Crystal Structures and Optical Properties of Two Novel 1,3,5-Trisubstituted Pyrazoline Derivatives
Abstract
:1. Introduction
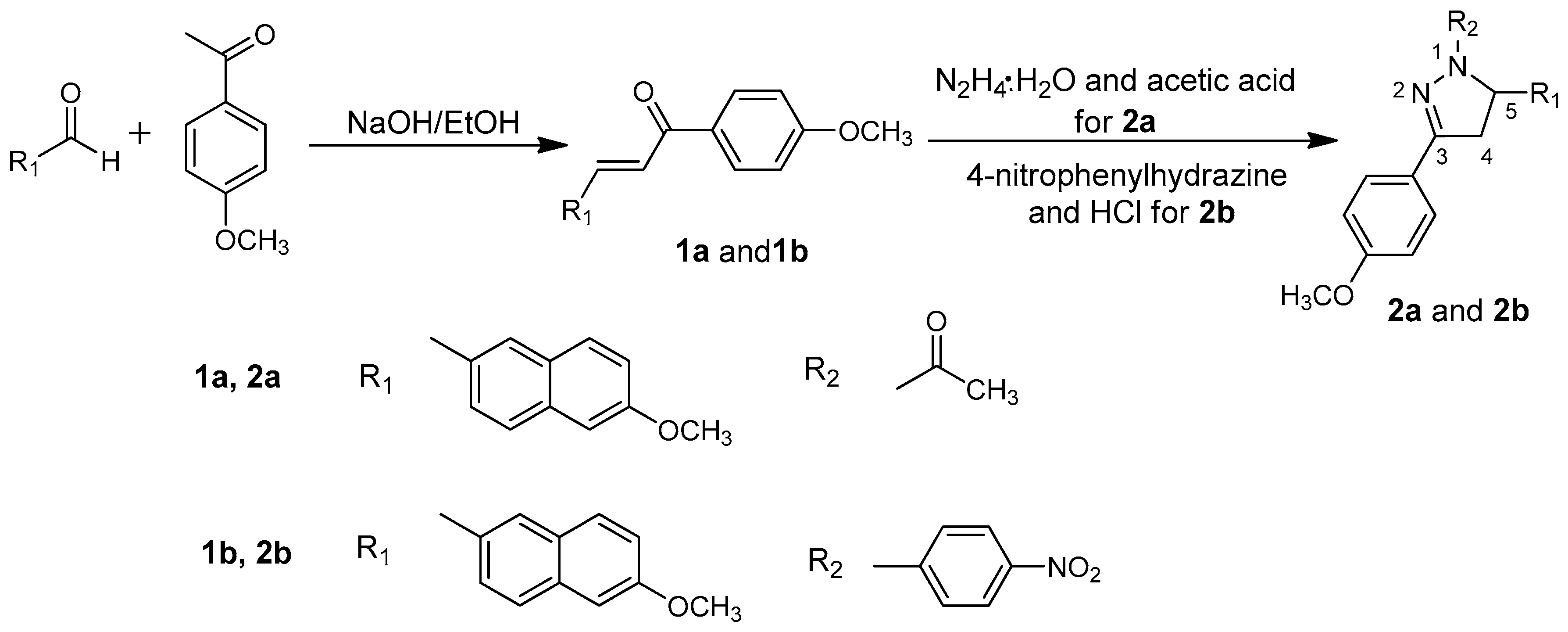
2. Materials and Methods
2.1. Synthesis
2.1.1. Synthesis of 2a
2.1.2. Synthesis of 2b
2.2. Crystals’ Preparation
2.3. Measurement
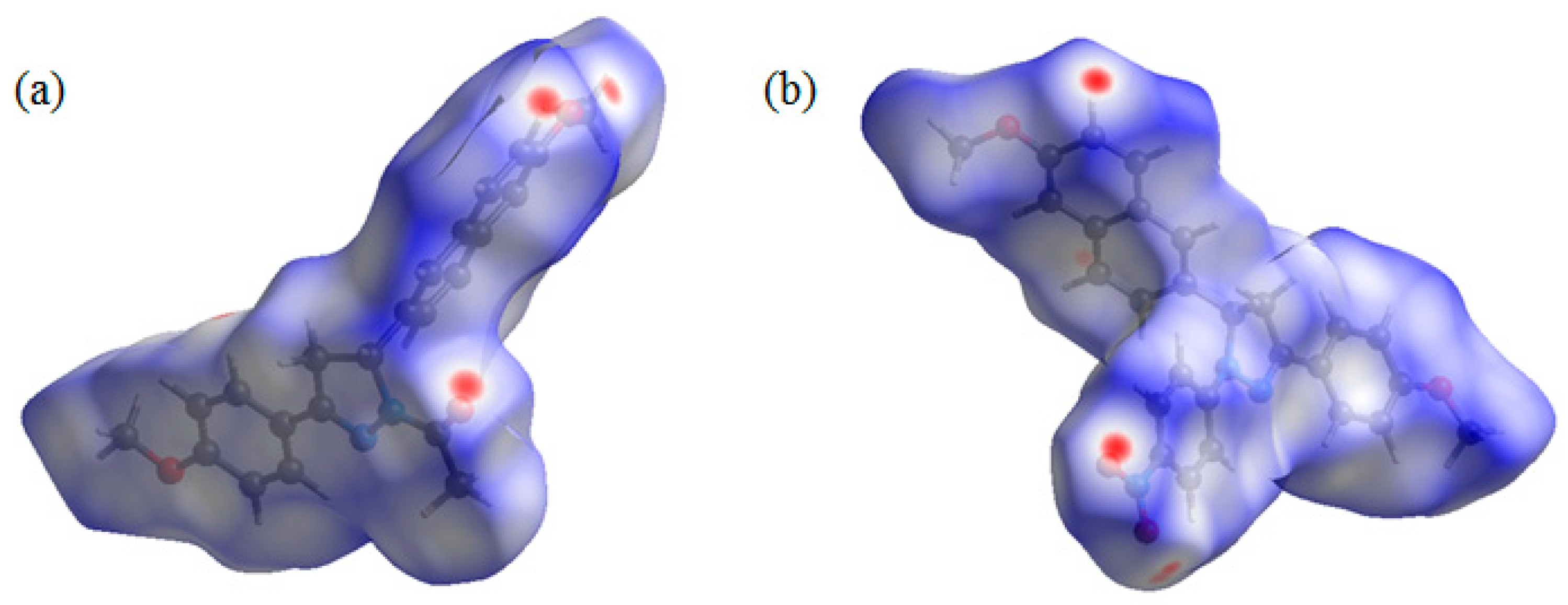
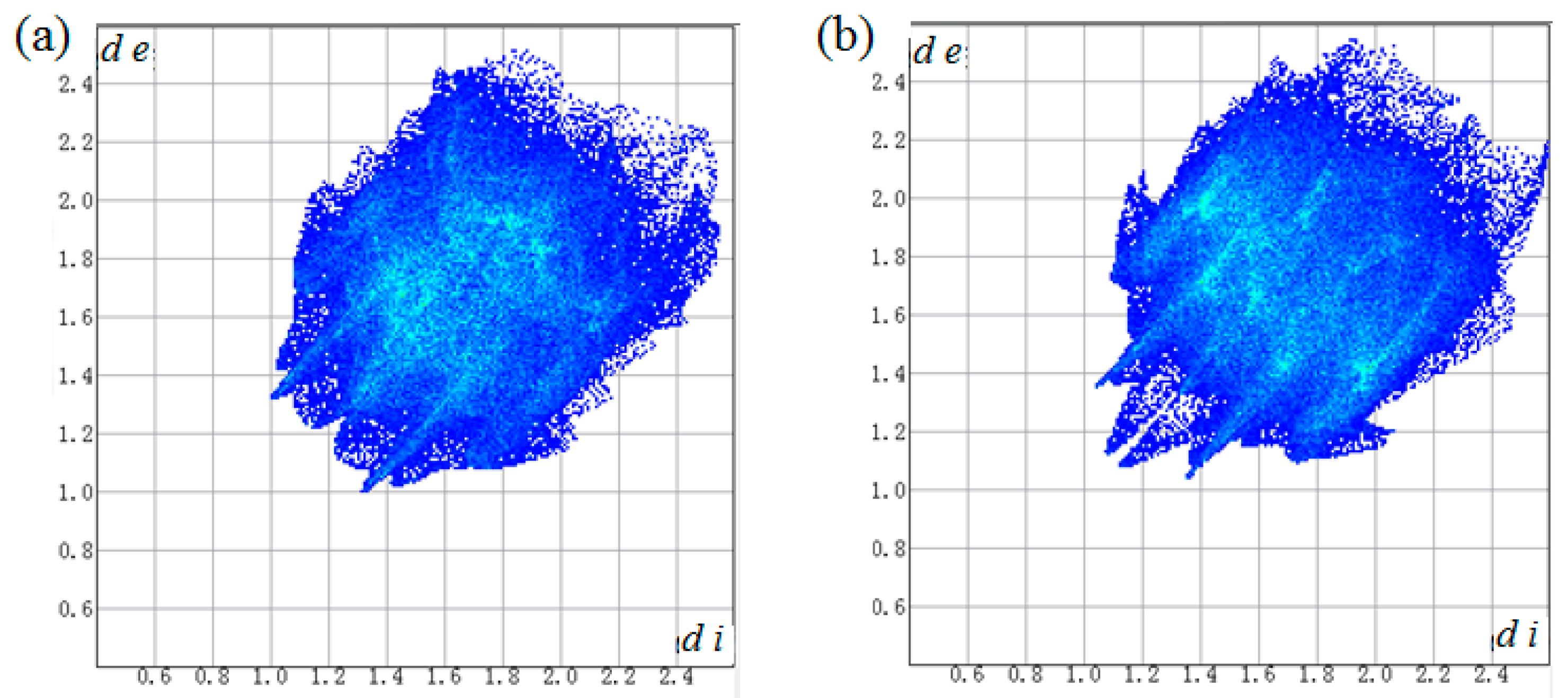
2.4. Hirshfeld Surface Analysis
3. Results
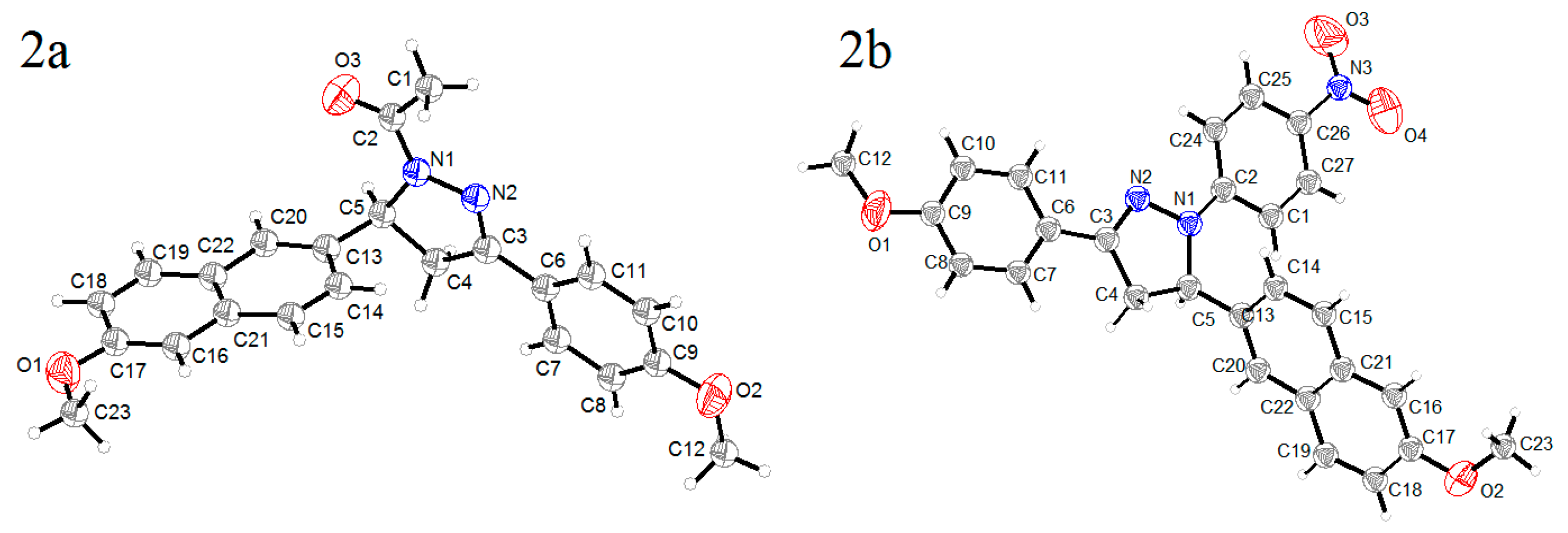
3.1. Crystal Structure
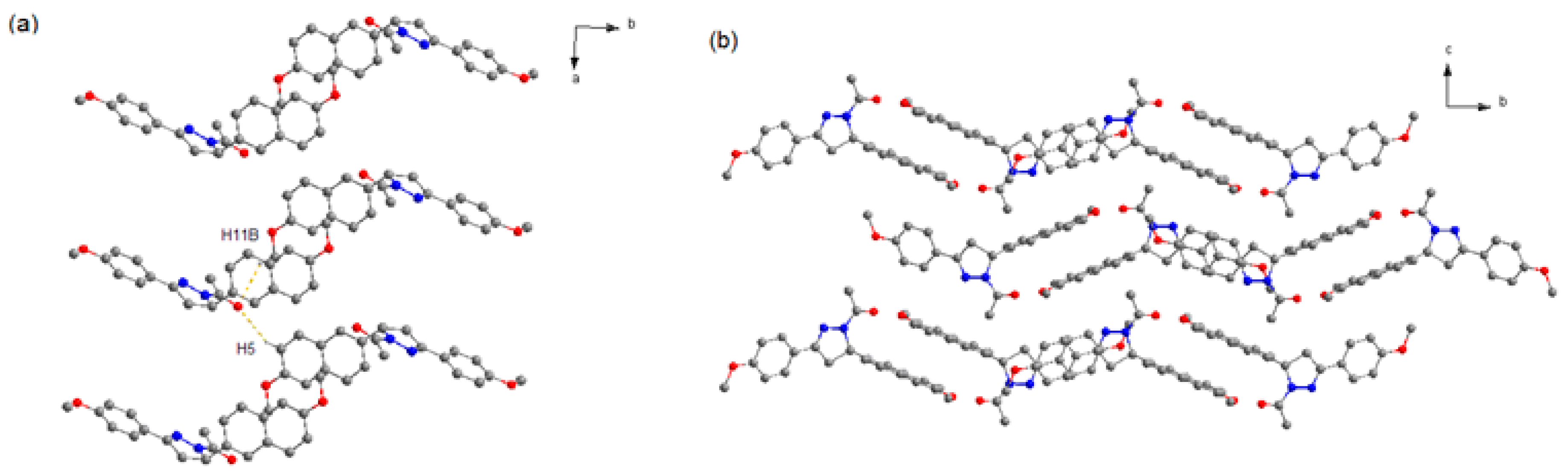
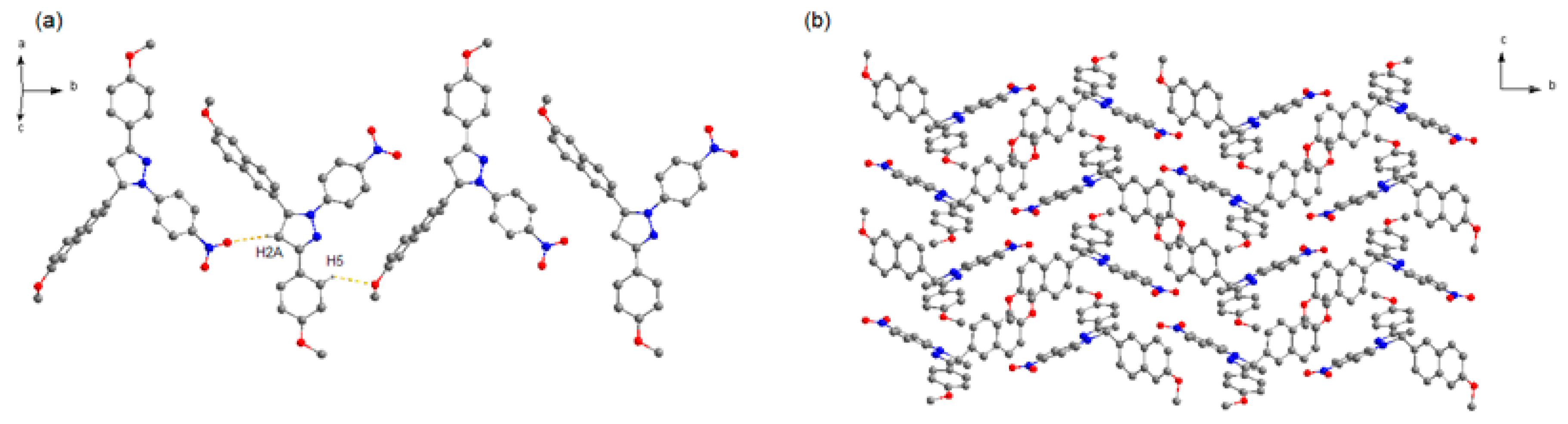
3.2. Hirshfeld Surface Analyses
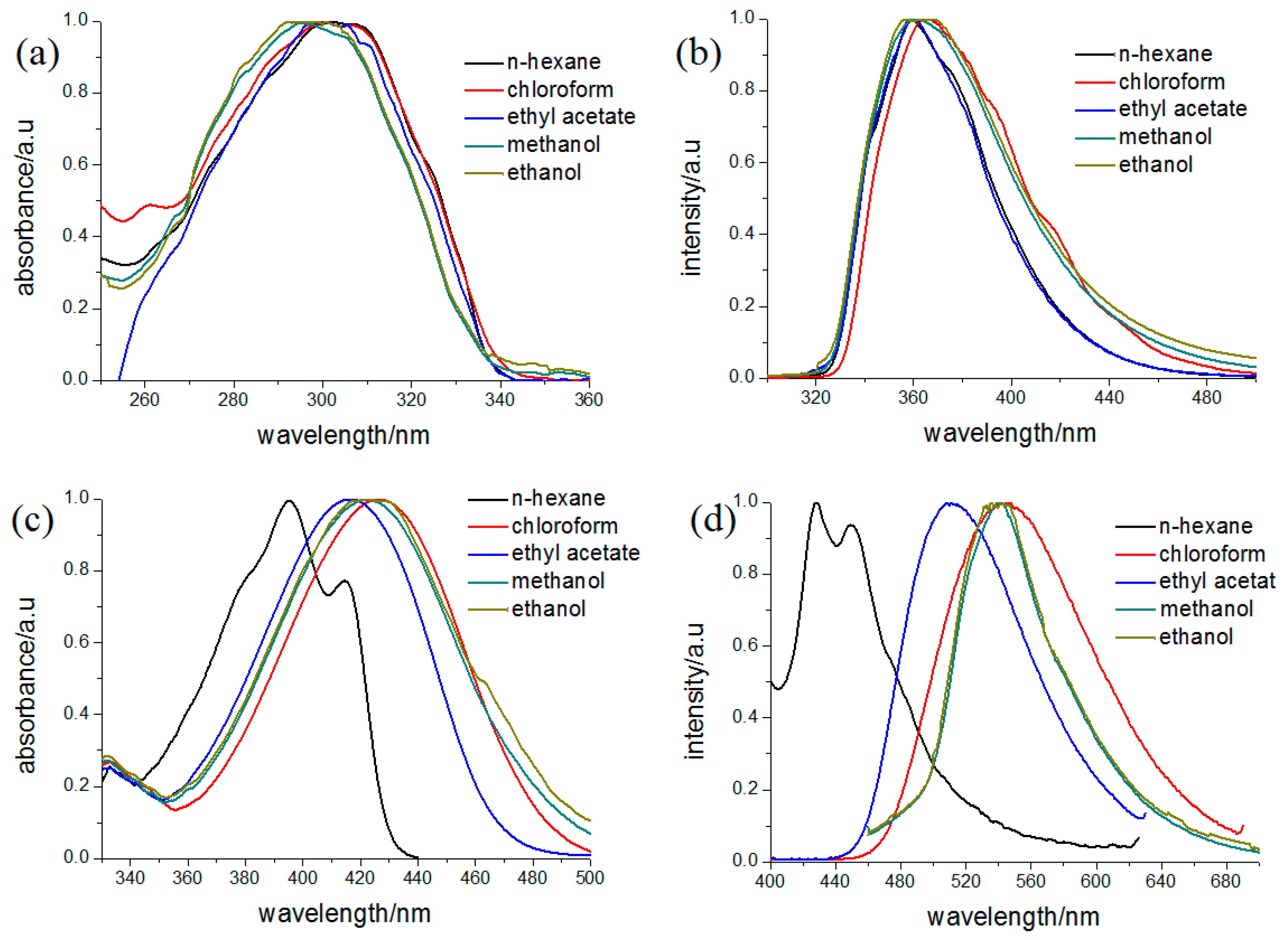
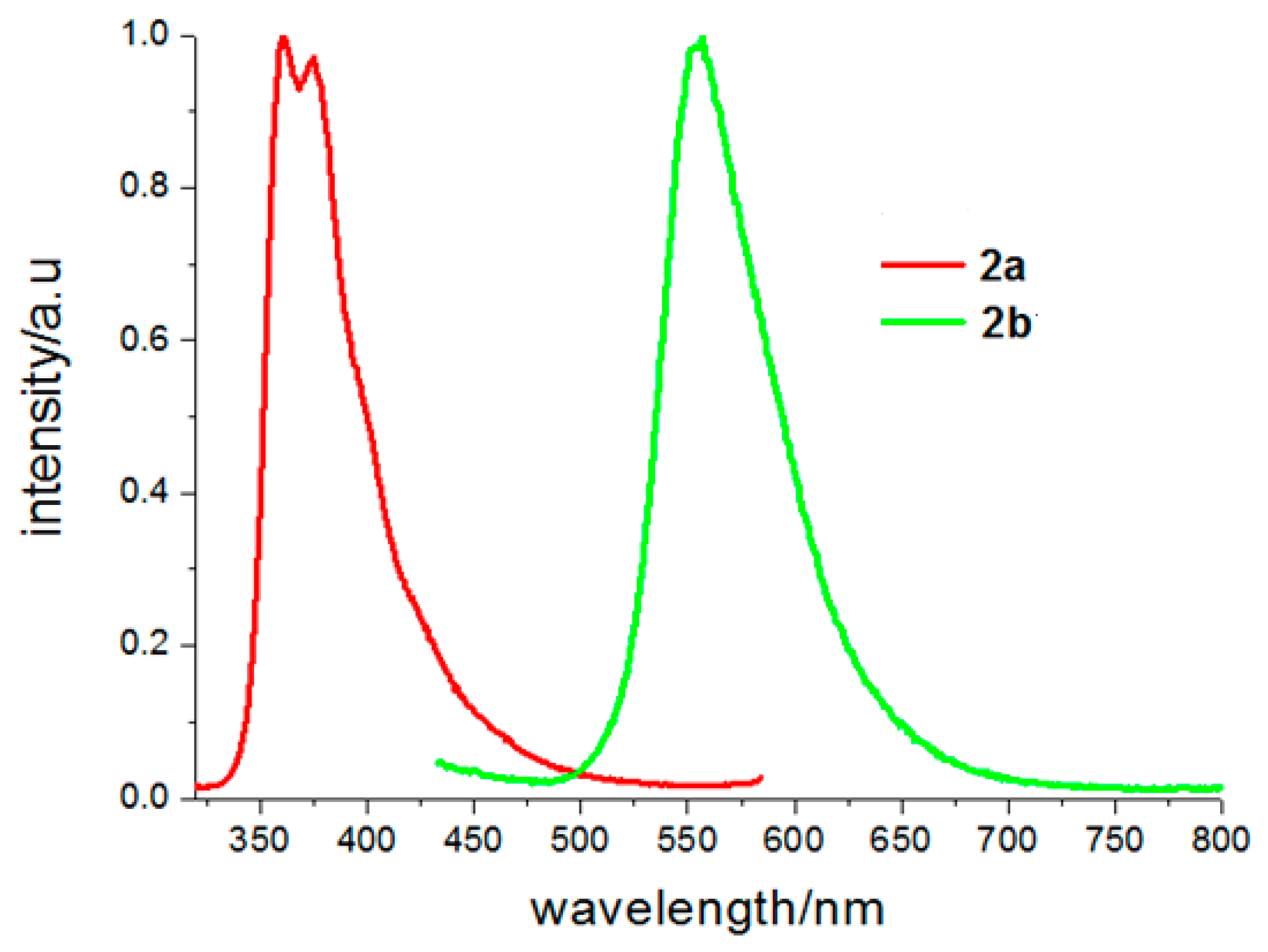
3.3. Photo-Physical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef]
- Gong, X.Q.; Cai, J.; Zhang, B.; Zhao, Q.; Piao, J.F.; Peng, W.P.; Gao, W.C.; Zhou, D.M.; Zhao, M.; Chang, J. A review of fluorescent signal-based lateral flow immunochromatographic strips. J. Mater. Chem. B 2017, 5, 5079–5091. [Google Scholar] [CrossRef]
