From Molecules to Carbon Materials—High Pressure Induced Polymerization and Bonding Mechanisms of Unsaturated Compounds
Abstract
1. Introduction
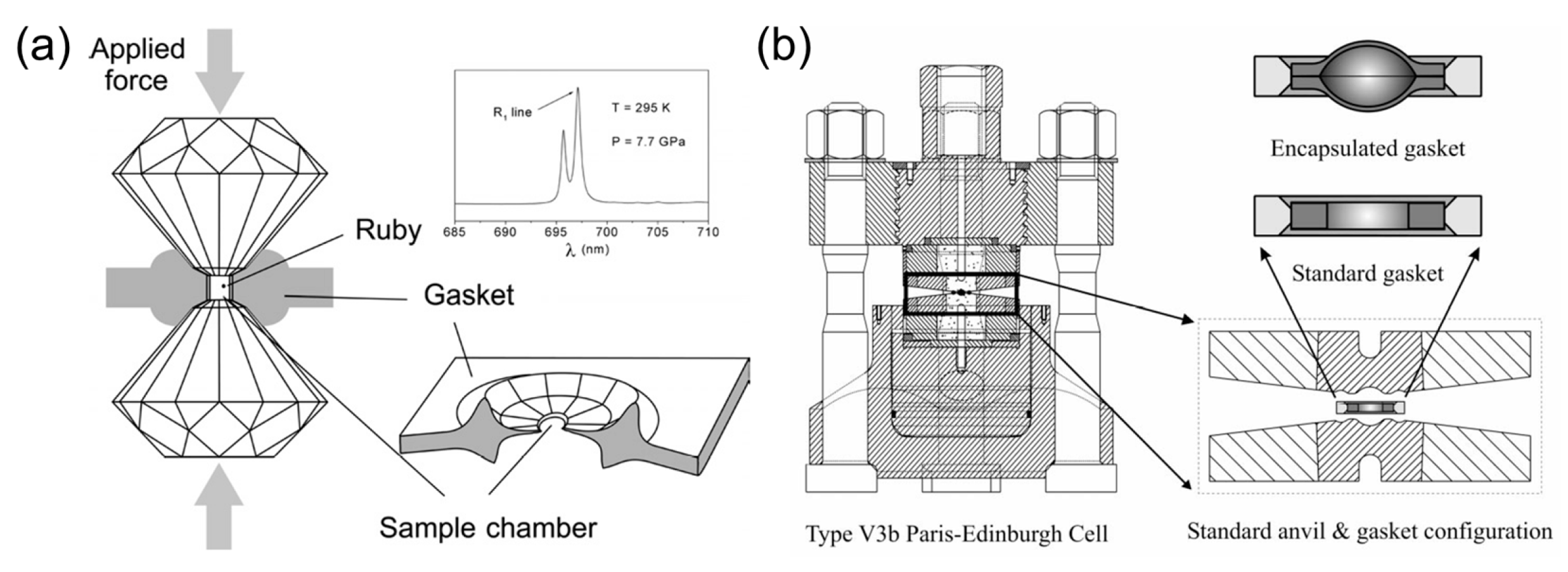
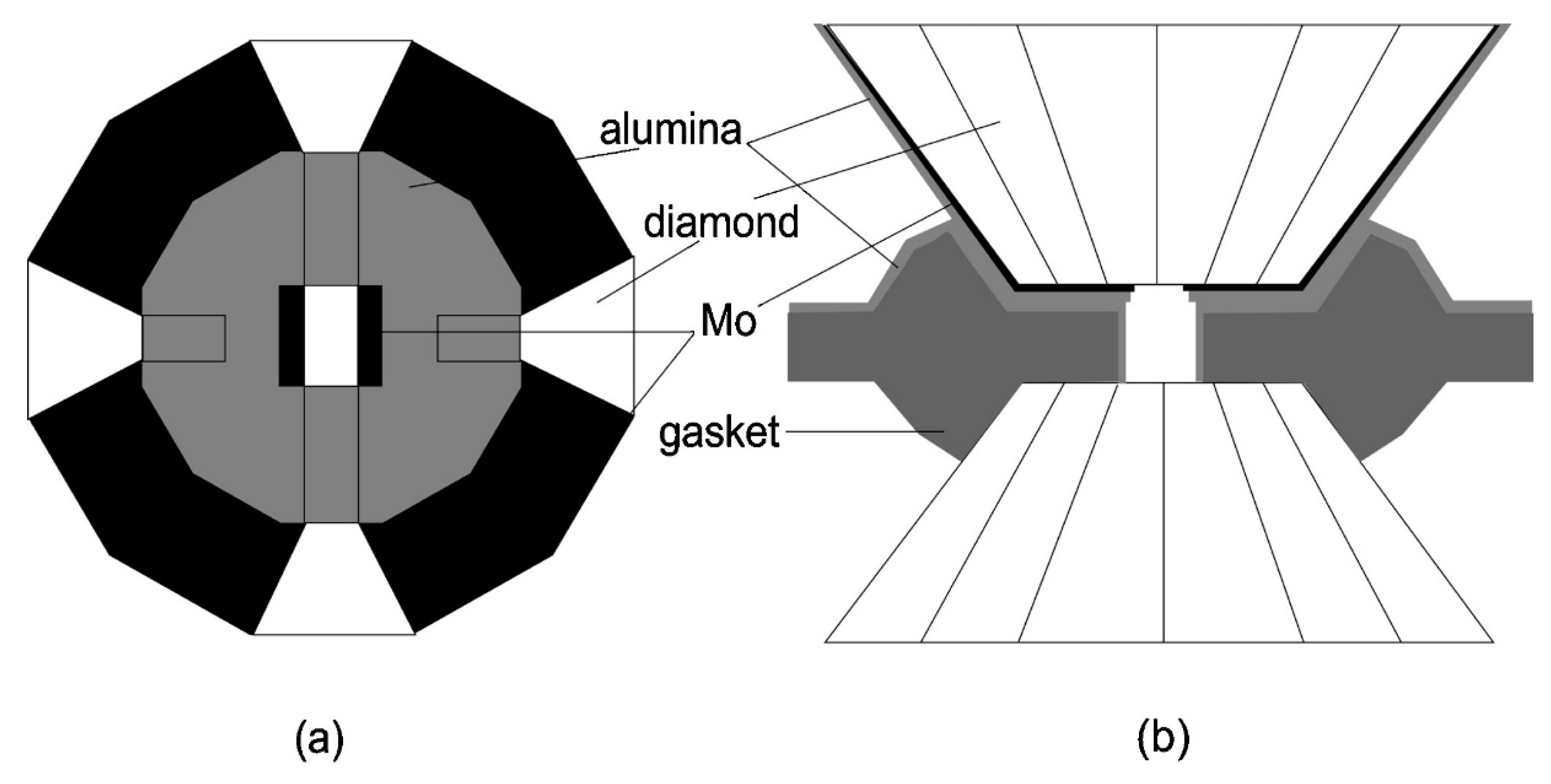
2. Generation of High Pressure and High Temperature
3. In Situ Characterization Under High Pressure
4. The Ionic Compounds with Unsaturated Bonds
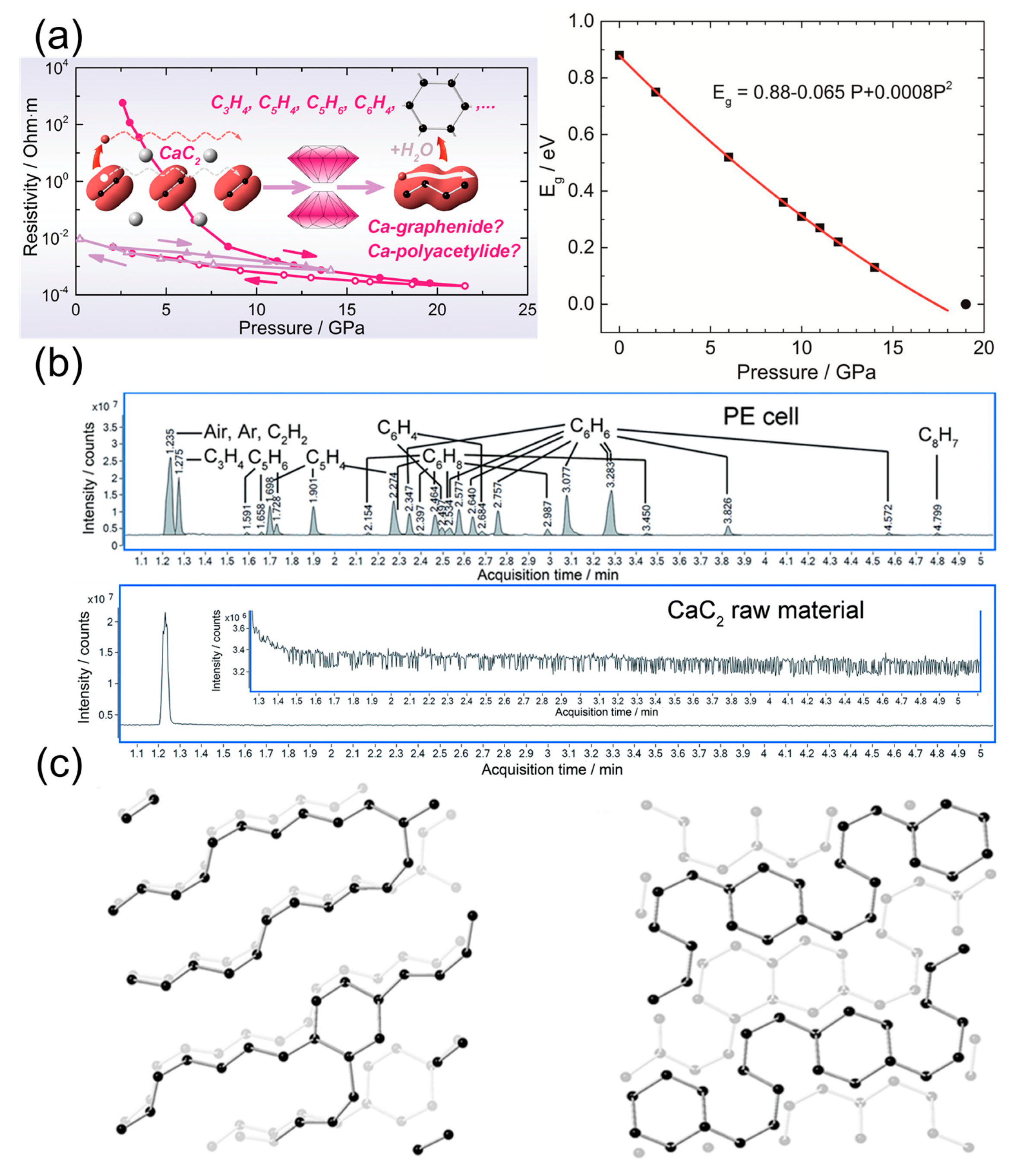
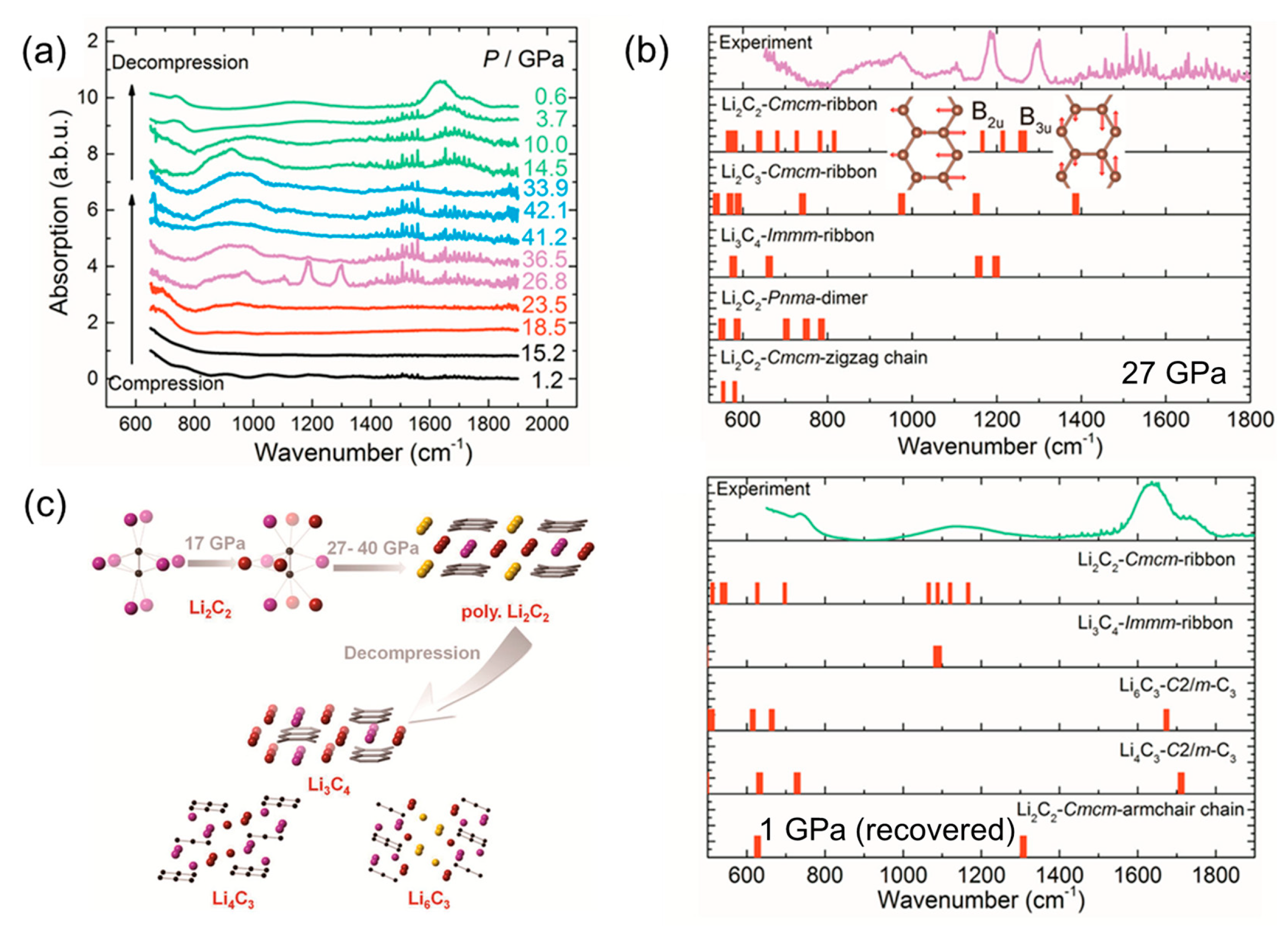
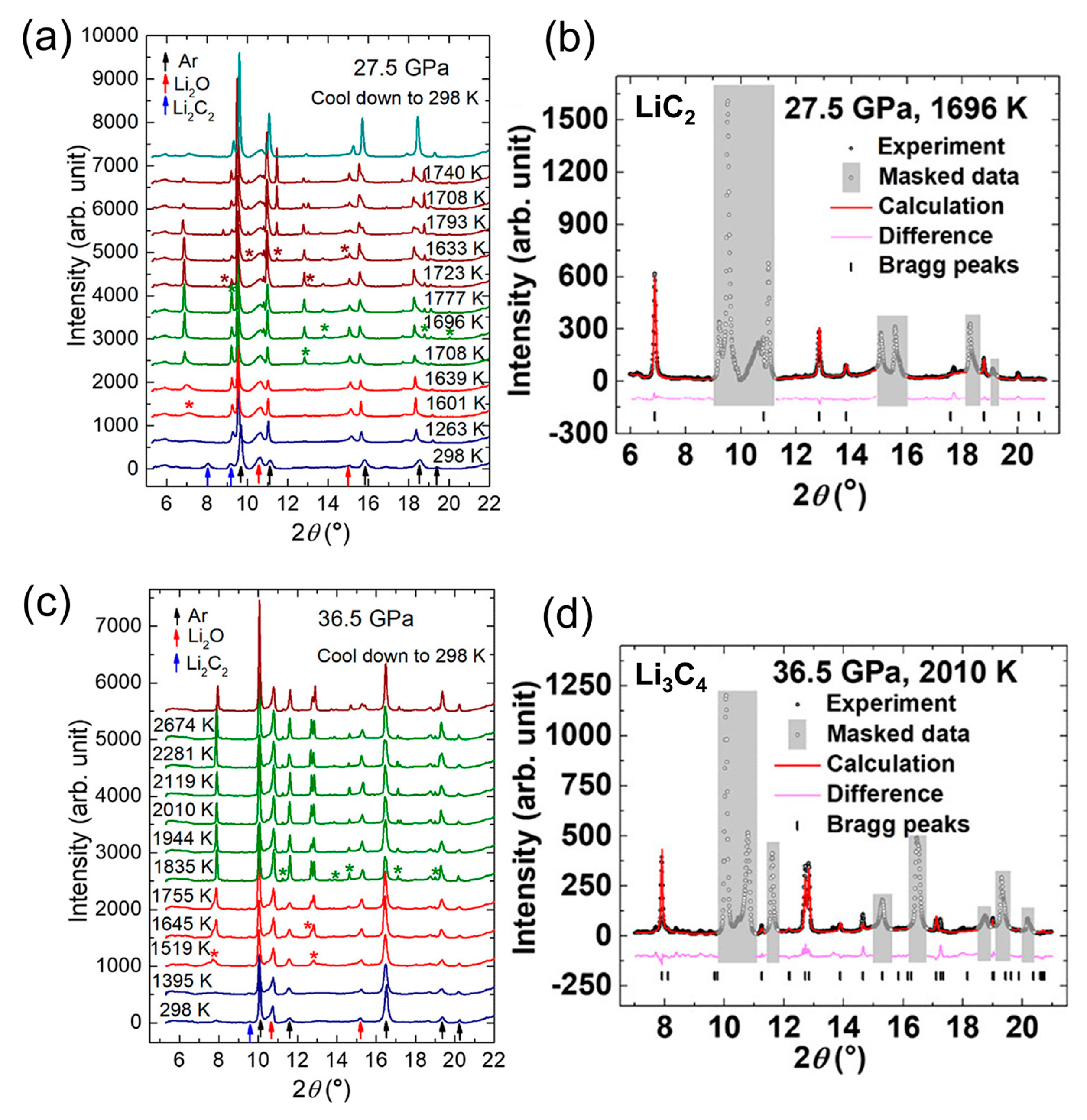
4.1. Metallic Carbide
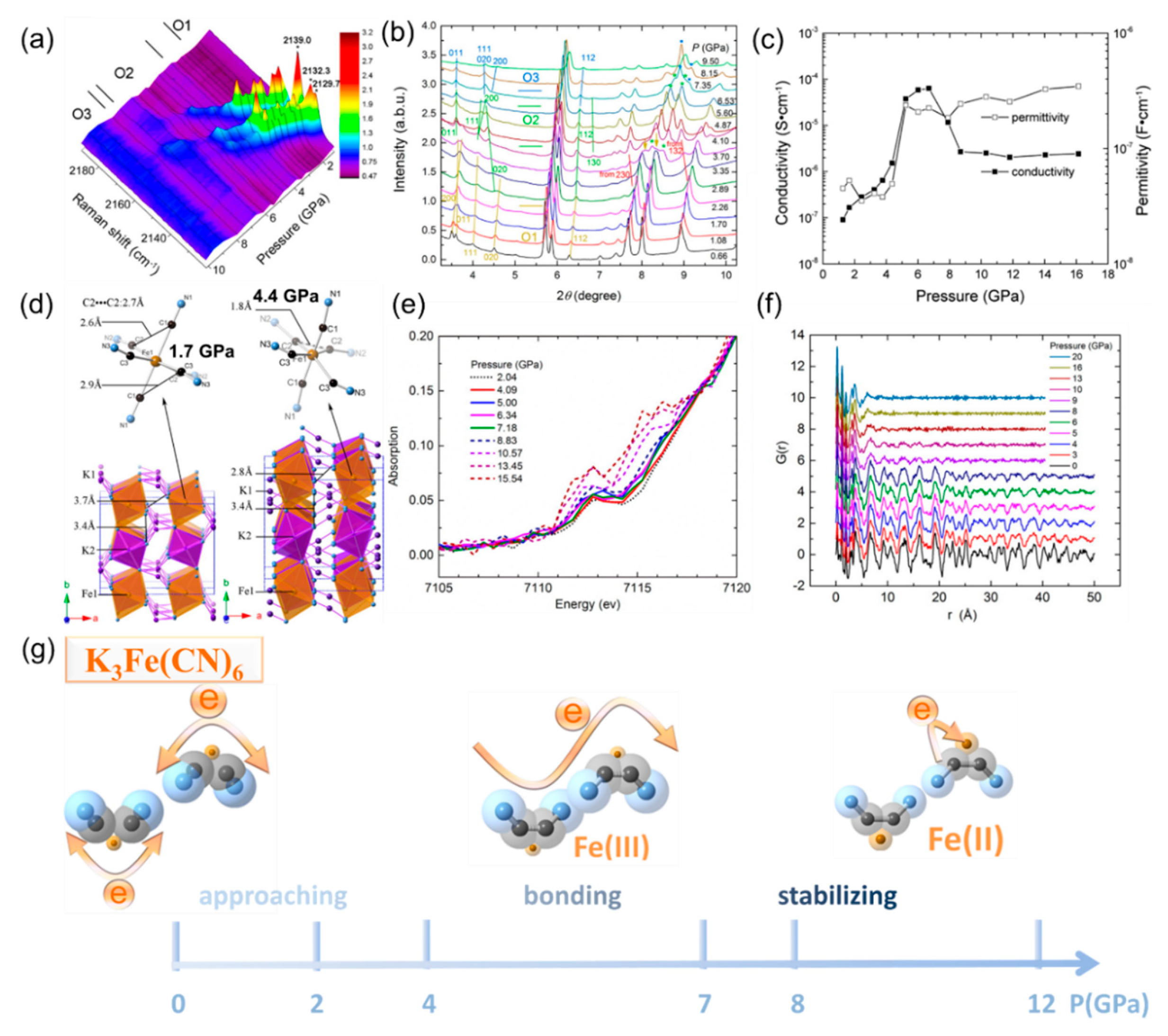
4.2. Cyanide
5. Unsaturated Organic Compounds
5.1. Alkene
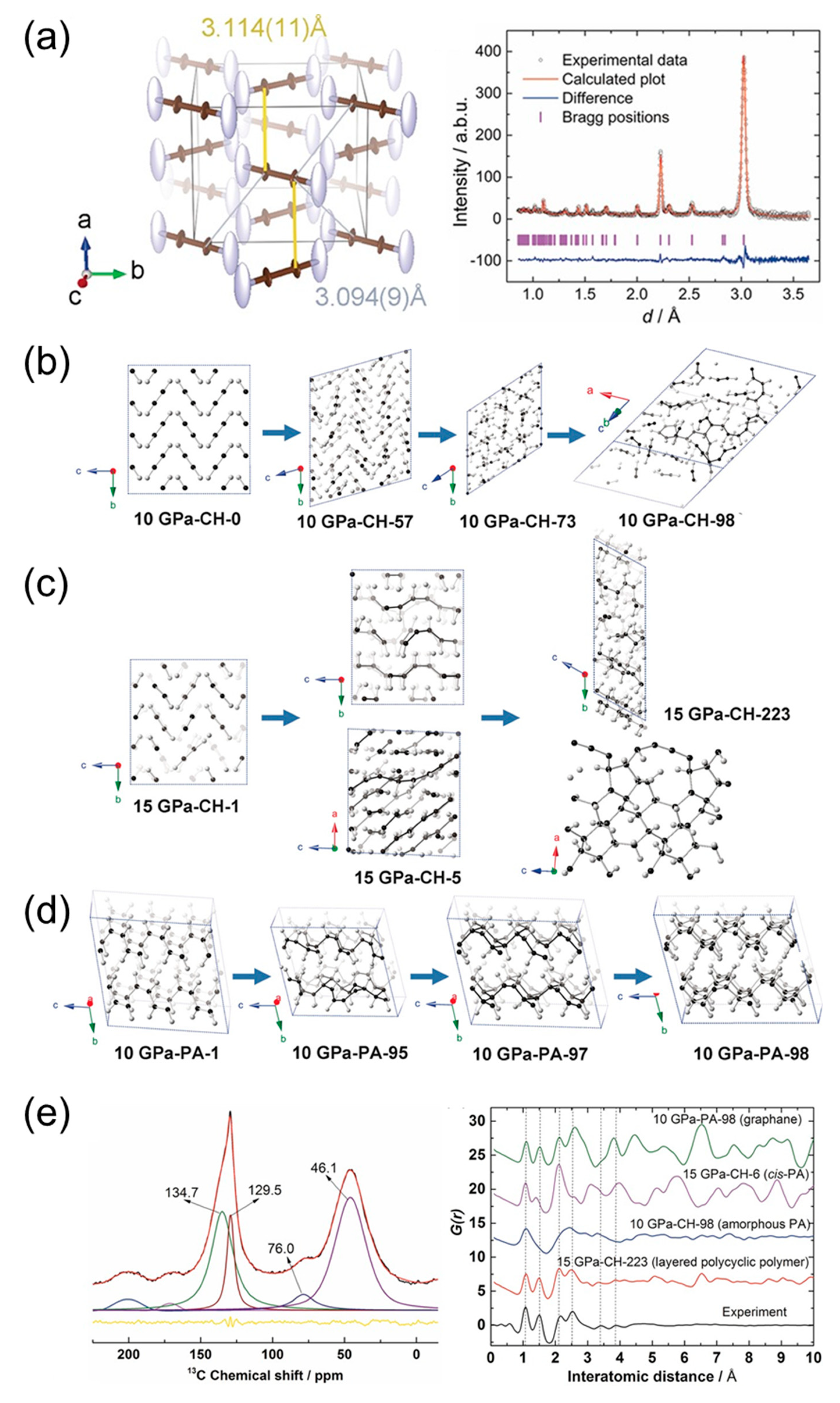
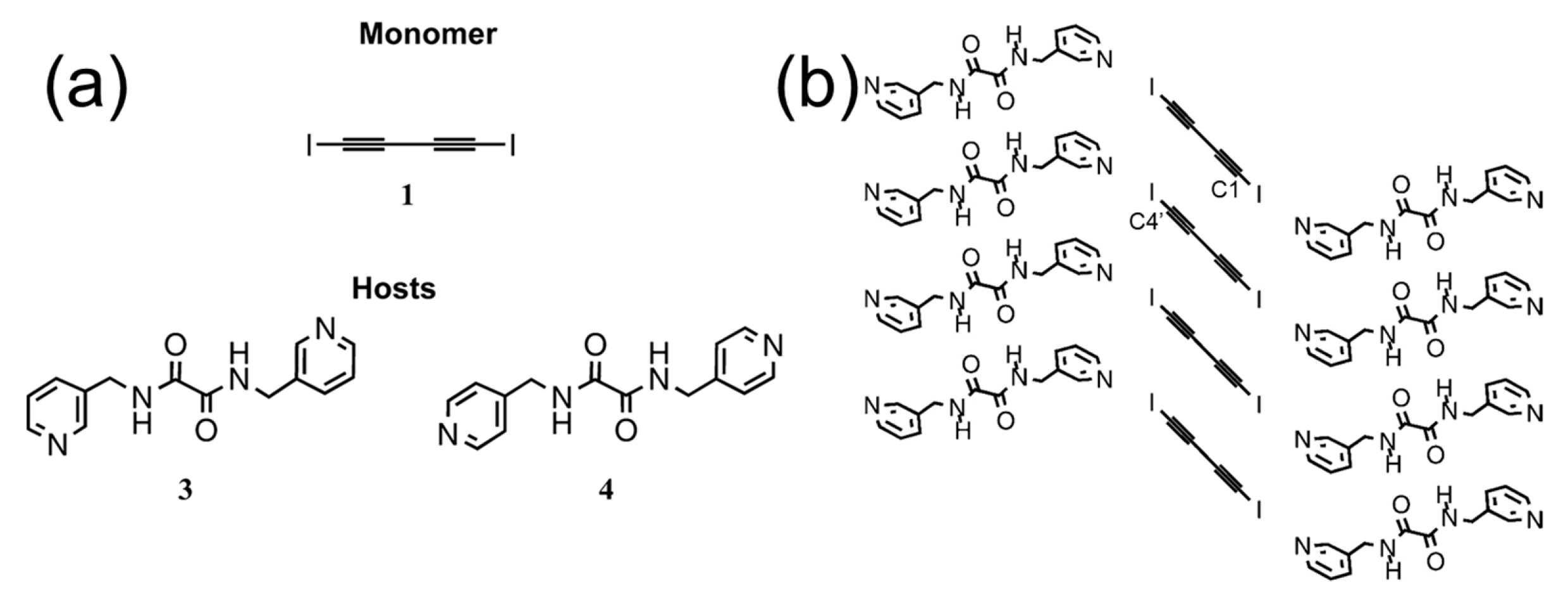
5.2. Alkyne
5.3. Aromatic Compounds
5.4. Nitriles
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mao, H.-K.; Chen, B.; Chen, J.; Li, K.; Lin, J.-F.; Yang, W.; Zheng, H. Recent advances in high-pressure science and technology. Matter Radiat. Extrem. 2016, 1, 59–75. [Google Scholar] [CrossRef]
- Lei, L.; Zhang, L. Recent advance in high-pressure solid-state metathesis reactions. Matter Radiat. Extrem. 2017, 3, 95–103. [Google Scholar] [CrossRef]
- Bini, R. Laser-assisted high-pressure chemical reactions. Acc. Chem. Res. 2004, 37, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Lipp, M.J.; Yoo, C.-S.; Cynn, H.; Herberg, J.L.; Maxwell, R.S. Pressure-induced polymerization of carbon monoxide: Disproportionation and synthesis of an energetic lactonic polymer. Chem. Mater. 2006, 18, 2520–2531. [Google Scholar] [CrossRef]
- Iota, V.; Yoo, C.-S.; Cynn, H. Quartzlike carbon dioxide: An optically nonlinear extended solid at high pressures and temperatures. Science 1999, 283, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Eremets, M.I.; Hemley, R.J.; Mao, H.-K.; Gregoryanz, E. Semiconducting non-molecular nitrogen up to 240 GPa and its low-pressure stability. Nature 2001, 411, 170–174. [Google Scholar] [CrossRef]
- Gorelli, F.A.; Santoro, M.; Ulivi, L.; Bini, R. The epsilon phase of solid oxygen: Evidence of an O4 molecule lattice. Phys. Rev. Lett. 1999, 83, 4093–4096. [Google Scholar] [CrossRef]
- Nobrega, M.M.; Temperini, M.L.A.; Bini, R. Probing the chemical stability of aniline under high pressure. J. Phys. Chem. C 2017, 121, 7495–7501. [Google Scholar] [CrossRef]
- Bassett, W.A. Diamond anvil cell, 50th birthday. High Pressure Res. 2009, 29, 163–186. [Google Scholar] [CrossRef]
- Xu, J.-A.; Mao, H.-K. Moissanite: A window for high-pressure experiments. Science 2000, 290, 783–785. [Google Scholar] [CrossRef]
- Xu, J.-A.; Huang, E. Graphite-diamond transition in gem anvil cells. Rev. Sci. Instrum. 1994, 65, 204–207. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Yan, Z.; Yuan, Y.; Li, X.; Pei, Z.H.; Wang, L. In situ high-pressure spectroscopic studies using moissanite (4H-SiC) anvils. AIP Adv. 2018, 8, 095012. [Google Scholar] [CrossRef]
- Xu, J.-A.; Mao, H.-K.; Bell, P.M. High-pressure ruby and diamond fluorescence: Observations at 0.21 to 0.55 terapascal. Science 1986, 232, 1404–1406. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.-K.; Xu, J.-A.; Bell, P.M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. 1986, 91, 4673–4676. [Google Scholar] [CrossRef]
- Barnett, J.D.; Block, S.; Piermarini, G.J. An optical fluorescence system for quantitative pressure measurement in the diamond-anvil cell. Rev. Sci. Instrum. 1973, 44, 1–4. [Google Scholar] [CrossRef]
- Forman, R.A.; Piermarini, G.J.; Barnett, J.D.; Block, S. Pressure measurement made by the utilization of ruby sharp-line luminescence. Science 1972, 176, 284–285. [Google Scholar] [CrossRef] [PubMed]
