Bioinspired Mineralization of Type I Collagen Fibrils with Apatite in Presence of Citrate and Europium Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Assembly and Mineralization of Collagen
2.3. Characterization Techniques
2.4. Luminescence Spectroscopy
2.5. Cytocompatibility Tests
3. Results and Discussion
3.1. Morphological, Chemical and Crystallographic Characteristics
3.2. FTIR and Raman Spectral Features
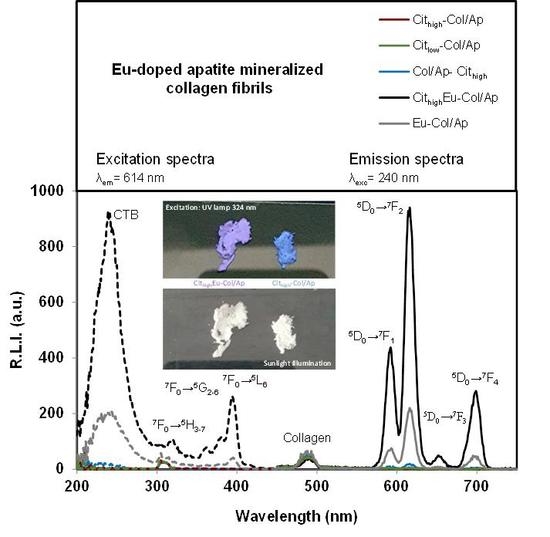
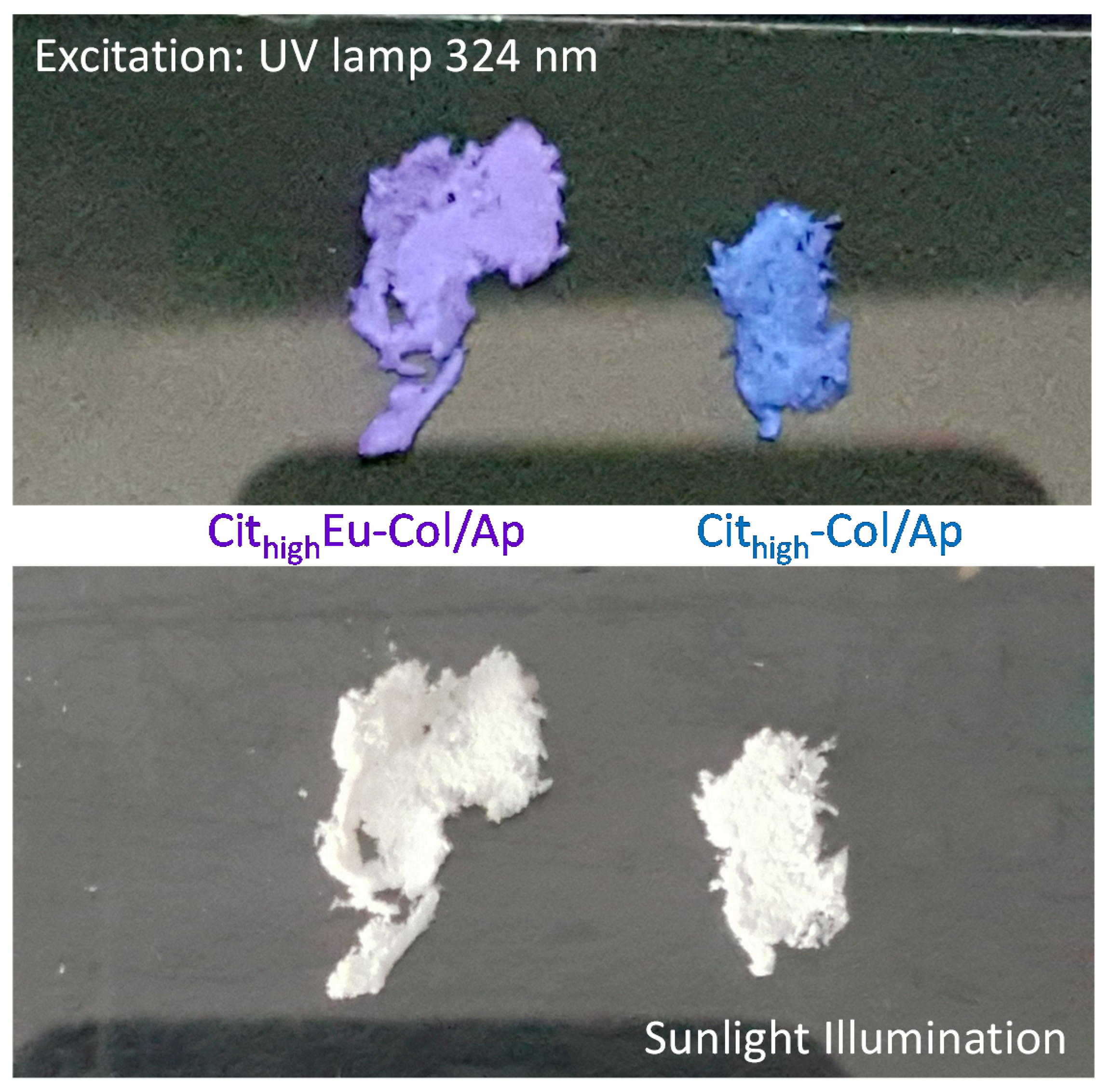
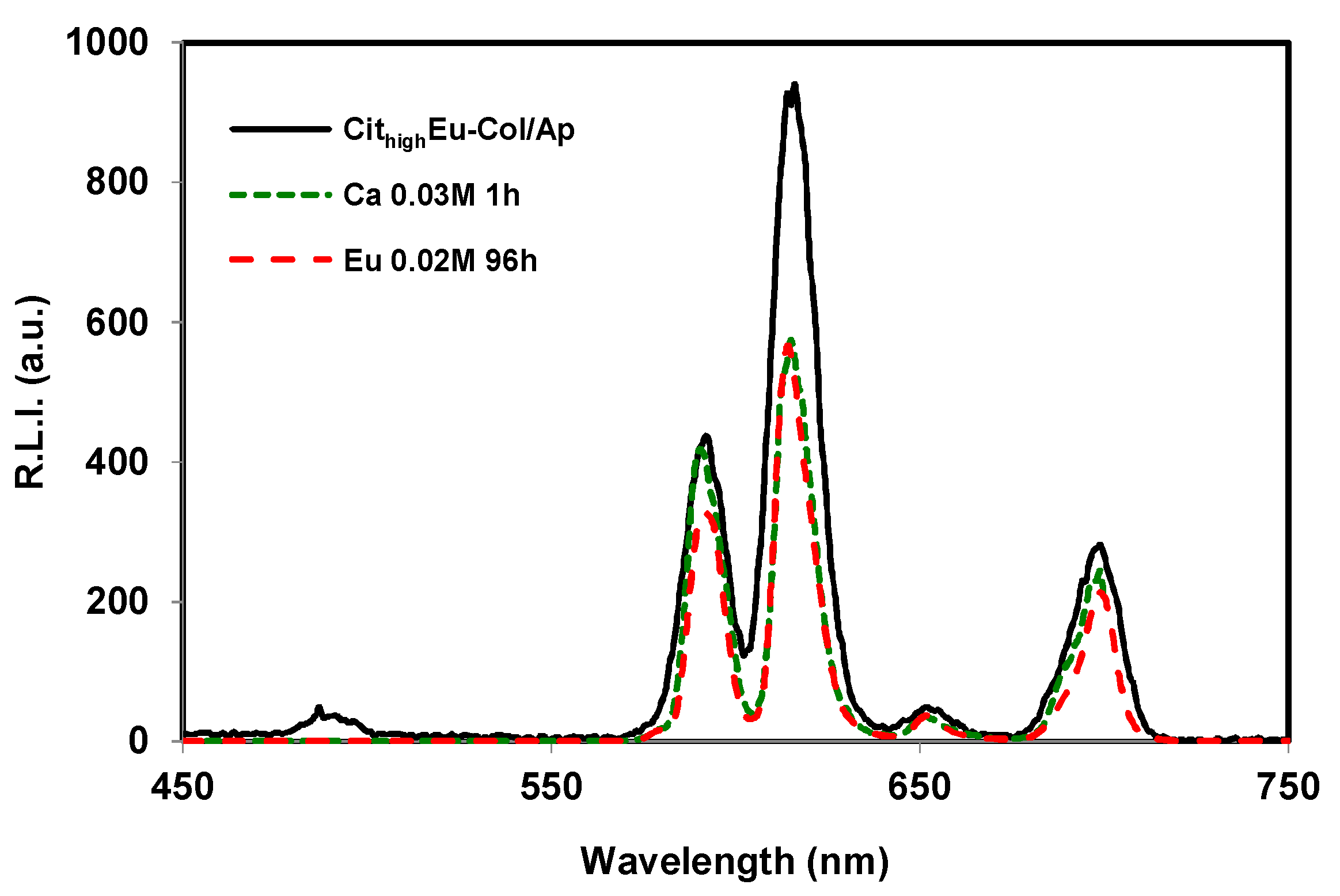
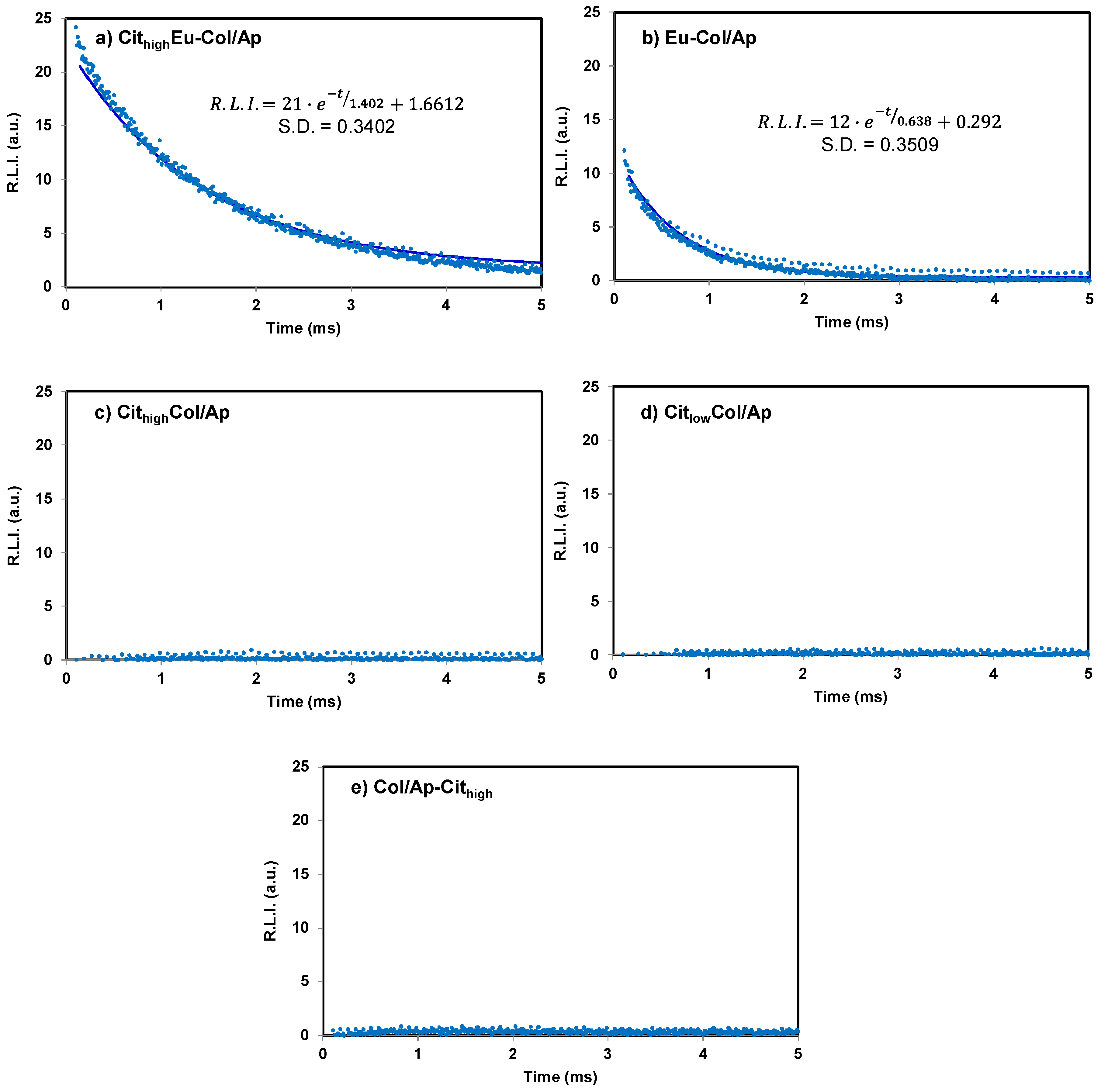
3.3. Luminescent Properties of Eu3+–Doped Mineralized Collagen Fibrils
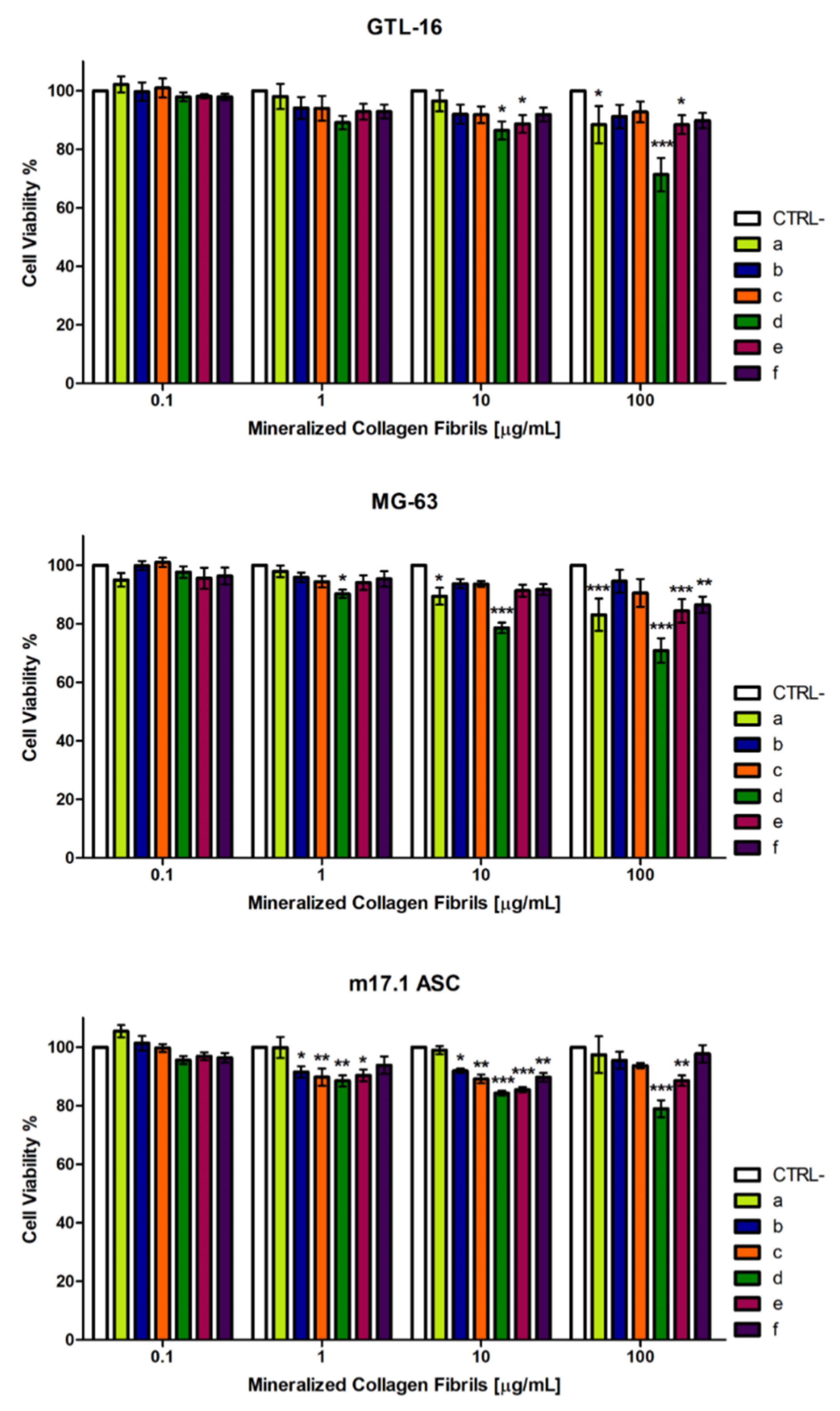
3.4. Cytocompatibility of the Mineralized Type I Collagen Fibrils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mat. 2015, 14, 23–36. [Google Scholar] [CrossRef]
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry; Oxford University Press: Oxford, UK, 2001; ISBN 9780198508823. [Google Scholar]
- Weiner, S.; Wagner, H.D. The material bone: Structure–mechanical functions relations. Ann. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Nudelman, F.; Pieterse, K.; George, A.; Bomans, P.H.; Friedrich, H.; Brylka, L.J.; Hilbers, P.A; de With, G.; Sommerdijk, N.A. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat. Mater. 2010, 9, 1004–1009. [Google Scholar] [CrossRef]
- Nudelman, F.; Lausch, A.J.; Sommerdijk, N.A.J.M.; Sone, E.D. In vitro models of collagen biomineralization. J. Struct. Biol. 2013, 183, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Ann. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Orgel, J.P.; Miller, A.; Irving, T.C.; Fischetti, R.F.; Hammersley, A.P.; Wess, T.J. The in situ supermolecular structure of type I collagen. Structure 2001, 9, 1061–1069. [Google Scholar] [CrossRef]
