Synthesis, Crystal Structures, Optical Properties and Theoretical Calculations of Two Metal Chalcogenides Ba2AlSbS5 and Ba2GaBiSe5
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. Ba2AlSbS5
2.1.2. Ba2GaBiSe5
2.2. Structure Determination
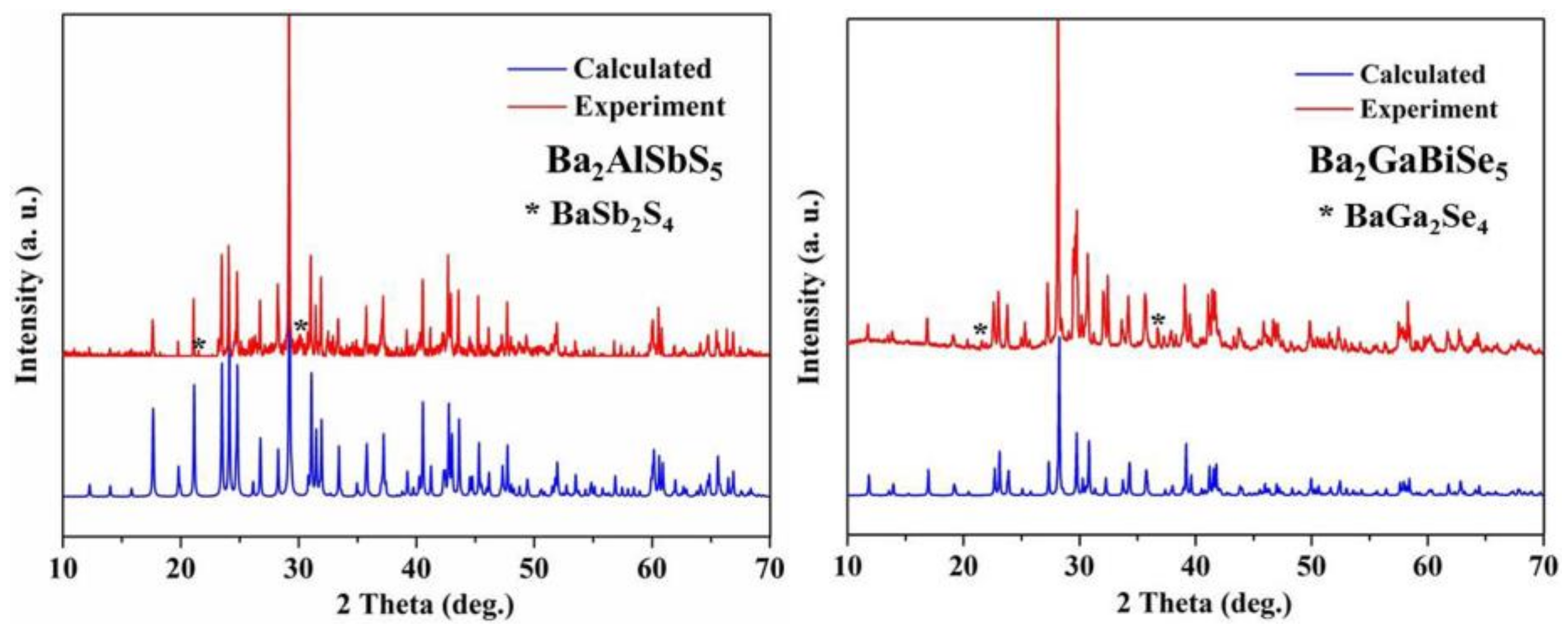
2.3. Powder XRD Measurement
2.4. UV–Vis–NIR Diffuse-Reflectance Spectroscopy
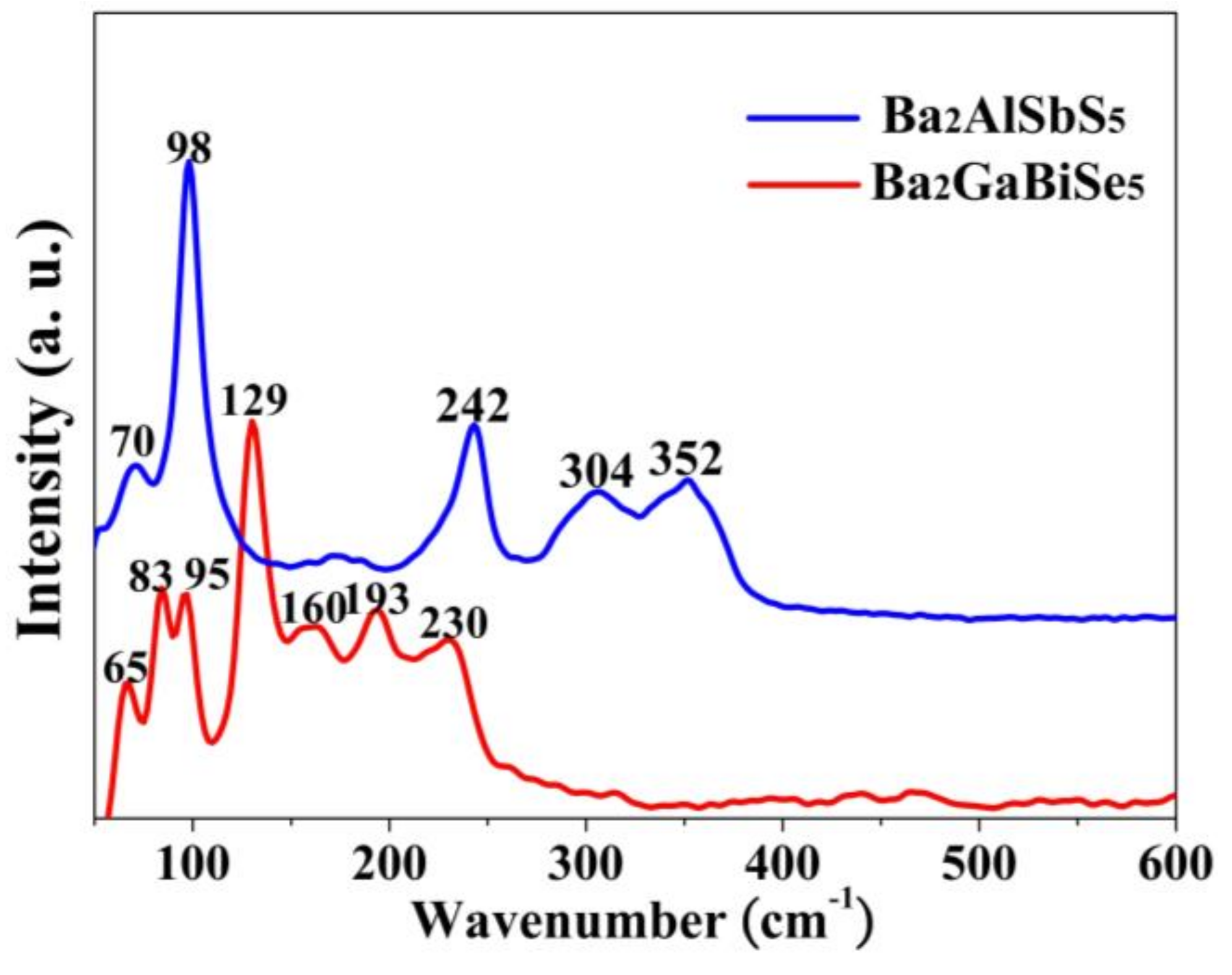
2.5. Raman Spectroscopy
2.6. Theoretical Calculation
3. Results and Discussion
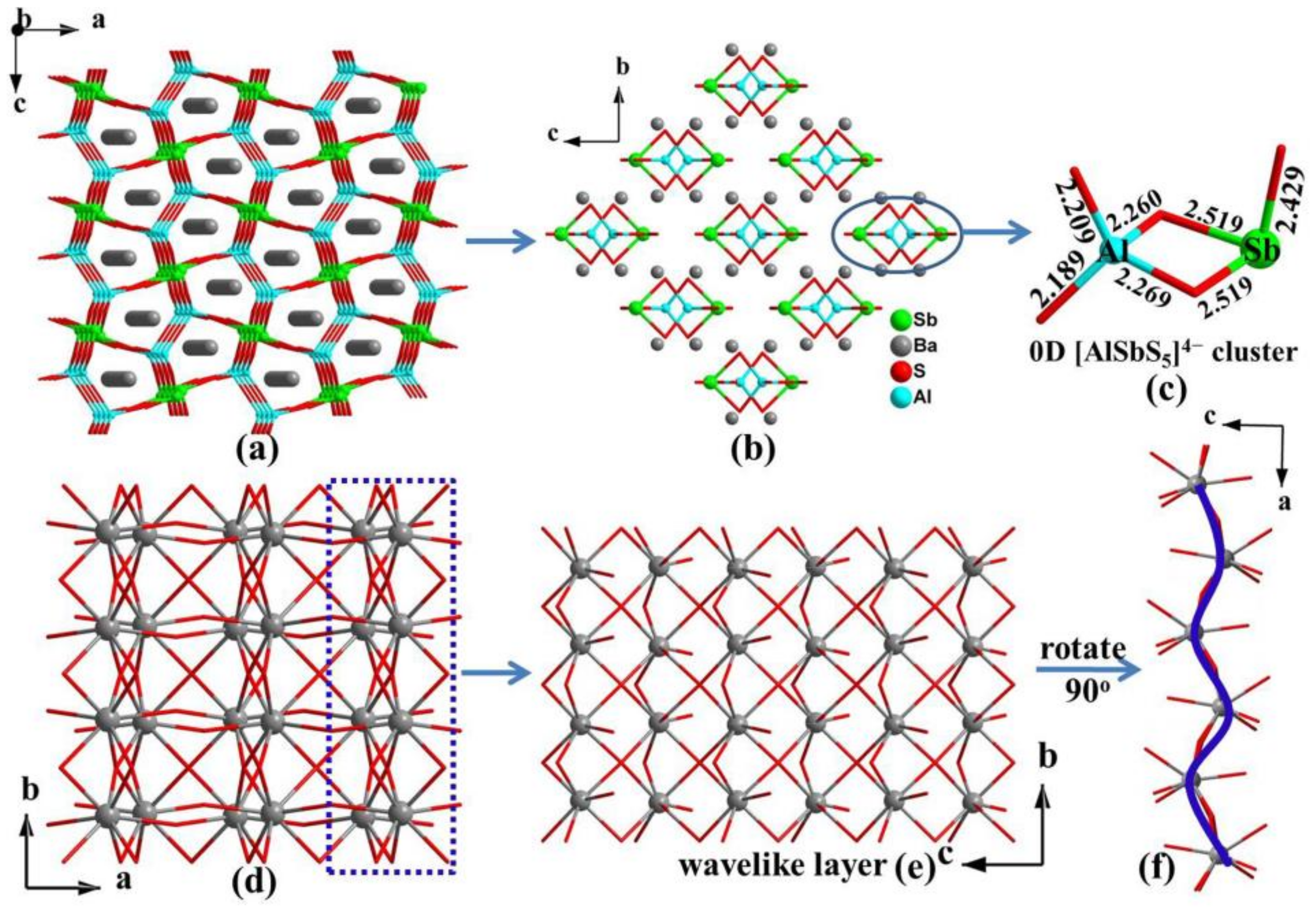
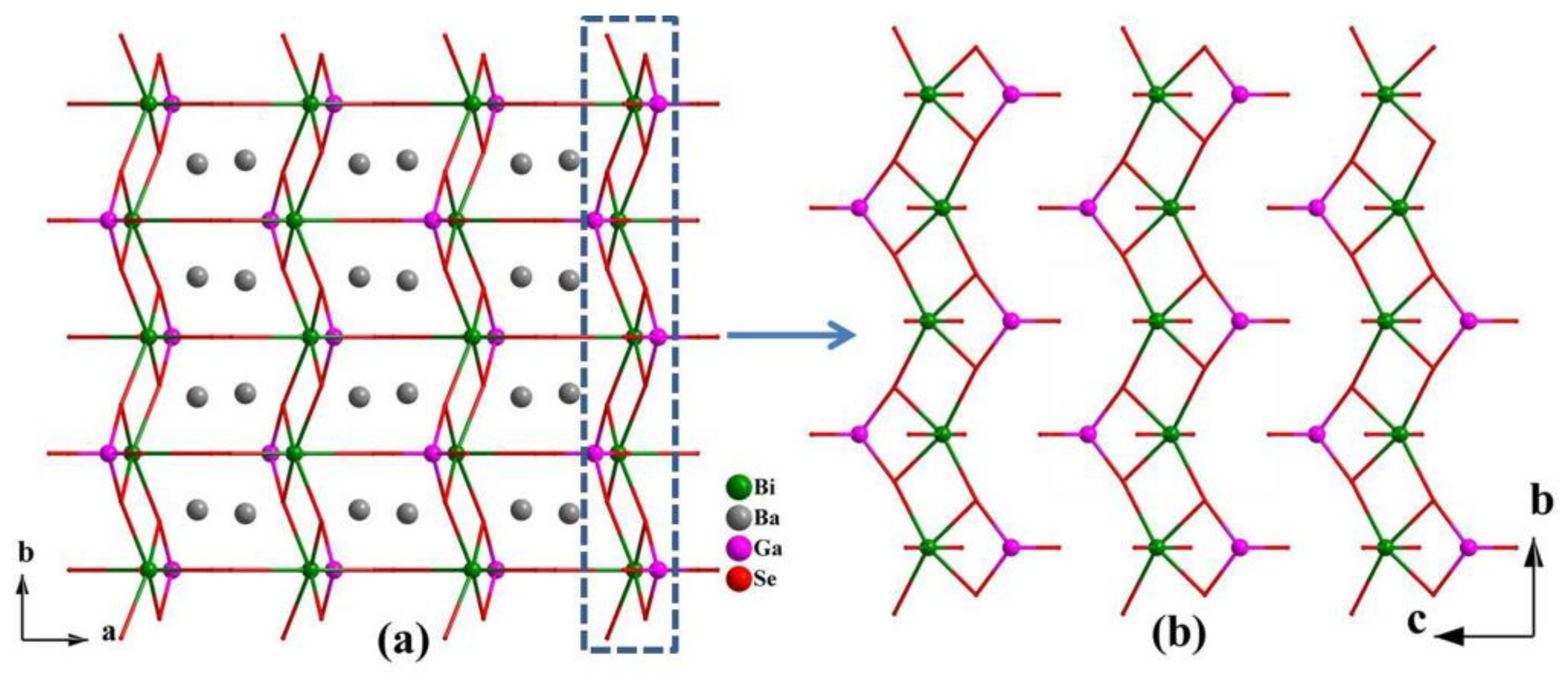
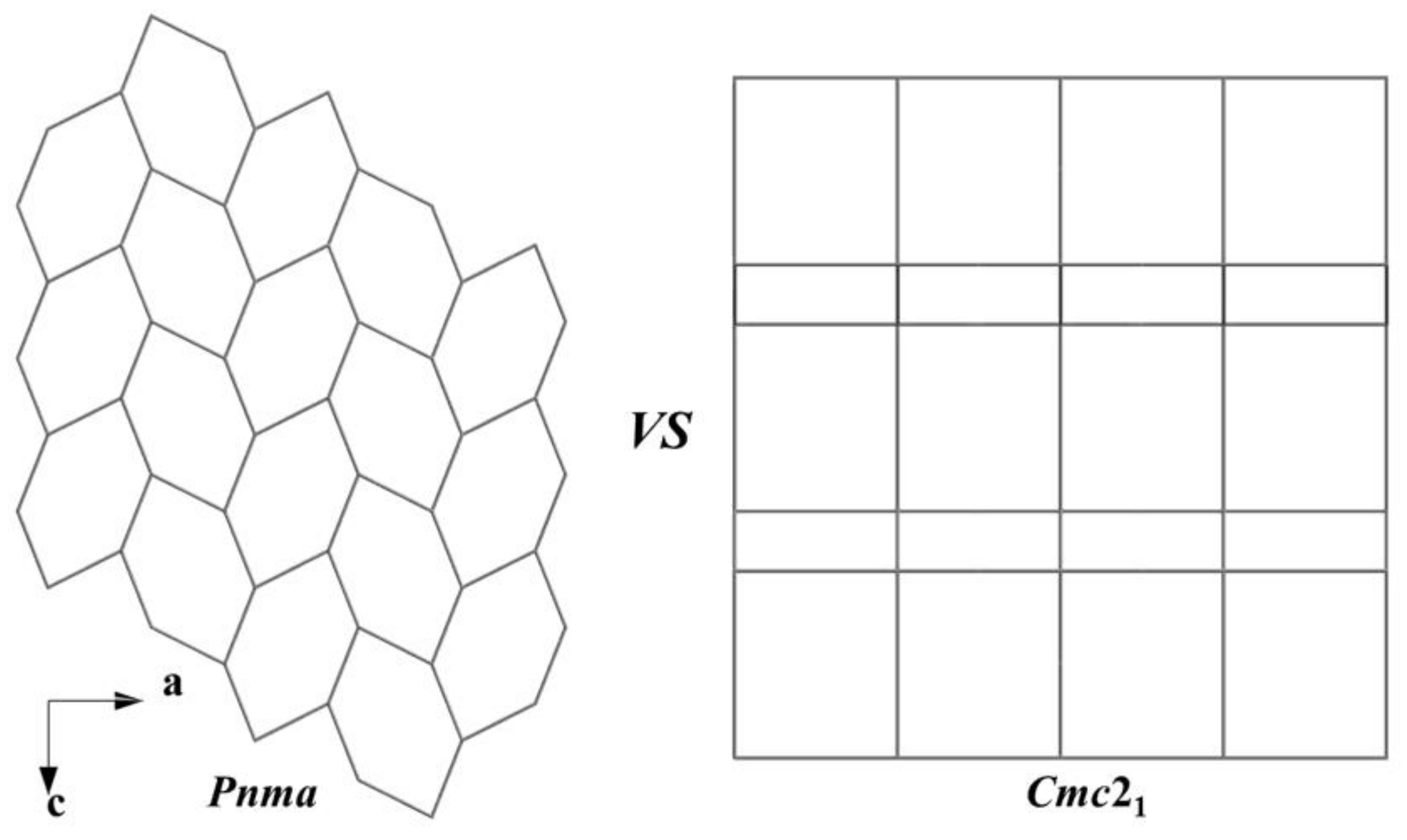
3.1. Crystal Structure
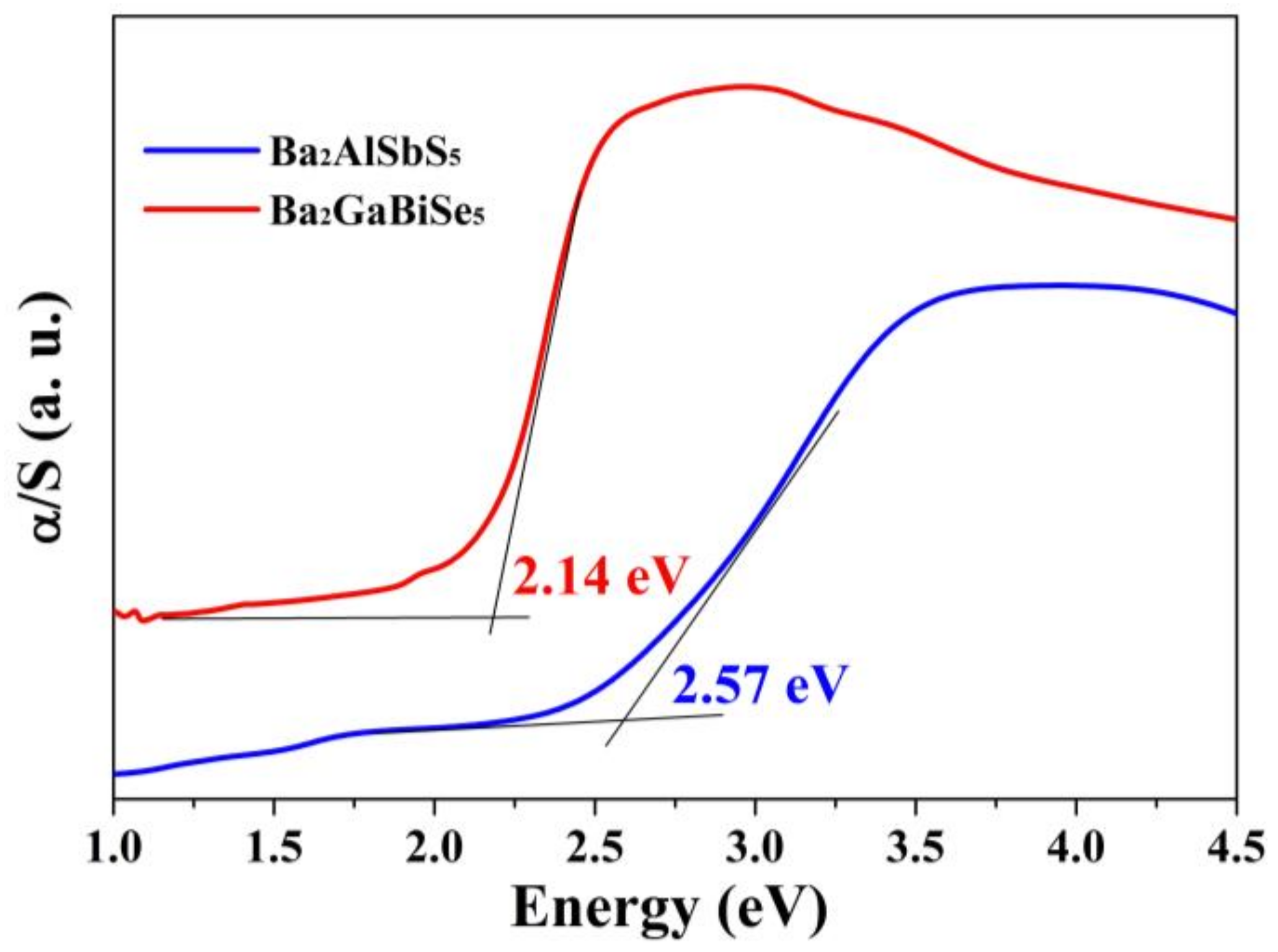
3.2. Optical Properties
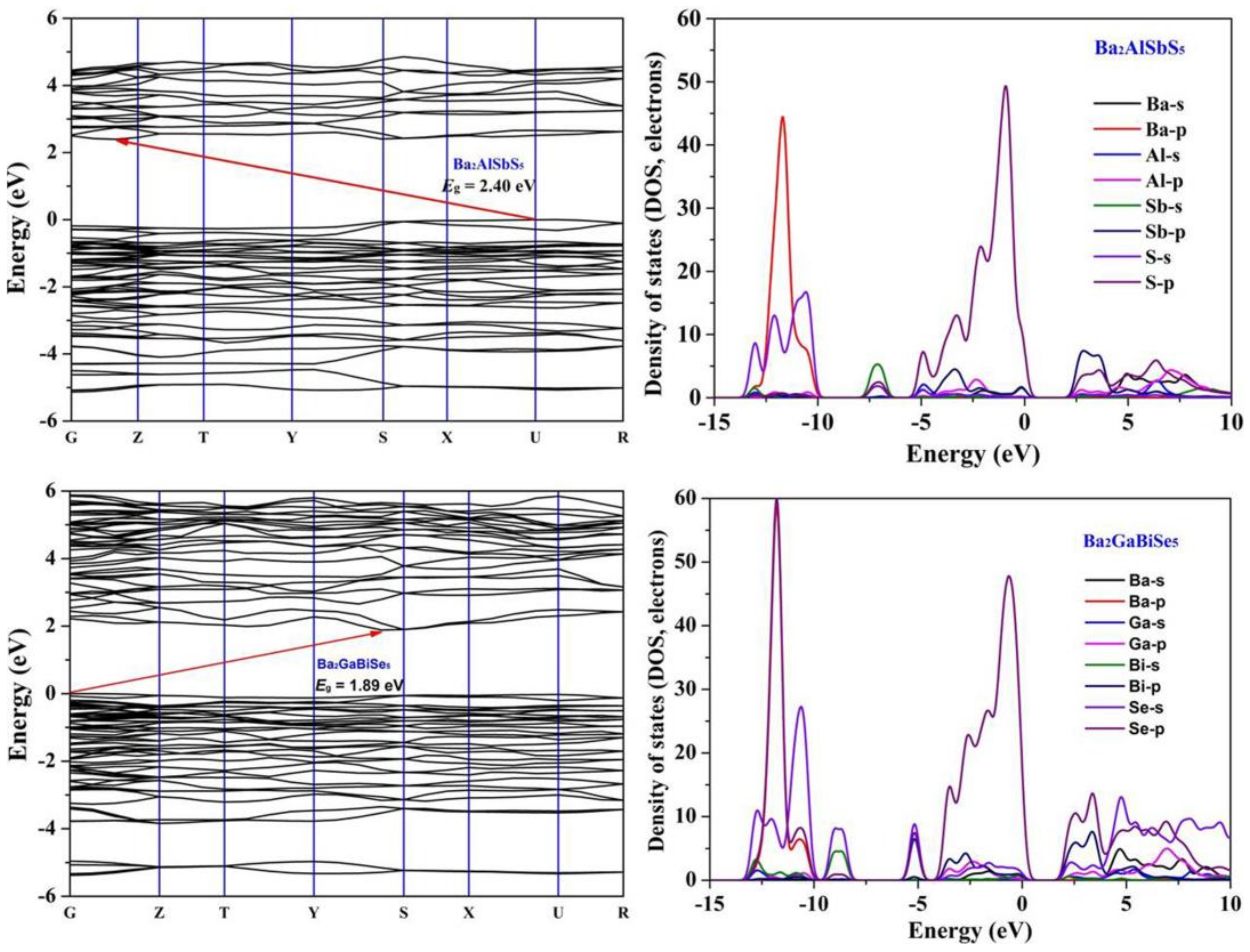
3.3. Theoretical Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kwasi, M.; Ibers, J.A. Rare-earth transition-metal chalcogenides. Chem. Rev. 2002, 102, 1929–1952. [Google Scholar]
- Chung, I.; Kanatzidis, M.G. Metal chalcogenides: A rich source of nonlinear optical materials. Chem. Mater. 2014, 26, 849–869. [Google Scholar] [CrossRef]
- Liang, F.; Kang, L.; Lin, Z.S.; Wu, Y.C.; Chen, C.T. Analysis and prediction of Mid-ir nonlinear optical metal sulfides with diamond-like structures. Coord. Chem. Rev. 2017, 333, 57–70. [Google Scholar] [CrossRef]
- Guo, S.P.; Chi, Y.; Guo, G.C. Recent achievements on middle and far-infrared second-order nonlinear optical materials. Coord. Chem. Rev. 2017, 335, 44–57. [Google Scholar] [CrossRef]
