Crystal Structure of the Catalytic Domain of MCR-1 (cMCR-1) in Complex with d-Xylose
Abstract
:1. Introduction
2. Materials and Methods
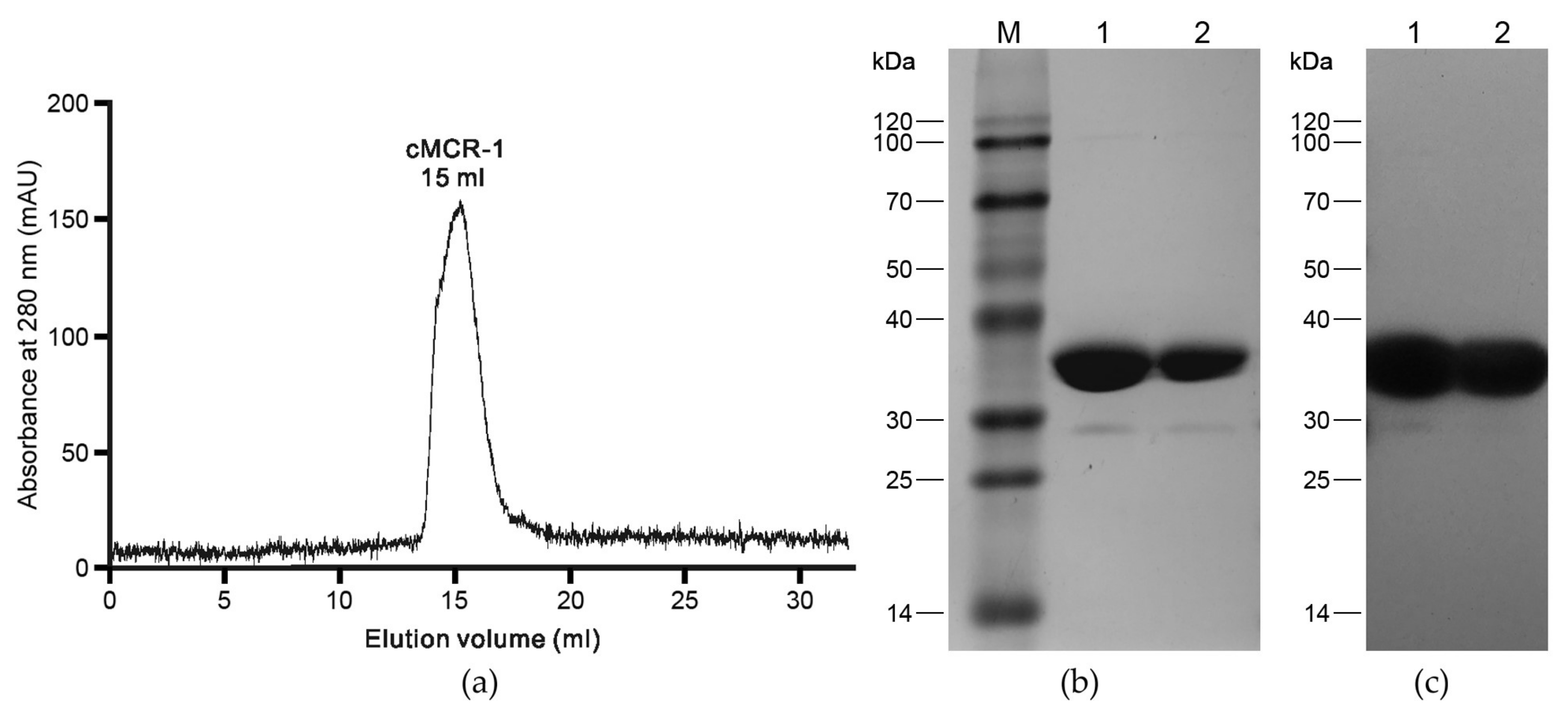
2.1. Recombinant cMCR-1 Production
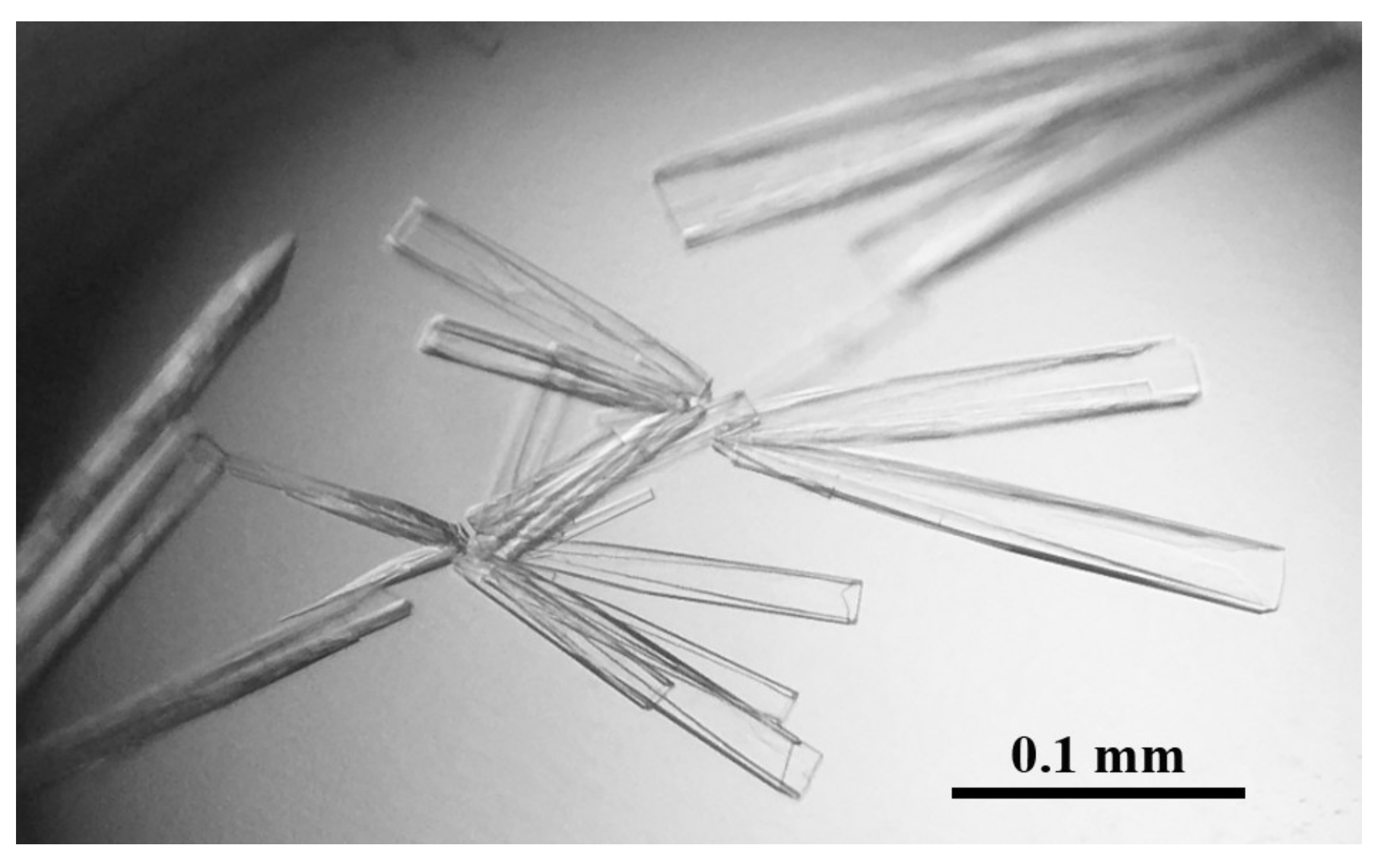
2.2. Crystallization
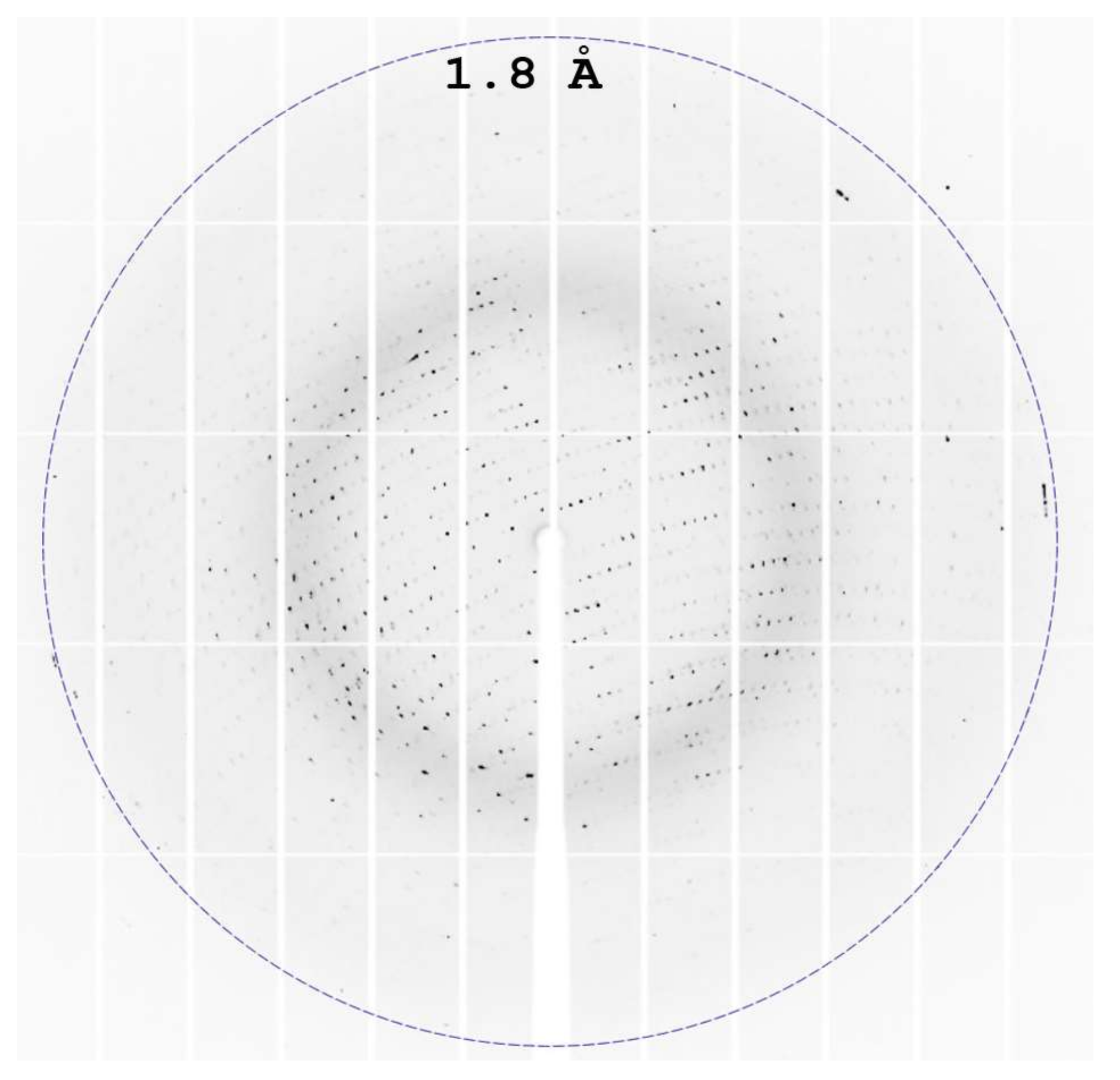
2.3. Data Collection, Structure Solution, and Refinement
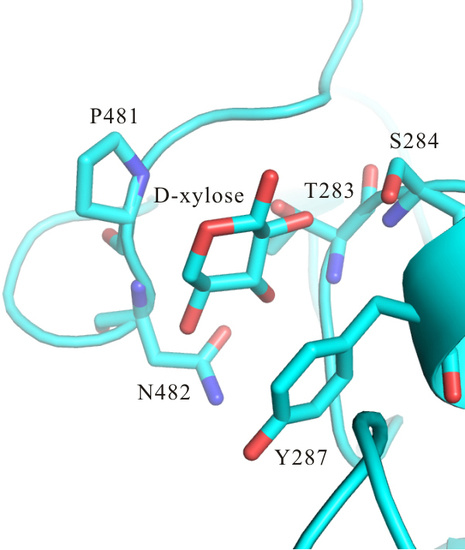
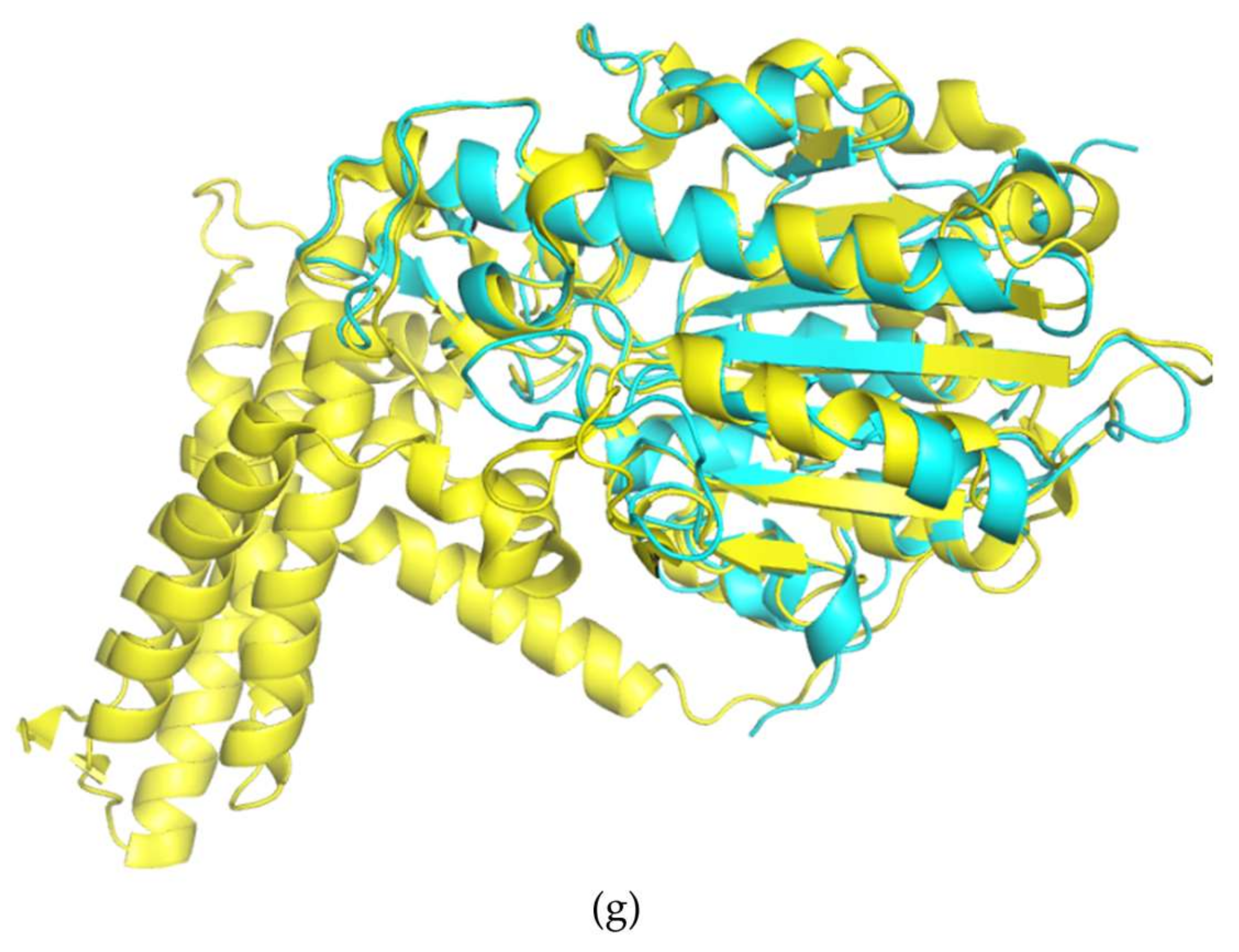
3. Results and Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Logan, L.K.; Weinstein, R.A. The epidemiology of carbapenem-resistant Enterobacteriaceae: The impact and evolution of a global menace. J. Infect. Dis. 2017, 215, S28–S36. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Jayol, A.; Nordmann, P. Polymyxins: Antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Wang, Y.L.; Sun, Q.L.; Huang, Z.X.; Wang, H.Y.; Zhang, R.; Chen, G.X. Colistin resistance gene mcr-1 in gut flora of children. Int. J. Antimicrob. Agents 2017, 50, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Hinchliffe, P.; Yang, Q.E.; Portal, E.; Young, T.; Li, H.; Tooke, C.L.; Carvalho, M.J.; Paterson, N.G.; Brem, J.; Niumsup, P.R.; et al. Insights into the mechanistic basis of plasmid-mediated colistin resistance from crystal structures of the catalytic domain of MCR-1. Sci. Rep. 2017, 7, 39392. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Guo, J.; Cheng, Q.; Yang, Z.; Chan, E.W.C.; Chen, S.; Hao, Q. Crystal structure of Escherichia coli originated MCR-1, a phosphoethanolamine transferase for colistin resistance. Sci. Rep. 2016, 6, 38793. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Zhu, Y.; Yu, Z.; Ahmad, A.; Zhang, H. High resolution crystal structure of the catalytic domain of MCR-1. Sci. Rep. 2016, 6, 39540. [Google Scholar] [CrossRef] [PubMed]
- Stojanoski, V.; Sankaran, B.; Prasad, B.V.; Poirel, L.; Nordmann, P.; Palzkill, T. Structure of the catalytic domain of the colistin resistance enzyme MCR-1. BMC Biol. 2016, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Song, G.; Shi, M.; Zhou, Y.; Liu, Y.; Lei, J.; Chen, P.; Yin, L. Substrate analog interaction with MCR-1 offers insight into the rising threat of the plasmid-mediated transferable colistin resistance. FASEB J. 2018, 32, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Otwinowski, Z.; Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997, 276, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Battye, T.G.; Kontogiannis, L.; Johnson, O.; Powell, H.R.; Leslie, A.G. iMOSFLM: A new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 271–281. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Joosten, R.P.; Long, F.; Murshudov, G.N.; Perrakis, A. The PDB_REDO server for macromolecular structure model optimization. IUCrJ 2014, 1, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.B.; Arendall, W.B.; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Anandan, A.; Evans, G.L.; Condic-Jurkic, K.; O’Mara, M.L.; John, C.M.; Phillips, N.J.; Jarvis, G.A.; Wills, S.S.; Stubbs, K.A.; Moraes, I.; et