Carboxylic Ester Hydrolases in Bacteria: Active Site, Structure, Function and Application
Abstract
1. Introduction
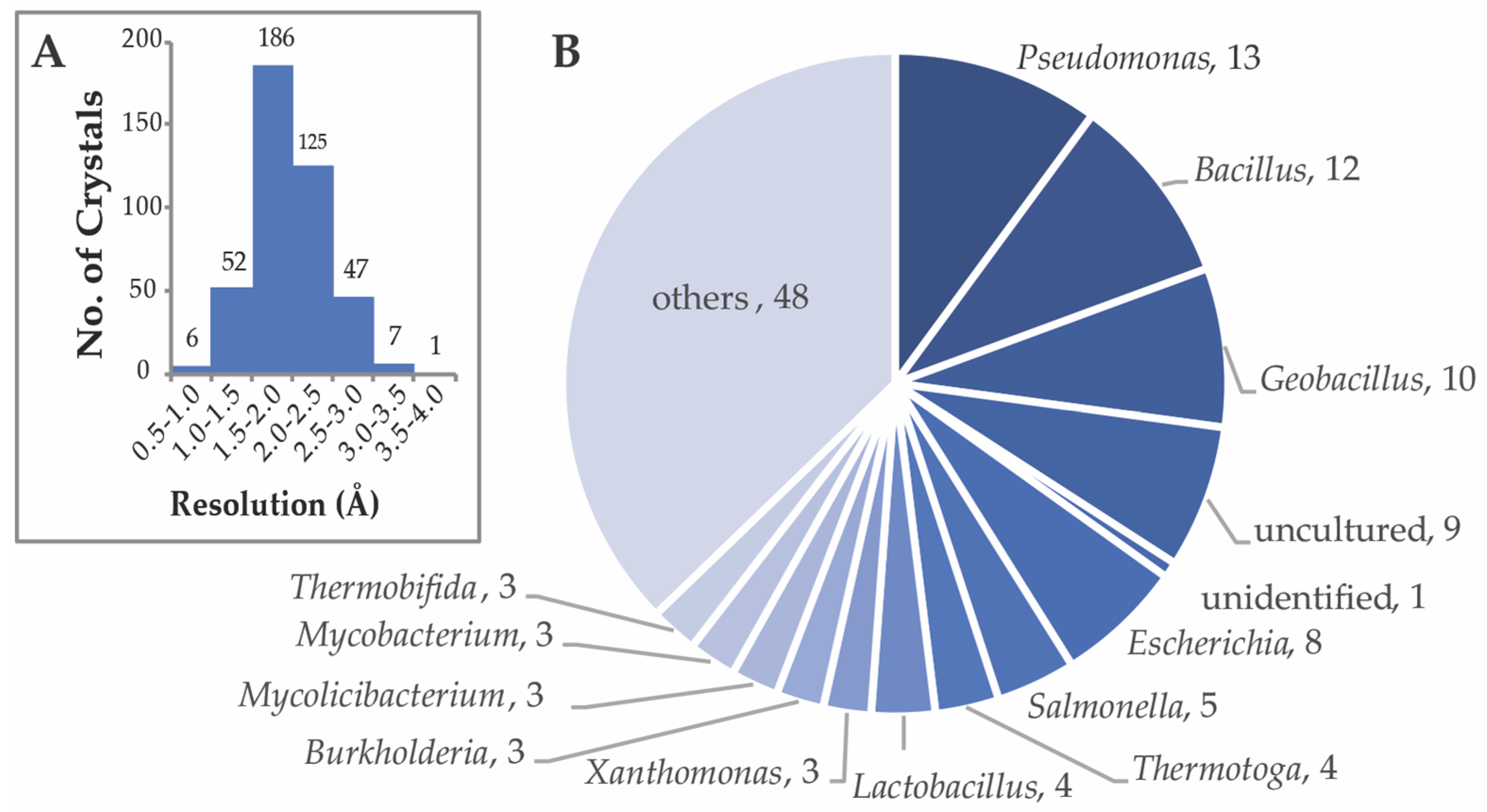
2. Sampling of Bacterial CEH Structures in the Protein Data Bank
3. Classification of CEHs Based on Substrates
4. Classification Based on Localization
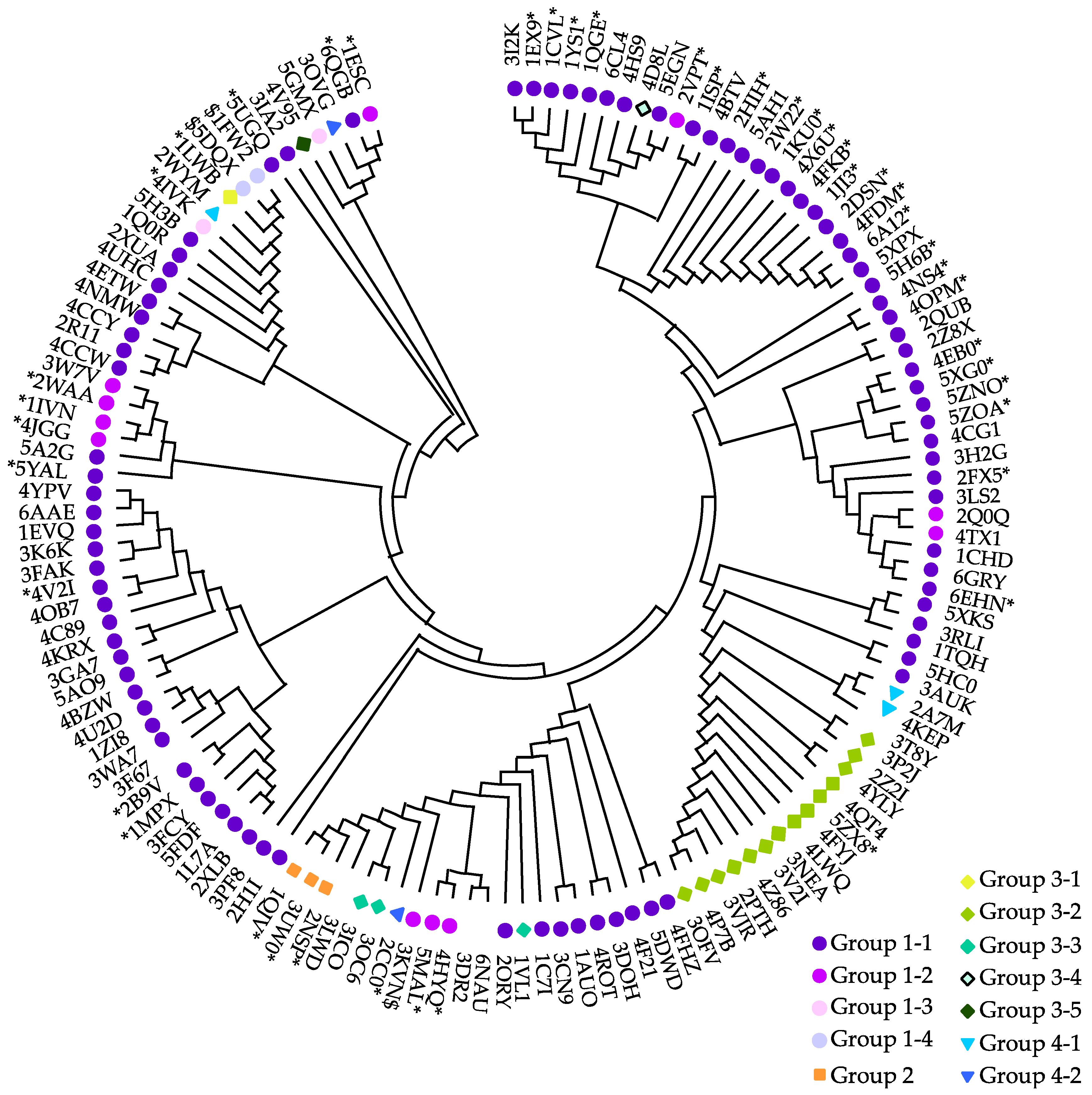
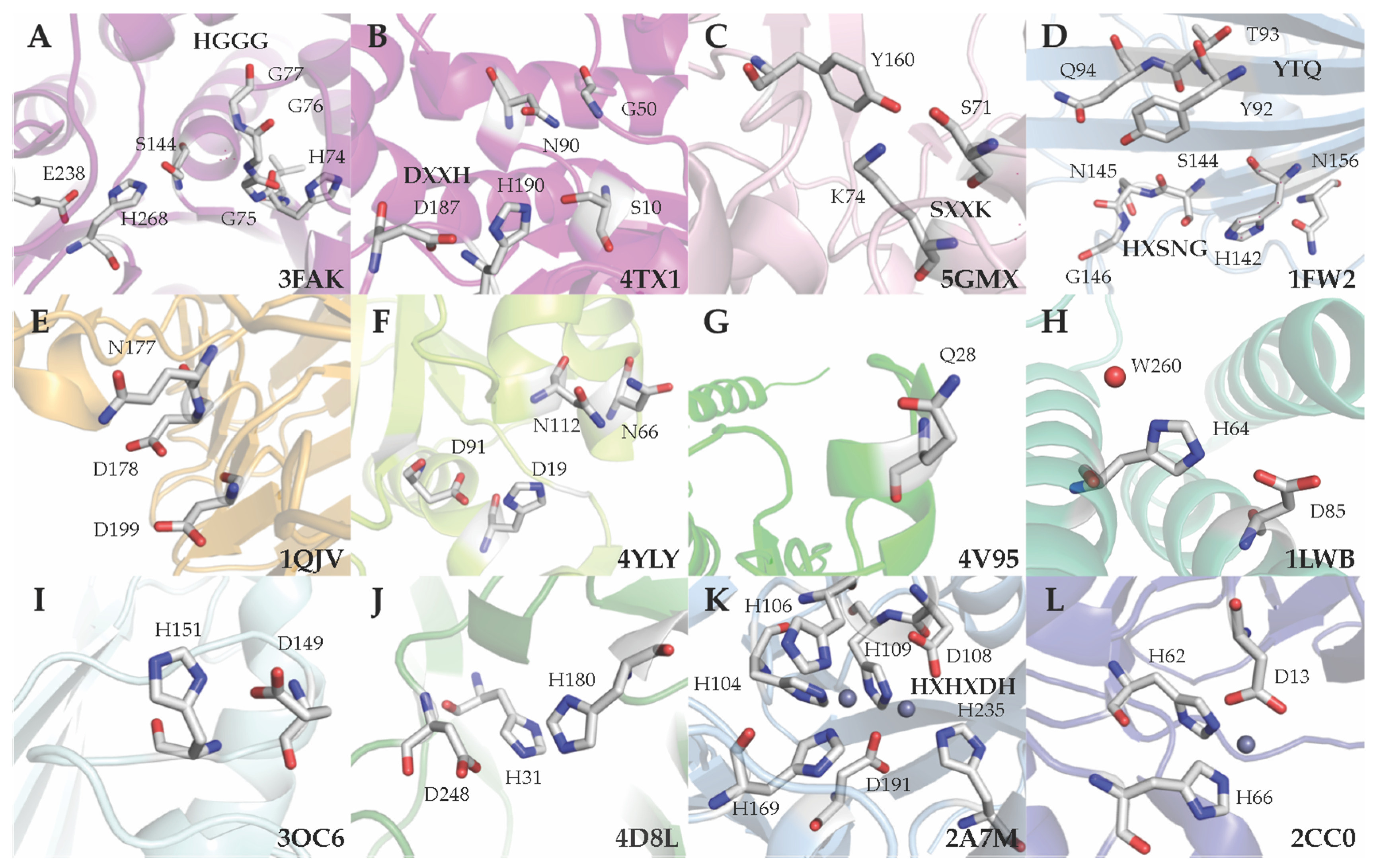
5. Classification Based on the Active Site Residues
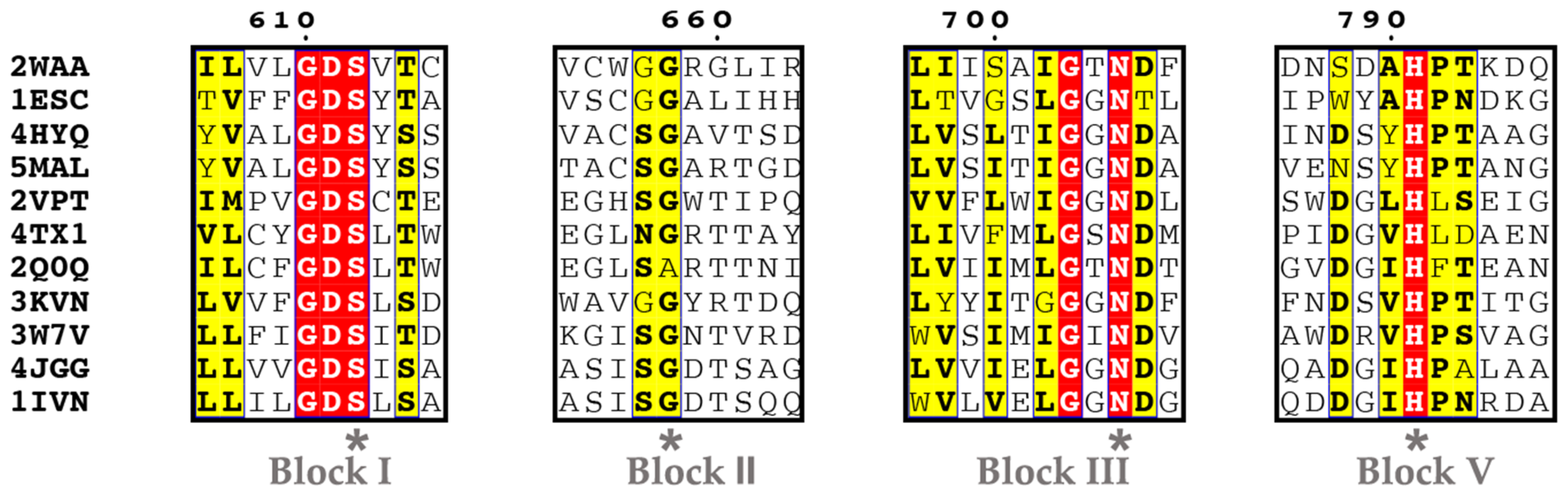
5.1. Ser Hydrolases (Group 1)
- Group 1-1
- Group 1-2
- Group 1-3
- Group 1-4
5.2. Aspartyl Hydrolases (Group 2)
5.3. Metal-independent Hydrolase with a Nucleophilic Water (Group 3)
- Group 3-1
- Group 3-2
- Group 3-3
- Group 3-4
- Group 3-5
5.4. Metal-dependent Metallohydrolases (Group 4)
- Group 4-1
- Group 4-2
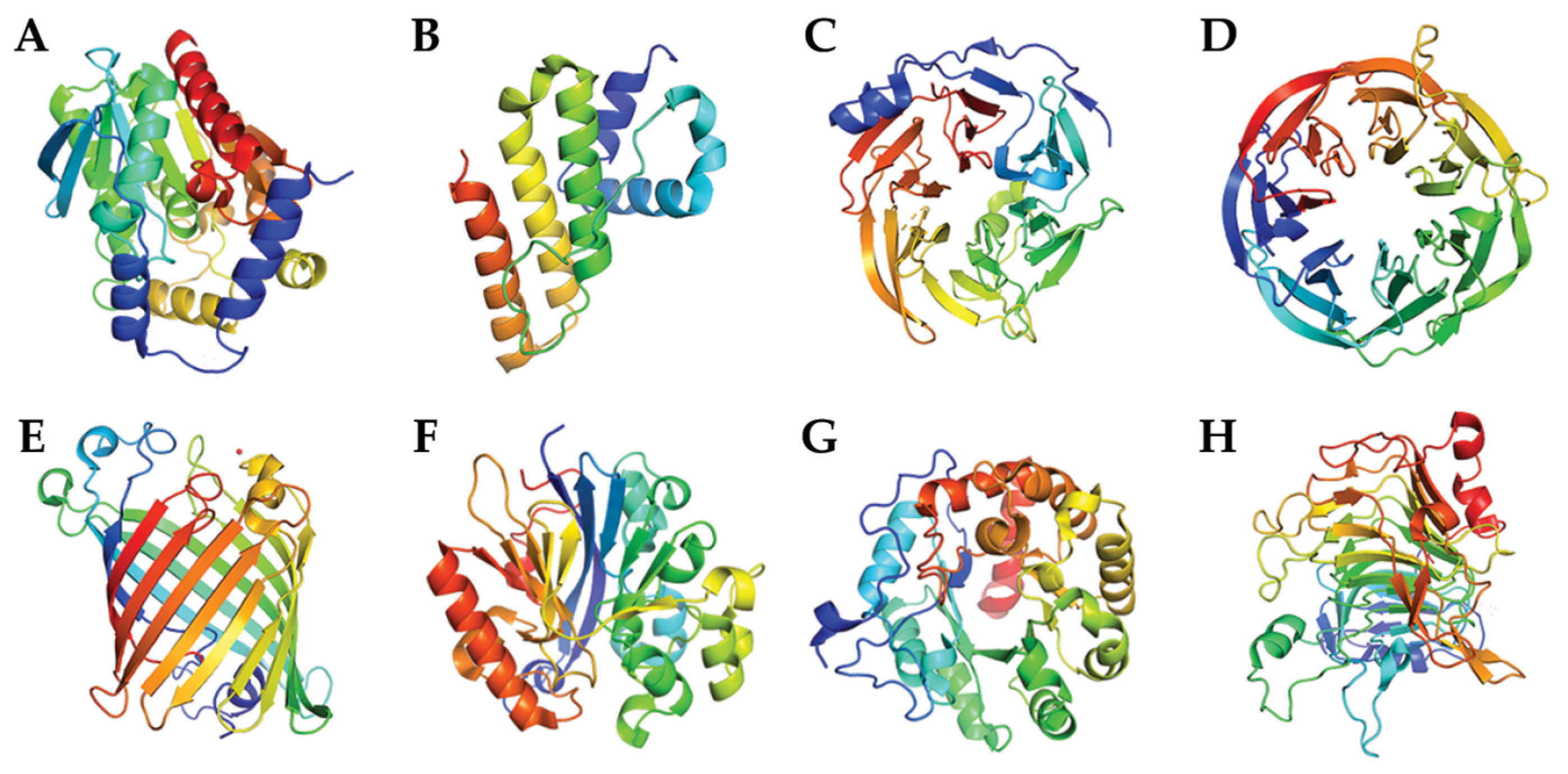
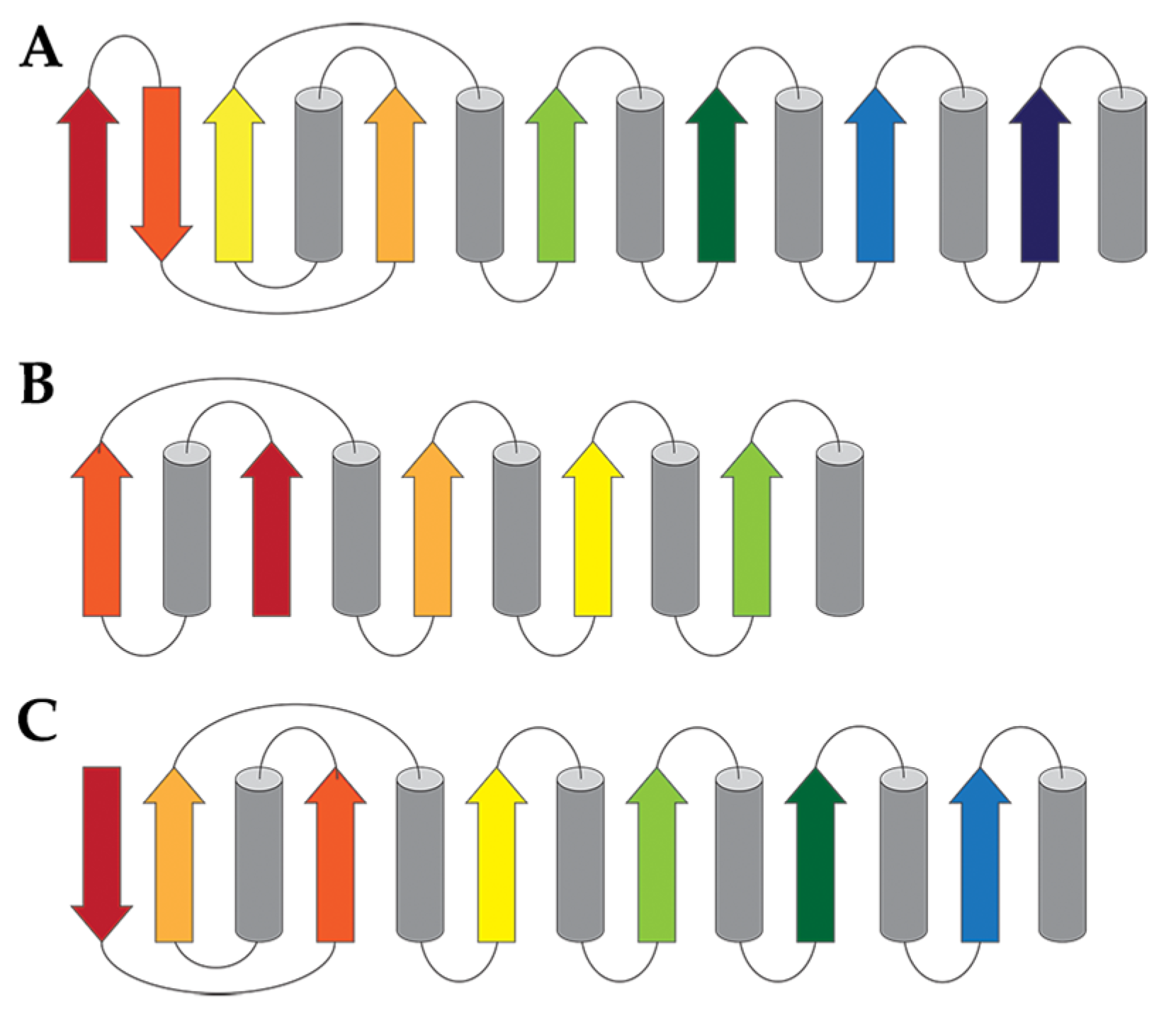
6. Classification Based on Tertiary Structure
7. Substrate-Structure Connection of CEHs
8. Physiological Functions of CEHs
9. Industrial Applications of CEHs
10. Perspectives on Identifying More CEHs and Their Functions
Funding
Conflicts of Interest
References
- Lian, J.; Nelson, R.; Lehner, R. Carboxylesterases in lipid metabolism: From mouse to human. Protein Cell 2018, 9, 178–195. [Google Scholar] [CrossRef]
- Soreq, H.; Seidman, S. Acetylcholinesterase--new roles for an old actor. Nat. Rev. Neurosci. 2001, 2, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Giudice, E.; Gillet, R. The task force that rescues stalled ribosomes in bacteria. Trends Biochem. Sci. 2013, 38, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Pilling, J.; Willmitzer, L.; Fisahn, J. Expression of a Petunia inflata pectin methyl esterase in Solanum tuberosum l. Enhances stem elongation and modifies cation distribution. Planta 2000, 210, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Bulgakov, V.P.; Jinn, T.L. Pectin methylesterases: Cell wall remodeling proteins are required for plant response to heat stress. Front. Plant Sci. 2018, 9, 1612. [Google Scholar] [CrossRef]
- Schafer, M.; Fischer, C.; Meldau, S.; Seebald, E.; Oelmuller, R.; Baldwin, I.T. Lipase activity in insect oral secretions mediates defense responses in arabidopsis. Plant Physiol. 2011, 156, 1520–1534. [Google Scholar] [CrossRef]
- Biely, P. Microbial carbohydrate esterases deacetylating plant polysaccharides. Biotechnol. Adv. 2012, 30, 1575–1588. [Google Scholar] [CrossRef]
- Gendrin, C.; Contreras-Martel, C.; Bouillot, S.; Elsen, S.; Lemaire, D.; Skoufias, D.A.; Huber, P.; Attree, I.; Dessen, A. Structural basis of cytotoxicity mediated by the type III secretion toxin exou from Pseudomonas aeruginosa. PLoS Pathog. 2012, 8, e1002637. [Google Scholar] [CrossRef]
- Sitkiewicz, I.; Stockbauer, K.E.; Musser, J.M. Secreted bacterial phospholipase A2 enzymes: Better living through phospholipolysis. Trends Microbiol. 2007, 15, 63–69. [Google Scholar] [CrossRef]
- Phillips, R.M.; Six, D.A.; Dennis, E.A.; Ghosh, P. In vivo phospholipase activity of the Pseudomonas aeruginosa cytotoxin exou and protection of mammalian cells with phospholipase A2 inhibitors. J. Biol. Chem. 2003, 278, 41326–41332. [Google Scholar] [CrossRef]
- Armendáriz-Ruiz, M.; Rodríguez-González, J.A.; Camacho-Ruíz, R.M.; Mateos-Díaz, J.C. Carbohydrate esterases: An overview. In Lipases and Phospholipases: Methods and Protocols; Sandoval, G., Ed.; Springer: New York, NY, USA, 2018; pp. 39–68. [Google Scholar]
- Casas-Godoy, L.; Duquesne, S.; Bordes, F.; Sandoval, G.; Marty, A. Lipases: An overview. In Lipases and Phospholipases: Methods and Protocols; Sandoval, G., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 3–30. [Google Scholar]
- West, A.H.; Martinez-Hackert, E.; Stock, A.M. Crystal structure of the catalytic domain of the chemotaxis receptor methylesterase, CheB. J. Mol. Biol. 1995, 250, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, S.; Goudreau, P.N.; Xu, Q.; Stock, A.M.; West, A.H. Structural basis for methylesterase CheB regulation by a phosphorylation-activated domain. Proc. Natl. Acad. Sci. USA 1998, 95, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Varshney, U. Peptidyl-tRNA hydrolase and its critical role in protein biosynthesis. Microbiology 2006, 152, 2191–2195. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.H.; Xu, J.L.; Li, X.Z.; Zhang, L.H. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA 2000, 97, 3526–3531. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Oh, C.; Ryu, B.; Yoo, W.; Nguyen, D.; Kim, T.; Ha, S.-C.; Kim, T.; Kim, K. Identification and crystallographic analysis of a new carbohydrate acetylesterase (SmAcE1) from Sinorhizobium meliloti. Crystals 2018, 8, 12. [Google Scholar] [CrossRef]
- Spiller, B.; Gershenson, A.; Arnold, F.H.; Stevens, R.C. A structural view of evolutionary divergence. Proc. Natl. Acad. Sci. USA 1999, 96, 12305–12310. [Google Scholar] [CrossRef]
- Wei, Y.; Schottel, J.L.; Derewenda, U.; Swenson, L.; Patkar, S.; Derewenda, Z.S. A novel variant of the catalytic triad in the Streptomyces scabies esterase. Nat. Struct. Biol. 1995, 2, 218–223. [Google Scholar] [CrossRef]
- Liu, D.; Lepore, B.W.; Petsko, G.A.; Thomas, P.W.; Stone, E.M.; Fast, W.; Ringe, D. Three-dimensional structure of the quorum-quenching N-acyl homoserine lactone hydrolase from Bacillus thuringiensis. Proc. Natl. Acad. Sci. USA 2005, 102, 11882–11887. [Google Scholar] [CrossRef]
- Montanier, C.; Money, V.A.; Pires, V.M.; Flint, J.E.; Pinheiro, B.A.; Goyal, A.; Prates, J.A.; Izumi, A.; Stalbrand, H.; Morland, C.; et al. The active site of a carbohydrate esterase displays divergent catalytic and noncatalytic binding functions. PLoS Biol. 2009, 7, e71. [Google Scholar] [CrossRef]
- Garces, F.; Fernandez, F.J.; Montella, C.; Penya-Soler, E.; Prohens, R.; Aguilar, J.; Baldoma, L.; Coll, M.; Badia, J.; Vega, M.C. Molecular architecture of the Mn2+-dependent lactonase ulag reveals an RNase-like metallo-beta-lactamase fold and a novel quaternary structure. J. Mol. Biol. 2010, 398, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Momb, J.; Wang, C.; Liu, D.; Thomas, P.W.; Petsko, G.A.; Guo, H.; Ringe, D.; Fast, W. Mechanism of the quorum-quenching lactonase (AiiA) from Bacillus thuringiensis. 2. Substrate modeling and active site mutations. Biochemistry 2008, 47, 7715–7725. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H.; Kim, M.Y.; Kim, S.J.; Priyadarshi, A.; Kwon, S.T.; Koo, B.S.; Yoon, S.H.; Hwang, K.Y. Structural and functional analysis of a novel hormone-sensitive lipase from a metagenome library. Proteins 2009, 74, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H.; Kim, M.Y.; Kim, S.J.; Priyadarshi, A.; Lee, W.H.; Hwang, K.Y. Structural and functional analysis of a novel EstE5 belonging to the subfamily of hormone-sensitive lipase. Biochem. Biophys. Res. Commun. 2009, 379, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.H.; Kim, S.J.; Priyadarshi, A.; Kim, H.S.; Hwang, K.Y. The crystal structure of an HSL-homolog EstE5 complex with PMSF reveals a unique configuration that inhibits the nucleophile Ser144 in catalytic triads. Biochem. Biophys. Res. Commun. 2009, 389, 247–250. [Google Scholar] [CrossRef]
- Aparna, G.; Chatterjee, A.; Sonti, R.V.; Sankaranarayanan, R. A cell wall-degrading esterase of Xanthomonas oryzae requires a unique substrate recognition module for pathogenesis on rice. Plant Cell 2009, 21, 1860–1873. [Google Scholar] [CrossRef]
- Narasimhan, D.; Nance, M.R.; Gao, D.; Ko, M.C.; Macdonald, J.; Tamburi, P.; Yoon, D.; Landry, D.M.; Woods, J.H.; Zhan, C.G.; et al. Structural analysis of thermostabilizing mutations of cocaine esterase. Protein Eng. Des. Sel. 2010, 23, 537–547. [Google Scholar] [CrossRef]
- Brim, R.L.; Nance, M.R.; Youngstrom, D.W.; Narasimhan, D.; Zhan, C.G.; Tesmer, J.J.; Sunahara, R.K.; Woods, J.H. A thermally stable form of bacterial cocaine esterase: A potential therapeutic agent for treatment of cocaine abuse. Mol. Pharmacol. 2010, 77, 593–600. [Google Scholar] [CrossRef]
- Alterio, V.; Aurilia, V.; Romanelli, A.; Parracino, A.; Saviano, M.; D’Auria, S.; De Simone, G. Crystal structure of an S-formylglutathione hydrolase from Pseudoalteromonas haloplanktis tac125. Biopolymers 2010, 93, 669–677. [Google Scholar]
- Lai, K.K.; Stogios, P.J.; Vu, C.; Xu, X.; Cui, H.; Molloy, S.; Savchenko, A.; Yakunin, A.; Gonzalez, C.F. An inserted alpha/beta subdomain shapes the catalytic pocket of Lactobacillus johnsonii cinnamoyl esterase. PLoS ONE 2011, 6, e23269. [Google Scholar] [CrossRef]
- Narasimhan, D.; Collins, G.T.; Nance, M.R.; Nichols, J.; Edwald, E.; Chan, J.; Ko, M.C.; Woods, J.H.; Tesmer, J.J.; Sunahara, R.K. Subunit stabilization and polyethylene glycolation of cocaine esterase improves in vivo residence time. Mol. Pharmacol. 2011, 80, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Filippova, E.V.; Weston, L.A.; Kuhn, M.L.; Geissler, B.; Gehring, A.M.; Armoush, N.; Adkins, C.T.; Minasov, G.; Dubrovska, I.; Shuvalova, L.; et al. Large scale structural rearrangement of a serine hydrolase from Francisella tularensis facilitates catalysis. J. Biol. Chem. 2013, 288, 10522–10535. [Google Scholar] [CrossRef] [PubMed]