- Wu, K.; Pan, S.L. Li2HgMS4 (M = Si, Ge, Sn): New quaternary diamond-like semiconductors for infrared laser frequency conversion. Crystals 2017, 7, 107. [Google Scholar] [CrossRef]
- Wu, K.; Yang, Z.H.; Pan, S.L. Na2BaMQ4 (M = Ge, Sn; Q = S, Se): Infrared nonlinear optical materials with excellent performances and that undergo structural transformations. Angew. Chem. Int. Ed. 2016, 55, 6713–6715. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Chen, L.; Yu, J.S.; Chen, H.; Wu, L.M. Infrared SHG materials CsM3Se6 (M = Ga/Sn, In/Sn): Phase matchability controlled by dipole moment of the asymmetric building unit. Chem. Mater. 2017, 29, 499–503. [Google Scholar] [CrossRef]
- Luo, Z.Z.; Lin, C.S.; Cui, H.H.; Zhang, W.L.; Zhang, H.; He, Z.Z.; Cheng, W.D. SHG materials SnGa4Q7 (Q = S, Se) appearing with large conversion efficiencies, high damage thresholds, and wide transparencies in the mid-infrared region. Chem. Mater. 2014, 26, 2743–2749. [Google Scholar] [CrossRef]
- Liang, F.; Kang, L.; Lin, Z.S.; Wu, Y.C. Mid-infrared nonlinear optical materials based on metal chalcogenides: Structure–property relationship. Cryst. Growth Des. 2017, 17, 2254–2289. [Google Scholar] [CrossRef]
- Brant, J.A.; Clark, D.J.; Kim, Y.S.; Jang, J.I.; Zhang, J.H.; Aitken, J.A. Li2CdGeS4, a diamond-like semiconductor with strong second-order optical nonlinearity in the infrared and exceptional laser damage threshold. Chem. Mater. 2014, 26, 3045–3048. [Google Scholar] [CrossRef]