- Schettino, V.; Bini, R. Molecules under extreme conditions: Chemical reactions at high pressure. Phys. Chem. 2003, 5, 1951–1965. [Google Scholar]
- Akahama, Y.; Kawamura, H. Pressure calibration of diamond anvil Raman gauge to 310 GPa. J. Appl. Phys. 2006, 100, 043516. [Google Scholar] [CrossRef]
- Piermarini, G.J.; Block, S.; Barnett, J.D. Hydrostatic limits in liquids and solids to 100 kbar. J. Appl. Phys. 1973, 44, 5377–5382. [Google Scholar] [CrossRef]
- Ragan, D.D.; Clarke, D.R.; Schiferl, D. Silicone fluid as a high-pressure medium in diamond anvil cells. Rev. Sci. Instrum. 1996, 67, 494. [Google Scholar] [CrossRef]
- Kenichi, T. Evaluation of the hydrostaticity of a helium-pressure medium with powder X-ray diffraction techniques. J. Appl. Phys. 2001, 89, 662–668. [Google Scholar]
- Besson, J.M.; Nelmes, R.J. New developments in neutron-scattering methods under high pressure with the Paris-Edinburgh cells. Physica B 1995, 231, 31–36. [Google Scholar] [CrossRef]
- Marshall, W.G.; Francis, D.J. Attainment of near-hydrostatic compression conditions using the Paris-Edinburgh cell. J. Appl. Cryst. 2002, 35, 122–125. [Google Scholar] [CrossRef]
- Kono, Y.; Park, C.; Kenney-Benson, C.; Shen, G.; Wang, Y. Toward comprehensive studies of liquids at high pressures and high temperatures: Combined structure, elastic wave velocity, and viscosity measurements in the Paris-Edinburgh cell. Phys. Earth Planet. Inter. 2014, 228, 269–280. [Google Scholar] [CrossRef]
- Wang, Y. Large volume presses for high-pressure studies using synchrotron radiation. In High-Pressure Crystallography; Boldyreva, E., Dera, P., Eds.; Springer: Berlin, Germany, 2010; pp. 81–96. [Google Scholar]
- Liebermann, R.C. Multi-anvil, high pressure apparatus: A half-century of development and progress. High Press. Res. 2011, 31, 493–532. [Google Scholar] [CrossRef]
- Mao, H.-K.; Chen, X.J.; Ding, Y.; Li, B.; Wang, L. Solids, liquids, and gases under high pressure. Rev. Mod. Phys. 2018, 90, 015007. [Google Scholar] [CrossRef]
- Shen, G.; Mao, H.-K.; Hemley, R.J. Laser-Heated Diamond Anvil Cell Technique: Double-Sided Heating with Multimode Nd:YAG Laser. In Advanced Materials ’96-New Trend in High Pressure Research, Proceedings of the 3rd NIRIM ISAM, Tsukuba, Japan, 4–8 March 1996; Akaishi, M., Ed.; National Institute for Research in Inorganic Materials: Tokyo, Japan, 1996; pp. 149–152. [Google Scholar]
- Smith, D.; Smith, J.S.; Childs, C.; Rod, E.; Hrubiak, R.; Shen, G.; Salamat, S. A CO2 laser heating system for in situ high pressure-temperature experiments at HPCAT. Rev. Sci. Instrum. 2018, 89, 083901. [Google Scholar] [CrossRef]
- Heinz, D.L.; Jeanloz, R. Measurement of the melting curve of Mg0.9Fe0.1SiO3 at lower mantle conditions and its geophysical implications. Phys. Earth Planet. Inter. 1987, 92, 11437–11444. [Google Scholar]
- Heinz, D.L.; Sweeney, J.S. A laser heating system that stabilizes and controls the temperature: Diamond anvil cell applications. Rev. Sci. Instrum. 1991, 62, 1568–1575. [Google Scholar] [CrossRef]
- Bassett, W.A.; Shen, A.H.; Bucknum, M. A new diamond anvil cell for hydrothermal studies to 2.5 GPa and from −190 °C to 1200 °C. Rev. Sci. Instrum. 1993, 64, 2340–2345. [Google Scholar] [CrossRef]