- Schiefner, A.; Gerber, K.; Brosig, A.; Boos, W. Structural and mutational analyses of Aes, an inhibitor of MalT in Escherichia coli. Proteins 2014, 82, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.; Kong, X.D.; Ma, B.D.; Chen, Q.; Zhang, J.; Zhou, J.; Xu, J.H. Crystal structures of Pseudomonas putida esterase reveal the functional role of residues 187 and 287 in substrate binding and chiral recognition. Biochem. Biophys. Res. Commun. 2014, 446, 1145–1150. [Google Scholar] [CrossRef]
- Perz, V.; Hromic, A.; Baumschlager, A.; Steinkellner, G.; Pavkov-Keller, T.; Gruber, K.; Bleymaier, K.; Zitzenbacher, S.; Zankel, A.; Mayrhofer, C.; et al. An esterase from anaerobic Clostridium hathewayi can hydrolyze aliphatic-aromatic polyesters. Environ. Sci. Technol. 2016, 50, 2899–2907. [Google Scholar] [CrossRef]
- Huang, J.; Huo, Y.Y.; Ji, R.; Kuang, S.; Ji, C.; Xu, X.W.; Li, J. Structural insights of a hormone sensitive lipase homologue Est22. Sci. Rep. 2016, 6, 28550. [Google Scholar] [CrossRef]
- Leščić Ašler, I.; Štefanić, Z.; Maršavelski, A.; Vianello, R.; Kojić-Prodić, B. Catalytic dyad in the sgnh hydrolase superfamily: In-depth insight into structural parameters tuning the catalytic process of extracellular lipase from Streptomyces rimosus. ACS Chem. Biol. 2017, 12, 1928–1936. [Google Scholar] [CrossRef]
- Naffin-Olivos, J.L.; Daab, A.; White, A.; Goldfarb, N.E.; Milne, A.C.; Liu, D.; Baikovitz, J.; Dunn, B.M.; Rengarajan, J.; Petsko, G.A.; et al. Structure determination of Mycobacterium tuberculosis serine protease Hip1 (Rv2224c). Biochemistry 2017, 56, 2304–2314. [Google Scholar] [CrossRef]
- De Santi, C.; Gani, O.A.; Helland, R.; Williamson, A. Structural insight into a CE15 esterase from the marine bacterial metagenome. Sci. Rep. 2017, 7, 17278. [Google Scholar] [CrossRef]
- Arnling Baath, J.; Mazurkewich, S.; Knudsen, R.M.; Poulsen, J.N.; Olsson, L.; Lo Leggio, L.; Larsbrink, J. Biochemical and structural features of diverse bacterial glucuronoyl esterases facilitating recalcitrant biomass conversion. Biotechnol. Biofuels 2018, 11, 213. [Google Scholar] [CrossRef]
- Kim, K.K.; Song, H.K.; Shin, D.H.; Hwang, K.Y.; Choe, S.; Yoo, O.J.; Suh, S.W. Crystal structure of carboxylesterase from Pseudomonas fluorescens, an alpha/beta hydrolase with broad substrate specificity. Structure 1997, 5, 1571–1584. [Google Scholar] [CrossRef]
- De Simone, G.; Galdiero, S.; Manco, G.; Lang, D.; Rossi, M.; Pedone, C. A snapshot of a transition state analogue of a novel thermophilic esterase belonging to the subfamily of mammalian hormone-sensitive lipase. J. Mol. Biol. 2000, 303, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.M.; Larsen, N.A.; Basran, A.; Barbas, C.F., 3rd; Bruce, N.C.; Wilson, I.A.; Lerner, R.A. Biochemical characterization and structural analysis of a highly proficient cocaine esterase. Biochemistry 2002, 41, 12297–12307. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.F.; Ewis, H.E.; Abdelal, A.T.; Lu, C.D.; Harrison, R.W.; Weber, I.T. Covalent reaction intermediate revealed in crystal structure of the Geobacillus stearothermophilus carboxylesterase Est30. J. Mol. Biol. 2004, 342, 551–561. [Google Scholar] [CrossRef]
- Mandrich, L.; Menchise, V.; Alterio, V.; De Simone, G.; Pedone, C.; Rossi, M.; Manco, G. Functional and structural features of the oxyanion hole in a thermophilic esterase from Alicyclobacillus acidocaldarius. Proteins 2008, 71, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Pesaresi, A.; Lamba, D. Insights into the fatty acid chain length specificity of the carboxylesterase PA3859 from Pseudomonas aeruginosa: A combined structural, biochemical and computational study. Biochimie 2010, 92, 1787–1792. [Google Scholar] [CrossRef]
- Levisson, M.; Sun, L.; Hendriks, S.; Swinkels, P.; Akveld, T.; Bultema, J.B.; Barendregt, A.; van den Heuvel, R.H.; Dijkstra, B.W.; van der Oost, J.; et al. Crystal structure and biochemical properties of a novel thermostable esterase containing an immunoglobulin-like domain. J. Mol. Biol. 2009, 385, 949–962. [Google Scholar] [CrossRef]
- Van den Berg, B. Crystal structure of a full-length autotransporter. J. Mol. Biol. 2010, 396, 627–633. [Google Scholar] [CrossRef]
- Benavente, R.; Esteban-Torres, M.; Acebron, I.; de Las Rivas, B.; Munoz, R.; Alvarez, Y.; Mancheno, J.M. Structure, biochemical characterization and analysis of the pleomorphism of carboxylesterase Cest-2923 from Lactobacillus plantarum wcfs1. FEBS J. 2013, 280, 6658–6671. [Google Scholar] [CrossRef]
- Alvarez, Y.; Esteban-Torres, M.; Cortes-Cabrera, A.; Gago, F.; Acebron, I.; Benavente, R.; Mardo, K.; de Las Rivas, B.; Munoz, R.; Mancheno, J.M. Esterase LpEst1 from Lactobacillus plantarum: A novel and atypical member of the alphabeta hydrolase superfamily of enzymes. PLoS ONE 2014, 9, e92257. [Google Scholar] [CrossRef]
- Rozeboom, H.J.; Godinho, L.F.; Nardini, M.; Quax, W.J.; Dijkstra, B.W. Crystal structures of two Bacillus carboxylesterases with different enantioselectivities. Biochim. Biophys. Acta 2014, 1844, 567–575. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, J.; Wu, L.; Guo, F.; Gu, J.; Tang, X.; Jiang, L.; Liu, J.; Zhou, J.; Yu, H. Enhanced enantioselectivity of a carboxyl esterase from Rhodobacter sphaeroides by directed evolution. Appl. Microbiol. Biotechnol. 2013, 97, 4897–4906. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.S.; An, Y.J.; Jeong, C.S.; Kim, M.K.; Jeon, J.H.; Lee, C.M.; Lee, H.S.; Kang, S.G.; Lee, J.H. Structural basis for the beta-lactamase activity of EstU1, a family VIII carboxylesterase. Proteins 2013, 81, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, F.; Granzin, J.; Wilhelm, S.; Kojic-Prodic, B.; Batra-Safferling, R.; Jaeger, K.E. Structural and functional characterisation of TesA—A novel lysophospholipase A from Pseudomonas aeruginosa. PLoS ONE 2013, 8, e69125. [Google Scholar] [CrossRef] [PubMed]
- Sayer, C.; Isupov, M.N.; Bonch-Osmolovskaya, E.; Littlechild, J.A. Structural studies of a thermophilic esterase from a new planctomycetes species, Thermogutta terrifontis. FEBS J. 2015, 282, 2846–2857. [Google Scholar] [CrossRef]
- De Santi, C.; Leiros, H.K.; Di Scala, A.; de Pascale, D.; Altermark, B.; Willassen, N.P. Biochemical characterization and structural analysis of a new cold-active and salt-tolerant esterase from the marine Bacterium thalassospira sp. Extremophiles 2016, 20, 323–336. [Google Scholar] [CrossRef]
- Pereira, M.R.; Maester, T.C.; Mercaldi, G.F.; de Macedo Lemos, E.G.; Hyvonen, M.; Balan, A. From a metagenomic source to a high-resolution structure of a novel alkaline esterase. Appl. Microbiol. Biotechnol. 2017, 101, 4935–4949. [Google Scholar] [CrossRef]
- Sayer, C.; Szabo, Z.; Isupov, M.N.; Ingham, C.; Littlechild, J.A. The structure of a novel thermophilic esterase from the Planctomycetes species, Thermogutta terrifontis reveals an open active site due to a minimal ‘cap’ domain. Front Microbiol. 2015, 6, 1294. [Google Scholar] [CrossRef]
- Cha, S.S.; An, Y.J. Crystal structure of EstSRT1, a family VIII carboxylesterase displaying hydrolytic activity toward oxyimino cephalosporins. Biochem. Biophys. Res. Commun. 2016, 478, 818–824. [Google Scholar] [CrossRef]
- Shi, J.; Cao, X.; Chen, Y.; Cronan, J.E.; Guo, Z. An atypical alpha/beta-hydrolase fold revealed in the crystal structure of pimeloyl-acyl carrier protein methyl esterase biog from Haemophilus influenzae. Biochemistry 2016, 55, 6705–6717. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, P.A.; Han, K.; Lee, S.W.; Rhee, S. Crystal structure of chloramphenicol-metabolizing enzyme EstDL136 from a metagenome. PLoS ONE 2019, 14, e0210298. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, J.D.; Tocilj, A.; Park, S.; Schrag, J.D.; Kazlauskas, R.J. Structure of an aryl esterase from Pseudomonas fluorescens. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Mathews, I.; Soltis, M.; Saldajeno, M.; Ganshaw, G.; Sala, R.; Weyler, W.; Cervin, M.A.; Whited, G.; Bott, R. Structure of a novel enzyme that catalyzes acyl transfer to alcohols in aqueous conditions. Biochemistry 2007, 46, 8969–8979. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.L.; Bernhardt, P.; Morley, K.L.; Jiang, Y.; Cheeseman, J.D.; Purpero, V.; Schrag, J.D.; Kazlauskas, R.J. Switching catalysis from hydrolysis to perhydrolysis in Pseudomonas fluorescens esterase. Biochemistry 2010, 49, 1931–1942. [Google Scholar] [CrossRef]
- Jiang, Y.; Morley, K.L.; Schrag, J.D.; Kazlauskas, R.J. Different active-site loop orientation in serine hydrolases versus acyltransferases. Chembiochem 2011, 12, 768–776. [Google Scholar] [CrossRef]