- Xia, Z.G.; Poeppelmeier, K.R. Chemistry-inspired adaptable framework structures. Acc. Chem. Res. 2017, 50, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Ok, K.M. Toward the rational design of novel noncentrosymmetric materials: Factors influencing the framework structures. Acc. Chem. Res. 2016, 49, 2774–2785. [Google Scholar] [CrossRef] [PubMed]
- Bera, T.K.; Jang, J.I.; Ketterson, J.B.; Kanatzidis, M.G. strong second harmonic generation from the tantalum thioarsenates A3Ta2AsS11 (A = K and Rb). J. Am. Chem. Soc. 2009, 131, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Li, L.H.; Chen, Y.B.; Chen, L. In-phase alignments of asymmetric building units in Ln4GaSbS9 (Ln = Pr, Nd, Sm, Gd− Ho) and their strong nonlinear optical responses in middle IR. J. Am. Chem. Soc. 2011, 133, 4617–4624. [Google Scholar] [CrossRef] [PubMed]
- Bera, T.K.; Jang, J.I.; Song, J.H.; Malliakas, C.D.; Freeman, A.J.; Ketterson, J.B.; Kanatzidis, M.G. Soluble semiconductors AAsSe2 (A = Li, Na) with a direct-band-gap and strong second harmonic generation: A combined experimental and theoretical study. J. Am. Chem. Soc. 2010, 132, 3484–3495. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J. Synthesis of heterometallic chalcogenides containing lanthanide and group 13–15 metal elements. Coord. Chem. Rev. 2016, 315, 112–134. [Google Scholar] [CrossRef]