- Kantor, I.; Prakapenka, V.; Kantor, A.; Dera, P.; Kurnosov, A. BX90: A new diamond anvil cell design for X-ray diffraction and optical measurements. Rev. Sci. Instrum. 2012, 83, 125102. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zheng, H.; Xiao, W. High-temperature and high-pressure cubic zirconia cell for Raman spectroscopy. Appl. Spectrosc. 2003, 57, 1295–1299. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, K.R.; Holzapfel, W.B. Effect of high pressure on the Raman spectra of ice VIII and evidence for ice X. J. Chem. Phys. 1986, 84, 2771–2775. [Google Scholar] [CrossRef]
- Gardiner, D.J.; Bowden, M.; Graves, P.R. Novel applications of Raman microscopy. Phil. Trans. R. Soc. Lond. A 1986, 320, 295–306. [Google Scholar] [CrossRef]
- Dreger, Z.A.; Gupta, Y.M. High pressure Raman spectroscopy of single crystals of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX). J. Phys. Chem. C 2007, 111, 3893–3903. [Google Scholar] [CrossRef] [PubMed]
- Zhuravlev, K.K.; Traikov, K.; Dong, Z.; Xie, S.; Song, Y. Raman and infrared spectroscopy of pyridine under high pressure. Phys. Rev. B 2010, 82, 064116. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, J.; Tan, X.; Wang, L.; Chen, J.; Li, B.; Ye, L.; Xu, B.; Zou, B.; Tian, W. Multi-stimuli responsive fluorescence switching: The reversible piezochromism and protonation effect of a divinylanthracene derivative. J. Mater. Chem. C 2013, 1, 7554–7559. [Google Scholar] [CrossRef]
- He, C.; Gao, C.; Ma, Y.; Li, M.; Hao, A.; Huang, X.; Liu, B.; Zhang, D.; Yu, C.; Zou, G.; et al. In situ electrical impedance spectroscopy under high pressure on diamond anvil cell. Appl. Phys. Lett. 2007, 91, 092124. [Google Scholar] [CrossRef]
- Han, Y.; Gao, C.; Ma, Y.; Liu, H.; Pan, Y.; Luo, J.; Li, M.; He, C.; Huang, X.; Zou, G.; et al. Integrated microcircuit on a diamond anvil for high-pressure electrical resistivity measurement. Appl. Phys. Lett. 2005, 86, 064104. [Google Scholar] [CrossRef]
- Mao, H.-K.; Bell, P.M. Electrical resistivity measurements of conductors in the diamond-window, high-pressure cell. Rev. Sci. Instrum. 1998, 52, 615–616. [Google Scholar] [CrossRef]
- Hamlin, J.J.; Jeffries, J.R.; Butch, N.P.; Syers, P.; Zocco, D.A.; Weir, S.T.; Vohra, Y.K.; Paglione, J.; Maple, M.B. High pressure transport properties of the topological insulator Bi2Se3. J. Phys. Condes. Matter. 2012, 24, 035602. [Google Scholar] [CrossRef][Green Version]
- Yamauchi, T.; Shimizu, K.; Takeshita, N.; Ishizuka, M.; Amaya, K.; Endo, S. Hall effect of iodine in high pressure. J. Phys. Soc. Jpn. 1994, 63, 3207–3209. [Google Scholar] [CrossRef]
- Lee, M.; Rosenbaum, T.F.; Saboungi, M.-L.; Schnyders, H.S. Band-gap tuning and linear magnetoresistance in the silver chalcogenides. Phys. Rev. Lett. 2002, 88, 066602. [Google Scholar] [CrossRef]
- Vaccari, M.; Aquilanti, G.; Pascarelli, S.; Mathon, O. A new EXAFS investigation of local structural changes in amorphous and crystalline GeO2 at high pressure. J. Phys. Condens. Matter. 2009, 21, 145403. [Google Scholar] [CrossRef]
- Rueff, J.-P.; Mattila, A.; Badro, J.; Vankó, G.; Shukla, A. Electronic properties of transition-metal oxides under high pressure revealed by X-ray emission spectroscopy. J. Phys. Condens. Matter. 2005, 17, S717–S726. [Google Scholar] [CrossRef]
- Lee, S.K.; Lin, J.-F.; Cai, Y.Q.; Hiraoka, N.; Eng, P.J.; Okuchi, T.; Mao, H.-K.; Meng, Y.; Hu, M.Y.; Chow, P.; et al. X-ray Raman scattering study of MgSiO3 glass at high pressure: Implication for triclustered MgSiO3 melt in Earth’s mantle. Proc. Natl. Acad. Sci. USA 2008, 105, 7925–7929. [Google Scholar] [CrossRef]
- Machida, S. Neutron diffraction experiments at high pressure in SNS. Rev. High. Pres. Sci. Tech. 2016, 26, 157–166. [Google Scholar] [CrossRef][Green Version]
