T. Monomeric and dimeric red/NIR-fluorescent dipyrrin-germanium complexes: Facile monomer-dimer interconversion driven by acid/base additions. Tetrahedron Lett. 2013, 54, 7049–7052. [Google Scholar] [CrossRef]
- Manna, A.K.; Mondal, J.; Chandra, R.; Rout, K.; Patra, G.K. A fluorescent colorimetric azo dye based chemosensor for detection of S2− in perfect aqueous solution and its applications in real sample analysis and molecular logic gate. Sens. Actuators B 2018, 10, 2317–2326. [Google Scholar] [CrossRef]
- Naik, A.D.; Bourbigot, S.; Bellayer, S.; Touati, N.; Ben-Tayeb, K.; Vezin, H.; Fontaine, G. Salen complexes as fire protective agents for thermoplastic polyurethane: Deep electron paramagnetic resonance spectroscopy investigation. ACS Appl. Mater. Interfaces 2018, 10, 24860–24875. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Z.; Wang, L.; Yu, M.; Zhao, Q.; Zhang, Y.; Sun, Y.X.; Dong, W.K. A highly sensitive and selective fluorescent “off-on-off” relay chemosensor based on a new bis(salamo)-type tetraoxime for detecting Zn2+ and CN−. Spectrochim. Acta A 2019, 222, 117209. [Google Scholar] [CrossRef]
- Zhang, L.W.; Liu, L.Z.; Wang, F.; Dong, W.K. Unprecedented fluorescent dinuclear CoII and ZnII coordination compounds with a symmetric bis(salamo)-like tetraoxime. Molecules 2018, 23, 1141. [Google Scholar] [CrossRef]
- Wang, L.; Ma, J.C.; Dong, W.K.; Zhu, L.C.; Zhang, Y. A novel self–assembled nickel(II)–cerium(III) heterotetranuclear dimer constructed from N2O2-type bisoxime and terephthalic acid: Synthesis, structure and photophysical properties. Z. Anorg. Allg. Chem. 2016, 642, 834–839. [Google Scholar] [CrossRef]
- Hao, J.; Li, X.Y.; Zhang, Y.; Dong, W.K. A reversible bis(salamo)-based fluorescence sensor for selective detection of Cd2+ in water-containing systems and food samples. Materials 2018, 11, 523. [Google Scholar] [CrossRef]
- Jia, H.R.; Li, J.; Sun, Y.X.; Guo, J.Q.; Yu, B.; Wen, N.; Xu, L. Two supramolecular cobalt(II) complexes: Syntheses, crystal structures, spectroscopic behaviors, and counter anion effects. Crystals 2017, 7, 247. [Google Scholar]
- Ledesma, G.N.; Anxolabéhère-Mallart, E.; Sabater, L.; Hureau, C.; Signorella, S.R. Functional modeling of the MnCAT active site with a dimanganese(III) complex of an unsymmetrical polydentate N3O3 ligand. J. Inorg. Biochem. 2018, 186, 10–16. [Google Scholar] [CrossRef]
- Hu, J.H.; Sun, Y.; Qi, J.; Li, Q.; Wei, T.B. A new unsymmetrical azine derivative based on coumarin group as dual-modal sensor for CN−, and fluorescent “off–on” for Zn2+. Spectrochim. Acta A 2017, 175, 125–133. [Google Scholar] [CrossRef]