- Ishikawa, K. Bone Substitute Fabrication Based on Dissolution-Precipitation Reactions. Materials 2010, 3, 1138–1155. [Google Scholar] [CrossRef]
- Gómez–Morales, J.; Iafisco, M.; Delgado–López, J.M.; Sarda, S.; Druet, C. Progress on the preparation of nanocrystalline apatites and surface characterization: Overview of fundamental and applied aspects. Prog. Cryst. Growth Charact. Mater. 2013, 59, 1–46. [Google Scholar] [CrossRef]
- Hara, E.S.; Okada, M.; Nagaoka, N.; Hattori, T.; Kuboki, T.; Nakano, T.; Matsumoto, T. Bioinspired Mineralization Using Chondrocyte Membrane Nanofragments. ACS Biomater. Sci. Eng. 2018, 4, 617–625. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Rawal, A.; Schmidt-Rohr, K. Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc. Natl. Acad. Sci. USA 2010, 107, 22425–22429. [Google Scholar] [CrossRef]
- Davies, E.; Müller, K.H.; Wong, W.C.; Pickard, C.J.; Reid, D.G.; Skepper, J.N.; Duer, M.J. Citrate bridges between mineral platelets in bone. Proc. Natl. Acad. Sci. USA 2014, 111, E1354–E1363. [Google Scholar] [CrossRef] [PubMed]
- Delgado-López, J.M.; Frison, R.; Cervellino, A.; Gómez-Morales, J.; Guagliardi, A.; Masciocchi, N. Crystal Size, Morphology, and Growth Mechanism in Bio-Inspired Apatite Nanocrystals. Adv. Funct. Mater. 2014, 24, 1090–1099. [Google Scholar] [CrossRef]
- Iafisco, M.; Ramírez–Rodríguez, G.B.; Sakhno, Y.; Tampieri, A.; Martra, G.; Gómez-Morales, J.; Delgado-López, J.M. The growth mechanism of apatite nanocrystals assisted by citrate: Relevance to bone biomineralization. CrystEngComm 2015, 17, 507–511. [Google Scholar] [CrossRef]
- Costello, L.C.; Chellaiah, M.; Zou, J.; Franklin, R.B.; Reynolds, M.A. The status of citrate in the hydroxyapatite/collagen complex of bone; and its role in bone formation. J. Regen. Med. Tissue Eng. 2014, 3, 4. [Google Scholar] [CrossRef]