- Hattori, T.; Sano-Furukawa, A.; Arima, H.; Komatsu, K.; Yamada, A.; Inamura, Y.; Nakatani, T.; Seto, Y.; Nagai, T.; Utsumi, W.; et al. Design and performance of high-pressure PLANET beamline at pulsed neutron source at J-PARC. Nucl. Instrum. Methods Phys. Res. A 2015, 780, 55–67. [Google Scholar] [CrossRef]
- Bull, C.L.; Funnell, N.P.; Tucker, M.G.; Hull, S.; Francis, D.J.; Marshall, W.G. Pearl: The high pressure neutron powder diffractometer at ISIS. High Press. Res. Int. J. 2016, 36, 493–511. [Google Scholar] [CrossRef]
- Klotz, S.; Besson, J.M.; Hamel, G.; Nelmes, R.J.; Loveday, J.S.; Marshall, W.G. High pressure neutron diffraction using the Paris-Edinburgh cell: Experimental possibilities and future prospects. High Press. Res. Int. J. 1996, 14, 249–255. [Google Scholar] [CrossRef]
- Goncharenko, I.N.; Mirebeau, I.; Ochiai, A. Magnetic neutron diffraction under pressures up to 43 GPa. Study of the EuX and GdX compounds. Hyperfine Interact. 2000, 128, 225–244. [Google Scholar] [CrossRef]
- Guthriea, M.; Boehlera, R.; Tulk, C.A.; Molaison, J.J.; Dos Santos, A.M.; Li, K.; Hemley, R.J. Neutron diffraction observations of interstitial protons in dense ice. Proc. Natl. Acad. Sci. USA 2013, 110, 10552–10556. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, W.; Kagi, H.; Komatsu, K.; Arima, H.; Nagai, T.; Okuchi, T.; Kamiyama, T.; Uwatoko, Y.; Matsubayashi, K.; Yagi, T. Neutron powder diffraction under high pressure at J-PARC. Nucl. Instrum. Methods Phys. Res. Sect. A 2009, 600, 50–52. [Google Scholar] [CrossRef]
- Goncharenko, I.N. Neutron diffraction experiments in diamond and sapphire anvil cells. High Press. Res. Int. J. 2004, 24, 193–204. [Google Scholar] [CrossRef]
- Presser, V.; Heon, M.; Gogotsi, Y. Carbide-derived carbons-from porous networks to nanotubes and graphene. Adv. Funct. Mater. 2011, 21, 810–833. [Google Scholar] [CrossRef]
- Benson, D.; Li, Y.; Luo, W.; Ahuja, R.; Svensson, G.; Haussermann, U. Lithium and calcium carbides with polymeric carbon structures. Inorg. Chem. 2013, 52, 6402–6406. [Google Scholar] [CrossRef]
- Srepusharawoot, P.; Blomqvist, A.; Araújo, C.M.; Scheicher, R.H.; Ahuja, R. One-dimensional polymeric carbon structure based on five-membered rings in alkaline earth metal dicarbides BeC2 and MgC2. Phys. Rev. B 2010, 82, 125439. [Google Scholar] [CrossRef]
- Li, Y.-L.; Wang, S.-N.; Oganov, A.R.; Gou, H.; Smith, J.S.; Strobel, T.A. Investigation of exotic stable calcium carbides using theory and experiment. Nat. Commun. 2015, 6, 6974. [Google Scholar] [CrossRef]
- Efthimiopoulos, I.; Benson, D.E.; Konar, S.; Nylen, J.; Svensson, G.; Haussermann, U.; Liebig, S.; Ruschewitz, U.; Vazhenin, G.V.; Loa, I.; et al. Structural transformations of Li2C2 at high pressures. Phys. Rev. B 2015, 92, 064111. [Google Scholar] [CrossRef]
- Efthimiopoulos, I.; Kunc, K.; Vazhenin, G.V.; Stavrou, E.; Syassen, K.; Hanfland, M.; Liebig, S.; Ruschewitz, U. Structural transformation and vibrational properties of BaC2 at high pressure. Phys. Rev. B 2012, 85, 054102. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, L.; Li, K.; Yang, Y.; Wang, Y.; Wu, J.; Dong, X.; Wang, C.-H.; Tulk, C.A.; Molaison, J.J.; et al. Pressure induced polymerization of acetylide anions in CaC2 and 107 fold enhancement of electrical conductivity. Chem. Sci. 2017, 8, 298–304. [Google Scholar] [CrossRef]
- Wang, L.; Dong, X.; Wang, Y.; Zheng, H.; Li, K.; Peng, X.; Mao, H.-K.; Jin, C.; Meng, Y.; Huang, M.; et al. Pressure-induced polymerization and disproportionation of Li2C2 accompanied with irreversible conductivity enhancement. J. Phys. Chem. Lett. 2017, 8, 4241–4245. [Google Scholar] [CrossRef]