- Wu, H.L.; Wang, H.; Wang, X.L.; Pan, G.L.; Shi, F.R.; Zhang, Y.H.; Bai, Y.C.; Kong, J. V-shaped ligand bis(2-benzimidazolylmethyl) amine containing three copper(II) ternary complexes: Synthesis, structure, DNA binding properties and antioxidant activity. New J. Chem. 2014, 38, 1052–1061. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.L.; Wu, H.L.; Aderinto, S.O.; Fan, X.Y. Mono-, bi- and multi-nuclear silver complexes constructed from bis(benzimidazole)-2-oxapropane ligands and methacrylate: Syntheses, crystal structures, DNA-binding properties and antioxidant activities. RSC Adv. 2016, 6, 83697–83708. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.D.; Wang, F.; Gao, L.; Dong, W.K. Structurally characterized dinuclear zinc(II) bis(salamo)-type tetraoxime complex possessing square pyramidal and trigonal bipyramidal geometries. J. Chin. Chem. Soc. 2018, 65, 893–899. [Google Scholar] [CrossRef]
- Novozhilova, M.V.; Smirnova, E.A.; Polozhentseva, J.A.; Danilova, J.A.; Chepurnaya, I.A.; Karushev, M.P.; Malev, V.V.; Timonov, A.M. Multielectron redox processes in polymeric cobalt complexes with N2O2 Schiff base ligands. Electrochim. Acta 2018, 282, 105–115. [Google Scholar] [CrossRef]
- Dong, X.Y.; Kang, Q.P.; Li, X.Y.; Ma, J.C.; Dong, W.K. Structurally characterized solvent-induced homotrinuclear cobalt(II) N2O2-donor bisoxime-type complexes. Crystals 2018, 8, 139. [Google Scholar] [CrossRef]
- Dong, W.K.; Ma, J.C.; Zhu, L.C.; Zhang, Y. Self-assembled zinc(II)-lanthanide(III) heteromultinuclear complexes constructed from 3-MeO salamo ligand: Syntheses, structures and luminescent properties. Cryst. Growth Des. 2016, 16, 6903–6914. [Google Scholar] [CrossRef]
- Bhowmik, P.; Nayek, H.P.; Corbella, M.; Aliaga-Alcalde, N.; Chattopadhyay, S. Control of molecular architecture by steric factors: Mononuclear vs polynuclear manganese(III) compounds with tetradentate N2O2 donor Schiff bases. Dalton Trans. 2011, 40, 7916–7926. [Google Scholar] [CrossRef]
- Nabeshima, T.; Yamamura, M. Cooperative formation and functions of multimetal supramolecular systems. Pure Appl. Chem. 2013, 85, 763–776. [Google Scholar] [CrossRef]
- Akine, S.; Hotate, S.; Nabeshima, T. A molecular leverage for helicity control and helix inversion. J. Am. Chem. Soc. 2011, 133, 13868–13871. [Google Scholar] [CrossRef]
- Akine, S.; Matsumoto, T.; Sairenji, S.; Nabeshima, T. Synthesis of acyclic tetrakis- and pentakis (N2O2) ligands for single-helical heterometallic complexes with a greater number of winding turns. Supramol. Chem. 2011, 23, 106–112. [Google Scholar] [CrossRef]
- Guo, J.Q.; Sun, Y.X.; Yu, B.; Li, J.; Jia, H.R. Syntheses, crystal structures and spectroscopic properties of copper(II) and nickel(II) complexes with oxime-type Schiff base ligands. Chin. J. Inorg. Chem. 2017, 33, 1481–1488. [Google Scholar]
- Maity, D.; Jana, A.D.; Debnath, M.; Hearns, N.G.R.; Sie, M.H.; Lee, H.M.; Clérac, R.; Ali, M. A μ3-alkoxo-bridged tetranuclear [Cu4L2] copper(II) complex of a hexadentate N2O4 donor ligand with a [6 + 0] Cu4O4 cubane core: Synthesis, crystal structure, and magnetic properties. Eur. J. Inorg. Chem. 2010, 22, 3484–3490. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, S.S.; Shi, X.K.; Shen, K.S.; Wu, H.L. Synthesis, crystal structure, DNA-binding properties and antioxidant activity of a copper(II) complex with naphthalimide Schiff base. Z. Anorg. Allg. Chem. 2017, 643, 1182–1190. [Google Scholar] [CrossRef]
