Crystal Structural Determination of SrAlD5 with Corner-Sharing AlD6 Octahedron Chains by X-ray and Neutron Diffraction
Abstract
:1. Introduction
2. Materials and Methods
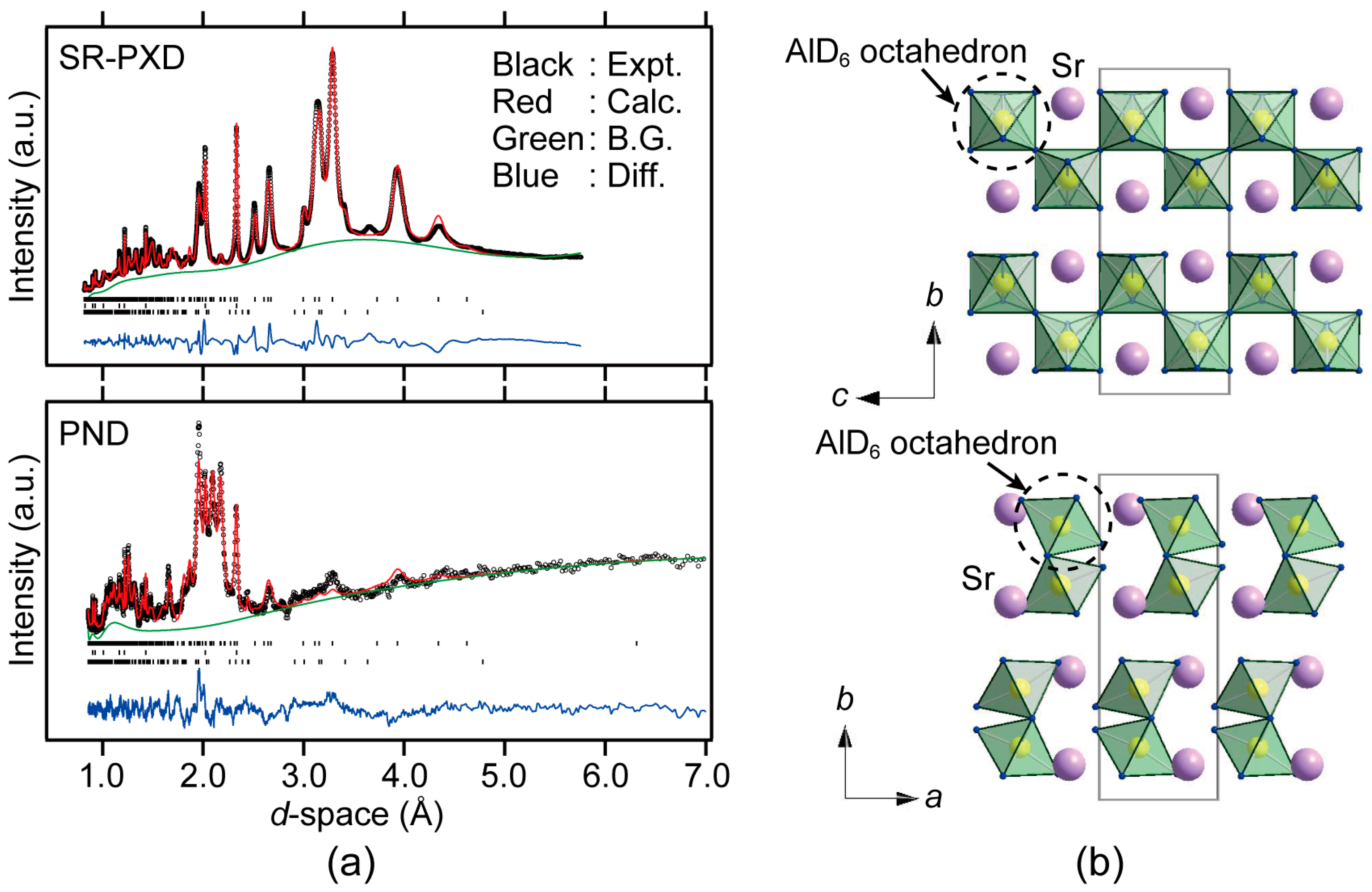
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bogdanović, B.; Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. J. Alloy. Compd. 1997, 253–254, 1–9. [Google Scholar] [CrossRef]
- Orimo, S.; Nakamori, Y.; Eliseo, R.J.; Züttel, A.; Jensen, C.M. Complex hydrides for hydrogen storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef] [PubMed]
- Hauback, B. Structures of aluminium–based light weight hydrides. Z. Krist. 2008, 223, 636–648. [Google Scholar] [CrossRef]
- Eberle, U.; Felderhoff, M.; Schüth, F. Chemical and physical solutions for hydrogen storage. Angew. Chem. Int. Ed. 2009, 48, 6608–6630. [Google Scholar] [CrossRef] [PubMed]
- Graetz, J.; Hauback, B.C. Recent developments in aluminum–based hydrides for hydrogen storage. MRS Bull. 2013, 38, 473–479. [Google Scholar] [CrossRef]
- Weidenthaler, C.; Frankcombe, T.J.; Felderhoff, M. First crystal structure studies of CaAlH5. Inorg. Chem. 2006, 45, 3849–3851. [Google Scholar] [CrossRef] [PubMed]
- Klaveness, A.; Vajeeston, P.; Ravindran, P.; Fjellvåg, H.; Kjekshus, A. Structure and bonding in BAlH5 (B = Be, Ca, Sr) from first–principle calculations. J. Alloy. Compd. 2007, 433, 225–232. [Google Scholar] [CrossRef]
- Sato, T.; Sørby, M.H.; Ikeda, K.; Sato, S.; Hauback, B.C.; Orimo, S. Syntheses, crystal structures, and thermal analyses of solvent–free Ca(AlD4)2 and CaAlD5. J. Alloy. Compd. 2009, 487, 472–478. [Google Scholar] [CrossRef]
- Zhang, Q.-A.; Nakamura, Y.; Oikawa, K.; Kamiyama, T.; Akiba, E. New alkaline earth aluminum hydride with one–dimensional zigzag chains of [AlH6]: Synthesis and crystal structure of BaAlH5. Inorg. Chem. 2002, 41, 6941–6943. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Takagi, S.; Deledda, S.; Hauback, B.C.; Orimo, S. Goldschmidt tolerance factor to arbitrary ionic compounds. Sci. Rep. 2016, 6, 23592. [Google Scholar] [CrossRef] [PubMed]
- Pommerin, A.; Wosylus, A.; Felderhoff, M.; Schüth, F.; Weidenthaler, C. Synthesis, crystal structures, and hydrogen-storage properties of Eu(AlH4)2 and Sr(AlH4)2 and of their decomposition intermediates, EuAlH5 and SrAlH5. Inorg. Chem. 2012, 51, 4143–4150. [Google Scholar] [CrossRef] [PubMed]
- Björling, T.; Noréus, D.; Jansson, K.; Andersson, M.; Leonova, E.; Edén, M.; Hålenius, U.; Häussermann, U. SrAlSiH: A polyanionic semiconductor hydride. Angew. Chem. Int. Ed. 2005, 44, 7269–7273. [Google Scholar] [CrossRef] [PubMed]