- Delgado-López, J.M.; Bertolotti, F.; Lyngsø, J.; Pedersen, J.S.; Cervellino, A.; Masciocchi, N.; Guagliardi, A. The synergic role of collagen and citrate in stabilizing amorphous calcium phosphate precursors with platy morphology. Acta Biomater. 2017, 49, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Zhao, R.; Jiang, S.; Yao, S.; Wu, Z.; Jin, B.; Yang, Y.; Pan, H.; Tang, R. Citrate Improves Collagen Mineralization via Interface Wetting: A Physicochemical Understanding of Biomineralization Control. Adv. Mater. 2018, 30, 1704876. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Nancollas, G.H. How to control the size and morphology of apatite nanocrystals in bone. Proc. Natl. Acad. Sci. USA 2010, 107, 22369–22370. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, I.; Delgado-López, J.M.; Durán-Olivencia, M.A.; Iafisco, M.; Tampieri, A.; Colangelo, D.; Prat, M.; Gómez-Morales, J. pH-Responsive Delivery of Doxorubicin from Citrate-Apatite Nanocrystals with Tailored Carbonate Content. Langmuir 2013, 29, 8213–8221. [Google Scholar] [CrossRef]
- Delgado-Lopez, J.M.; Iafisco, M.; Rodriguez-Ruiz, I.; Tampieri, A.; Prat, M.; Gómez–Morales, J. Crystallization of bioinspired citrate-functionalized nanoapatite with tailored carbonate content. Acta Biomater. 2012, 8, 3491–3499. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Blaker, J.J. Bioactive composite materials for tissue engineering scaffolds. Expert Rev. Med. Devices 2005, 2, 303–317. [Google Scholar] [CrossRef]
- Sprio, S.; Sandri, M.; Panseri, S.; Cunha, C.; Tampieri, A. Hybrid Scaffolds for Tissue Regeneration: Chemotaxis and Physical Confinement as Sources of Biomimesis. J. Nanomater. 2012, 418281. [Google Scholar] [CrossRef]