- Wu, A.L.; Sun, P.P.; Sun, N.; Yu, Y.; Zheng, L.Q. Coassembly of a Polyoxometalate and a Zwitterionic Amphiphile into a Luminescent Hydrogel with Excellent Stimuli Responsiveness. Chem.-Eur. J. 2018, 24, 16857–16864. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Goubard, F.; Ibrahim-Ouali, M.; Gigmes, D.; Dumur, F. Recent advances on organic blue thermally activated delayed fluorescence (TADF) emitters for organic light-emitting diodes (OLEDs). Beilstein. J. Org. Chem. 2018, 14, 282–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Liu, J.; Gao, J.K.; Zhang, F.; Ding, L. High Solid Fluorescence of a Pyrazoline Derivative through Hydrogen Bonding. Molecules 2017, 22, 1304. [Google Scholar] [CrossRef] [PubMed]
- Varghese, B.; Al-Busafi, S.N.; Suliman, F.O.; Al-Kindy, S.M.Z. Unveiling a versatile heterocycle: Pyrazoline a review. RSC Adv. 2017, 7, 46999–47016. [Google Scholar] [CrossRef]
- Haupa, K.A.; Szukalski, A.; Mysliwiec, J. Low-Molecular Push Pull Pyrazoline Derivatives: Solvatochromic Effect and Theoretical Insights into Dye-based Molecular Engineering. J. Chem. Phys. 2018, 122, 7808–7818. [Google Scholar] [CrossRef] [PubMed]