- Kim, K.; Ryu, B.H.; Kim, S.S.; An, D.R.; Ngo, T.D.; Pandian, R.; Kim, K.K.; Kim, T.D. Structural and biochemical characterization of a carbohydrate acetylesterase from Sinorhizobium meliloti 1021. FEBS Lett. 2015, 589, 117–122. [Google Scholar] [CrossRef]
- Lang, D.; Hofmann, B.; Haalck, L.; Hecht, H.J.; Spener, F.; Schmid, R.D.; Schomburg, D. Crystal structure of a bacterial lipase from Chromobacterium viscosum ATCC 6918 refined at 1.6 Å resolution. J. Mol. Biol. 1996, 259, 704–717. [Google Scholar] [CrossRef]
- Nardini, M.; Lang, D.A.; Liebeton, K.; Jaeger, K.E.; Dijkstra, B.W. Crystal structure of Pseudomonas aeruginosa lipase in the open conformation. The prototype for family I.1 of bacterial lipases. J. Biol. Chem. 2000, 275, 31219–31225. [Google Scholar] [CrossRef]
- Luic, M.; Tomic, S.; Lescic, I.; Ljubovic, E.; Sepac, D.; Sunjic, V.; Vitale, L.; Saenger, W.; Kojic-Prodic, B. Complex of Burkholderia cepacia lipase with transition state analogue of 1-phenoxy-2-acetoxybutane: Biocatalytic, structural and modelling study. Eur. J. Biochem. 2001, 268, 3964–3973. [Google Scholar] [CrossRef]
- Van Pouderoyen, G.; Eggert, T.; Jaeger, K.E.; Dijkstra, B.W. The crystal structure of Bacillus subtilis lipase: A minimal alpha/beta hydrolase fold enzyme. J. Mol. Biol. 2001, 309, 215–226. [Google Scholar] [CrossRef]
- Kawasaki, K.; Kondo, H.; Suzuki, M.; Ohgiya, S.; Tsuda, S. Alternate conformations observed in catalytic serine of Bacillus subtilis lipase determined at 1.3 Å resolution. Acta. Crystallogr. D Biol. Crystallogr. 2002, 58, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Tyndall, J.D.; Sinchaikul, S.; Fothergill-Gilmore, L.A.; Taylor, P.; Walkinshaw, M.D. Crystal structure of a thermostable lipase from Bacillus stearothermophilus P1. J. Mol. Biol. 2002, 323, 859–869. [Google Scholar] [CrossRef]
- Jeong, S.T.; Kim, H.K.; Kim, S.J.; Chi, S.W.; Pan, J.G.; Oh, T.K.; Ryu, S.E. Novel zinc-binding center and a temperature switch in the Bacillus stearothermophilus L1 lipase. J. Biol. Chem. 2002, 277, 17041–17047. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Song, H.K.; Shin, D.H.; Hwang, K.Y.; Suh, S.W. The crystal structure of a triacylglycerol lipase from Pseudomonas cepacia reveals a highly open conformation in the absence of a bound inhibitor. Structure 1997, 5, 173–185. [Google Scholar] [CrossRef]
- Dröge, M.J.; Boersma, Y.L.; van Pouderoyen, G.; Vrenken, T.E.; Rüggeberg, C.J.; Reetz, M.T.; Dijkstra, B.W.; Quax, W.J. Directed evolution of Bacillus subtilis lipase a by use of enantiomeric phosphonate inhibitors: Crystal structures and phage display selection. ChemBioChem 2006, 7, 149–157. [Google Scholar] [CrossRef]
- Acharya, P.; Rajakumara, E.; Sankaranarayanan, R.; Rao, N.M. Structural basis of selection and thermostability of laboratory evolved Bacillus subtilis lipase. J. Mol. Biol. 2004, 341, 1271–1281. [Google Scholar] [CrossRef]
- Mezzetti, A.; Schrag, J.D.; Cheong, C.S.; Kazlauskas, R.J. Mirror-image packing in enantiomer discrimination: molecular basis for the enantioselectivity of B. cepacia lipase toward 2-methyl-3-phenyl-1-propanol. Chem. Biol. 2005, 12, 427–437. [Google Scholar] [CrossRef]
- Matsumura, H.; Yamamoto, T.; Leow, T.C.; Mori, T.; Salleh, A.B.; Basri, M.; Inoue, T.; Kai, Y.; Rahman, R.N. Novel cation-pi interaction revealed by crystal structure of thermoalkalophilic lipase. Proteins 2008, 70, 592–598. [Google Scholar] [CrossRef]
- Pauwels, K.; Lustig, A.; Wyns, L.; Tommassen, J.; Savvides, S.N.; Van Gelder, P. Structure of a membrane-based steric chaperone in complex with its lipase substrate. Nat. Struct. Mol. Biol. 2006, 13, 374–375. [Google Scholar] [CrossRef]
- Tiesinga, J.J.; van Pouderoyen, G.; Nardini, M.; Ransac, S.; Dijkstra, B.W. Structural basis of phospholipase activity of Staphylococcus hyicus lipase. J. Mol. Biol. 2007, 371, 447–456. [Google Scholar] [CrossRef]
- Schrag, J.D.; Li, Y.; Cygler, M.; Lang, D.; Burgdorf, T.; Hecht, H.J.; Schmid, R.; Schomburg, D.; Rydel, T.J.; Oliver, J.D.; et al. The open conformation of a Pseudomonas lipase. Structure 1997, 5, 187–202. [Google Scholar] [CrossRef]
- Luic, M.; Stefanic, Z.; Ceilinger, I.; Hodoscek, M.; Janezic, D.; Lenac, T.; Asler, I.L.; Sepac, D.; Tomic, S. Combined X-ray diffraction and QM/MM study of the Burkholderia cepacia lipase-catalyzed secondary alcohol esterification. J. Phys. Chem. B 2008, 112, 4876–4883. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.K.; Jeong, D.G.; Lee, M.S.; Lee, J.K.; Kim, H.K.; Ryu, S.E.; Park, B.C.; Kim, J.H.; Kim, S.J. Structural basis for the cold adaptation of psychrophilic M37 lipase from Photobacterium lipolyticum. Proteins 2008, 71, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Drepper, T.; Svensson, V.; Jaeger, K.E.; Baumann, U. A calcium-gated lid and a large beta-roll sandwich are revealed by the crystal structure of extracellular lipase from Serratia marcescens. J. Biol. Chem. 2007, 282, 31477–31483. [Google Scholar] [CrossRef]
- Rajakumara, E.; Acharya, P.; Ahmad, S.; Sankaranaryanan, R.; Rao, N.M. Structural basis for the remarkable stability of Bacillus subtilis lipase (Lip A) at low pH. Biochim. Biophys. Acta 2008, 1784, 302–311. [Google Scholar] [CrossRef]
- Carrasco-Lopez, C.; Godoy, C.; de Las Rivas, B.; Fernandez-Lorente, G.; Palomo, J.M.; Guisan, J.M.; Fernandez-Lafuente, R.; Martinez-Ripoll, M.; Hermoso, J.A. Activation of bacterial thermoalkalophilic lipases is spurred by dramatic structural rearrangements. J. Biol. Chem. 2009, 284, 4365–4372. [Google Scholar] [CrossRef]
- Angkawidjaja, C.; You, D.J.; Matsumura, H.; Kuwahara, K.; Koga, Y.; Takano, K.; Kanaya, S. Crystal structure of a family I.3 lipase from Pseudomonas sp. MIS38 in a closed conformation. FEBS Lett. 2007, 581, 5060–5064. [Google Scholar] [CrossRef]
- Kuwahara, K.; Angkawidjaja, C.; Matsumura, H.; Koga, Y.; Takano, K.; Kanaya, S. Importance of the Ca2+-binding sites in the N-catalytic domain of a family I.3 lipase for activity and stability. Protein Eng. Des. Sel. 2008, 21, 737–744. [Google Scholar] [CrossRef]
- Angkawidjaja, C.; Matsumura, H.; Koga, Y.; Takano, K.; Kanaya, S. X-ray crystallographic and MD simulation studies on the mechanism of interfacial activation of a family I.3 lipase with two lids. J. Mol. Biol. 2010, 400, 82–95. [Google Scholar] [CrossRef]
- Ahmad, S.; Kamal, M.Z.; Sankaranarayanan, R.; Rao, N.M. Thermostable Bacillus subtilis lipases: In vitro evolution and structural insight. J. Mol. Biol. 2008, 381, 324–340. [Google Scholar] [CrossRef]
- Kamal, M.Z.; Ahmad, S.; Molugu, T.R.; Vijayalakshmi, A.; Deshmukh, M.V.; Sankaranarayanan, R.; Rao, N.M. In vitro evolved non-aggregating and thermostable lipase: Structural and thermodynamic investigation. J. Mol. Biol. 2011, 413, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Augustyniak, W.; Brzezinska, A.A.; Pijning, T.; Wienk, H.; Boelens, R.; Dijkstra, B.W.; Reetz, M.T. Biophysical characterization of mutants of Bacillus subtilis lipase evolved for thermostability: Factors contributing to increased activity retention. Protein Sci. 2012, 21, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Ruslan, R.; Abd Rahman, R.N.; Leow, T.C.; Ali, M.S.; Basri, M.; Salleh, A.B. Improvement of thermal stability via outer-loop ion pair interaction of mutated T1 lipase from Geobacillus zalihae strain T1. Int. J. Mol. Sci. 2012, 13, 943–960. [Google Scholar] [CrossRef] [PubMed]
- Abd Rahman, R.N.; Shariff, F.M.; Basri, M.; Salleh, A.B. 3D structure elucidation of thermostable L2 lipase from thermophilic Bacillus sp. L2. Int. J. Mol. Sci. 2012, 13, 9207–9217. [Google Scholar] [CrossRef] [PubMed]
- Korman, T.P.; Bowie, J.U. Crystal structure of Proteus mirabilis lipase, a novel lipase from the Proteus/psychrophilic subfamily of lipase family I.1. PLoS ONE 2012, 7, e52890. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.A.; Mannesse, M.L.; de Haas, G.H.; Verheij, H.M.; Dijkstra, B.W. Structural basis of the chiral selectivity of Pseudomonas cepacia lipase. Eur. J. Biochem. 1998, 254, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Dror, A.; Kanteev, M.; Kagan, I.; Gihaz, S.; Shahar, A.; Fishman, A. Structural insights into methanol-stable variants of lipase T6 from Geobacillus stearothermophilus. Appl. Microbiol. Biotechnol. 2015, 99, 9449–9461. [Google Scholar] [CrossRef]
- Perz, V.; Baumschlager, A.; Bleymaier, K.; Zitzenbacher, S.; Hromic, A.; Steinkellner, G.; Pairitsch, A.; Lyskowski, A.; Gruber, K.; Sinkel, C.; et al. Hydrolysis of synthetic polyesters by Clostridium botulinum esterases. Biotechnol. Bioeng. 2016, 113, 1024–1034. [Google Scholar] [CrossRef]
- Nordwald, E.M.; Plaks, J.G.; Snell, J.R.; Sousa, M.C.; Kaar, J.L. Crystallographic investigation of imidazolium ionic liquid effects on enzyme structure. Chembiochem 2015, 16, 2456–2459. [Google Scholar] [CrossRef]