- Sprio, S.; Sandri, M.; Iafisco, M.; Panseri, S.; Adamaiano, A.; Camparodoni, E.; Tampieri, A. Bio-inspired assembling/mineralization process as a flexible approach to develop new smart scaffolds for the regeneration of complex anatomical regions. J. Eur. Ceram. Soc. 2016, 36, 2857–2867. [Google Scholar] [CrossRef]
- Irizarry, A.R.; Yan, G.; Zeng, Q.; Lucchesi, J.; Hamang, M.J.; Ma, Y.L.; Rong, J.X. Defective enamel and bone development in sodium-dependent citrate transporter (NaCT) Slc13a5 deficient mice. PLoS ONE 2017, 12, e0175465. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Morales, J.; Verdugo-Escamilla, C.; Fernández-Penas, R.; Parra-Milla, C.M.; Drouet, C.; Maube-Bosc, F.; Oltolina, F.; Prat, M.; Fernández-Sánchez, J.F. Luminescent biomimetic citrate–coated europium doped carbonated apatite nanoparticles for use in bioimaging: Physico-chemistry and cytocompatibility. RSC Adv. 2018, 8, 2385–2397. [Google Scholar] [CrossRef]
- Feng, W.; Wee Beng, T.; Yong, Z.; Xianping, F.; Minquan, W. Luminescent nanomaterials for biological labelling. Nanotechnology 2006, 17, R1–R13. [Google Scholar] [CrossRef]
- Piccirillo, C.; Adamiano, A.; Tobaldi, D.M.; Montalti, V.; Manzi, J.; Lima Castro, P.M.; Panseri, S.; Montesi, M.; Sprio, S.; Tampieri, A.; et al. Luminescent calcium phosphate bioceramics doped with europium derived from fish industry byproducts. J. Am. Ceram. Soc. 2017, 100, 3402–3414. [Google Scholar] [CrossRef]
- Tampieri, A.; Celotti, G.; Landi, E.; Sandri, M.; Roveri, N.; Falini, G. Biologically inspired synthesis of bone-like composite: Self-assembled collagen fibers/hydroxyapatite nanocrystals. J. Biomed. Mater. Res. Part A 2003, 67, 618–625. [Google Scholar] [CrossRef]
- Tampieri, A.; Sandri, M.; Landi, E.; Pressato, D.; Francioli, S.; Quarto, R.; Martin, I. Design of graded biomimetic osteochondral composite scaffolds. Biomaterials 2008, 29, 3539–3546. [Google Scholar] [CrossRef]