- Liu, L.Z.; Yu, M.; Li, X.Y.; Kang, Q.P.; Dong, W.K. Syntheses, structures, Hirshfeld analyses and fluorescent properties of two Ni(II) and Zn(II) complexes constructed from a bis(salamo)-like ligand. Chin. J. Inorg. Chem. 2019, 35, 1283–1294. [Google Scholar]
- Wang, L.; Wei, Z.L.; Yu, M.; Pan, Y.Q.; Zhang, Y.; Dong, W.K. Multihalogen-substituted salamo-type Mn(II) complex: Syntheses crystal structure, Hirshfeld analyses and fluorescence properties. Chin. J. Inorg. Chem. 2019, 35, 1791–1804. [Google Scholar]
- Hao, J.; Li, L.H.; Zhang, J.T.; Akogun, S.F.; Wang, L.; Dong, W.K. Four homo- and hetero-bismetallic 3d/3d-2s complexes constructed from a naphthalenediol-based acyclic bis(salamo)-type tetraoxime ligand. Polyhedron 2017, 134, 1–10. [Google Scholar] [CrossRef]
- Akine, S.; Piao, S.J.; Miyashita, M.; Nabeshima, T. Cage-like tris(salen)-type metallocryptand for cooperative guest recognition. Tetrahedron Lett. 2013, 54, 6541–6544. [Google Scholar] [CrossRef]
- Sun, Y.X.; Liu, L.Z.; Wang, F.; Shang, X.Y.; Chen, L.; Dong, W.K. Structural and Hirshfeld surface analyses of a novel hetero-tetranuclear CuII-NaI bis(salamo)-based coordination compound. Crystals 2018, 8, 227. [Google Scholar] [CrossRef]
- Sun, Y.X.; Zhao, Y.Y.; Li, C.Y.; Yu, B.; Guo, J.Q.; Li, J. Supramolecular cobalt(II) and copper(II) complexes with Schiff base ligand: Syntheses, characterizations and crystal structures. Chin. J. Inorg. Chem. 2016, 32, 913–920. [Google Scholar]
- Gao, L.; Liu, C.; Wang, F.; Dong, W.K. Tetra-, penta- and hexa-coordinated transition metal complexes constructed from coumarin-containing N2O2 ligand. Crystals 2018, 8, 77. [Google Scholar] [CrossRef]
- Sakamoto, N.; Ikeda, C.; Yamamura, M.; Nabeshima, T. Structural interconversion and regulation of optical properties of stable hypercoordinate dipyrrin-silicon complexes. J. Am. Chem. Soc. 2011, 133, 4726–4729. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.R.; Chang, J.; Zhang, H.J.; Li, J.; Sun, Y.X. Three polyhydroxyl-bridged defective dicubane tetranuclear MnIII complexes: Synthesis, crystal structures, and spectroscopic properties. Crystals 2018, 8, 272. [Google Scholar] [CrossRef]
- Sun, Y.X.; Li, C.Y.; Yang, C.J.; Zhao, Y.Y.; Guo, J.Q.; Yu, B. Two Cu(II) complexes with Schiff base ligands: Syntheses, crystal structures, spectroscopic properties, and substituent effect. Chin. J. Inorg. Chem. 2016, 32, 327–335. [Google Scholar]
- Hu, J.H.; Li, J.B.; Qi, J.; Sun, Y. Selective colorimetric and “turn-on” fluorimetric detection of cyanide using an acylhydrazone sensor in aqueous media. New J. Chem. 2015, 39, 4041–4046. [Google Scholar] [CrossRef]
- Hu, J.H.; Li, J.B.; Qi, J.; Chen, J.J. Highly selective and effective mercury(II) fluorescent sensor. New J. Chem. 2015, 39, 843–848. [Google Scholar] [CrossRef]