- Vandana, T.; Ramkumar, V.; Kannan, P. Synthesis and fluorescent properties of poly(arylpyrazoline)’s for organic-electronics. Opt. Mater. 2016, 58, 514–523. [Google Scholar] [CrossRef]
- Zhang, R.-R.; Zhang, J.-F.; Wang, S.-Q.; Cheng, Y.-L.; Miao, J.-Y.; Zhao, B.-X. Novel pyrazoline-based fluorescent probe for detecting thiols and its application in cells. Spectrochim. Acta. A 2015, 137, 450–455. [Google Scholar] [CrossRef]
- Singh, P.; Rawat, B.; Rawat, B.S.; Pant, G.J.N.; Rawat, M.S.M.; Joshi, G.C. Synthesis, centrifugal chromatographic separation and fluorescence study of a 2-pyrazoline. J. Indian Chem. Soc. 2014, 91. [Google Scholar]
- Jin, M.; Liang, Y.J.; Lu, R.; Chuai, X.H.; Yi, Z.H.; Zhao, Y.Y.; Hong, J.Z. Synthesis and properties of photoluminescence and electroluminescence of pyrazoline derivatives. Synth. Met. 2004, 140, 37–41. [Google Scholar] [CrossRef]
- Bai, G.; Li, J.F.; Li, D.X.; Dong, C.; Han, X.Y.; Lin, P.H. Synthesis and spectrum characteristic of four new organic fluorescent dyes of pyrazoline compounds. Dyes Pigment. 2007, 75, 93–98. [Google Scholar] [CrossRef]