- Giordano, S.; Ponzetto, C.; Di Renzo, M.F.; Cooper, C.S.; Comoglio, P.M. Tyrosine kinase receptor indistinguishable from the c-met protein. Nature 1989, 339, 155–156. [Google Scholar] [CrossRef]
- Zamperone, A.; Pietronave, S.; Merlin, S.; Colangelo, D.; Ranaldo, G.; Medico, E.; Di Scipio, F.; Berta, G.N.; Follenzi, A.; Prat, M. Isolation and characterization of a spontaneously immortalized multipotent mesenchymal cell line derived from mouse subcutaneous adipose tissue. Stem Cells Dev. 2013, 22, 2873–2884. [Google Scholar] [CrossRef]
- Sugiura, Y.; Makita, Y. Sodium Induces Octacalcium Phosphate Formation and EnhancesIts Layer Structure by Affecting the Hydrous Layer Phosphate. Cryst. Growth Des. 2018, 18, 6165–6171. [Google Scholar] [CrossRef]
- Sugiura, Y.; Makita, Y. Ammonium Substitutional Solid Solution of Octacalcium Phosphate (OCP). Chem. Lett. 2018, 47, 1371–1374. [Google Scholar] [CrossRef]
- López-Macipe, A.; Gómez-Morales, J.; Rodríguez-Clemente, R. Nanosized hydroxyapatite precipitation from homogeneous calcium/citrate/phosphate solutions using microwave and conventional heating. Adv. Mater. 1998, 10, 49–53. [Google Scholar] [CrossRef]
- Torrent-Burgués, J.; Gómez-Morales, J.; López-Macipe, A.; Rodríguez-Clemente, R. Continuous precipitation of hydroxyapatite from Ca/citrate/phosphate solutions using microwave heating. Cryst. Res. Technol. 1999, 34, 757–762. [Google Scholar] [CrossRef]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Bi, X.; Li, G.; Doty, S.B.; Camacho, N.P. A novel method for determination of collagen orientation in cartilage by Fourier transform infrared imaging spectroscopy (FT–IRIS). Osteoarthr. Cartil. 