- Gingl, F.; Vogt, T.; Akiba, E. Trigonal SrAl2H2: The first Zintl phase hydride. J. Alloy. Compd. 2000, 306, 127–132. [Google Scholar] [CrossRef]
- Zhang, Q.-A.; Nakamura, Y.; Oikawa, K.; Kamiyama, T.; Akiba, E. Synthesis and crystal structure of Sr2AlH7: A new structural type of alkaline earth aluminum hydride. Inorg. Chem. 2002, 41, 6547–6549. [Google Scholar] [CrossRef] [PubMed]
- Brower, F.M.; Matzek, N.E.; Reigler, P.F.; Rinn, H.W.; Roberts, C.B.; Schmidt, D.L.; Snover, J.A.; Terada, K. Preparation and properties of aluminum hydride. J. Am. Chem. Soc. 1976, 98, 2450–2453. [Google Scholar] [CrossRef]
- Ikeda, K.; Muto, S.; Tatsumi, K.; Menjo, M.; Kato, S.; Bielmann, M.; Züttel, A.; Jensen, C.M.; Orimo, S. Dehydriding reaction of AlH3: In situ microscopic observations combined with thermal and surface analysis. Nanotechnology 2009, 20, 204004. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Ikeda, K.; Li, H.-W.; Yukawa, H.; Morinaga, M.; Orimo, S. Direct dry syntheses and thermal analyses of a series of aluminum complex hydrides. Mater. Trans. 2009, 50, 182–186. [Google Scholar] [CrossRef]
- Werner, P.-E.; Eriksson, L.; Westdahl, M. TREOR, a semi-exhaustive trial-and-error powder indexing program for all symmetries. J. Appl. Crystallogr. 1985, 18, 367–370. [Google Scholar] [CrossRef]
- Favre-Nicolin, V.; Černý, R. FOX, ‘Free objects for crystallography’: A modular approach to ab initio structure determination from powder diffraction. J. Appl. Crystallogr. 2002, 35, 734–743. [Google Scholar] [CrossRef]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Van Laar, B.; Yelon, W.B. The peak in neutron powder diffraction. J. Appl. Crystallogr. 1984, 17, 47–54. [Google Scholar] [CrossRef]
- Thompson, P.; Cox, D.E.; Hastings, J.B. Rietveld refinement of Deby-Scherrer synchrotron X-ray data from Al2O3. J. Appl. Crystallogr. 1987, 20, 79–83. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M.; Von Dreele, R.B. Synthesis and crystal structure of SrD2 and SrND and bond valence parameters for hydrides. J. Solid State Chem. 1990, 88, 571–576. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryatallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Weidenthaler, C.; Pommerin, A.; Felderhoff, M.; Sun, W.; Wolverton, C.; Bogdanović, B.; Schüth, F. Complex rare-earth aluminum hydrides: Mechanochemical preparation, crystal structure and potential for hydrogen storage. J. Am. Chem. Soc. 2009, 131, 16735–16743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.A.; Nakamura, Y.; Oikawa, K.; Kamiyama, T.; Akiba, E. Hydrogen–induced phase decomposition of Ba7Al13 and the crystal structure of Ba2AlH7. J. Alloy. Compd. 2003, 361, 180–186. [Google Scholar] [CrossRef]