- Maslakci, N.N.; Eren, E.; Topel, S.D.; Cin, G.T.; Oksuz, A.U. Electrospun plasma-modified chitosan/poly(ethylene terephthalate)/ferrocenyl-substituted N-acetyl-2-pyrazoline fibers for phosphate anion sensing. J. Appl. Polym. Sci. 2016, 133, 43344. [Google Scholar] [CrossRef]
- Yang, A.B.; Xu, Z.X.; Wu, J.; Feng, Y.Y.; Wang, B.X. Synthesis of 1-Acetyl-3-(2-hydroxyl-4,6-dimethoxylphenyl)-5-phenyl-2-pyrazoline and Studies on Its Zinc Ion Probe. Chem. J. Chin. Univ. 2010, 31, 1365–1368. [Google Scholar]
- Fahrni, C.J.; Yang, L.C.; VanDerveer, D.G. Tuning the photoinduced electron-transfer thermodynamics in 1,3,5-triaryl-2-pyrazoline fluorophores: X-ray structures, photophysical characterization, computational analysis, and in vivo evaluation. J. Am. Chem. Soc. 2003, 125, 3799–3812. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Kinoshita, Y.; Tamiaki, H. Synthesis of chlorophyll-a derivatives possessing the 3-(2-acylethenyl) group by cross-aldol condensation and their optical properties. Tetrahedron 2018, 74, 2703–2715. [Google Scholar] [CrossRef]