- Zhao, Z.; Hou, S.; Lan, D.; Wang, X.; Liu, J.; Khan, F.I.; Wang, Y. Crystal structure of a lipase from Streptomyces sp. Strain W007—Implications for thermostability and regiospecificity. FEBS J. 2017, 284, 3506–3519. [Google Scholar] [CrossRef]
- Moharana, T.R.; Pal, B.; Rao, N.M. X-ray structure and characterization of a thermostable lipase from Geobacillus thermoleovorans. Biochem. Biophys. Res. Commun. 2019, 508, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Gihaz, S.; Kanteev, M.; Pazy, Y.; Fishman, A. Filling the void: Introducing aromatic interactions into solvent tunnels to enhance lipase stability in methanol. Appl. Microbiol. Biotechnol. 2018, 84, e02143-18. [Google Scholar] [CrossRef] [PubMed]
- Matoba, Y.; Katsube, Y.; Sugiyama, M. The crystal structure of prokaryotic phospholipase A2. J. Biol. Chem. 2002, 277, 20059–20069. [Google Scholar] [CrossRef] [PubMed]
- Matoba, Y.; Sugiyama, M. Atomic resolution structure of prokaryotic phospholipase A2: Analysis of internal motion and implication for a catalytic mechanism. Proteins 2003, 51, 453–469. [Google Scholar] [CrossRef]
- Snijder, H.J.; Ubarretxena-Belandia, I.; Blaauw, M.; Kalk, K.H.; Verheij, H.M.; Egmond, M.R.; Dekker, N.; Dijkstra, B.W. Structural evidence for dimerization-regulated activation of an integral membrane phospholipase. Nature 1999, 401, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Snijder, H.J.; Kingma, R.L.; Kalk, K.H.; Dekker, N.; Egmond, M.R.; Dijkstra, B.W. Structural investigations of calcium binding and its role in activity and activation of outer membrane phospholipase A from Escherichia coli. J. Mol. Biol. 2001, 309, 477–489. [Google Scholar] [CrossRef][Green Version]
- Snijder, H.J.; Van Eerde, J.H.; Kingma, R.L.; Kalk, K.H.; Dekker, N.; Egmond, M.R.; Dijkstra, B.W. Structural investigations of the active-site mutant Asn156Ala of outer membrane phospholipase A: Function of the Asn-his interaction in the catalytic triad. Protein Sci. 2001, 10, 1962–1969. [Google Scholar] [CrossRef]
- Lo, Y.C.; Lin, S.C.; Shaw, J.F.; Liaw, Y.C. Crystal structure of Escherichia coli thioesterase I/protease I/lysophospholipase L1: Consensus sequence blocks constitute the catalytic center of SGNH-hydrolases through a conserved hydrogen bond network. J. Mol. Biol. 2003, 330, 539–551. [Google Scholar] [CrossRef]
- Lo, Y.C.; Lin, S.C.; Shaw, J.F.; Liaw, Y.C. Substrate specificities of Escherichia coli thioesterase I/protease I/lysophospholipase L1 are governed by its switch loop movement. Biochemistry 2005, 44, 1971–1979. [Google Scholar] [CrossRef]
- Grisewood, M.J.; Hernandez Lozada, N.J.; Thoden, J.B.; Gifford, N.P.; Mendez-Perez, D.; Schoenberger, H.A.; Allan, M.F.; Floy, M.E.; Lai, R.Y.; Holden, H.M.; et al. Computational redesign of acyl-ACP thioesterase with improved selectivity toward medium-chain-length fatty acids. ACS Catal. 2017, 7, 3837–3849. [Google Scholar] [CrossRef]
- Montoro-García, S.; Gil-Ortiz, F.; García-Carmona, F.; Polo, L.M.; Rubio, V.; Sánchez-Ferrer, Á. The crystal structure of the cephalosporin deacetylating enzyme acetyl xylan esterase bound to paraoxon explains the low sensitivity of this serine hydrolase to organophosphate inactivation. Biochem. J. 2011, 436, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.; Mayans, O.; Smith, D.; Worboys, K.; Pickersgill, R.W. Three-dimensional structure of Erwinia chrysanthemi pectin methylesterase reveals a novel esterase active site. J. Mol. Biol. 2001, 305, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Fries, M.; Ihrig, J.; Brocklehurst, K.; Shevchik, V.E.; Pickersgill, R.W. Molecular basis of the activity of the phytopathogen pectin methylesterase. EMBO J. 2007, 26, 3879–3887. [Google Scholar] [CrossRef] [PubMed]
- Boraston, A.B.; Abbott, D.W. Structure of a pectin methylesterase from Yersinia enterocolitica. Acta Crystallogr. F Struct Biol. Commun. 2012, 68, 129–133. [Google Scholar] [CrossRef]
- Chen, C.N.; Chin, K.H.; Wang, A.H.; Chou, S.H. The first crystal structure of gluconolactonase important in the glucose secondary metabolic pathways. J. Mol. Biol. 2008, 384, 604–614. [Google Scholar] [CrossRef]
- Matoba, Y.; Tanaka, N.; Noda, M.; Higashikawa, F.; Kumagai, T.; Sugiyama, M. Crystallographic and mutational analyses of tannase from Lactobacillus plantarum. Proteins 2013, 81, 2052–2058. [Google Scholar] [CrossRef]
- Rengachari, S.; Bezerra, G.A.; Riegler-Berket, L.; Gruber, C.C.; Sturm, C.; Taschler, U.; Boeszoermenyi, A.; Dreveny, I.; Zimmermann, R.; Gruber, K.; et al. The structure of monoacylglycerol lipase from Bacillus sp. H257 reveals unexpected conservation of the cap architecture between bacterial and human enzymes. Biochim. Biophys. Acta 2012, 1821, 1012–1021. [Google Scholar] [CrossRef]
- Rengachari, S.; Aschauer, P.; Schittmayer, M.; Mayer, N.; Gruber, K.; Breinbauer, R.; Birner-Gruenberger, R.; Dreveny, I.; Oberer, M. Conformational plasticity and ligand binding of bacterial monoacylglycerol lipase. J. Biol. Chem. 2013, 288, 31093–31104. [Google Scholar] [CrossRef]
- Tsurumura, T.; Tsuge, H. Substrate selectivity of bacterial monoacylglycerol lipase based on crystal structure. J. Struct. Funct. Genom. 2014, 15, 83–89. [Google Scholar] [CrossRef]
- Bains, J.; Kaufman, L.; Farnell, B.; Boulanger, M.J. A product analog bound form of 3-oxoadipate-enol-lactonase (PcaD) reveals a multifunctional role for the divergent cap domain. J. Mol. Biol. 2011, 406, 649–658. [Google Scholar] [CrossRef]
- Schmitt, E.; Mechulam, Y.; Fromant, M.; Plateau, P.; Blanquet, S. Crystal structure at 1.2 Å resolution and active site mapping of Escherichia coli peptidyl-tRNA hydrolase. EMBO J. 1997, 16, 4760–4769. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, M.; Roy, S.; Singh, N.S.; Sangeetha, R.; Varshney, U.; Vijayan, M. Structural plasticity and enzyme action: Crystal structures of Mycobacterium tuberculosis peptidyl-tRNA hydrolase. J. Mol. Biol. 2007, 372, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.E.; Romanov, V.; Lam, R.; Gothe, S.A.; Peddi, S.R.; Razumova, E.B.; Lipman, R.S.; Branstrom, A.A.; Chirgadze, N.Y. Structure of Francisella tularensis peptidyl-tRNA hydrolase. Acta Crystallogr. F Struct. Biol. Commun. 2011, 67, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, M.; Ahmad, R.; Varshney, U.; Vijayan, M. Structures of new crystal forms of Mycobacterium tuberculosis peptidyl-tRNA hydrolase and functionally important plasticity of the molecule. Acta Crystallogr. F Struct. Biol. Commun. 2012, 68, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Baugh, L.; Gallagher, L.A.; Patrapuvich, R.; Clifton, M.C.; Gardberg, A.S.; Edwards, T.E.; Armour, B.; Begley, D.W.; Dieterich, S.H.; Dranow, D.M.; et al. Combining functional and structural genomics to sample the essential Burkholderia structome. PLoS ONE 2013, 8, e53851. [Google Scholar] [CrossRef]
- Ito, K.; Murakami, R.; Mochizuki, M.; Qi, H.; Shimizu, Y.; Miura, K.; Ueda, T.; Uchiumi, T. Structural basis for the substrate recognition and catalysis of peptidyl-tRNA hydrolase. Nucleic Acids Res. 2012, 40, 10521–10531. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumar, A.; Gautam, L.; Sharma, P.; Sinha, M.; Bhushan, A.; Kaur, P.; Sharma, S.; Arora, A.; Singh, T.P. Structural and binding studies of peptidyl-tRNA hydrolase from Pseudomonas aeruginosa provide a platform for the structure-based inhibitor design against peptidyl-tRNA hydrolase. Biochem. J. 2014, 463, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Singh, N.; Yamini, S.; Singh, A.; Sinha, M.; Arora, A.; Kaur, P.; Sharma, S.; Singh, T.P. The mode of inhibitor binding to peptidyl-tRNA hydrolase: Binding studies and structure determination of unbound and bound peptidyl-tRNA hydrolase from Acinetobacter baumannii. PLoS ONE 2013, 8, e67547. [Google Scholar] [CrossRef]
- Hughes, R.C.; McFeeters, H.; Coates, L.; McFeeters, R.L. Recombinant production, crystallization and X-ray crystallographic structure determination of the peptidyl-tRNA hydrolase of Pseudomonas aeruginosa. Acta Crystallogr. F Struct. Biol. Commun. 2012, 68, 1472–1476. [Google Scholar] [CrossRef]
- Vandavasi, V.; Taylor-Creel, K.; McFeeters, R.L.; Coates, L.; McFeeters, H. Recombinant production, crystallization and X-ray crystallographic structure determination of peptidyl-tRNA hydrolase from Salmonella typhimurium. Acta Crystallogr. F Struct. Biol. Commun. 2014, 70, 872–877. [Google Scholar] [CrossRef]