- Li, C.; Li, X.S.; Huang, H.W.; Yao, J.Y.; Wu, Y.C. Ba2AsGaSe5: A new quaternary selenide with the novel [AsGaSe5]4− cluster and interesting photocatalytic properties. Inorg. Chem. 2015, 54, 9785–9789. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.Y.; Mei, D.J.; Yin, W.L.; Feng, K.; Yao, J.Y.; Wu, Y.C. Synthesis, structural characterization and optical properties of new compounds: Centrosymmetric Ba2GaMQ5 (M = Sb, Bi; Q = Se, Te), Ba2InSbTe5 and noncentrosymmetric Ba2InSbSe5. J. Solid State Chem. 2013, 198, 81–86. [Google Scholar] [CrossRef]
- Lin, C.S.; Luo, Z.Z.; Cheng, W.D.; Zhang, H.; Zhang, W.L. Design of SHG materials with mid-infrared transparency based on genetic engineering for Ba2BiInA5 (A = Se, Te). J. Mater. Chem. 2012, 22, 21713–21719. [Google Scholar] [CrossRef]
- Geng, L.; Zhang, H.; Cheng, W.D. Ba2SbGaS5: Solid-state synthesis, crystal and electronic structures, and property characterization. Chin. J. Struct. Chem. 2013, 32, 538–544. [Google Scholar]
- Geng, L.; Cheng, W.D.; Lin, C.S.; Zhang, W.L.; Zhang, H.; He, Z.Z. Syntheses and characterization of new mid-infrared transparency compounds: Centric Ba2BiGaS5 and acentric Ba2BiInS5. Inorg. Chem. 2011, 50, 5679–5686. [Google Scholar] [CrossRef] [PubMed]
- Iordanidis, L.; Brazis, P.W.; Kyratsi, T.; Ireland, J.; Lane, M.; Kannewurf, C.R.; Chen, W.; Dyck, J.S.; Uher, C.; Ghelani, N.A.; et al. A2Bi8Se13 (A = Rb, Cs), CsBi3.67Se6, and BaBi2Se4: New ternary semiconducting bismuth selenides. Chem. Mater. 2001, 13, 622–633. [Google Scholar] [CrossRef]