- Dong, X.; Wang, L.; Li, K.; Zheng, H.; Wang, Y.; Meng, Y.; Shu, H.; Mao, H.-K.; Feng, S.; Jin, C. Tailored synthesis of the narrowest zigzag graphene nanoribbon structure by compressing the lithium acetylide under high temperature. J. Phys. Chem. C 2018, 122, 20506–20512. [Google Scholar] [CrossRef]
- Chena, J.-Y.; Yoo, C.-S. Physical and chemical transformations of sodium cyanide at high pressures. J. Chem. Phys. 2009, 131, 144507. [Google Scholar] [CrossRef]
- Catafesta, J.; Haines, J.; Zorzi, J.E.; Pereira, A.S.; Perottoni, C.A. Pressure-induced amorphization and decomposition of Fe[Co(CN)6]. Phys. Rev. B 2008, 77, 064104. [Google Scholar] [CrossRef]
- Hara, Y.; Shirotani, I.; Minomura, S. Changes of the electronic state and the electrical resistance of some iron compounds at high pressures. Inorg. Chem. 1975, 14, 1834–1837. [Google Scholar] [CrossRef]
- Li, K.; Zheng, H.; Ivanov, I.N.; Guthrie, M.; Xiao, Y.; Yang, W.; Tulk, C.A.; Zhao, Y.; Mao, H.-K. K3Fe(CN)6: Pressure-induced polymerization and enhanced conductivity. J. Phys. Chem. C 2013, 117, 24174–24180. [Google Scholar] [CrossRef]
- Li, K.; Zheng, H.; Wang, L.; Tulk, C.A.; Molaison, J.J.; Feygenson, M.; Yang, W.; Guthrie, M.; Mao, H.-K. K3Fe(CN)6 under external pressure: Dimerization of CN− coupled with electron transfer to Fe(III). J. Phys. Chem. C 2015, 119, 22351–22356. [Google Scholar] [CrossRef]
- Li, K.; Zheng, H.; Hattori, T.; Sano-Furukawa, A.; Tulk, C.A.; Molaison, J.; Feygenson, M.; Ivanov, I.N.; Yang, W.; Mao, H.-K. Synthesis, structure, and pressure-Induced polymerization of Li3Fe(CN)6 accompanied with enhanced conductivity. Inorg. Chem. 2015, 54, 11276–11282. [Google Scholar] [CrossRef]
- Jurgens, B.; Irran, E.; Schneider, J.; Schnick, W. Trimerization of NaC2N3 to Na3C6N9 in the Solid: Ab initio crystal structure determination of two polymorphs of NaC2N3 and of Na3C6N9 from X-ray powder diffractometry. Inorg. Chem. 2000, 39, 665–670. [Google Scholar] [CrossRef]
- Cairns, T.L.; Larchar, A.W.; McKusick, B.C. The trimerization of nitriles at high pressures. J. Am. Chem. Soc. 1952, 74, 5633–5636. [Google Scholar] [CrossRef]
- Purdy, A.P.; Houser, E.; George, C.F. Lithium dicyanamide, its reactions with cyanuric chloride, and the crystal structures of LiN(CN)2(MeCN)2 and LiCN(C5H5N)2. Polyhedron 1997, 16, 3671–3679. [Google Scholar] [CrossRef]
- Keefer, D.W.; Gou, H.; Purdy, A.P.; Epshteyn, A.; Kim, D.Y.; Badding, J.V.; Strobel, T.A. Pressure-induced polymerization of LiN(CN)2. J. Phys. Chem. A 2016, 120, 9370–9377. [Google Scholar] [CrossRef]
- Lotsch, B.V.; Schnick, W. From triazines to heptazines: Novel nonmetal tricyanomelaminates as precursors for graphitic carbon nitride materials. Chem. Mater. 2006, 18, 1891–1900. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Shen, L.; Ma, Y.; Lei, W.; Cui, Q.; Zou, G. Preparation and characterization of graphitic carbon nitride through pyrolysis of melamine. Appl. Phys. A Mater. Sci. Process. 2009, 94, 387–392. [Google Scholar] [CrossRef]
- Koglin, E.; Kip, B.J.; Meier, R.J. Adsorption and displacement of melamine at the Ag/electrolyte interface probed by surface-enhanced Raman microprobe spectroscopy. J. Phys. Chem. 1996, 100, 5078–5089. [Google Scholar] [CrossRef]
- Andreyev, A.; Akaishi, M.; Golberg, D. Synthesis of nanocrystalline nitrogen-rich carbon nitride powders at high pressure. Diamond. Relat. Mater. 2002, 11, 1885–1889. [Google Scholar] [CrossRef]