- Mellado, M.; Madrid, A.; Martinez, U.; Mella, J.; Salas, C.O.; Cuellar, M. Hansch’s analysis application to chalcone synthesis by Claisen-Schmidt reaction based in DFT methodology. Chem. Pap. 2018, 72, 703–709. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer 3.1; The University of Western Australia: Perth, Australia, 2012. [Google Scholar]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr. Sect. B Struct. Sci. 2004, 60, 627–668. [Google Scholar] [CrossRef]
- Bozkurt, E.; Gul, H.I.; Mete, E. Solvent and substituent effect on the photophysical properties of pyrazoline derivatives: A spectroscopic study. J. Photoch. Photobio. A 2018, 72, 703–709. [Google Scholar] [CrossRef]
- Wang, M.L.; Zhang, J.X.; Liu, J.Z.; Xu, C.X.; Ju, H.X. Intramolecular energy and charge transfer in 5-(9-anthryl)-3-(4-nitrophenyl)-1-phenyl-2-pyrazoline. J. Lumin. 2002, 99, 79–83. [Google Scholar] [CrossRef]
- Kucukoz, B.; Sevinc, G.; Yildiz, E.; Karatay, A.; Zhong, F.; Yilmaz, H.; Tutel, Y.; Hayvali, M.; Zhao, J.; Yaglioglu, H.G. Enhancement of two photon absorption properties and intersystem crossing by charge transfer in pentaaryl boron-dipyrromethene (BODIPY) derivatives. Phys. Chem. Chem. Phys. 2016, 18, 13546–13553. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Wang, M.L.; Usman, R.; Lu, J.; Sun, H.; Du, M.; Zhang, R.M.; Xu, C.X. Organic charge-transfer complexes for the selective accommodation of aromatic isomers using anthracene derivatives and TCNQ. New J. Chem. 2016, 40, 5277–5284. [Google Scholar] [CrossRef]
| Crystal | 2a | 2b |
|---|---|---|
| Molecular formula | C23H22N2O3 | C27H23N3O4 |
| Formula weight | 374.43 | 452.48 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/n | P21/c |
| a (Å) | 8.5757 (17) | 10.356 (2) |
| b (Å) | 17.422 (4) | 20.342 (4) |
| c (Å) | 13.279 (3) | 11.787 (2) |
| α (°) | 90.00 | 90.00 |
| β (°) | 97.65 (3) | 112.81 (3) |
| γ (°) | 90.00 | 90.00 (3) |
| volume (Å3) | 1966.3 (7) | 2288.7 (8) |
| density (g/cm3) | 1.265 | 1.316 |
| Z | 4 | 4 |
| F(000) | 792 | 952 |
| Absorption coefficient/mm−1 | 0.084 | 0.090 |
| Parameters | 256 | 309 |
| θ Range for data collection/° | 3.02–27.48 | 3.00–25.00 |
| R1 (I > 2σ(I)) | 0.0726 | 0.0856 |
| w R2 (I > 2σ(I)) | 0.1569 | 0.1655 |
| GOF | 1.024 | 1.016 |