- Singh, A.; Gautam, L.; Sinha, M.; Bhushan, A.; Kaur, P.; Sharma, S.; Singh, T.P. Crystal structure of peptidyl-tRNA hydrolase from a gram-positive bacterium, Streptococcus pyogenes at 2.19 Å resolution shows the closed structure of the substrate-binding cleft. FEBS Open Bio. 2014, 4, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, M.G.; Seetharaman, S.V.; Bulkley, D.; Steitz, T.A. Structural basis for the rescue of stalled ribosomes: Structure of YaeJ bound to the ribosome. Science 2012, 335, 1370–1372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Song, Y.; Niu, L.; Teng, M.; Li, X. Crystal structure of Staphylococcus aureus peptidyl-tRNA hydrolase at a 2.25 Å resolution. Acta Biochim. Biophys. Sin. (Shanghai) 2015, 47, 1005–1010. [Google Scholar] [PubMed]
- Kabra, A.; Shahid, S.; Pal, R.K.; Yadav, R.; Pulavarti, S.V.; Jain, A.; Tripathi, S.; Arora, A. Unraveling the stereochemical and dynamic aspects of the catalytic site of bacterial peptidyl-tRNA hydrolase. RNA 2017, 23, 202–216. [Google Scholar] [CrossRef]
- Kaushik, S.; Iqbal, N.; Singh, N.; Sikarwar, J.S.; Singh, P.K.; Sharma, P.; Kaur, P.; Sharma, S.; Owais, M.; Singh, T.P. Search of multiple hot spots on the surface of peptidyl-tRNA hydrolase: Structural, binding and antibacterial studies. Biochem. J. 2018, 475, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Uehara, Y.; Shimizu, Y.; Ueda, T.; Uchiumi, T.; Ito, K. High-resolution crystal structure of peptidyl-tRNA hydrolase from Thermus thermophilus. Proteins 2019, 87, 226–235. [Google Scholar] [CrossRef]
- Baugh, L.; Phan, I.; Begley, D.W.; Clifton, M.C.; Armour, B.; Dranow, D.M.; Taylor, B.M.; Muruthi, M.M.; Abendroth, J.; Fairman, J.W.; et al. Increasing the structural coverage of tuberculosis drug targets. Tuberculosis (Edinb) 2015, 95, 142–148. [Google Scholar] [CrossRef]
- Fujieda, N.; Schätti, J.; Stuttfeld, E.; Ohkubo, K.; Maier, T.; Fukuzumi, S.; Ward, T.R. Enzyme repurposing of a hydrolase as an emergent peroxidase upon metal binding. Chem. Sci. 2015, 6, 4060–4065. [Google Scholar] [CrossRef]
- Murayama, K.; Kano, K.; Matsumoto, Y.; Sugimori, D. Crystal structure of phospholipase A1 from Streptomyces albidoflavus NA297. J. Struct. Biol. 2013, 182, 192–196. [Google Scholar] [CrossRef]
- Vincent, F.; Charnock, S.J.; Verschueren, K.H.; Turkenburg, J.P.; Scott, D.J.; Offen, W.A.; Roberts, S.; Pell, G.; Gilbert, H.J.; Davies, G.J.; et al. Multifunctional xylooligosaccharide/cephalosporin c deacetylase revealed by the hexameric structure of the Bacillus subtilis enzyme at 1.9 Å resolution. J. Mol. Biol. 2003, 330, 593–606. [Google Scholar] [CrossRef]
- Levisson, M.; Han, G.W.; Deller, M.C.; Xu, Q.; Biely, P.; Hendriks, S.; Ten Eyck, L.F.; Flensburg, C.; Roversi, P.; Miller, M.D.; et al. Functional and structural characterization of a thermostable acetyl esterase from Thermotoga maritima. Proteins 2012, 80, 1545–1559. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Manoj, N. An extended loop in CE7 carbohydrate esterase family is dispensable for oligomerization but required for activity and thermostability. J. Struct. Biol. 2016, 194, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Manoj, N. Structural role of a conserved active site cis proline in the Thermotoga maritima acetyl esterase from the carbohydrate esterase family 7. Proteins 2017, 85, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Manoj, N. Crystal structure of Thermotoga maritima acetyl esterase complex with a substrate analog: Insights into the distinctive substrate specificity in the CE7 carbohydrate esterase family. Biochem. Biophys. Res. Commun. 2016, 476, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Barends, T.R.; Polderman-Tijmes, J.J.; Jekel, P.A.; Hensgens, C.M.; de Vries, E.J.; Janssen, D.B.; Dijkstra, B.W. The sequence and crystal structure of the alpha-amino acid ester hydrolase from Xanthomonas citri define a new family of beta-lactam antibiotic acylases. J. Biol. Chem. 2003, 278, 23076–23084. [Google Scholar] [CrossRef]
- Barends, T.R.; Polderman-Tijmes, J.J.; Jekel, P.A.; Williams, C.; Wybenga, G.; Janssen, D.B.; Dijkstra, B.W. Acetobacter turbidans alpha-amino acid ester hydrolase: How a single mutation improves an antibiotic-producing enzyme. J. Biol. Chem. 2006, 281, 5804–5810. [Google Scholar] [CrossRef]
- Pathak, D.; Ollis, D. Refined structure of dienelactone hydrolase at 1.8 Å. J. Mol. Biol. 1990, 214, 497–525. [Google Scholar] [CrossRef]
- Robinson, A.; Edwards, K.J.; Carr, P.D.; Barton, J.D.; Ewart, G.D.; Ollis, D.L. Structure of the C123S mutant of dienelactone hydrolase (DLH) bound with the PMS moiety of the protease inhibitor phenylmethylsulfonyl fluoride (PMSF). Acta Crystallogr. D Biol. Crystallogr. 2000, 56, 1376–1384. [Google Scholar] [CrossRef]
- Kim, H.K.; Liu, J.W.; Carr, P.D.; Ollis, D.L. Following directed evolution with crystallography: Structural changes observed in changing the substrate specificity of dienelactone hydrolase. Acta Crystallogr. D Biol. Crystallogr. 2005, 61, 920–931. [Google Scholar] [CrossRef]
- Porter, J.L.; Carr, P.D.; Collyer, C.A.; Ollis, D.L. Crystallization of dienelactone hydrolase in two space groups: Structural changes caused by crystal packing. Acta Crystallogr. F Struct. Biol. Commun. 2014, 70, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.L.; Boon, P.L.; Murray, T.P.; Huber, T.B.; Collyer, C.A.; Ollis, D.L. Directed evolution of new and improved enzyme functions using an evolutionary intermediate and multidirectional search. ACS Chem. Biol. 2015, 10, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, M.E.; Malashkevich, V.; Williams, H.J.; Xu, C.; Sauder, J.M.; Burley, S.K.; Almo, S.C.; Raushel, F.M. Structure and catalytic mechanism of LigI: Insight into the amidohydrolase enzymes of cog3618 and lignin degradation. Biochemistry 2012, 51, 3497–3507. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Crane, B.R.; Park, S.Y. An insight into the interaction mode between CheB and chemoreceptor from two crystal structures of CheB methylesterase catalytic domain. Biochem. Biophys. Res. Commun. 2011, 411, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Crane, B.R. Structural insight into the low affinity between Thermotoga maritima CheA and CheB compared to their Escherichia coli/Salmonella typhimurium counterparts. Int. J. Biol. Macromol. 2011, 49, 794–800. [Google Scholar] [CrossRef][Green Version]
- Taylor, E.J.; Gloster, T.M.; Turkenburg, J.P.; Vincent, F.; Brzozowski, A.M.; Dupont, C.; Shareck, F.; Centeno, M.S.; Prates, J.A.; Puchart, V.; et al. Structure and activity of two metal ion-dependent acetylxylan esterases involved in plant cell wall degradation reveals a close similarity to peptidoglycan deacetylases. J. Biol. Chem. 2006, 281, 10968–10975. [Google Scholar] [CrossRef]
- Correia, M.A.; Prates, J.A.; Bras, J.; Fontes, C.M.; Newman, J.A.; Lewis, R.J.; Gilbert, H.J.; Flint, J.E. Crystal structure of a cellulosomal family 3 carbohydrate esterase from Clostridium thermocellum provides insights into the mechanism of substrate recognition. J. Mol. Biol. 2008, 379, 64–72. [Google Scholar] [CrossRef]
- Lansky, S.; Alalouf, O.; Solomon, H.V.; Alhassid, A.; Govada, L.; Chayen, N.E.; Belrhali, H.; Shoham, Y.; Shoham, G. A unique octameric structure of Axe2, an intracellular acetyl-xylooligosaccharide esterase from Geobacillus stearothermophilus. Acta Crystallogr. D Biol. Crystallogr. 2014, 70, 261–278. [Google Scholar] [CrossRef]
- Uraji, M.; Tamura, H.; Mizohata, E.; Arima, J.; Wan, K.; Ogawa, K.; Inoue, T.; Hatanaka, T. Loop of streptomyces feruloyl esterase plays an important role in the enzyme’s catalyzing the release of ferulic acid from biomass. Appl. Environ. Microbiol. 2018, 84, e02300–e02317. [Google Scholar] [CrossRef]
- Roth, C.; Wei, R.; Oeser, T.; Then, J.; Follner, C.; Zimmermann, W.; Strater, N. Structural and functional studies on a thermostable polyethylene terephthalate degrading hydrolase from Thermobifida fusca. Appl. Microbiol. Biotechnol. 2014, 98, 7815–7823. [Google Scholar] [CrossRef]
- Numoto, N.; Kamiya, N.; Bekker, G.J.; Yamagami, Y.; Inaba, S.; Ishii, K.; Uchiyama, S.; Kawai, F.; Ito, N.; Oda, M. Structural dynamics of the PET-degrading cutinase-like enzyme from Saccharomonospora viridis AHK190 in substrate-bound states elucidates the Ca(2+)-driven catalytic cycle. Biochemistry 2018, 57, 5289–5300. [Google Scholar] [CrossRef]
