Abstract
The development of photocatalytic materials with specific morphologies promises to be a good opportunity to discover geometrically relevant properties. Herein, this paper reported a facile hydrothermal method to directly synthesize TiO2 crystals with flower-like structures using tetrabutyl titanate (TBT) as a titanium source and ethylene glycol as an additive. We also proposed a reasonable growth mechanism by controlling reaction time in detail. The as-prepared samples were analyzed by using X-ray diffraction, scanning electron microscopy and transmission electron microscopy for structure and morphology characterization. The N2 adsorption-desorption isotherm results showed that the surface area of flower-like TiO2 with 10 h reaction time can reach 297 m2/g. We evaluated the photocatalytic performance of samples by using the degradation rate of methylene blue (MB) solution under UV-vis light. The TiO2 with 10 h reaction time exhibited a superior photocatalytic property than other samples in degrading MB under UV-vis light irradiation. More importantly, the catalyst could be reused many times. These results could benefit from the special morphology, high crystallinity and large specific surface area of the samples.
1. Introduction
With the increasing use of energy resources around the world, it has become a major topic of research to develop a high-efficiency, environmentally-friendly, low-cost energy conversion and storage system. Especially, the requirements for materials with unique designs and advanced functions are very high, which could overcome the limitations of current traditional energy sources [1]. TiO2, as a crucial component of energy conversion and storage systems, has attracted much attention because of its excellent physical-chemical characteristic and versatility. TiO2 has extensive applications in many energy fields as well [2,3,4,5]. For the many applications of TiO2, it is very important to precise control TiO2 morphology, size, specific surface area and crystalline phase because the properties of TiO2 are determined by these structural characteristics [6,7,8,9,10].
At present, TiO2 as one of the critical semiconductor oxides, has been widely used in the field of photocatalysis [11,12,13]. Particularly, TiO2 with special morphological structures has been intensively explored by researchers due to its unique properties [14,15,16,17]. A variety of TiO2 with different morphologies have been prepared, such as nanosheets, nanowires, nanotubes, and nanoflowers [18,19,20,21]. One dimensional (1D) TiO2 nanostructures with direct transport pathways, such as nanorods [22] or nanosheets [23], show promise of enhanced charge transport efficiency. However, these one-dimensional TiO2 structures have relatively small specific surface areas, hence impeding light harvesting efficiency [24]. For example, Chen et al. [25] reported the synthesis of hierarchical sphere TiO2 assembled from ultrathin nanosheets with exposed {001} facets. However, the use of surfactants may passivate the surface of the crystal, which leads to a rapid decrease in photocatalytic activity. Recently, the photocatalytic ability of three-dimensional (3D) hierarchical flower-like TiO2 structures has been extensively studied by researchers [26]. In this case, the nanoflower structure facilitates exposure of the specific surface area and an increase in the number of active sites. Furthermore, they are generally used as a scattering layer to improve the light absorption ability [27]. However, the synthesis process of this structure usually requires the use of templates, which may make it very tedious and difficult to separate unintended impurities [28]. For example, Xiang group [29] reported the fabrication of flower-like TiO2 by no template and no surfactant method. However, the route was so complicated that it needs two steps to prepare. Réti et al. [30] reported the synthesis of TiO2 hollow sphere via a hydrothermal and annealing route. However, carbon sphere templates affect the size of the hollow TiO2 structures, which caused a decrease in photocatalytic activity. From these new findings, we were really eager to develop a synthetic process for an easy fabrication of anatase TiO2 nanostructure having high specific surface area, so as to achieve efficient catalysis of pollutants.
Herein, we present a simple and template-free method using tetrabutyl titanate (TBT) as titanium source to synthesize novel flower-like TiO2 nanostructure assembled from nanosheets. The morphological characteristics and basic properties were obtained by using X-ray diffraction, scanning electron microscopy, N2 adsorption-desorption isotherms and photocatalytic activity. Subsequently, we have explored exhaustively the morphological growth and formation mechanism of flower-like structures. The results demonstrated that the flower-like TiO2 with 10 h reaction time exhibits the higher crystallinity and high photocatalytic activity for degradation of MB under UV-vis light irradiation.