2005, 13, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Lakshmana, B.R. Infrared Absorption spectrum of sodium citrate. J. Indian Inst. Sci. 1957, 39A, 27–29. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristics Group Frequencies: Tables and Charts, 3rd ed.; John Wiley and Sons, Ltd.: Chichester, UK, 2001. [Google Scholar]
- Nguyen, T.T.; Gobinet, C.; Lakshmana, B.R.; Feru, J.; Brassart-Pasco, S.; Manfait, M.; Piot, O. Characterization of type I and IV collagens by Raman microspectroscopy: identification of spectral markers of the dermo-epidermal junction. Spectrosc. Int. J. 2012, 27, 421–427. [Google Scholar] [CrossRef]
- Chatzipanagis, K.; Iafisco, M.; Roncal-Herrero, T.; Bilton, M.; Tampieri, A.; Kröger, R.; Delgado-López, J.M. Crystallization of citrate-stabilized amorphous calcium phosphate to nanocrystalline apatite: A surface–mediated transformation. CrystEngComm 2016, 18, 3170–3173. [Google Scholar] [CrossRef]
- Long, C. (Ed.) Biochemists’ Handbook; Spon: London, UK, 1971. [Google Scholar]
- López-Macipe, A.; Gómez-Morales, J.; Rodríguez Clemente, R. The Role of pH in the Adsorption of Citrate Ions on Hydroxyapatite. J. Colloid Interface Sci. 1998, 200, 114–120. [Google Scholar] [CrossRef]
- Hemmilä, I.; Dakubu, S.; Mukkala, V.-M.; Siitaria, H.; Lövgrena, T. Europium as a label in time-resolved immunofluorometric assays. Anal. Biochem. 1984, 137, 335–343. [Google Scholar] [CrossRef]
- Siqueira, K.P.F.; Lima, P.P.; Ferreira, R.A.S.; Carlos, L.D.; Bittar, E.M.; Matinaga, F.M.; Paniago, R.; Krambrock, K.; Moreira, R.L.; Dias, A. Influence of the Matrix on the Red Emission in Europium Self-Activated Orthoceramics. J. Phys. Chem. C 2015, 119, 17825–17835. [Google Scholar] [CrossRef]