- Jendrossek, D.; Hermawan, S.; Subedi, B.; Papageorgiou, A.C. Biochemical analysis and structure determination of Paucimonas lemoignei poly(3-hydroxybutyrate) (PHB) depolymerase PhaZ7 muteins reveal the PHB binding site and details of substrate-enzyme interactions. Mol. Microbiol. 2013, 90, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Choi, W.C.; Kang, H.O.; Lee, J.S.; Kang, B.S.; Kim, K.J.; Derewenda, Z.S.; Oh, T.K.; Lee, C.H.; Lee, J.K. The molecular structure and catalytic mechanism of a quorum-quenching N-acyl-L-homoserine lactone hydrolase. Proc. Natl. Acad. Sci. USA 2005, 102, 17606–17611. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Momb, J.; Thomas, P.W.; Moulin, A.; Petsko, G.A.; Fast, W.; Ringe, D. Mechanism of the quorum-quenching lactonase (AiiA) from Bacillus thuringiensis. 1. Product-bound structures. Biochemistry 2008, 47, 7706–7714. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Liu, D.; Momb, J.; Thomas, P.W.; Lajoie, A.; Petsko, G.A.; Fast, W.; Ringe, D. A phenylalanine clamp controls substrate specificity in the quorum-quenching metallo-gamma-lactonase from Bacillus thuringiensis. Biochemistry 2013, 52, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Hong, N.; Baier, F.; Jackson, C.J.; Tokuriki, N. Conformational tinkering drives evolution of a promiscuous activity through indirect mutational effects. Biochemistry 2016, 55, 4583–4593. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.A.; Turner, J.M.; Stevens, J.; Rosser, S.J.; Basran, A.; Lerner, R.A.; Bruce, N.C.; Wilson, I.A. Crystal structure of a bacterial cocaine esterase. Nat. Struct. Biol. 2002, 9, 17–21. [Google Scholar] [CrossRef]
- Fang, L.; Chow, K.M.; Hou, S.; Xue, L.; Chen, X.; Rodgers, D.W.; Zheng, F.; Zhan, C.G. Rational design, preparation, and characterization of a therapeutic enzyme mutant with improved stability and function for cocaine detoxification. ACS Chem. Biol. 2014, 9, 1764–1772. [Google Scholar] [CrossRef]
- Agarwal, V.; Lin, S.; Lukk, T.; Nair, S.K.; Cronan, J.E. Structure of the enzyme-acyl carrier protein (ACP) substrate gatekeeper complex required for biotin synthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 17406–17411. [Google Scholar] [CrossRef]
- Jansson, A.; Niemi, J.; Mantsala, P.; Schneider, G. Crystal structure of aclacinomycin methylesterase with bound product analogues: Implications for anthracycline recognition and mechanism. J. Biol. Chem. 2003, 278, 39006–39013. [Google Scholar] [CrossRef]
- Han, X.; Liu, W.; Huang, J.W.; Ma, J.; Zheng, Y.; Ko, T.P.; Xu, L.; Cheng, Y.S.; Chen, C.C.; Guo, R.T. Structural insight into catalytic mechanism of pet hydrolase. Nat. Commun. 2017, 8, 2106. [Google Scholar] [CrossRef]
- Joo, S.; Cho, I.J.; Seo, H.K.; Son, H.F.; Sagong, H.Y.; Shin, T.J.; Choi, S.Y.; Lee, S.Y.; Kim, K.J. Structural insight into molecular mechanism of poly(ethylene terephthalate) degradation. Nat. Commun. 2018, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; He, L.; Wang, L.; Li, T.; Li, C.; Liu, H.; Luo, Y.; Bao, R. Protein crystallography and site-direct mutagenesis analysis of the poly(ethylene terephthalate) hydrolase petase from Ideonella sakaiensis. Chembiochem 2018, 19, 1471–1475. [Google Scholar] [CrossRef] [PubMed]
- Fecker, T.; Galaz-Davison, P.; Engelberger, F.; Narui, Y.; Sotomayor, M.; Parra, L.P.; Ramírez-Sarmiento, C.A. Active site flexibility as a hallmark for efficient PET degradation by I. sakaiensis PETase. Biophys. J. 2018, 114, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shi, C.; Zhu, S.; Wei, R.; Yin, C.C. Structural and functional characterization of polyethylene terephthalate hydrolase from Ideonella sakaiensis. Biochem. Biophys. Res. Commun. 2019, 508, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Palm, G.J.; Reisky, L.; Bottcher, D.; Muller, H.; Michels, E.A.P.; Walczak, M.C.; Berndt, L.; Weiss, M.S.; Bornscheuer, U.T.; Weber, G. Structure of the plastic-degrading Ideonella sakaiensis mhetase bound to a substrate. Nat. Commun. 2019, 10, 1717. [Google Scholar] [CrossRef]
- Ali, Y.B.; Verger, R.; Abousalham, A. Lipases or esterases: Does it really matter? Toward a new bio-physico-chemical classification. In Lipases and Phospholipases: Methods and Protocols; Sandoval, G., Ed.; Humana Press: Totowa, NJ, USA, 2012; Volume 861, pp. 31–51. [Google Scholar]
- Jaeger, K.E.; Ransac, S.; Koch, H.B.; Ferrato, F.; Dijkstra, B.W. Topological characterization and modeling of the 3D structure of lipase from Pseudomonas aeruginosa. FEBS Lett. 1993, 332, 143–149. [Google Scholar] [CrossRef]
- Lesuisse, E.; Schanck, K.; Colson, C. Purification and preliminary characterization of the extracellular lipase of Bacillus subtilis 168, an extremely basic pH-tolerant enzyme. Eur. J. Biochem. 1993, 216, 155–160. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The carbohydrate-active enzymes database (CAZy): An expert resource for glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef]
- Ray, S.K.; Rajeshwari, R.; Sonti, R.V. Mutants of Xanthomonas oryzae pv. Oryzae deficient in general secretory pathway are virulence deficient and unable to secrete xylanase. Mol. Plant Microbe Interact. 2000, 13, 394–401. [Google Scholar] [CrossRef]
- Sugiyama, M.; Ohtani, K.; Izuhara, M.; Koike, T.; Suzuki, K.; Imamura, S.; Misaki, H. A novel prokaryotic phospholipase A2. Characterization, gene cloning, and solution structure. J. Biol. Chem. 2002, 277, 20051–20058. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Grishin, N.V. PROMALS3D: Multiple protein sequence alignment enhanced with evolutionary and three-dimensional structural information. Methods Mol. Biol. 2014, 1079, 263–271. [Google Scholar] [PubMed]
- Pei, J.; Kim, B.H.; Grishin, N.V. PROMALS3D: A tool for multiple protein sequence and structure alignments. Nucleic Acids Res. 2008, 36, 2295–2300. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Tang, M.; Grishin, N.V. PROMALS3D web server for accurate multiple protein sequence and structure alignments. Nucleic Acids Res. 2008, 36, W30–W34. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Rauwerdink, A.; Kazlauskas, R.J. How the same core catalytic machinery catalyzes 17 different reactions: The serine-histidine-aspartate catalytic triad of α/β-hydrolase fold enzymes. ACS Catal. 2015, 5, 6153–6176. [Google Scholar] [CrossRef]
- Dodson, G.; Wlodawer, A. Catalytic triads and their relatives. Trends Biochem. Sci. 1998, 23, 347–352. [Google Scholar] [CrossRef]
- Gariev, I.A.; Varfolomeev, S.D. Hierarchical classification of hydrolases catalytic sites. Bioinformatics 2006, 22, 2574–2576. [Google Scholar] [CrossRef]
- DeLano, W.L. The Pymol Molecular Graphics System; Delano Scientific: San Carlos, CA, USA, 2002. [Google Scholar]
- Walker, I.; Easton, C.J.; Ollis, D.L. Site-directed mutagenesis of dienelactone hydrolase produces dienelactone isomerase. ChemComm 2000, 671–672. [Google Scholar] [CrossRef]
- Borrelli, G.M.; Trono, D. Recombinant lipases and phospholipases and their use as biocatalysts for industrial applications. Int. J. Mol. Sci. 2015, 16, 20774–20840. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new endscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [PubMed]
- Akoh, C.C.; Lee, G.C.; Liaw, Y.C.; Huang, T.H.; Shaw, J.F. GDSL family of serine esterases/lipases. Prog. Lipid Res. 2004, 43, 534–552. [Google Scholar] [CrossRef] [PubMed]
- Goffin, C.; Ghuysen, J.M. Biochemistry and comparative genomics of SXXK superfamily acyltransferases offer a clue to the mycobacterial paradox: Presence of penicillin-susceptible target proteins versus lack of efficiency of penicillin as therapeutic agent. Microbiol. Mol. Biol. Rev. 2002, 66, 702–738. [Google Scholar] [CrossRef] [PubMed]
- Arpigny, J.L.; Jaeger, K.E. Bacterial lipolytic enzymes: Classification and properties. Biochem. J. 1999, 343 Pt 1, 177–183. [Google Scholar] [CrossRef]
- Hausmann, S.; Jaeger, K.-E. Lipolytic enzymes from bacteria. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1099–1126. [Google Scholar]
- Wang, X.; Jiang, F.; Zheng, J.; Chen, L.; Dong, J.; Sun, L.; Zhu, Y.; Liu, B.; Yang, J.; Yang, G.; et al. The outer membrane phospholipase A is essential for membrane integrity and type III secretion in Shigella flexneri. Open Biol. 2016, 6, 160073. [Google Scholar] [CrossRef]
- Istivan, T.S.; Coloe, P.J. Phospholipase A in gram-negative bacteria and its role in pathogenesis. Microbiology 2006, 152, 1263–1274. [Google Scholar] [CrossRef]
- Van Loo, B.; Kingma, J.; Arand, M.; Wubbolts, M.G.; Janssen, D.B. Diversity and biocatalytic potential of epoxide hydrolases identified by genome analysis. Appl. Environ. Microbiol. 2006, 72, 2905–2917. [Google Scholar] [CrossRef]
- Davies, G.; Henrissat, B. Structures and mechanisms of glycosyl hydrolases. Structure 1995, 3, 853–859. [Google Scholar] [CrossRef]
- Burroughs, A.M.; Aravind, L. The origin and evolution of release factors: Implications for translation termination, ribosome rescue, and quality control pathways. Int. J. Mol. Sci. 2019, 20, 1981. [Google Scholar] [CrossRef]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 2009, 50, S237–S242. [Google Scholar] [CrossRef]
- Crichton, R.R. Chapter 12—Zinc—lewis acid and gene regulator. In Biological Inorganic Chemistry, 2nd ed.; Crichton, R.R., Ed.; Elsevier: Oxford, UK, 2012; pp. 229–246. [Google Scholar]
- Dawson, N.L.; Lewis, T.E.; Das, S.; Lees, J.G.; Lee, D.; Ashford, P.; Orengo, C.A.; Sillitoe, I. CATH: An expanded resource to predict protein function through structure and sequence. Nucleic Acids Res. 2016, 45, D289–D295. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Black, D.S.; Reilly, P.J. Carboxylic ester hydrolases: Classification and database derived from their primary, secondary, and tertiary structures. Protein Sci. 2016, 25, 1942–1953. [Google Scholar] [CrossRef] [PubMed]