2. Experiment Section
2.1. Synthesis of Flower-Like TiO2 Nanostructures
In a typical synthesis, a certain amount of hydrofluoric acid and 5 mL of ethylene glycol were added into 40 mL of ethanol under constant stirring. Subsequently, 3.3 mL of TBT were added drop wise into the above mixed solution. It was stirring for 0.5 h to obtain a clear homogeneous solution. Next, the solution was transferred into a Teflon-lined stainless steel autoclave (100 mL) and heated at 180 °C for 10 h. Then the autoclave was cooled down to room temperature naturally. The precipitate was treated by suction filtration and washed 3 times with water so as to remove organic impurities. Finally, we dried the sample at 80 °C for 10 h. In order to better explore the formation mechanism of TiO2 with flower-like morphology, the experiments procedures with different reaction times were also carried out under same conditions. In addition, we also performed the corresponding calcination treatment on the samples (550 °C/4 h).
2.2. Characterization
The phase composition and crystal structure of all samples obtained were characterized by using a Bruker D8 advanced X-ray powder diffractometer (Bruker Corporation, Billerica, MA, USA) with Cu-Ka radiation (λ = 1.5418 Å). The morphologies of TiO2 structures were observed by a field emission scanning electron microscope (SEM, S-4800, Hitachi Ltd., Tokyo, Japan) and transmission electron microscope (TEM, JEOL-2100, JEOL Ltd., Tokyo, Japan). Furthermore, the Brunauer-Emmett-Teller (BET) specific surface areas of the powders were measured by nitrogen adsorption in a nitrogen adsorption apparatus (TriStar II 3020 instrument, Micromeritics, Norcross, GA, USA). In particular, all products were degassed at 100 °C before the N2 adsorption measurements. We use adsorption data to determine BET surface area and use Barrett-Joyner-Halenda (BJH) method to achieve pore size distribution. Thermogravimetric (TG) analyses were conducted using a Shimadzu TGA-50 (Shimadzu, Tokyo, Japan) analyser with temperature programmed at a constant heating rate of 10.0 K min−1 in air with gas flow of 100 mL min−1. Photocurrent densities of samples were measured with electrochemical system (CHI660E, Shanghai Chenhua Instrumental Co., Ltd., Shanghai, China) in electrolyte including 0.1 M Na2SO4. The obtained sample was the working electrode, Pt worked as counter electrode, and Ag/AgCl worked as reference electrode. A 300 W Xe lamp (CEL-PF300-T8E, Beijing China Education Au-light Co., Ltd., Beijing, China) was utilized as a light source.
2.3. Measurement of Photocatalytic Activity
We performed the degradation of MB under UV-vis light irradiation at ambient temperature to explore the photocatalytic activities of the as-prepared samples. In this process, first, 0.1 g of the samples were added to a 150 mL glass beaker containing 100 mL MB with concentration of 20 mg/L aqueous solution. Then the beaker was stirred continuously in the dark for a certain period of time to achieve an equilibrium adsorption state. Next, we used a 300 W xenon lamp (PLS-SXE300UV, Beijing Perfectlight Co. Ltd., Beijing, China) as a light source to initiate a photocatalytic reaction. The UV lamp has an average light intensity of about 2000 mW/cm2 on the surface of the solution. We measured the absorbance at 664 nm by UV-2600 UV-Vis spectroscopy (Shimadzu Corporation, Kyoto, Japan) in the wavelength range of 200–800 nm.
In order to identify the generated active species during the reaction process, we carried out a set of radical trapping experiments. A certain amount (0.1 mmol) of ammonium oxalate (AO), 1, 4-benzoquinone (BQ) and 2-propanol (IPA) were used as the hole (h+) scavenger, superoxide radical (∙O2−) scavenger and hydroxyl radical (∙OH) scavenger, respectively. Subsequently, the active substance was detected by exploring the change of degradation rate of the solution.
3. Results and Discussion
3.1. Structures and Morphologies of the TiO2 Samples
In general, the crystallinity of the samples may be important for its photocatalytic performance [31]. Therefore, we conducted a study on the characterization of the prepared samples structures by XRD method and the results were shown in Figure 1. And the position of each peak corresponds to pure anatase TiO2 phase, which is consistent with the International Center of Diffraction Data Card (JCPDS No. 21-1272). It illustrated that the as-prepared products were in accordance with pure phase structures because no other impurity peaks appeared. It is worth noting that the XRD pattern of the sample with 0.5 h reaction time showed only one protrusion at 25.3°, which due to the presence of amorphous TiO2 particles. With the increase of hydrothermal synthesis time, we found that the diffraction peaks of TiO2 become more and more sharp. The diffraction peaks at the positions of 2θ = 48.05° and 55.06° became more obvious, which showed that the degree of crystallization was increasing.