- Citroni, M.; Ceppatelli, M.; Bini, R.; Schettino, V. Laser-induced selectivity for dimerization versus polymerization of butadiene under pressure. Science 2002, 295, 2058–2060. [Google Scholar] [CrossRef]
- Bartlett, R.K.; Neill, G.O.; Turner, H.S.; Wall, W.F. High pressure polymerisation of acetylenic compounds. Br. Polym. J. 1972, 4, 503–509. [Google Scholar] [CrossRef]
- Yamawaki, H.; Aoki, K.; Kakudate, Y.; Yoshida, M.; Usuba, S.; Fujiwara, S. Infrared study of phase transition and chemical reaction in tetracyanoethylene under high pressure. Chem. Phys. Lett. 1992, 198, 183–187. [Google Scholar] [CrossRef]
- Bernard, S.; Chiarotti, G.L.; Scandolo, S.; Tosatti, E. Decomposition and polymerization of solid carbon monoxide under pressure. Phys. Rev. Lett. 1998, 81, 2092. [Google Scholar] [CrossRef]
- Wen, X.-D.; Hoffmann, R.; Ashcroft, N.W. Benzene under high pressure: A story of molecular crystals transforming to saturated networks, with a possible intermediate metallic phase. J. Am. Chem. Soc. 2011, 133, 9023–9035. [Google Scholar] [CrossRef]
- Scelta, D.; Ceppatelli, M.; Bini, R. Pressure induced polymerization of fluid ethylene. J. Chem. Phys. 2016, 145, 164504. [Google Scholar] [CrossRef]
- Chelazzi, D.; Ceppatelli, M.; Santoro, M.; Bini, R.; Schettino, V. Pressure-induced polymerization in solid ethylene. J. Phys. Chem. B 2005, 109, 21658–21663. [Google Scholar] [CrossRef]
- Chelazzi, D.; Ceppatelli, M.; Santoro, M.; Bini, R.; Schettino, V. High-pressure synthesis of crystalline polyethylene using optical catalysis. Nature Mater. 2004, 3, 470. [Google Scholar] [CrossRef]
- Citroni, M.; Ceppatelli, M.; Bini, R.; Schettino, V. The high-pressure chemistry of butadiene crystal. J. Chem. Phys. 2003, 118, 1815. [Google Scholar] [CrossRef]
- Shirakawa, H.; Ito, T.; Ikeda, S. Raman scattering and electronic spectra of poly(acetylene). Polym. J. 1973, 4, 460–462. [Google Scholar] [CrossRef]
- Sakashita, M.; Yamawaki, H.; Aoki, K. FT-IR study of the solid state polymerization of acetylene under pressure. J. Phys. Chem. 1996, 100, 9943–9947. [Google Scholar] [CrossRef]
- Ceppatelli, M.; Santoro, M.; Bini, R.; Schettino, V. Fourier transform infrared study of the pressure and laser induced polymerization of solid acetylene. J. Chem. Phys. 2000, 113, 5991–6000. [Google Scholar] [CrossRef]
- Trout, C.C.; Badding, J.V. Solid state polymerization of acetylene at high pressure and low temperature. J. Phys. Chem. A 2000, 104, 8142–8145. [Google Scholar] [CrossRef]
- Aoki, K.; Kakudate, Y.; Yoshida, M.; Usuba, S.; Tanaka, K.; Fujiwara, S. Solid-state polymerization of acetylene under pressure. Synth. Met. 1989, 28, D91–D98. [Google Scholar] [CrossRef]
- Sun, J.; Dong, X.; Wang, Y.; Li, K.; Zheng, H.; Wang, L.; Cody, G.D.; Tulk, C.A.; Molaison, J.J.; Lin, X.; et al. Pressure-induced polymerization of acetylene: Structure-directed stereoselectivity and a possible route to graphane. Angew. Chem. Int. Ed. 2017, 56, 1–6. [Google Scholar] [CrossRef]
- Ward, M.D.; Huang, H.-T.; Zhu, L.; Biswas, A.; Popov, D.; Badding, J.V.; Strobel, T.A. Chemistry through cocrystals: Pressure-induced polymerization of C2H2∙C6H6 to an extended crystalline hydrocarbon. Phys. Chem. Chem. Phys. 2018, 20, 7282–7294. [Google Scholar] [CrossRef]
- Ward, M.D.; Huang, H.-T.; Zhu, L.; Popov, D.; Strobel, T.A. High-pressure behavior of C2I2 and polymerization to a conductive polymer. J. Phys. Chem. C 2019, 123, 11369–11377. [Google Scholar] [CrossRef]
- Sharma, S.K.; Rezan, G.S.A.; Misra, V.N.; Tripathi, K.N. Third order nonlinear polymer materials for photonics. J. Mater. Sci. Lett. 2003, 22, 737–738. [Google Scholar] [CrossRef]