- Siqueira, K.P.F.; Lima, P.P.; Ferreira, R.A.S.; Carlos, R.D.; Bittar, E.M.; Granado, E.; González, J.C.; Abelenda, A.; Moreira, R.L.; Dias, A. Lanthanide Orthoantimonate Light Emitters: Structural, Vibrational, and Optical Properties. Chem. Mater. 2014, 26, 6351–6360. [Google Scholar] [CrossRef]
- Richardson, F.S. Terbium(III) and europium(III) ions as luminescent probes and stains for biomolecular systems. Chem. Rev. 1982, 82, 541–552. [Google Scholar] [CrossRef]
- Huang, R.H.; Huang, P.R.H. Phosphorescence of five amino acids. Prog. Biochem. Biophys. 1997, 24, 60–63. [Google Scholar]
- Ward, J.L.; Walden, G.L.; Winefordner, J.D. A review of recent uses of phosphorimetry for organic analysis. Talanta 1981, 28, 201–206. [Google Scholar] [CrossRef]
- Zollfrank, C.; Scheel, H.; Brungs, S.; Greil, P. Europium(III) Orthophosphates: Synthesis, characterization, and optical Properties. Cryst. Growth Des. 2008, 8, 766–770. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 2nd ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999; pp. 130–131. ISBN 978-0-387-46312-4. [Google Scholar]
- ISO 10993-5:2009. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. Available online: https://www.iso.org/standard/36406.html (accessed on 22 December 2018).
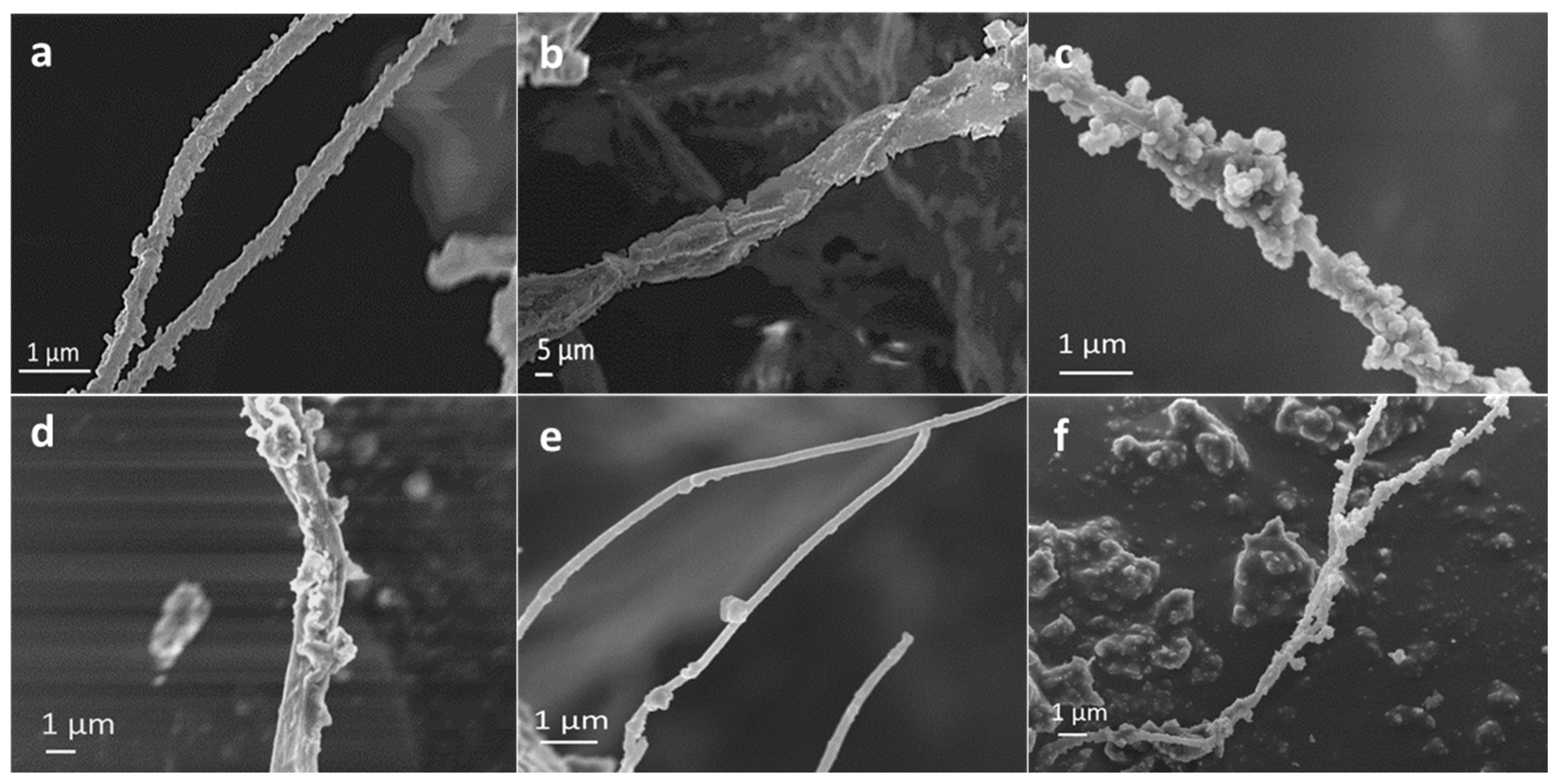
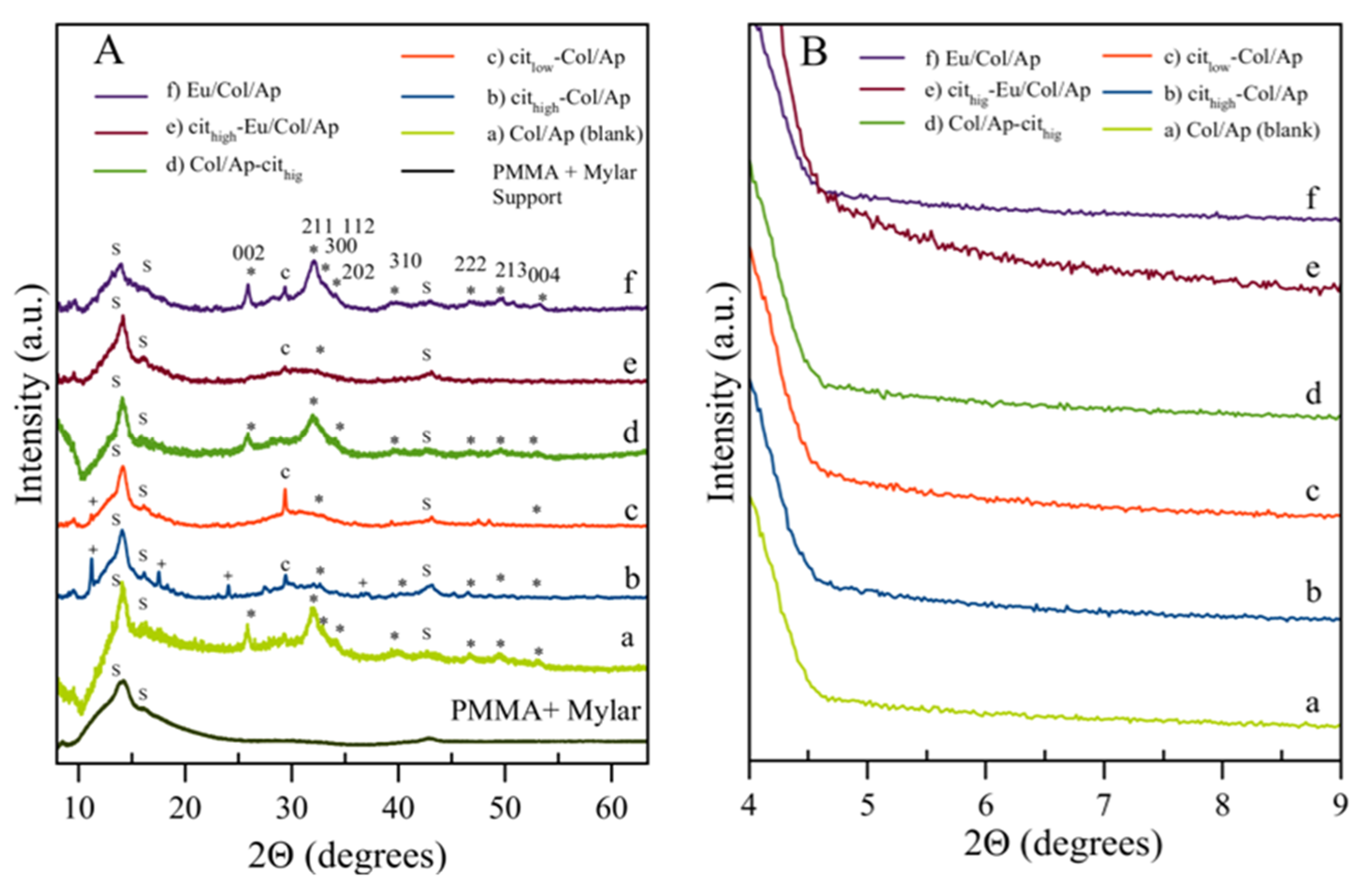

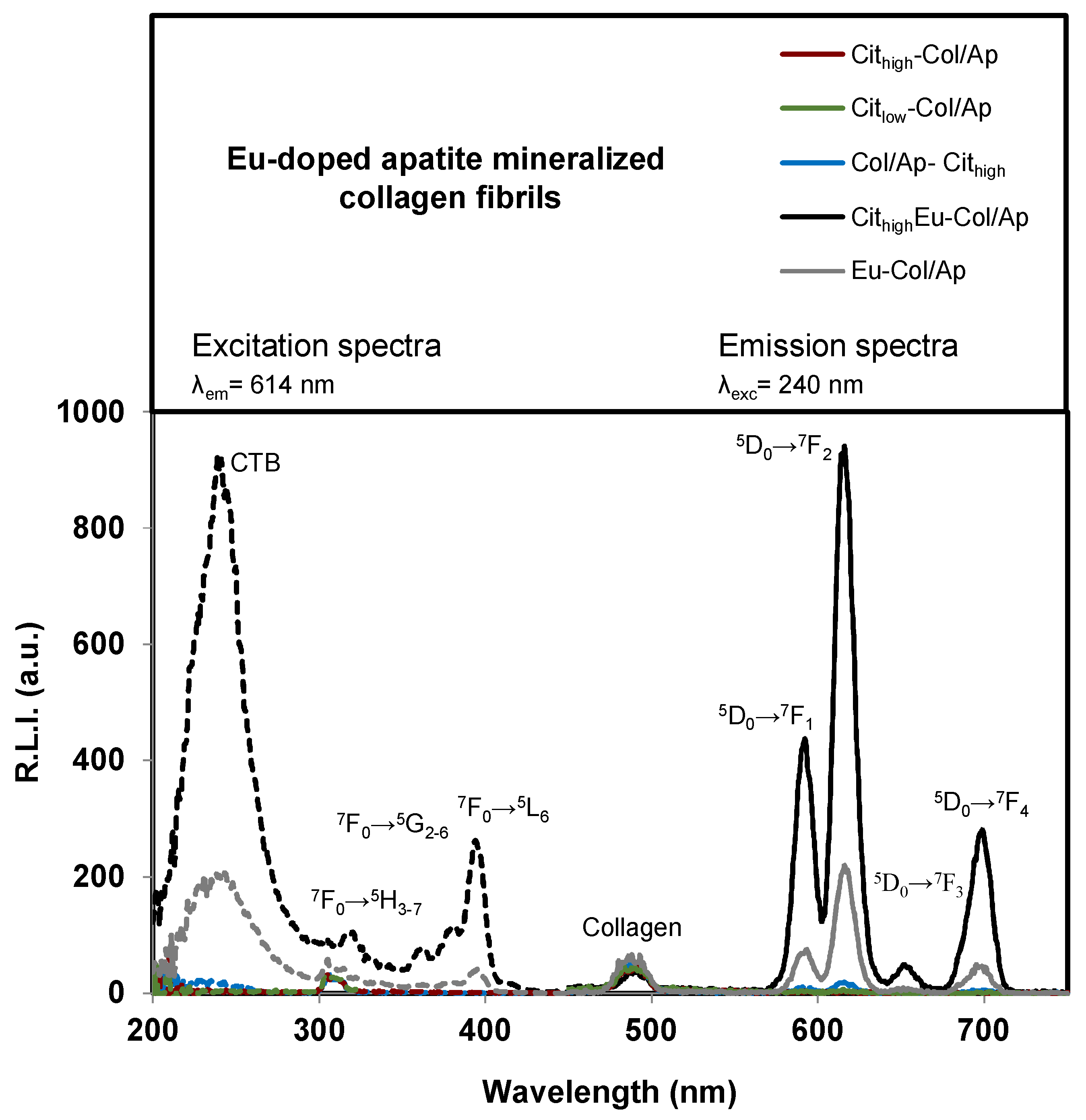
| Sample Identificación | Basic Titrant Solution | Mineral Phase | pHs (Initial; Final) | Eu (wt %) | Collagen (wt %) | Citrate (wt %) | Inorganic Residues (wt %) | Carbo–nate (wt %) |
|---|---|---|---|---|---|---|---|---|
| a. Col/Ap | 0.495 M Ca(OH)2 | Ap | 12.5; 7.3 | 0 | 7.6 ± 0.8 | 0 | 83.5 ± 8.0 | 3.1 ± 0.3 |
| b. cithigh–Col/Ap | 0.495 M Ca(OH)2 + +0.990 M Na3cit | ACP | 13.5; 7.3 | 0 | 23.8 ± 2.0 | 5.3 ± 0.5 | 50.7 ± 5.0 | 8.5 ± 0.9 |
| c. citlow–Col/Ap | 0.495 M Ca(OH)2 + 0.495 M Na3cit | ACP | 13.6; 7.1 | 0 | 34.7 ± 3.0 | 1.7 ± 0.2 | 59.3 ± 6.0 | 1.5 ± 0.2 |