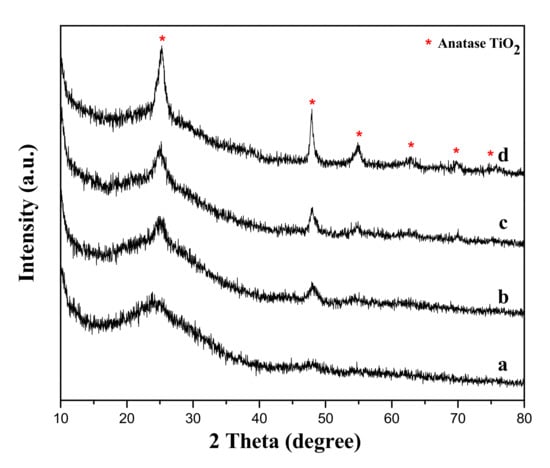
Figure 1.
XRD patterns of the samples with different reaction time: (a) 0.5 h; (b) 2 h; (c) 6 h; (d) 10 h.
In order to explore the morphological evolution of anatase TiO2 with flower-like nanostructures by our prepared methods. We conducted a series of exploratory experiments based on time changes and keeping other conditions constant. The SEM images of TiO2 nanostructures synthesized with different hydrothermal times were shown in Figure 2. As shown in Figure 2a, only some irregular nanoparticles are gathered together when the reaction time was 0.5 h. Subsequently, many nano-spheres with a rough surface appeared. When the reaction time reached 2 h, the rough sphere surface indicated that the thorn-like structures appear firstly (Figure 2b). With time prolonged, the thorn structures gradually grown into nanosheets and they still maintained the spherical morphology in Figure 2c and this unique flower-like structure was assembled from nanosheets. In addition, the sample with 10 h hydrothermal reaction time was illustrated in Figure 2d. We can clearly see that the 3D flower-like nanostructures are completely formed. The size of the flower-like TiO2 structure is about 600–800 nm. In particular, the nanosheets are relatively thicker and more compact than the sample with 6 h reaction time. The results correspond to our previous XRD characterization (Figure 1).
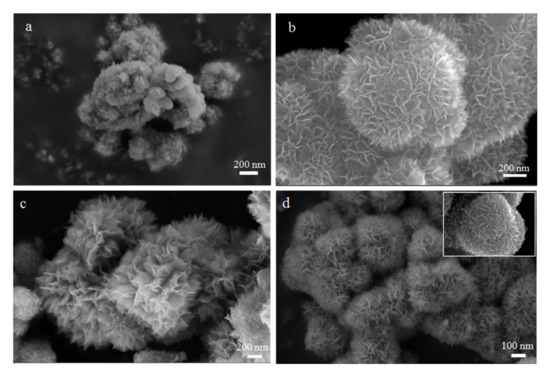
Figure 2.
SEM images showing the morphological evolution of the obtained samples prepared from different solvothermal reaction time: (a) 0.5 h; (b) 2 h; (c) 6 h and (d) 10 h.
Based on the above experiments, we have calcined the samples of 10 h reaction time. As shown in Figure 3a, the sample after calcination has good crystallinity. Figure 3b showed the TG curve analysis of the sample with 10 h reaction time. According to Figure 3b, we can clearly observe that the sample has almost no weight loss at 550 °C. Therefore, we determined the calcination temperature to be 550 °C. It can be seen from the Figure 3c that the samples after calcination still keep the original morphology. However, the nanosheets on the spherical surface grown into tiny nanorods due to the aggregation at high temperatures. Moreover, we can clearly see the hollow structure of the TiO2 microspheres from the SEM image of the broken microspheres (Figure 3d). A representative TEM micrograph further indicated the resulting microspheres possess hollow structures (Figure 3e). Furthermore, in the high-resolution TEM image of TiO2 after calcination (Figure 3f), the lattice spacing (0.235 nm) and its corresponding FFT image (Figure 3f, inset) indicated that facet is the {001} reactive facet of anatase TiO2 [32,33]. It was reported that the exposure of the {001} plane is contributed to the improvement of photocatalytic performance.