| CCDC No. | 911423 | 911424 |
| Parameter | 2a | 2b |
|---|---|---|
| N1–N2 | 1.396 | 1.407 |
| N1–C2 | 1.362 | 1.380 |
| N1–C5 | 1.483 | 1.479 |
| N2–C3 | 1.293 | 1.299 |
| C3–C6 | 1.460 | 1.461 |
| C3–C4 | 1.507 | 1.501 |
| C4–C5 | 1.539 | 1.540 |
| C5–C13 | 1.510 | 1.515 |
| N2–C3–C6 (degree) | 122.1 | 122.5 |
| C2–N1–N2 (degree) | 121.9 | 117.5 |
| N1–C5–C13 (degree) | 111.0 | 115.6 |
| Crystal | D–H (Å) | H···A (Å) | D···A (Å) | D–H···A (°) |
|---|---|---|---|---|
| 2a | ||||
| C14–H1···O2 | 0.930 | 2.453 | 3.178 | 134.93 |
| C18–H5···O3 | 0.929 | 2.527 | 3.378 | 152.42 |
| C12–H21B···O1 | 0.960 | 2.566 | 3.503 | 165.36 |
| C23–H11B···O3 | 0.960 | 2.699 | 3.438 | 134.18 |
| C7–H16···O1 | 0.929 | 2.603 | 3.458 | 153.09 |
| 2b | ||||
| C11–H5···O2 | 0.930 | 2.710 | 3.393 | 130.97 |
| C4–H2A···O3 | 0.970 | 2.521 | 3.133 | 120.97 |
| C18–H14···O4 | 0.931 | 2.526 | 3.370 | 150.73 |
| Crystal | H···H % | C···H % | O···H % | N···H % | C···C % | C···O % | C···N % | O···N % | O···O % |
|---|---|---|---|---|---|---|---|---|---|
| 2a | 39.9 | 31.5 | 22.2 | 3.2 | 0.5 | 0.6 | 1.7 | 0.3 | 0.2 |