al. Structure of a lipid A phosphoethanolamine transferase suggests how conformational changes govern substrate binding. Proc. Natl. Acad. Sci. USA 2017, 114, 2218–2223. [Google Scholar] [CrossRef] [PubMed]
- Holm, L.; Laakso, L.M. Dali server update. Nucleic Acids Res. 2016, 44, W351–W355. [Google Scholar] [CrossRef] [PubMed]

| Source | Escherichia coli |
|---|---|
| DNA | Synthesized DNA |
| Forward primer 1 | 5′-CATGCCATGGCCAAAAGATACCATTTATCAC-3′ |
| Reverse primer 2 | 5′-CCCTCGAGGCGGATGAATGCGGTGCGGTC-3′ |
| Expression vector | pET-28a(+) |
| Host | E. coli BL21(DE3)pLysS |
| Recombinant protein sequence 3 | MGPKDTIYHAKDAVQATKPDMRKPRLVVFVVGETARADHVSFNGYERDTFPQLAKIDGVTNFSNVTSCGTSTAYSVPCMFSYLGADEYDVDTAKYQENVLDTLDRLGVSILWRDNNSDSKGVMDKLPKAQFADYKSATNNAICNTNPYNECRDVGMLVGLDDFVAANNGKDMLIMLHQMGNHGPAYFKRYDEKFAKFTPVCEGNELAKCEHQSLINAYDNALLATDDFIAQSIQWLQTHSNAYDVSMLYVSDHGESLGENGVYLHGMPNAFAPKEQRSVPAFFWTDKQTGITPMATDTVLTHDAITPTLLKLFDVTADKVKDRTAFIRLEHHHHHH |