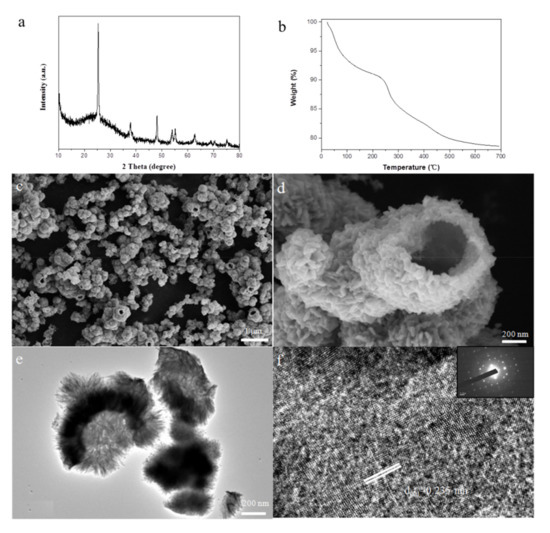
Figure 3.
Surface morphology of the TiO2 after calcination: (a) XRD patterns of the samples after calcination; (b) TG of the sample after calcination; (c,d) SEM images of the sample after calcination; (e,f) TEM images of the sample after calcination.
3.2. Formation Mechanism of the Flower-Like TiO2
Based on the above exploration of the morphology of flower-like TiO2, we propose a simple growth mechanism diagram as follows: (I) Nucleation of nanoparticles aggregating; (II) Dissolution-recrystallization and ostwald ripening; (III) Formation of flower-like structure. We made a simple illustration in Scheme 1. Importantly, we noticed that time was an imperative controlling factor in the whole reaction. At early stages of the reaction, ethylene glycol acts as a ligand to form coordination complexes with titanium (IV) [34,35]. The weak O–H bond caused the O–H bond to cleave and react with another O–H bond to produce H2O. Hydrolysis of tetrabutyl titanate formed TiO2 particles with poor crystallinity. Many nanoparticles aggregated into irregular spheres because of the high surface energy. Afterward, under the control of F−, the sheet-like structure appeared and nanosheets undergo ostwald ripening and dissolution-recrystallization growth to induce the formation of nanosheets having exposed {001} facets [36]. Next, the TiO2 with flower-like structure was formed. Then, under the action of high temperature calcination, the low crystallinity particles inside the flower-sphere gradually dissolved and recrystallized on the surface of the sphere, and the ethylene glycol gas was blocked when it diffused from the inside to the edge of the sphere. Finally, high crystallinity TiO2 hollow spheres were obtained. The broken spheres were caused by the gas not escaping in time. Interestingly, high-temperature calcination caused the sheet structure to agglomerate into nanorods resulting in the increased density of the spherical surface.

Scheme 1.
Schematic illustration of the morphological evolution.
3.3. BET Surface Areas and Pore Size Distributions
Figure 4 showed the specific surface area of our preparing different TiO2 products. The result illustrated that the specific surface area of unique flower-like TiO2 is 297.23 m2 g−1. Large specific surface area is advantageous for the improvements of photocatalytic activity. It could provide more reactive sites to adsorb reactant molecules, so as to make the photocatalytic process more efficient. In particular, the sample after calcination has hollow structures, but its specific surface area is extremely small, because annealing caused the sheet on the surface of the spherical structure to become denser. Next, we analyzed the structural properties of the flower-like TiO2 using nitrogen adsorption-desorption isotherms and related pore size distribution curves. As shown in Figure 5, the isotherm of the sample with 10 h reaction time is type IV, and its hysteresis loop occurred at a relative pressure range of 0.5–1.0, which illustrated the presence of mesopores (2–50 nm). Its hysteresis loops shape is type H3. The mesopores in these samples are formed by the aggregation of nanosheets. All these results are consistent with the SEM of the flower-like TiO2 morphology (Figure 2d).
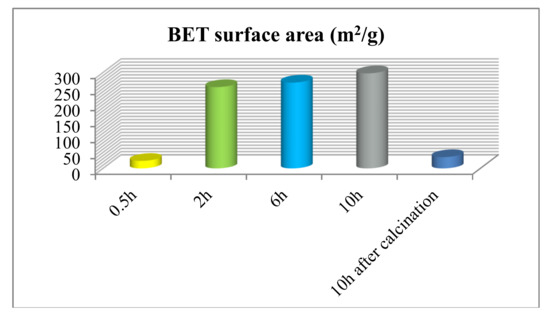
Figure 4.