- Wilhelm, C.; Boyd, S.A.; Chawda, S.; Fowler, F.W.; Goroff, N.S.; Halada, G.P.; Grey, C.P.; Lauher, J.W.; Luo, L.; Martin, C.D.; et al. Pressure-induced polymerization of diiodobutadiyne in assembled cocrystals. J. Am. Chem. Soc. 2008, 130, 4415–4420. [Google Scholar] [CrossRef]
- Pruzan, P.; Chervin, J.C.; Thiery, M.M.; Itie, J.P.; Besson, J.M. Transformation of benzene to a polymer after static pressurization to 30 GPa. J. Chem. Phys. 1990, 92, 6910–6915. [Google Scholar] [CrossRef]
- Ciabini, L.; Santoro, M.; Bini, R.; Schettino, V. High pressure reactivity of solid benzene probed by infrared spectroscopy. J. Chem. Phys. 2002, 116, 2928. [Google Scholar] [CrossRef]
- Cansell, F.; Fabre, D.; Petitet, J.-P. Phase transitions and chemical transformations of benzene up to 550 °C and 30 GPa. J. Chem. Phys. 1993, 99, 7300–7304. [Google Scholar] [CrossRef]
- Jackson, B.R.; Trout, C.C.; Badding, J.V. UV Raman analysis of the C:H network formed by Compression of benzene. Chem. Mater. 2003, 15, 1820–1824. [Google Scholar] [CrossRef]
- Fitzgibbons, T.C.; Guthrie, M.; Xu, E.-S.; Crespi, V.H.; Davidowski, S.K.; Cody, G.D.; Alem, N.; Badding, J.V. Benzene-derived carbon nanothreads. Nat. Mater. 2015, 14, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Baldini, M.; Wang, T.; Chen, B.; Xu, E.-S.; Vermilyea, B.; Crespi, V.H.; Hoffmann, R.; Molaison, J.J.; Tulk, C.A.; et al. Mechanochemical synthesis of carbon nanothread single crystals. J. Am. Chem. Soc. 2017, 139, 16343–16349. [Google Scholar] [CrossRef] [PubMed]
- Ciabini, L.; Santoro, M.; Gorelli, F.A.; Bini, R.; Schettino, V.; Raugei, S. Triggering dynamics of the high-pressure benzene amorphization. Nat. Mater. 2007, 6, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Hoffmann, R.; Ashcroft, N.W.; Badding, J.; Xu, E.; Crespi, V. Linearly polymerized benzene arrays as intermediates, tracing pathways to carbon nanothreads. J. Am. Chem. Soc. 2015, 137, 14373–14386. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Zheng, H.; Li, K.; Andrzejewski, M.; Hattori, T.; Sano-Furukawa, A.; Katrusiak, A.; Meng, Y.; Liao, F.; et al. Phase transitions and polymerization of C6H6-C6F6 cocrystal under extreme conditions. J. Phys. Chem. C 2016, 120, 29510–29519. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, X.; Tang, X. Pressure-induced Diels-Alder reactions in C6H6-C6F6 cocrystal towards graphane structure. Angew. Chem. Int. Ed. 2019, 58, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Nobrega, M.M.; Teixeira-Neto, E.; Cairns, A.B.; Temperini, M.L.A.; Bini, R. One-dimensional diamondoid polyaniline-like nanothreads from compressed crystal aniline. Chem. Sci. 2018, 9, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Fanetti, S.; Nobrega, M.M.; Teixeira-Neto, E.; Temperini, M.L.A.; Bini, R. Effect of structural anisotropy in high-pressure reaction of aniline. J. Phys. Chem. C 2018, 122, 29158–29164. [Google Scholar] [CrossRef]
- Citroni, M.; Fanetti, S.; Bazzicalupi, C.; Dziubek, K.; Pagliai, M.; Nobrega, M.M.; Mezouar, M.; Bini, R. Structural and electronic competing mechanisms in the formation of amorphous carbon nitride by compressing s-triazine. J. Phys. Chem. C 2015, 119, 28560–28569. [Google Scholar] [CrossRef]
- Fanetti, S.; Citroni, M.; Bini, R. Structure and reactivity of pyridine crystal under pressure. J. Phys. Chem. C 2011, 134, 204504. [Google Scholar] [CrossRef]