- Chung, D.Y.; Jobic, S.; Hogan, T.; Kannewurf, C.R.; Brec, R.; Rouxel, R.; Kanatzidis, M.G. Oligomerization versus polymerization of texn- in the polytelluride compound babite3. Structural characterization, electronic structure, and thermoelectric properties. J. Am. Chem. Soc. 1997, 119, 2505–2515. [Google Scholar] [CrossRef]
- Kim, J.H.; Chung, D.Y.; Bilc, D.; Loo, S.; Short, J.; Mahanti, S.D.; Hogan, T.; Kanatzidis, M.G. Crystal growth, thermoelectric properties, and electronic structure of AgBi3S5 and AgSbxBi3-xS5 (x = 0.3). Chem. Mater. 2005, 17, 3606–3614. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Shelxtl; Version 6.14; Bruker Analytical X-Ray Instruments, Inc.: Madison, WI, USA, 2008. [Google Scholar]
- Spek, A.L. Single-crystal structure validation with the program platon. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. First principles methods using castep. Z. Kristallogr. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3870. [Google Scholar] [CrossRef] [PubMed]
- Rappe, A.M.; Rabe, K.M.; Kaxiras, E.; Joannopoulos, J.D. Optimized pseudopotentials. Phys. Rev. B 1990, 41, 1227–1230. [Google Scholar] [CrossRef]
- Lin, J.S.; Qteish, A.; Payne, M.C.; Heine, V. Optimized and transferable non-local separable pseudopotentials. Phys. Rev. B 1993, 47, 4174–4178. [Google Scholar] [CrossRef]