The surface area of the different TiO2 products: 0.5 h; 2 h; 6 h; 10 h; 10 h after calcination.
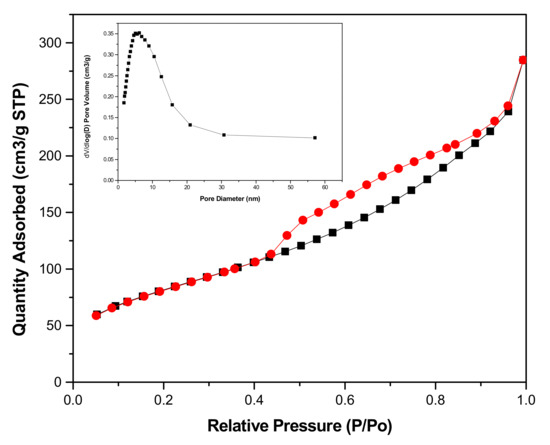
Figure 5.
N2 adsorption-desorption isotherm curves of the TiO2 sample with 10 h reaction time and the inset showing its pore size distribution.
3.4. Photocatalytic Activity of Different Samples
TiO2 as a semiconductor photocatalyst can be used for photocatalytic degradation of organic pollutants in aqueous solution [37]. Hu et al. [38] synthesized 3D flower-like TiO2 by a simple hydrothermal method and annealing treatment and explored their photocatalytic performance. The sample showed excellent photocatalytic activity toward the degradation of methyl orange (MO) solution up to 99% for 120 min of irradiation. Herein, to investigate the photocatalytic properties of our preparing samples, the experiment to evaluate the photocatalytic degradation of MB aqueous solution was carried out under UV-vis light. We also analyzed photocatalytic tests with other samples obtained under different conditions. For the control, the MB without photocatalyst was also investigated under similar conditions. For other conditions, it kept the same as before. Figure 6 showed the curves about the concentration of MB solution degradation changed under UV-vis light irradiation. The results showed that the absorption peak at 664 nm decrease rapidly with the extension of the irradiation time. After some time, the presence of absorption peak was hardly observed, indicating that the MB molecule was almost completely decomposed. The results revealed flower-like TiO2 with 10 h reaction time exhibit superior photocatalytic activity (degradation 98–99% in 30 min) than other samples. The sample with 0.5 h reaction time exhibited the worst photocatalytic performance due to the poor crystallinity of the sample.
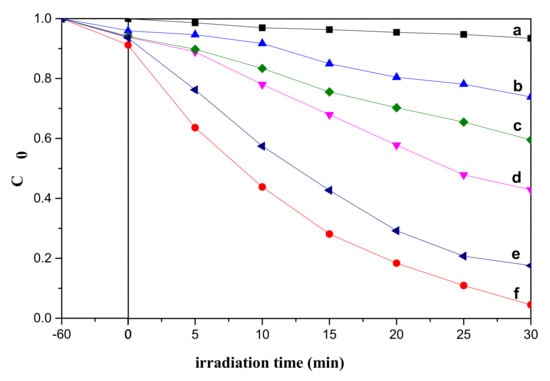
Figure 6.
Photocatalytic degradation of MB by different photocatalysts under UV-vis light irradiation: (a) without catalyst; (b) 0.5 h; (c) 2 h; (d) 6 h; (e) 10 h after calcination; (f) 10 h.
From Figure 7 we get the pseudo-first-rate order kinetic curves, ln(C0/C) = kt, for the photocatalytic degradation kinetic reaction [39]. According to the formula of pseudo-first-rate, k and t denote the pseudo-first-rate kinetic constant and irradiation time. The corresponding apparent degradation rate constant for sample are (b) 0.0085 min−1, (c) 0.0168 min−1, (d) is 0.0291 min−1 and (f) 0.0717 min−1 by calculating, and the flower-like TiO2 with 10 h reaction time (e) is 0.1133 min−1, which is significantly higher than other as-prepared samples. It further confirmed that the TiO2 with flower-like nanostructures exhibit more excellent photocatalytic efficiency than other samples. This is attributed to the flower-like TiO2 having higher specific surface area than other samples (Figure 4). Additionally, the unique nanostructure could provide more active catalytic sites [40,41]. Flower-like TiO2 also showed good crystallinity (Figure 1d), and its photocatalytic property was related to the crystallinity of anatase. We also found that the sample after calcination showed significant photocatalytic activity, but it was slightly lower than the sample with 10 h reaction time. It further demonstrated that photocatalytic performance is not only related to the specific surface area of the samples, but also to the crystallinity of the samples.