| 2b | 51.5 | 25.5 | 17.3 | 2.6 | 2.7 | 0.2 | 0.1 | 0 | 0 |
| 2a | 2b | |||
|---|---|---|---|---|
| Solvents | λab (nm) | λem (nm) | λab (nm) | λem (nm) |
| n-Hexane | 302 | 358 | 395 | 428 |
| Chloroform | 300 | 364 | 426 | 547 |
| Ethyl acetate | 300 | 359 | 416 | 508 |
| Ethanol | 294 | 358 | 422 | 533 |
| Methanol | 294 | 360 | 422 | 540 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Q.; Huan, W.; Wang, J.; Lu, J.; Diao, G.; Shan, Y. Crystal Structures and Optical Properties of Two Novel 1,3,5-Trisubstituted Pyrazoline Derivatives. Crystals 2018, 8, 467. https://doi.org/10.3390/cryst8120467
Feng Q, Huan W, Wang J, Lu J, Diao G, Shan Y. Crystal Structures and Optical Properties of Two Novel 1,3,5-Trisubstituted Pyrazoline Derivatives. Crystals. 2018; 8(12):467. https://doi.org/10.3390/cryst8120467
Chicago/Turabian StyleFeng, Qi, Wenhui Huan, Jiali Wang, Jiadan Lu, Guowang Diao, and Yaqi Shan. 2018. "Crystal Structures and Optical Properties of Two Novel 1,3,5-Trisubstituted Pyrazoline Derivatives" Crystals 8, no. 12: 467. https://doi.org/10.3390/cryst8120467