| Diffraction Source | BL17U1, SSRF |
|---|---|
| Wavelength (Å) | 0.9792 |
| Temperature (K) | 100 |
| Detector | ADSC Q315r |
| Crystal-to-detector distance (mm) | 350 |
| Total rotation range (°) | 360 |
| Rotation range per image (°) | 1.0 |
| Exposure time per image (s) | 0.5 |
| Space group | P212121 |
| a, b, c (Å) | 51.6, 73.1, 82.2 |
| α, β, γ (°) | 90, 90, 90 |
| Resolution range (Å) | 43.71–1.82 (1.88–1.82) 1 |
| Total number of reflections | 56014 (5514) |
| Number of unique reflections | 28258 (2789) |
| Mosaicity (°) | 0.5 |
| Multiplicity | 2.0 (2.0) |
| Completeness (%) | 99.0 (99.0) |
| Mean I/σ(I) | 10.61 (4.94) |
| Rmerge (%) | 3.5 (11.1) |
| CC1/2 | 0.997 (0.949) |
| Wilson plot overall B factor (Å2) | 13.74 |
| Reflection number, working set | 28223 (2785) |
| Reflection number, test set | 1997 (196) |
| Rwork | 0.139 |
| Rfree | 0.178 |
| Ramachandran favored region (%) | 98 |
| Ramachandran allowed region (%) | 1.75 |
| Ramachandran outliers (%) | 0.25 |
| Rotamer outliers (%) | 0.65 |