- Hauback, B.C.; Brinks, H.W.; Fjellvåg, H. Accurate structure of LiAlD4 studied by combined powder neutron and X-ray diffraction. J. Alloy. Compd. 2002, 346, 184–189. [Google Scholar] [CrossRef]
- Hauback, B.C.; Brinks, H.W.; Jensen, C.M.; Murphy, K.; Maeland, A.J. Neutron diffraction structure determination of NaAlD4. J. Alloy. Compd. 2003, 358, 142–145. [Google Scholar] [CrossRef]
- Hauback, B.C.; Brinks, H.W.; Heyn, R.H.; Blom, R.; Fjellvåg, H. The crystal structure of KAlD4. J. Alloy. Compd. 2005, 394, 35–38. [Google Scholar] [CrossRef]
- Fossdal, A.; Brinks, H.W.; Fichtner, M.; Hauback, B.C. Determination of the crystal structure of Mg(AlH4)2 by combined X-ray and neutron diffraction. J. Alloy. Compd. 2005, 387, 47–51. [Google Scholar] [CrossRef]
- Grove, H.; Brinks, H.W.; Heyn, R.H.; Wu, F.-J.; Opalka, S.M.; Tang, X.; Laube, B.L.; Hauback, B.C. The structure of LiMg(AlD4)3. J. Alloy. Compd. 2008, 455, 249–254. [Google Scholar] [CrossRef]
- Brinks, H.W.; Hauback, B.C. The structure of Li3AlD6. J. Alloy. Compd. 2003, 354, 143–147. [Google Scholar] [CrossRef]
- Rönnebro, E.; Noréus, D.; Kadir, K.; Reiser, A.; Bogdanovic, B. Investigation of the perovskite related structures of NaMgH3, NaMgF3 and Na3AlH6. J. Alloy. Compd. 2000, 299, 101–106. [Google Scholar] [CrossRef]
- Grove, H.; Brinks, H.W.; Løvvik, O.M.; Heyn, R.H.; Hauback, B.C. The structure of LiMgAlD6 from combined neutron and synchrotron X-ray powder diffraction. J. Alloy. Compd. 2008, 460, 64–68. [Google Scholar] [CrossRef]
- Brinks, H.W.; Hauback, B.C.; Jensen, C.M.; Zidan, R. Synthesis and crystal structure of Na2LiAlD6. J. Alloy. Compd. 2005, 392, 27–30. [Google Scholar] [CrossRef]
- Sørby, M.H.; Brinks, H.W.; Fossdal, A.; Thorshaug, K.; Hauback, B.C. The crystal structure and stability of K2NaAlH6. J. Alloy. Compd. 2006, 415, 284–287. [Google Scholar] [CrossRef]
- Lee, M.H.; Börling, T.; Hauback, B.C.; Utsumi, T.; Moser, D.; Bull, D.; Noréus, D.; Sankey, O.F.; Häussermann, U. Crystal structure, electronic structure, and vibrational properties of MAlSiH (M = Ca,Sr,Ba): Hydrogenation-induced semiconductors from the AlB2-type alloys MAlSi. Phys. Rev. B 2008, 78, 195209. [Google Scholar] [CrossRef]
- Jenkins, H.D.B.; Thakur, K.P. Reappraisal of thermochemical radii for complex ions. J. Chem. Educ. 1979, 56, 576–577. [Google Scholar] [CrossRef]