- Kourist, R.; Jochens, H.; Bartsch, S.; Kuipers, R.; Padhi, S.K.; Gall, M.; Bottcher, D.; Joosten, H.J.; Bornscheuer, U.T. The alpha/beta-hydrolase fold 3DM database (ABHDB) as a tool for protein engineering. Chembiochem 2010, 11, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Heikinheimo, P.; Goldman, A.; Jeffries, C.; Ollis, D.L. Of barn owls and bankers: A lush variety of alpha/beta hydrolases. Structure 1999, 7, R141–R146. [Google Scholar] [CrossRef]
- Korman, T.P.; Sahachartsiri, B.; Charbonneau, D.M.; Huang, G.L.; Beauregard, M.; Bowie, J.U. Dieselzymes: Development of a stable and methanol tolerant lipase for biodiesel production by directed evolution. Biotechnol. Biofuels 2013, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Solomon, K.V.; Haitjema, C.H.; Thompson, D.A.; O’Malley, M.A. Extracting data from the muck: Deriving biological insight from complex microbial communities and non-model organisms with next generation sequencing. Curr. Opin. Biotechnol. 2014, 28, 103–110. [Google Scholar] [CrossRef]
- Mukherjee, S.; Seshadri, R.; Varghese, N.J.; Eloe-Fadrosh, E.A.; Meier-Kolthoff, J.P.; Goker, M.; Coates, R.C.; Hadjithomas, M.; Pavlopoulos, G.A.; Paez-Espino, D.; et al. 1003 reference genomes of bacterial and archaeal isolates expand coverage of the tree of life. Nat. Biotechnol. 2017, 35, 676–683. [Google Scholar] [CrossRef]
- Lewin, H.A.; Robinson, G.E.; Kress, W.J.; Baker, W.J.; Coddington, J.; Crandall, K.A.; Durbin, R.; Edwards, S.V.; Forest, F.; Gilbert, M.T.P.; et al. Earth biogenome project: Sequencing life for the future of life. Proc. Natl. Acad. Sci. USA 2018, 115, 4325–4333. [Google Scholar] [CrossRef]
- Mukherjee, S.; Stamatis, D.; Bertsch, J.; Ovchinnikova, G.; Katta, H.Y.; Mojica, A.; Chen, I.A.; Kyrpides, N.C.; Reddy, T. Genomes Online database (GOLD) v.7: Updates and new features. Nucleic Acids Res. 2019, 47, D649–D659. [Google Scholar] [CrossRef]
- Koutsandreas, T.; Ladoukakis, E.; Pilalis, E.; Zarafeta, D.; Kolisis, F.N.; Skretas, G.; Chatziioannou, A.A. Anastasia: An automated metagenomic analysis pipeline for novel enzyme discovery exploiting next generation sequencing data. Front. Genet. 2019, 10, 469. [Google Scholar] [CrossRef]
- Prosser, G.A.; Larrouy-Maumus, G.; de Carvalho, L.P. Metabolomic strategies for the identification of new enzyme functions and metabolic pathways. EMBO Rep. 2014, 15, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Von Grotthuss, M.; Plewczynski, D.; Vriend, G.; Rychlewski, L. 3D-Fun: Predicting enzyme function from structure. Nucleic Acids Res. 2008, 36, W303–W307. [Google Scholar] [CrossRef] [PubMed]
- Latino, D.A.; Aires-de-Sousa, J. Assignment of EC numbers to enzymatic reactions with MOLMAP reaction descriptors and random forests. J. Chem. Inf. Model. 2009, 49, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.N.; Zhu, H.; Li, X.; Zhang, M.; Deng, Z.; Yang, X.; Deng, Z. Assignment of EC numbers to enzymatic reactions with reaction difference fingerprints. PLoS ONE 2012, 7, e52901. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rahman, S.A.; Cuesta, S.M.; Furnham, N.; Holliday, G.L.; Thornton, J.M. EC-BLAST: A tool to automatically search and compare enzyme reactions. Nat. Methods 2014, 11, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.Y.; Kim, H.U.; Lee, S.Y. Deep learning enables high-quality and high-throughput prediction of enzyme commission numbers. Proc. Natl. Acad. Sci. USA 2019, 116, 13996–14001. [Google Scholar] [CrossRef] [PubMed]
- Gerlt, J.A. Genomic enzymology: Web tools for leveraging protein family sequence-function space and genome context to discover novel functions. Biochemistry 2017, 56, 4293–4308. [Google Scholar] [CrossRef]
- Martinez-Martinez, M.; Coscolin, C.; Santiago, G.; Chow, J.; Stogios, P.J.; Bargiela, R.; Gertler, C.; Navarro-Fernandez, J.; Bollinger, A.; Thies, S.; et al. Determinants and prediction of esterase substrate promiscuity patterns. ACS Chem. Biol. 2018, 13, 225–234. [Google Scholar] [CrossRef]
- Martinez Cuesta, S.; Rahman, S.A.; Furnham, N.; Thornton, J.M. The classification and evolution of enzyme function. Biophys. J. 2015, 109, 1082–1086. [Google Scholar] [CrossRef]
- Wadhams, G.H.; Armitage, J.P. Making sense of it all: Bacterial chemotaxis. Nat. Rev. Mol. Cell Biol. 2004, 5, 1024–1037. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Weng, L.X.; Dong, Y.H.; Zhang, L.H. Specificity and enzyme kinetics of the quorum-quenching N-acyl homoserine lactone lactonase (AHL-lactonase). J. Biol. Chem. 2004, 279, 13645–13651. [Google Scholar] [CrossRef] [PubMed]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Dong, Y.H.; Wang, L.H.; Xu, J.L.; Zhang, H.B.; Zhang, X.F.; Zhang, L.H. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 2001, 411, 813–817. [Google Scholar] [CrossRef]
- Remy, B.; Mion, S.; Plener, L.; Elias, M.; Chabriere, E.; Daude, D. Interference in bacterial quorum sensing: A biopharmaceutical perspective. Front. Pharmacol. 2018, 9, 203. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive lipids and chronic inflammation: Managing the fire within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef]
- Bonventre, J.V. Phospholipase A2 and signal transduction. J. Am. Soc. Nephrol. 1992, 3, 128–150. [Google Scholar]
- Rameshwaram, N.R.; Singh, P.; Ghosh, S.; Mukhopadhyay, S. Lipid metabolism and intracellular bacterial virulence: Key to next-generation therapeutics. Future Microbiol. 2018, 13, 1301–1328. [Google Scholar] [CrossRef]
- Tilg, H.; Zmora, N.; Adolph, T.E.; Elinav, E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2019, 1–15. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Backhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Keiler, K.C.; Feaga, H.A. Resolving nonstop translation complexes is a matter of life or death. J. Bacteriol. 2014, 196, 2123–2130. [Google Scholar] [CrossRef] [PubMed]
- Keiler, K.C. Mechanisms of ribosome rescue in bacteria. Nat. Rev. Microbiol. 2015, 13, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Littlechild, J.A. Enzymes from extreme environments and their industrial applications. Front. Bioeng. Biotechnol. 2015, 3, 161. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Sheldon, R.A. Biocatalysis and green chemistry. In Green Biocatalysis, 1st ed.; Patel, R.N., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 1–15. [Google Scholar]
- Sweeney, M.D.; Xu, F. Biomass converting enzymes as industrial biocatalysts for fuels and chemicals: Recent developments. Catalysts 2012, 2, 244–263. [Google Scholar] [CrossRef]
- Yeoman, C.J.; Han, Y.; Dodd, D.; Schroeder, C.M.; Mackie, R.I.; Cann, I.K.O. Chapter 1—Thermostable enzymes as biocatalysts in the biofuel industry. In Advances in Applied Microbiology; Academic Press: San Diego, CA, USA, 2010; Volume 70, pp. 1–55. [Google Scholar]
- Mukherjee, J.; Gupta, M.N. Biocatalysis for biomass valorization. Sustain. Chem. Process. 2015, 3, 7. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W.H. The role of microbes in coffee fermentation and their impact on coffee quality. J. Food Qual. 2019, 2019, 6. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Hao, G.; Yu, H.; Tian, H.; Zhao, G. Role of lactic acid bacteria on the yogurt flavour: A review. Int. J. Food Prop. 2017, 20, S316–S330. [Google Scholar] [CrossRef]
- Holland, R.; Liu, S.Q.; Crow, V.L.; Delabre, M.L.; Lubbers, M.; Bennett, M.; Norris, G. Esterases of lactic acid bacteria and cheese flavour: Milk fat hydrolysis, alcoholysis and esterification. Int. Dairy J. 2005, 15, 711–718. [Google Scholar] [CrossRef]
- Kawai, F.; Kawabata, T.; Oda, M. Current knowledge on enzymatic PET degradation and its possible application to waste stream management and other fields. Appl. Microbiol. Biotechnol. 2019, 103, 4253–4268. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Huisman, G.W.; Kazlauskas, R.J.; Lutz, S.; Moore, J.C.; Robins, K. Engineering the third wave of biocatalysis. Nature 2012, 485, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.L.; Naebe, M.; Barrow, C.J.; Puri, M. Enzyme immobilisation on amino-functionalised multi-walled carbon nanotubes: Structural and biocatalytic characterisation. PLoS ONE 2013, 8, e73642. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; van Rantwijk, F.; Sheldon, R.A. Cross-linked enzyme aggregates: A simple and effective method for the immobilization of penicillin acylase. Org. Lett. 2000, 2, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Li, G.; Fan, Y.; Yan, Y. Enhanced performance of lipase via microcapsulation and its application in biodiesel preparation. Sci. Rep. 2016, 6, 29670. [Google Scholar] [CrossRef]