- Godby, R.W.; Schluter, M.; Sham, L.J. Self-energy operators and exchange-correlation potentials in semiconductors. Phys. Rev. B 1988, 37, 10159–10175. [Google Scholar] [CrossRef]
- Schevciw, O.; White, W.B. The optical absorption edge of rare earth sesquisulfides and alkaline earth-rare earth sulfides. Mater. Res. Bull. 1983, 18, 1059–1068. [Google Scholar] [CrossRef]
- Boyd, G.D.; Storz, F.G.; McFee, J.H.; Kasper, H.M. Linear and nonlinear optical properties of some ternary selenides. IEEE J. Quantum Electron. 1972, 8, 900–908. [Google Scholar] [CrossRef]
- Boyd, G.D.; Buehler, E.; Storz, F.G. Linear and nonlinear optical properties of ZnGeP2 and CdSe. Appl. Phys. Lett. 1971, 18, 301–304. [Google Scholar] [CrossRef]
- Al-Bloushi, M.; Davaasuren, B.; Emwas, A.H.; Rothenberger, A. Synthesis and characterization of the quaternary thioaluminogermanates A(AlS2)(GeS2) (A = Na, K). Z. Anorg. Allg. Chem. 2015, 641, 1352–1356. [Google Scholar] [CrossRef]
- Yohannan, J.P.; Vidyasagar, K. Syntheses and characterization of one-dimensional alkali metal antimony (III) thiostannates (IV), A2Sb2Sn3S10 (A = K, Rb, Cs). J. Solid State Chem. 2015, 221, 426–432. [Google Scholar] [CrossRef]
- Kuo, S.M.; Chang, Y.M.; Chung, I.; Jang, J.I.; Her, B.H.; Yang, S.H.; Ketterson, J.B.; Kanatzidis, M.G.; Hsu, K.F. New metal chalcogenides Ba4CuGa5Q12 (Q = S, Se) displaying strong infrared nonlinear optical response. Chem. Mater. 2013, 25, 2427–2433. [Google Scholar] [CrossRef]