- Yasuzuka, T.; Komatsu, K.; Kagi, H. A revisit to high-pressure transitions of pyridine: A new phase transition at 5 GPa and formation of a crystalline phase over 20 GPa. Chem. Lett. 2011, 40, 733–735. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Duan, P.; Baldini, M.; Huang, H.-T.; Chen, B.; Juhl, S.J.; Koeplinger, D.; Crespi, V.H.; Schmidt-Rohr, K.; et al. Carbon nitride nanothread crystals derived from pyridine. J. Am. Chem. Soc. 2018, 140, 4969–4972. [Google Scholar] [CrossRef] [PubMed]
- Ceppatelli, M.; Santoro, M.; Bini, R.; Schettino, V. High pressure reactivity of solid furan probed by infrared and Raman spectroscopy. J. Chem. Phys. 2003, 118, 1499–1506. [Google Scholar] [CrossRef]
- Santoro, M.; Ceppatelli, M.; Bini, R.; Schettino, V. High-pressure photochemistry of furane crystal. J. Chem. Phys. 2003, 118, 8321–8325. [Google Scholar] [CrossRef]
- Aoki, K.; Baer, B.J.; Cynn, H.C.; Bicol, M. High-pressure Raman study of one-dimensional crystals of the very polar molecule hydrogen cyanide. Phys. Rev. B 1990, 42, 4298–4303. [Google Scholar] [CrossRef] [PubMed]
- Völker, T. Polymere Blausäure. Angew. Chem. 1960, 72, 379–384. [Google Scholar] [CrossRef]
- Zheng, H.; Li, K.; Cody, G.D.; Tulk, C.A.; Dong, X.; Gao, G.; Molaison, J.J.; Liu, Z.; Feygenson, M.; Yang, W.; et al. Polymerization of acetonitrile via a hydrogen transfer reaction from CH3 to CN under extreme conditions. Angew. Chem. Int. Ed. 2016, 55, 12040–12044. [Google Scholar] [CrossRef]
- Yoo, C.-S.; Nicol, M. Chemical and phase transformations of cyanogen at high pressures. J. Phys. Chem. 1986, 90, 6726–6731. [Google Scholar] [CrossRef]
- Yamawaki, H.; Sakashita, M.; Aoki, K. Reversible phase transition between the metastable phases of tetracyanoethylene under high pressure. Phys. Rev. B 1996, 53, 11403–11407. [Google Scholar] [CrossRef]
- Gou, H.; Yonke, B.L.; Epshteyn, A.; Kim, D.Y.; Smith, J.S.; Strobel, T.A. Pressure-induced polymerization of P(CN)3. J. Chem. Phys. 2015, 142, 194503. [Google Scholar] [CrossRef]
- Keefer, D.W.; Gou, H.; Wang, Q.; Purdy, A.; Epshteyn, A.; Juhl, S.J.; Cody, G.D.; Badding, J.; Strobel, T.A. Tetracyanomethane under pressure: Extended CN polymers from precursors with built-in sp3 centers. J. Phys. Chem. A 2018, 122, 2858–2863. [Google Scholar] [CrossRef] [PubMed]
- Gou, H.; Zhu, L.; Huang, H.-T.; Biswas, A.; Keefer, D.W.; Chaloux, B.L.; Prescher, C.; Yang, L.; Kim, D.Y.; Ward, M.D.; et al. From linear molecular chains to extended polycyclic networks: Polymerization of dicyanoacetylene. Chem. Mater. 2017, 29, 6706–6718. [Google Scholar] [CrossRef]
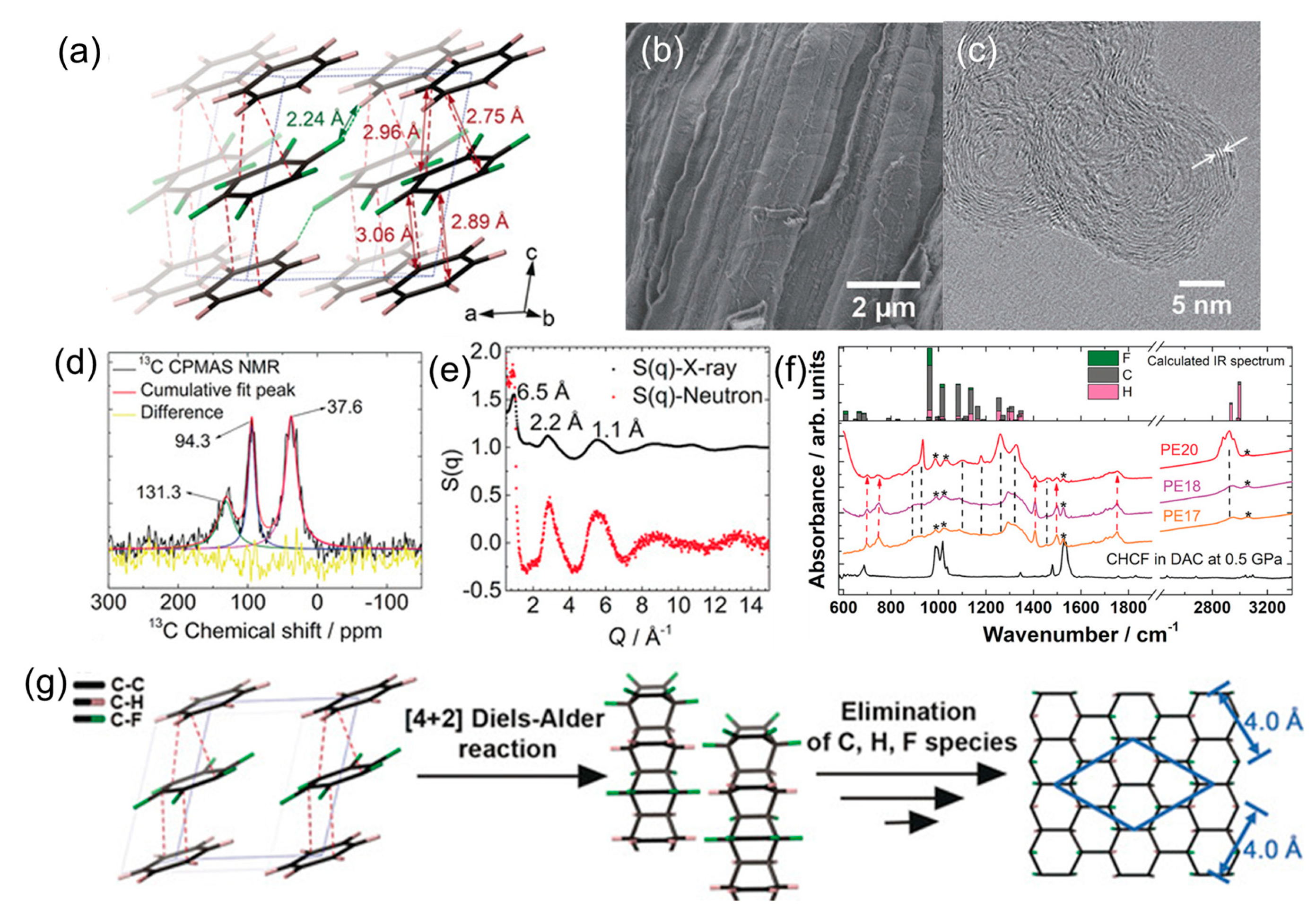
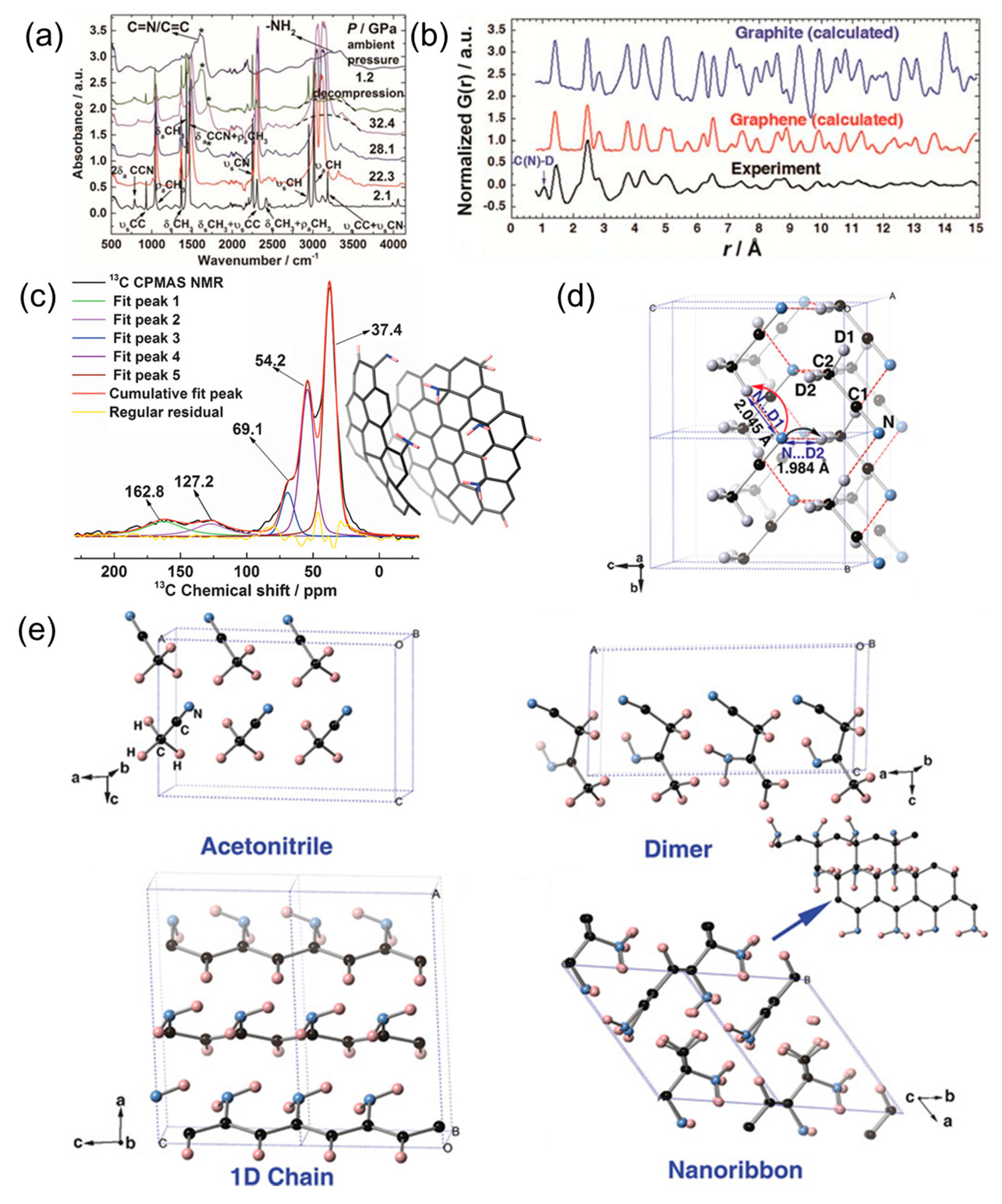
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Wang, X.; Wang, Y.; Li, K.; Zheng, H. From Molecules to Carbon Materials—High Pressure Induced Polymerization and Bonding Mechanisms of Unsaturated Compounds. Crystals 2019, 9, 490. https://doi.org/10.3390/cryst9100490
Yang X, Wang X, Wang Y, Li K, Zheng H. From Molecules to Carbon Materials—High Pressure Induced Polymerization and Bonding Mechanisms of Unsaturated Compounds. Crystals. 2019; 9(10):490. https://doi.org/10.3390/cryst9100490
Chicago/Turabian StyleYang, Xin, Xuan Wang, Yida Wang, Kuo Li, and Haiyan Zheng. 2019. "From Molecules to Carbon Materials—High Pressure Induced Polymerization and Bonding Mechanisms of Unsaturated Compounds" Crystals 9, no. 10: 490. https://doi.org/10.3390/cryst9100490
APA StyleYang, X., Wang, X., Wang, Y., Li, K., & Zheng, H. (2019). From Molecules to Carbon Materials—High Pressure Induced Polymerization and Bonding Mechanisms of Unsaturated Compounds. Crystals, 9(10), 490. https://doi.org/10.3390/cryst9100490