- Mingarro, I.; Gonzalez-Navarro, H.; Braco, L. Trapping of different lipase conformers in water-restricted environments. Biochemistry 1996, 35, 9935–9944. [Google Scholar] [CrossRef]
- Singh, R.K.; Tiwari, M.K.; Singh, R.; Lee, J.K. From protein engineering to immobilization: Promising strategies for the upgrade of industrial enzymes. Int. J. Mol. Sci. 2013, 14, 1232–1277. [Google Scholar] [CrossRef]
- Amin, S.R.; Erdin, S.; Ward, R.M.; Lua, R.C.; Lichtarge, O. Prediction and experimental validation of enzyme substrate specificity in protein structures. Proc. Natl. Acad. Sci. USA 2013, 110, E4195–E4202. [Google Scholar] [CrossRef]
- Kingsley, L.J.; Lill, M.A. Substrate tunnels in enzymes: Structure–function relationships and computational methodology. Proteins 2015, 83, 599–611. [Google Scholar] [CrossRef]
- Chen, L.; Vitkup, D. Distribution of orphan metabolic activities. Trends Biotechnol. 2007, 25, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Waller, A.S.; Raes, J.; Zelezniak, A.; Perchat, N.; Perret, A.; Salanoubat, M.; Patil, K.R.; Weissenbach, J.; Bork, P. Prediction and identification of sequences coding for orphan enzymes using genomic and metagenomic neighbours. Mol. Syst. Biol. 2012, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Hadadi, N.; MohammadiPeyhani, H.; Miskovic, L.; Seijo, M.; Hatzimanikatis, V. Enzyme annotation for orphan and novel reactions using knowledge of substrate reactive sites. Proc. Natl. Acad. Sci. USA 2019, 116, 7298–7307. [Google Scholar] [CrossRef] [PubMed]
| EC Number | Enzyme Function | PDB Codes |
|---|---|---|
| EC 3.1.1.- | carboxylic ester hydrolases | 1C7I[19], 1C7J[19], 1ESC[20], 1ESD[20], 1ESE[20], 1QE3[19], 2A7M[21], 2WAA[22], 2WYL[23], 2WYM[23], 3DHA[24], 3DHB[24], 3DHC[24], 3DNM[25], 3F67, 3FAK[26], 3G9T, 3G9U, 3G9Z, 3GA7, 3H17[27], 3H18[27], 3H19, 3H1A, 3H1B, 3H2G[28], 3H2H[28], 3H2I[28], 3H2J[28], 3H2K[28], 3I2F[29], 3I2G[29], 3I2H[29], 3I2I[29], 3I2J[29], 3I2K[29], 3IDA[30], 3K6K, 3L1H, 3L1I, 3L1J, 3LS21[31], 3PF8[32], 3PF9[32], 3PFB[32], 3PFC[32], 3PUH[33], 3PUI[33], 3QM1[32], 3S2Z[32], 4F21[34], 4KRX[35], 4KRY[35], 4OB6[36], 4OB7[36], 4OB8[36], 5A2G[37], 5HC0[38], 5HC2[38], 5HC3[38], 5HC4[38], 5HC5[38], 5MAL2[39], 5UGQ[40], 5UNO[40], 5UOH[40], 6EHN[41], 6GRY[42] |
| EC 3.1.1.1 | carboxylesterase | 1AUO[43], 1AUR[43], 1EVQ[44], 1L7Q[45], 1L7R[45], 1R1D, 1TQH[46], 2H1I, 2HM7[47], 2R11, 3CN7[48], 3CN9[48], 3DOH[49], 3DOI[49], 3KVN[50], 4BZW[51], 4BZZ[51], 4C01[51], 4C87[52], 4C88[52], 4C89[52], 4CCW[53], 4CCY[53], 4FHZ[54], 4FTW[54], 4IVI[55], 4IVK[55], 4JGG[56], 4OU4[36], 4OU5[36], 4ROT, 4UHC[57], 4UHD[57], 4UHE[57], 4UHF[57], 4UHH[57], 4V2I[58], 4YPV[59], 5AO9[60], 5AOA[60], 5AOB[60], 5AOC[60], 5DWD, 5EGN, 5GMX[61], 5H3B[62], 6AAE[63], 6IEY[63] |
| EC 3.1.1.2 | arylesterase | 1VA43[64], 2Q0Q[65], 2Q0S[65], 3HEA3[64,66], 3HI43[66], 3IA23[67], 3T4U3, 3T523, 4ROT, 4TX1[68] |
| EC 3.1.1.3 | triacylglycerol lipase | 1CVL[69], 1EX9[70], 1HQD[71], 1I6W[72], 1ISP4[73], 1JI3[74], 1KU0[75], 1OIL[76], 1QGE, 1R4Z[77], 1R50[77], 1T2N[78], 1T4M[78], 1TAH[78], 1YS1[79], 1YS2[79], 2DSN[80], 2ES4[81], 2FX5, 2HIH[82], 2LIP[83], 2NW6[84], 2ORY[85], 2QUA[86], 2QUB[86], 2QXT[87], 2QXU[87], 2W22[88], 2Z5G[80], 2Z8X[89], 2Z8Z[89], 2ZJ6[90], 2ZJ7[90], 2ZVD[91], 3A6Z[91], 3A70[91], 3AUK, 3D2A[92], 3D2B[92], 3D2C[92], 3LIP[83], 3QMM[93], 3QZU[94], 3UMJ[95], 3W9U, 4FDM[96], 4FKB, 4FMP, 4GW3[97], 4GXN[97], 4HS9[97], 4LIP[98], 4OPM, 4X6U[99], 4X71[99], 4X7B[99], 4X85[99], 5AH1[100], 5CE5[100], 5CRI[101], 5CT4[101], 5CT6[101], 5CT9[101], 5CTA[101], 5CUR[101], 5H6B[102], 5LIP[98], 5MAL2[39], 5XPX, 6A12[103], 6CL4[103], 6FZ1[104], 6FZ7[104], 6FZ8[104], 6FZ9[104], 6FZA[104], 6FZC[104], 6FZD[104] |
| EC 3.1.1.4 | phospholipase A2 | 1FAZ[105], 1KP4[105], 1LWB[106], 1QD5[107], 1QD6[107], 1FW2[108], 1FW3[108], 1ILD[109], 1ILZ[109], 1IM0[109], 5DQX |
| EC 3.1.1.5 | lysophospholipase | 1IVN5[110], 1J005[110], 1JRL5[110], 1U8U5[111], 1V2G5[111], 5TIC5[112], 5TID5[112], 5TIE5[112], 5TIF5[112] |
| EC 3.1.1.6 | acetylesterase | 2XLB[113], 2XLC[113], 3FVR, 3FVT, 3FYT, 3FYU, 4NS4 |
| EC 3.1.1.11 | pectinesterase | 1QJV[114], 2NSP[115], 2NST[115], 2NT6[115], 2NT9[115], 2NTB[115], 2NTP[115], 2NTQ[115], 3UW0[116] |
| EC 3.1.1.17 | gluconolactonase | 3DR2[117] |
| EC 3.1.1.20 | tannase | 3WA6[118], 3WA7[118] |
| EC 3.1.1.23 | acylglycerol lipase | 3RLI[119], 3RM3[119], 4KE6[120], 4KE7[120], 4KE8[120], 4KE9[120], 4KEA[120], 4LHE[121], 5XKS |
| EC 3.1.1.24 | 3-oxoadipate enol-lactonase | 2XUA[122] |
| EC 3.1.1.25 | 1,4-lactonase | 3MSR, 3OVG |
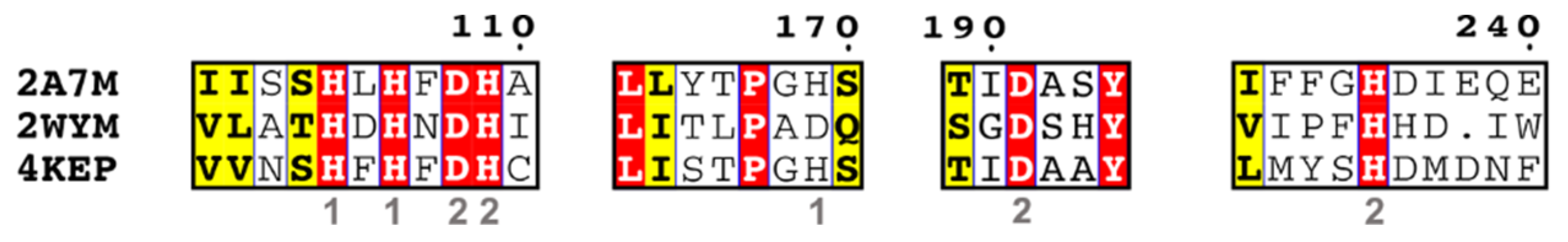
| EC 3.1.1.27 | 4-pyridoxolactonase | 3AJ3, 4KEP, 4KEQ |
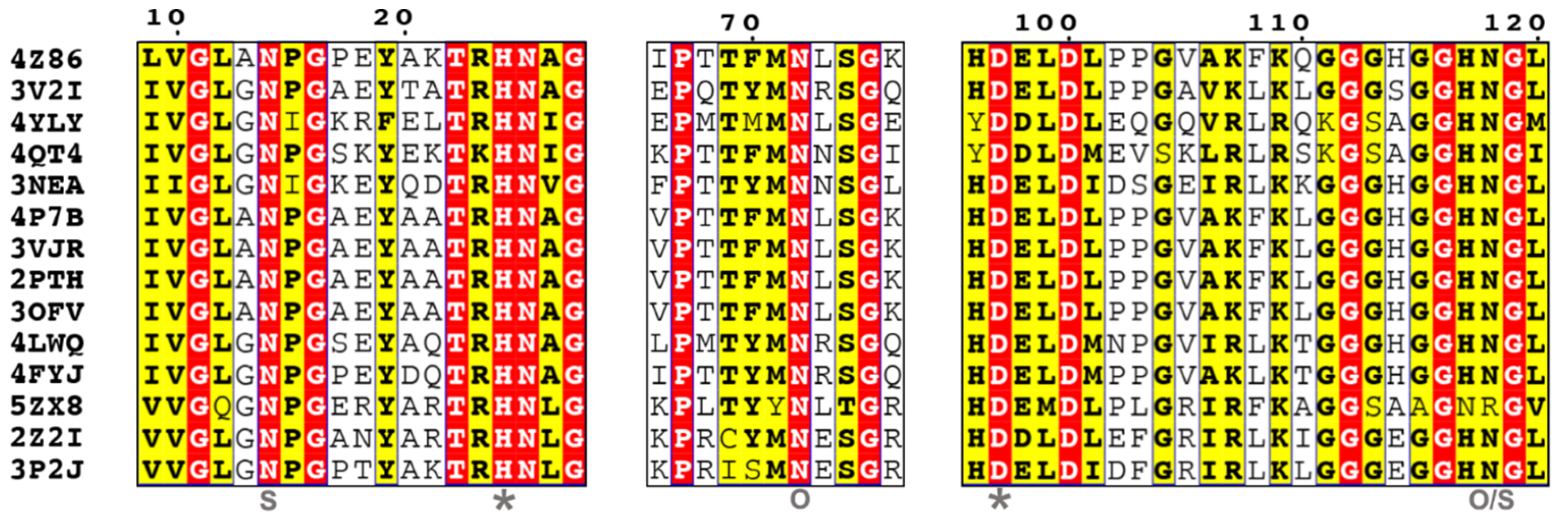
| EC 3.1.1.29 | aminoacyl-tRNA hydrolase | 2PTH[123], 2Z2I[124], 2Z2J[124], 2Z2K[124], 3KJZ, 3KK0, 3NEA[125], 3OFV, 3P2J, 3TCK[126], 3TCN[126], 3TD2[126], 3TD6[126], 3V2I[127], 3VJR[128], 4DHW, 4DJJ, 4ERX, 4FNO[129], 4FOP[130], 4FOT[130], 4FYJ[131], 4HOY[130], 4IKO[130], 4JC4[129], 4JWK[130], 4JX9[130], 4JY7[130], 4LIP[98], 4LWQ, 4OLJ, 4P7B[132], 4QAJ[129], 4QBK[129], 4QD3[129], 4QT4[133], 4V95[134], 4YLY[135], 4Z86[136], 4ZXP[136], 5B6J[136], 5EKT, 5GVZ, 5IKE[136], 5IMB[136], 5IVP, 5Y98[137], 5Y9A[137], 5YL8, 5YLA, 5YN4, 5ZK0, 5ZX8[138], 5ZZV, 6A31, 6IVV, 6IX6, 6IYE, 6J93, 6JGU, 6JJ1, 6JJQ, 6JKX, 6JQT |
| EC 3.1.1.31 | 6-phosphoglucono-lactonase | 1PBT, 1VL1, 3ICO[139], 3LWD, 3OC6[139], 4TM7[140], 4TM8[140], 6NAU |
| EC 3.1.1.32 | phospholipase A1 | 1QD5[107], 1QD6[107], 1FW2[108], 1FW3[108], 1ILD[109], 1ILZ[109], 1IM0[109], 4HYQ[141], 5DQX |
| EC 3.1.1.41 | cephalosporin-C deacetylase | 1L7A, 1ODS[142], 1ODT[142], 1VLQ[143], 3FCY, 3M81[143], 3M82[143], 3M83[143], 5FDF[144], 5GMA[145], 5HFN[144], 5JIB[146] |
| EC 3.1.1.43 | alpha-amino-acid esterase | 1MPX[147], 1NX9[148], 1RYY[148], 2B4K[148], 2B9V[148] |
| EC 3.1.1.45 | carboxymethylene-butenolidase | 1DIN[149], 1GGV[150], 1ZI6[151], 1ZI8[151], 1ZI9[151], 1ZIC[151], 1ZIX[151], 1ZIY[151], 1ZJ4[151], 1ZJ5[151], 4P92[152], 4P93[152], 4U2B[153], 4U2C[153], 4U2D[153], 4U2E[153], 4U2F[153], 4U2G[153] |
| EC 3.1.1.57 | 2-pyrone-4,6-dicarboxylate lactonase | 4D8L[154] |
| EC 3.1.1.61 | protein-glutamate methylesterase | 1CHD[13], 3SFT[155], 3T8Y[156] |
| EC 3.1.1.72 | acetylxylan esterase | 2CC0[157], 2VPT6[158], 2WAA[22], 2XLB[113], 2XLC[113], 3FCY, 3W7V[159], 4JHL[159], 4JJ4, 4JJ6, 4JKO[159], 4OAO, 4OAP, 5BN1, 5FDF[144], 5GMA[145], 5HFN[144], 5JIB[146] |
| EC 3.1.1.73 | feruloyl esterase | 5YAE[160], 5YAL[160] |
| EC 3.1.1.74 | cutinase | 4CG1[161], 4CG2[161], 4CG3[161], 4EB0, 5ZOA, 5ZNO[162] |
| EC 3.1.1.75 | poly(3-hydroxybutyrate) depolymerase | 4BRS[163], 4BTV[163], 4BVJ[163], 4BVK[163], 4BVL[163], 4BYM[163] |
| EC 3.1.1.81 | quorum-quenching N-acyl-homoserine lactonase | 2A7M[21], 2BR6[164], 2BTN[164], 3DHA[165], 3DHB[165], 3DHC[165], 4J5F[166], 4J5H[166], 5EH9[167], 5EHT[167] |
| EC 3.1.1.84 | cocaine esterase | 1JU3[168], 1JU4[168], 1L7Q[45], 1L7R[45], 3IDA[30], 4P08[169] |
| EC 3.1.1.85 | pimeloyl-[acyl-carrier protein] methyl ester esterase | 4ETW[170], 4NMW |
| EC 3.1.1.95 | aclacinomycin methylesterase | 1Q0R[171], 1Q0Z[171] |
| EC 3.1.1.101 | poly(ethylene terephthalate) hydrolase | 5XFY[172], 5XFZ[172], 5XG0[172], 5XH2[172], 5XH3[172], 5XJH[173], 5YFE[174], 5YNS[173], 6ANE[175], 6EQD[174], 6EQE[174], 6EQF[174], 6EQG[174], 6EQH[174], 6ILW[176], 6ILX[176], 6QGC[177] |
| EC 3.1.1.102 | mono(ethylene terephthalate) hydrolase | 6QG9[177], 6QGA[177], 6QGB[177] |
| Clan Types | PDB Codes |
|---|---|
| Clan A | 1C7I[19], 1EVQ[44], 1L7A, 1Q0R[171], 2R11, 2XLB[113], 2XUA[122], 3FAK[26], 3FCY, 3GA7, 3K6K, 4CCW[53], 4CCY[53], 4CG1[161], 4EB0, 4KRX[35], 4OB7[36] |
| Clan B | 1CVL[69], 1ESC[20], 1EX9[70], 1JI3[74], 1KU0[75], 1QGE, 1YS1[79], 2DSN[80], 2HIH[82], 2W22[88], 3AUK, 4FDM[96], 4FKB, 4HS9[211], 4HYQ[141], 4X6U[99] |
| Clan C | 1AUO[43], 1TQH[46], 1ZI8 [151], 3CN9[48], 3F67, 3IA2[67], 3RLI[119], 4ETW[170], 4F21[34], 4FHZ[54], 4NMW, 4U2D[153], 4UHC[57] |
| Clan D | 3DR2[117] |
| Clan E | N/A |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, C.; Kim, T.D.; Kim, K.K. Carboxylic Ester Hydrolases in Bacteria: Active Site, Structure, Function and Application. Crystals 2019, 9, 597. https://doi.org/10.3390/cryst9110597
Oh C, Kim TD, Kim KK. Carboxylic Ester Hydrolases in Bacteria: Active Site, Structure, Function and Application. Crystals. 2019; 9(11):597. https://doi.org/10.3390/cryst9110597
Chicago/Turabian StyleOh, Changsuk, T. Doohun Kim, and Kyeong Kyu Kim. 2019. "Carboxylic Ester Hydrolases in Bacteria: Active Site, Structure, Function and Application" Crystals 9, no. 11: 597. https://doi.org/10.3390/cryst9110597
APA StyleOh, C., Kim, T. D., & Kim, K. K. (2019). Carboxylic Ester Hydrolases in Bacteria: Active Site, Structure, Function and Application. Crystals, 9(11), 597. https://doi.org/10.3390/cryst9110597