- Beck, J.; Schlüter, S.; Zotov, N. The cube-shaped main group element clusters (Bi4S4)4+ and (Bi4Se4)4+—Synthesis from chloroaluminate melts, crystal structures and vibrational spectra. Z. Anorg. Allg. Chem. 2004, 630, 2512–2519. [Google Scholar] [CrossRef]
- Wooten, F. Optical Properties of Solid; Academic Press: New York, NY, USA, 1972. [Google Scholar]
| Empirical Formula | Ba2AlSbS5 | Ba2GaBiSe5 |
|---|---|---|
| fw | 583.71 | 948.18 |
| crystal system | orthorhombic | orthorhombic |
| space group | Pnma | Pnma |
| a (Å) | 12.140(3) | 12.691(4) |
| b (Å) | 8.8911(17) | 9.190(3) |
| c (Å) | 8.9552(17) | 9.245(3) |
| Z, V (Å3) | 4, 966.6(4) | 4, 1078.2(6) |
| Dc (g/cm3) | 4.011 | 5.841 |
| μ (mm−1) | 11.922 | 42.757 |
| GOF on F2 | 1.080 | 0.997 |
| R1, wR2 (I > 2σ(I)) a | 0.0202, 0.0473 | 0.0273, 0.0499 |
| R1, wR2 (all data) | 0.0225, 0.0485 | 0.0371, 0.0535 |
| largest diff. peak and hole (e Å−3) | 1.351, −0.780 | 1.570, −1.648 |
| Compounds | Crystal System | Space Group | Anion Connection Mode | Ref. |
|---|---|---|---|---|
| Ba2GaSbS5 | orthorhombic | Pnma | ∞[SbS3]n chain and GaS4 | [20] |
| Ba2GaSbSe5 | orthorhombic | Pnma | ∞[SbSe3]n chain and GaSe4 | [18] |
| Ba2GaSbTe5 | orthorhombic | Pnma | ∞[SbTe3]n chain and GaTe4 | [18] |
| Ba2GaBiS5 | orthorhombic | Pnma | ∞[BiS3]n chain and GaS4 | [21] |
| Ba2GaBiSe5 | orthorhombic | Pnma | ∞[BiSe3]n chain and GaSe4 | [18], this work |
| Ba2GaBiTe5 | orthorhombic | Pnma | ∞[BiTe3]n chain and GaTe4 | [18] |