- Hu, J.H.; Li, J.B.; Qi, J.; Sun, Y. Acylhydrazone based fluorescent chemosensor for zinc in aqueous solution with high selectivity and sensitivity. Sens. Actuators B 2015, 208, 581–587. [Google Scholar] [CrossRef]
- Zhang, L.W.; Li, X.Y.; Kang, Q.P.; Liu, L.Z.; Ma, J.C.; Dong, W.K. Structures and fluorescent and magnetic behaviors of newly synthesized NiII and CuII coordination compounds. Crystals 2018, 8, 173. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.Z.; Peng, Y.D.; Li, N.; Dong, W.K. Structurally characterized trinuclear nickel(II) and copper(II) salamo-type complexes: Syntheses, Hirshfeld analyses and fluorescent properties. Transit. Met. Chem. 2019, 44, 627–639. [Google Scholar] [CrossRef]
- Ma, J.C.; Dong, X.Y.; Dong, W.K.; Zhang, Y.; Zhu, L.C.; Zhang, J.T. An unexpected dinuclear Cu(II) complex with a bis(salamo) chelating ligand: Synthesis, crystal structure, and photophysical properties. J. Coord. Chem. 2016, 69, 149–159. [Google Scholar] [CrossRef]
- Delasi, R.; Holt, S.L.; Post, B. Crystal structure of the oxygeninactive form of bis(salicylaldehyde)ethylenediiminecobalt(II). Inorg. Chem. 1971, 10, 1498–1500. [Google Scholar]
- Akine, S.; Sunaga, S.; Nabeshima, T. Multistep oligometal complexation of the macrocyclic tris(N2O2) hexaoxime ligand. Chem. Eur. J. 2011, 17, 6853–6861. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Sairenji, S.; Taniguchi, T.; Nabeshima, T. Stepwise helicity inversions by multisequential metal exchange. J. Am. Chem. Soc. 2013, 135, 12948–12951. [Google Scholar] [CrossRef] [PubMed]
- Rohl, A.L.; Moret, M.; Kaminsky, W.; Claborn, K.; McKinnon, J.J.; Kahr, B. Hirshfeld surfaces identify inadequacies in computations of intermolecular interactions in crystals: Pentamorphic 1,8 dihydroxyanthraquinone. Cryst. Growth Des. 2008, 8, 4517–4525. [Google Scholar] [CrossRef]
- Kupcewicz, B.; Małecka, M.; Zapadka, M.; Krajewska, U.; Rozalski, M.; Budzisz, E. Quantitative relationships between structure and cytotoxic activity of flavonoid derivatives. An application of Hirshfeld surface derived descriptors. Bioorg. Med. Chem. Lett. 2016, 26, 3336–3341. [Google Scholar] [CrossRef]
- Hu, J.H.; Sun, Y.; Qi, J.; Pei, P.X.; Lin, Q.; Zhang, Y.M. A colorimetric and “turn-on” fluorimetric chemosensor for the selective detection of cyanide and its application in food samples. RSC Adv. 2016, 6, 100401–100406. [Google Scholar] [CrossRef]
- Dong, W.K.; Li, X.L.; Wang, L.; Zhang, Y.; Ding, Y.J. A new application of salamo-type bisoximes: As a relay-sensor for Zn2+/Cu2+ and its novel complexes for successive sensing of H+/OH−. Sens. Actuators B 2016, 229, 370–378. [Google Scholar] [CrossRef]

| Compound | The CuII Complex |
|---|---|
| Empirical formula | C32H20Cl8Cu2N4O8 |
| Molecular weight, g·mol−1 | 999.22 |
| Temperature (K) | 273 K |
| Wavelength (Å) | 0.71073 |
| Color | Brown |