| d. Col/Ap–cithigh | 0.495 M Ca(OH)2 +0.990 M Na3cit* | Ap | 12.5; 9.8 | 0 | 13.8 ± 2.0 | 0.7 ± 0.1 | 76.6 ± 8.0 | 3.9 ± 0.4 |
| e. cithigh–Eu/Col/Ap | 0.495 M Ca(OH)2 + +0.990 M Na3cit+ +0.010 M EuCl3 | ACP | 13.4; 7.2 | 0.6 ± 0.3 | 21.6 ± 2.0 | 5.9 ± 0.6 | 51.3 ± 5.0 | 8.1 ± 0.8 |
| f. Eu/Col/Ap | 0.495 M Ca(OH)2 + +0.010 M EuCl3 | Ap | 13.03; 7.3 | 2.4 ± 0.2 | 7.5 ± 0.8 | 0 | 79.5 ± 8.0 | 1.6 ± 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez Morales, J.; Fernández Penas, R.; Verdugo-Escamilla, C.; Degli Esposti, L.; Oltolina, F.; Prat, M.; Iafisco, M.; Fernández Sánchez, J.F. Bioinspired Mineralization of Type I Collagen Fibrils with Apatite in Presence of Citrate and Europium Ions. Crystals 2019, 9, 13. https://doi.org/10.3390/cryst9010013
Gómez Morales J, Fernández Penas R, Verdugo-Escamilla C, Degli Esposti L, Oltolina F, Prat M, Iafisco M, Fernández Sánchez JF. Bioinspired Mineralization of Type I Collagen Fibrils with Apatite in Presence of Citrate and Europium Ions. Crystals. 2019; 9(1):13. https://doi.org/10.3390/cryst9010013
Chicago/Turabian StyleGómez Morales, Jaime, Raquel Fernández Penas, Cristóbal Verdugo-Escamilla, Lorenzo Degli Esposti, Francesca Oltolina, Maria Prat, Michele Iafisco, and Jorge Fernando Fernández Sánchez. 2019. "Bioinspired Mineralization of Type I Collagen Fibrils with Apatite in Presence of Citrate and Europium Ions" Crystals 9, no. 1: 13. https://doi.org/10.3390/cryst9010013
APA StyleGómez Morales, J., Fernández Penas, R., Verdugo-Escamilla, C., Degli Esposti, L., Oltolina, F., Prat, M., Iafisco, M., & Fernández Sánchez, J. F. (2019). Bioinspired Mineralization of Type I Collagen Fibrils with Apatite in Presence of Citrate and Europium Ions. Crystals, 9(1), 13. https://doi.org/10.3390/cryst9010013