| Ba2InSbTe5 | orthorhombic | Pnma | ∞[SbTe3]n chain and InTe4 | [18] |
| Ba2InSbSe5 | orthorhombic | Cmc21 | [InS3]n and [SbS3]nchains | [18] |
| Ba2InBiS5 | orthorhombic | Cmc21 | [InS3]n and [BiS3]nchains | [21] |
| Ba2InBiSe5 | orthorhombic | Cmc21 | [InSe3]n and [BiSe3]nchains | [19] |
| Ba2GaAsSe5 | orthorhombic | Pnma | 0D [GaAsSe5]4− cluster | [17] |
| Ba2AlSbS5 | orthorhombic | Pnma | 0D [AlSbS5]4− cluster | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Gu, X.; Pan, H.; Hu, Y.; Wu, K. Synthesis, Crystal Structures, Optical Properties and Theoretical Calculations of Two Metal Chalcogenides Ba2AlSbS5 and Ba2GaBiSe5. Crystals 2018, 8, 165. https://doi.org/10.3390/cryst8040165
Wu X, Gu X, Pan H, Hu Y, Wu K. Synthesis, Crystal Structures, Optical Properties and Theoretical Calculations of Two Metal Chalcogenides Ba2AlSbS5 and Ba2GaBiSe5. Crystals. 2018; 8(4):165. https://doi.org/10.3390/cryst8040165
Chicago/Turabian StyleWu, Xiaowen, Xiaofeng Gu, Hui Pan, Yi Hu, and Kui Wu. 2018. "Synthesis, Crystal Structures, Optical Properties and Theoretical Calculations of Two Metal Chalcogenides Ba2AlSbS5 and Ba2GaBiSe5" Crystals 8, no. 4: 165. https://doi.org/10.3390/cryst8040165
APA StyleWu, X., Gu, X., Pan, H., Hu, Y., & Wu, K. (2018). Synthesis, Crystal Structures, Optical Properties and Theoretical Calculations of Two Metal Chalcogenides Ba2AlSbS5 and Ba2GaBiSe5. Crystals, 8(4), 165. https://doi.org/10.3390/cryst8040165