| Crystal size, mm3 | 0.25 × 0.18 × 0.13 |
| Crystal shape | Parallelepiped |
| Crystal system | Triclinic |
| Space group | P-1 |
| Unit cell dimension | |
| a (Å) | 10.7507(5) |
| b (Å) | 13.5169(6) |
| c (Å) | 14.3611(6) |
| α (°) | 86.689(2) |
| β (°) | 68.572(2) |
| γ (°) | 67.681(2) |
| V (Å3) | 1789.15(14) |
| Z | 2 |
| Dc (g cm−3) | 1.855 |
| μ (mm−1) | 1.845 |
| F(000) | 996 |
| θ Range (°) | 3.024–27.186 |
| Index ranges | −13 ≤ h ≤ 13, −17 ≤ k ≤ 16, −18 ≤ l ≤ 17 |
| Reflections collected | 17905 |
| Completeness (%) | 97.0 |
| Data/restraints/parameters | 7728/0/487 |
| Final R1/wR2 [I > 2σ(I)] | R1 = 0.0349, wR2 = 0.0919 |
| Final R1/wR2 (all data) | R1 = 0.0459, wR2 = 0.0969 |
| ∆ρmax/min (e·Å−3) | 0.381 and −0.337 |
| Compound | ν(O–H) | ν(C=N) | ν(Ar–O) | ν(C=C) |
|---|---|---|---|---|
| HL1 | 3438 | 1615 | 1214 | 1470, 1449 |
| The CuII complex | – | 1609 | 1205 | 1507, 1443 |
| Bond | Lengths | Bond | Lengths |
| Cu1–O1 | 1.914(2) | Cu2–O5 | 1.923(2) |
| Cu1–O4 | 1.921(2) | Cu2–O8 | 1.897(2) |
| Cu1–O6 | 2.508(2) | Cu2–N3 | 1.958(3) |
| Cu1–N1 | 2.014(3) | Cu2–N4 | 2.007(3) |
| Cu1–N2 | 1.964(3) | ||
| Bond | Angles | Bond | Angles |
| O1–Cu1–O4 | 84.20(10) | O6–Cu1–N2 | 86.93(9) |
| O1–Cu1–O6 | 92.95(9) | N1–Cu1–N2 | 98.06(12) |
| O1–Cu1–N1 | 88.86(11) | O5–Cu2–O8 | 84.87(10) |
| O1–Cu1–N2 | 173.03(11) | O5–Cu2–N3 | 88.47(10) |
| O4–Cu1–O6 | 97.49(9) | O5–Cu2–N4 | 168.37(10) |
| O4–Cu1–N1 | 172.79(11) | O8–Cu2–N3 | 172.25(10) |
| O4–Cu1–N2 | 88.91(10) | O8–Cu2–N3 | 88.93(10) |
| O6–Cu1–N1 | 84.76(9) | N3–Cu2–N4 | 98.30(11) |
| D–H···A | d(H···A) | d(D···A) | ∠DHA | Symmetry Code |
|---|---|---|---|---|
| C24−H24A···O3 | 2.51 | 3.234(5) | 131 | |
| C8−H8A···O1 | 2.52 | 3.488(5) | 173 | – x, 1 – y, 2 – z |
| C24−H24A···Cl5 | 2.71 | 3.427(4) | 132 | –1 + x, y, z |
| C25−H25A···O8 | 2.46 | 3.394(6) | 163 | 1 – x, 1 – y, 1 – z |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.-X.; Pan, Y.-Q.; Xu, X.; Zhang, Y. Unprecedented Dinuclear CuII N,O-Donor Complex: Synthesis, Structural Characterization, Fluorescence Property, and Hirshfeld Analysis. Crystals 2019, 9, 607. https://doi.org/10.3390/cryst9120607
Sun Y-X, Pan Y-Q, Xu X, Zhang Y. Unprecedented Dinuclear CuII N,O-Donor Complex: Synthesis, Structural Characterization, Fluorescence Property, and Hirshfeld Analysis. Crystals. 2019; 9(12):607. https://doi.org/10.3390/cryst9120607
Chicago/Turabian StyleSun, Yin-Xia, Ying-Qi Pan, Xin Xu, and Yang Zhang. 2019. "Unprecedented Dinuclear CuII N,O-Donor Complex: Synthesis, Structural Characterization, Fluorescence Property, and Hirshfeld Analysis" Crystals 9, no. 12: 607. https://doi.org/10.3390/cryst9120607
APA StyleSun, Y.-X., Pan, Y.-Q., Xu, X., & Zhang, Y. (2019). Unprecedented Dinuclear CuII N,O-Donor Complex: Synthesis, Structural Characterization, Fluorescence Property, and Hirshfeld Analysis. Crystals, 9(12), 607. https://doi.org/10.3390/cryst9120607





