Naked Metal Cations Swimming in Protein Crystals
Abstract
1. Introduction
2. Materials and Methods
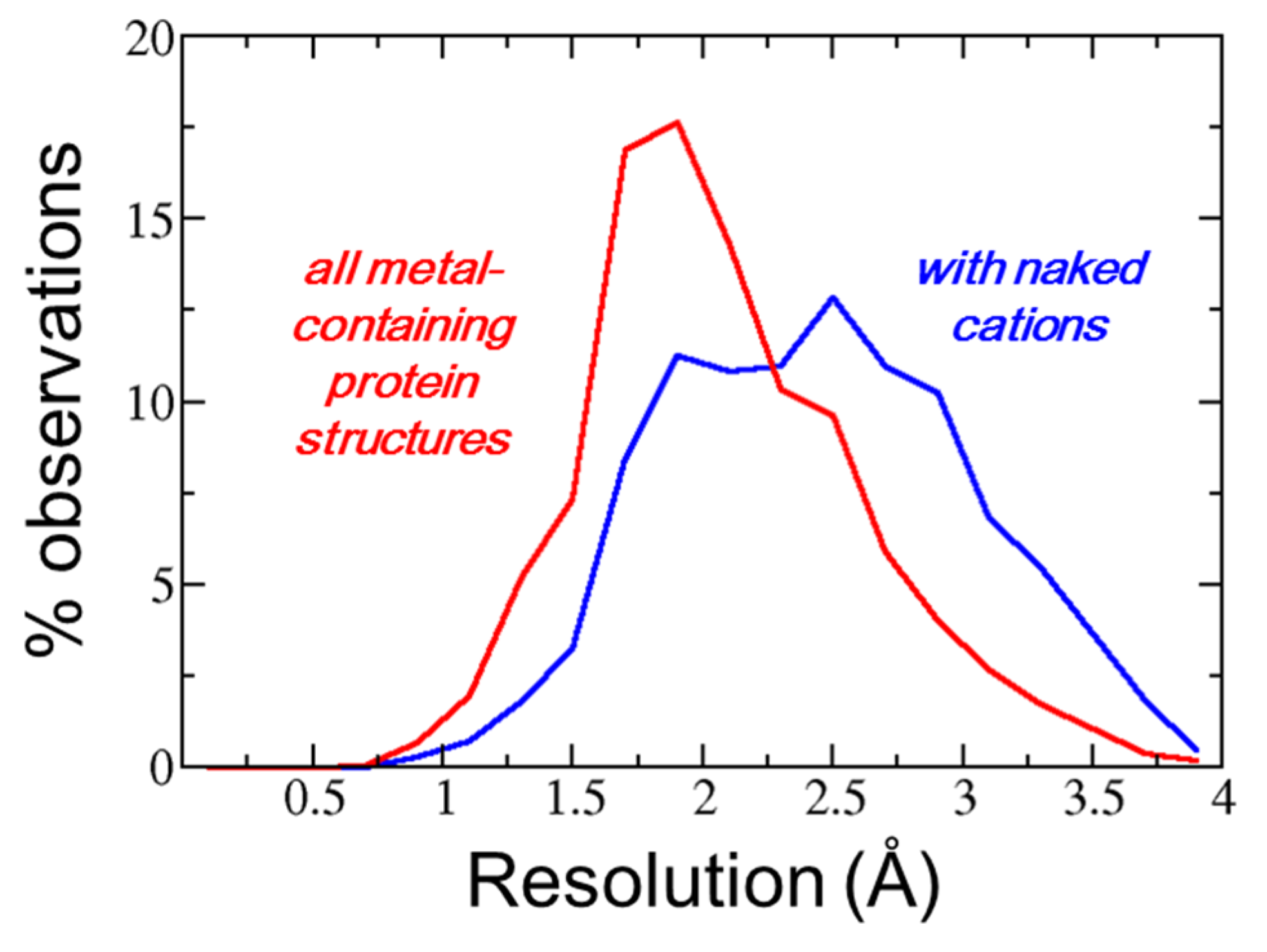
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Djinovic-Carugo, K.; Carugo, O. Structural biology of the lanthanides-mining rare earths in the Protein Data Bank. J. Inorg. Biochem. 2015, 143, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Carugo, O. Silver and gold in the Protein Data Bank. J. Inorg. Biochem. 2017, 175, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Carugo, O. Structural features of uranium-protein complexes. J. Inorg. Biochem. 2018, 189, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, F.C.; Koetzle, T.F.; Williams, G.J.B.; Meyer, E.F.J.; Brice, M.D.; Rodgers, J.R.; Kennard, O.; Shimanouchi, T.; Tasumi, M.J. The Protein Data Bank: A computer-based archival file for macromolecular structures. Mol. Biol. 1977, 112, 535–542. [Google Scholar] [CrossRef]
- Carugo, O.; Djinović-Carugo, K. How many packing contacts are observed in protein crystals? J. Struct. Biol. 2012, 180, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Carugo, O.; Djinovic-Carugo, K. Packing bridges in protein crystal structures. J. Appl. Cryst. 2014, 47, 458–461. [Google Scholar] [CrossRef]
- Gagné, O.C.; Hawthorne, F.C. Comprehensive derivation of bond-valence parameters for ion pairs involving oxygen. Acta Cryst. 2016, B72, 601–625. [Google Scholar] [CrossRef] [PubMed]
- Joosten, R.P.; Long, F.; Murshudov, G.N.; Perrakis, A. The PDB_REDO server for macromolecular structure model optimization. IUCrJ 2014, 1, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Dieckmann, C.; Panjikar, S.; Schmidt, A.; Mueller, S.; Kuper, J.; Geerlof, A.; Wilmanns, M.; Singh, R.K.; Tucker, P.A.; Weiss, M.S. On the routine use of soft X-rays in macromolecular crystallography. Part IV. Efficient determination of anomalous substructures in biomacromolecules using longer X-ray wavelengths. Acta Crystallogr. 2007, D63, 366–380. [Google Scholar] [CrossRef] [PubMed]
- van Heuveln, F.; Meijering, H.; Wieling, J. Inductively coupled plasma-MS in drug development: Bioanalytical aspects and applications. Bioanalysis 2012, 4, 1933–1965. [Google Scholar] [CrossRef] [PubMed]
- Arcovito, A.; della Longa, S. Local structure and dynamics of hemeproteins by X-ray absorption near edge structure spectroscopy. J. Inorg. Biochem. 2012, 112, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. X-Ray Absorption Spectroscopy of Metalloproteins. Methods Mol. Biol. 2019, 1876, 179–195. [Google Scholar] [PubMed]
- Hummer, A.A.; Rompel, A. The use of X-ray absorption and synchrotron based micro-X-ray fluorescence spectroscopy to investigate anti-cancer metal compounds in vivo and in vitro. Metallomics 2013, 5, 597–614. [Google Scholar] [CrossRef] [PubMed]