| Atom | Wyckoff Position | x | y | z | 100 × Uiso (Å2) |
|---|---|---|---|---|---|
| Sr | 4d | 0.2532(7) | 0.8925(3) | 0.2500 | 0.13(3) |
| Al | 4d | 0.3296(11) | 0.1597(3) | 0.2500 | 1.00 |
| D1 | 4c | 0.4366(13) | 0.2500 | 0.0000 | 4.75(15) |
| D2 | 4d | 0.3461(13) | 0.5790(5) | 0.2500 | 4.75(15) |
| D3 | 4d | 0.0311(13) | 0.7146(3) | 0.2500 | 4.75(15) |
| D4 | 8e | 0.1914(7) | 0.0718(3) | 0.4986(9) | 4.75(15) |
| Inter-Atomic Distances (Å) | |
|---|---|
| Sr–D1 | 2.621(4) × 2 |
| Sr–D2 | 2.5776(19) × 2 2.996(7) |
| Sr–D3 | 2.4686(24) 3.035(4) × 2 |
| Sr–D4 | 2.4549(17) × 2 2.602(4) × 2 2.898(5) × 2 |
| Al–D1 | 1.7683(30) × 2 |
| Al–D2 | 1.812(5) |
| Al–D3 | 1.806(4) |
| Al–D4 | 1.7901(29) × 2 |
| Crystal System (Space Group) | Unit Cell Parameters | Z | Avg. Al–D Distances | D–Al–D Angles | Avg. M–D Distances | Avg. M–Al Distances | |
|---|---|---|---|---|---|---|---|
| CaAlD5 [8] | Monoclinic (P21/c) | a = 9.800 Å b = 6.908 Å c = 12.450 Å β = 137.94° V = 564.69 Å3 | 8 | 1.75 Å | 78.0°–101.8° 166.6°–177.9° | 2.43 Å | 3.50 Å |
| SrAlD5 (present result) | Orthorhombic (Pbcm) | a = 4.6226(10) Å b = 12.6213(30) Å c = 5.0321(10) Å V = 293.59(12) Å3 | 4 | 1.79(2) Å | 84.7(2)°–97.5(3)° 168.4(3)°–175.3(3)° | 2.70(21) Å | 3.55(26) Å |
| BaAlD5 [9] | Orthorhombic (Pna21) | a = 9.194 Å b = 7.040 Å c = 5.106 Å V = 330.51 Å3 | 4 | 1.77 Å | 75.7°–103.7° 161.4°–169.9° | 2.82 Å | 3.68 Å |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, T.; Takagi, S.; Sørby, M.H.; Deledda, S.; Hauback, B.C.; Orimo, S.-i. Crystal Structural Determination of SrAlD5 with Corner-Sharing AlD6 Octahedron Chains by X-ray and Neutron Diffraction. Crystals 2018, 8, 89. https://doi.org/10.3390/cryst8020089
Sato T, Takagi S, Sørby MH, Deledda S, Hauback BC, Orimo S-i. Crystal Structural Determination of SrAlD5 with Corner-Sharing AlD6 Octahedron Chains by X-ray and Neutron Diffraction. Crystals. 2018; 8(2):89. https://doi.org/10.3390/cryst8020089
Chicago/Turabian StyleSato, Toyoto, Shigeyuki Takagi, Magnus H. Sørby, Stefano Deledda, Bjørn C. Hauback, and Shin-ichi Orimo. 2018. "Crystal Structural Determination of SrAlD5 with Corner-Sharing AlD6 Octahedron Chains by X-ray and Neutron Diffraction" Crystals 8, no. 2: 89. https://doi.org/10.3390/cryst8020089
APA StyleSato, T., Takagi, S., Sørby, M. H., Deledda, S., Hauback, B. C., & Orimo, S.-i. (2018). Crystal Structural Determination of SrAlD5 with Corner-Sharing AlD6 Octahedron Chains by X-ray and Neutron Diffraction. Crystals, 8(2), 89. https://doi.org/10.3390/cryst8020089