| R.m.s.d. bond lengths (Å) | 0.006 |
| R.m.s.d. bond angles (°) | 0.86 |
| Average B factor (Å2) | 17.55 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.-X.; Han, Z.; Yu, X.-L.; Wen, G.; Zeng, C. Crystal Structure of the Catalytic Domain of MCR-1 (cMCR-1) in Complex with d-Xylose. Crystals 2018, 8, 172. https://doi.org/10.3390/cryst8040172
Liu Z-X, Han Z, Yu X-L, Wen G, Zeng C. Crystal Structure of the Catalytic Domain of MCR-1 (cMCR-1) in Complex with d-Xylose. Crystals. 2018; 8(4):172. https://doi.org/10.3390/cryst8040172
Chicago/Turabian StyleLiu, Zhao-Xin, Zhenggang Han, Xiao-Li Yu, Guoyuan Wen, and Chi Zeng. 2018. "Crystal Structure of the Catalytic Domain of MCR-1 (cMCR-1) in Complex with d-Xylose" Crystals 8, no. 4: 172. https://doi.org/10.3390/cryst8040172
APA StyleLiu, Z.-X., Han, Z., Yu, X.-L., Wen, G., & Zeng, C. (2018). Crystal Structure of the Catalytic Domain of MCR-1 (cMCR-1) in Complex with d-Xylose. Crystals, 8(4), 172. https://doi.org/10.3390/cryst8040172