- Read, R.J.; Adams, P.D.; Arendall, W.B.r.; Brunger, A.T.; Emsley, P.; Joosten, R.P.; Kleywegt, G.J.; Krissinel, E.B.; Lütteke, T.; Otwinowski, Z.; et al. A new generation of crystallographic validation tools for the protein data bank. Structure 2011, 19, 1395–1412. [Google Scholar] [CrossRef] [PubMed]
- Terwilliger, T.C.; Bricogne, G. Continuous mutual improvement of macromolecular structure models in the PDB and of X-ray crystallographic software: The dual role of deposited experimental data. Acta Cryst. 2014, D70, 2533–2543. [Google Scholar] [CrossRef] [PubMed]
- Domagalski, M.J.; Zheng, H.; Zimmerman, M.D.; Dauter, Z.; Wlodawer, A.; Minor, W. The quality and validation of structures from structural genomics. Methods Mol. Biol. 2014, 2091, 297–314. [Google Scholar]
- Zheng, H.; Chordia, M.D.; Cooper, D.R.; Chruszcz, M.; Müller, P.; Sheldrick, G.M.; Minor, W. Validation of metal-binding sites in macromolecular structures with the CheckMyMetal web server. Nat. Protoc. 2014, 9, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Cooper, D.R.; Porebski, P.J.; Shabalin, I.G.; Handing, K.B.; Minor, W. CheckMyMetal: A macromolecular metal-binding validation tool. Acta Cryst. 2017, D73, 223–233. [Google Scholar] [CrossRef] [PubMed]
| Cation | Total Number | Naked | % Naked |
|---|---|---|---|
| Mg | 61,887 (60,495) | 10,434 (10,252) | 17 (17) |
| Fe | 36,809 (35,486) | 63 (46) | 0 (0) |
| Zn | 36,327 (34,102) | 290 (236) | 1 (1) |
| Ca | 31,968 (30,986) | 1061 (1013) | 3 (3) |
| Na | 23,342 (22,810) | 1826 (1786) | 8 (8) |
| Mn | 11,352 (10,773) | 959 (932) | 8 (9) |
| K | 7312 (6607) | 1368 (1309) | 19 (20) |
| Cu | 6147 (5279) | 20 (13) | 0 (0) |
| Cd | 4706 (3665) | 138 (108) | 3 (3) |
| Ni | 3583 (3271) | 130 (108) | 4 (3) |
| Co | 2786 (2515) | 125 (100) | 4 (4) |
| Hg | 1840 (1122) | 118 (68) | 6 (6) |
| Sr | 1428 (1373) | 822 (815) | 58 (59) |
| Mo | 955 (749) | 57 (24) | 6 (3) |
| W | 702 (532) | 89 (50) | 13 (9) |
| Pt | 614 (300) | 81 (42) | 13 (14) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djinović-Carugo, K.; Carugo, O. Naked Metal Cations Swimming in Protein Crystals. Crystals 2019, 9, 581. https://doi.org/10.3390/cryst9110581
Djinović-Carugo K, Carugo O. Naked Metal Cations Swimming in Protein Crystals. Crystals. 2019; 9(11):581. https://doi.org/10.3390/cryst9110581
Chicago/Turabian StyleDjinović-Carugo, Kristina, and Oliviero Carugo. 2019. "Naked Metal Cations Swimming in Protein Crystals" Crystals 9, no. 11: 581. https://doi.org/10.3390/cryst9110581
APA StyleDjinović-Carugo, K., & Carugo, O. (2019). Naked Metal Cations Swimming in Protein Crystals. Crystals, 9(11), 581. https://doi.org/10.3390/cryst9110581