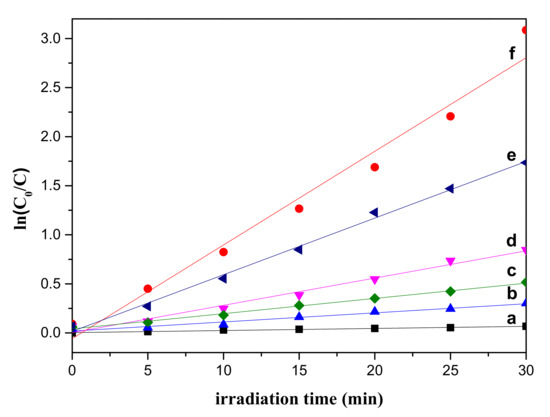
Figure 7.
Linear fit between ln(C0/C) and time of photocatalysis degradation of MB solution by using different catalysts: (a) without catalyst; (b) 0.5 h; (c) 2 h; (d) 6 h; (e) 10 h after calcination; (f) 10 h.
In order to investigate the photocatalytic stability of flower-like TiO2, we evaluated the photodegradation of MB in several successive recycling experiments using the same photocatalyst under the same conditions. Figure 8 showed that the sample still maintain excellent photocatalytic performance after repeated cycles of degradation, indicating that the flower-like TiO2 nanostructures are stable throughout the photodegradation process.
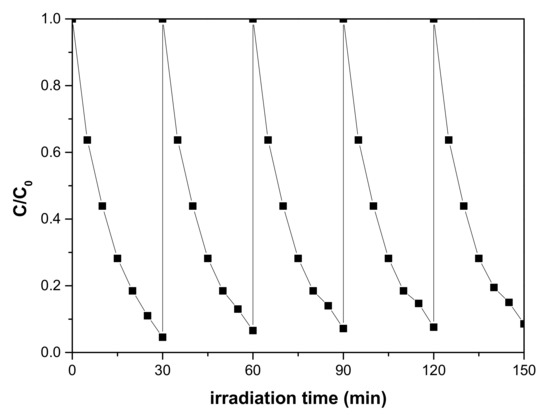
Figure 8.
Cycling degradation MB of flower-like TiO2 with 10 h reaction time under the UV-vis light irradiation.
To further explore the role of active species in the reaction process, a set of radical trapping experiments were carried out. From Figure 9, we can clearly observe the distinct decrease in photodegradation rate in the presence of IPA. The photocatalytic degradation rates of flower-like TiO2 are almost identical whether BQ or AO was present or not. Therefore, we can confirm that ·OH contributes the most to photocatalytic activity of flower-like TiO2.
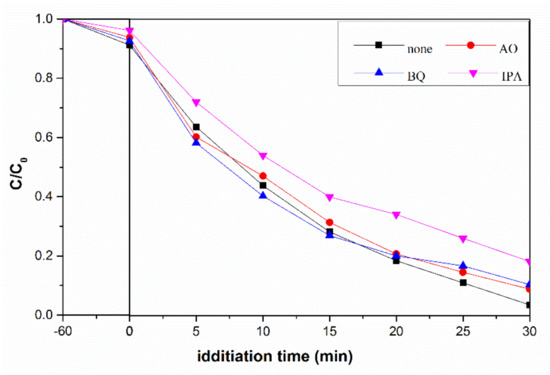
Figure 9.
Photodegradation rate of MB by flower-like TiO2 catalyst with different scavengers under UV-vis light irradiation.
From Figure 10, we can observe the photocurrent response of TiO2 prepared under different reaction conditions. All samples except the calcined sample showed an enhanced photocurrent densities as the hydrothermal reaction time increased. As we all know, the larger the specific surface area, the more favorable the propagation and scattering of incident light, thereby improving the absorption and utilization of light. The thinner nanosheets facilitated the separation of photogenerated electrons and holes, so flower-like TiO2 exhibited excellent electrochemical property. In particular, the photocurrent density of the calcined sample was only lower than that of the sample with 10 h reaction time, higher than the other samples. The high crystallinity is advantageous for the transport of charge. These results were in accordance with their photocatalytic activities, respectively.
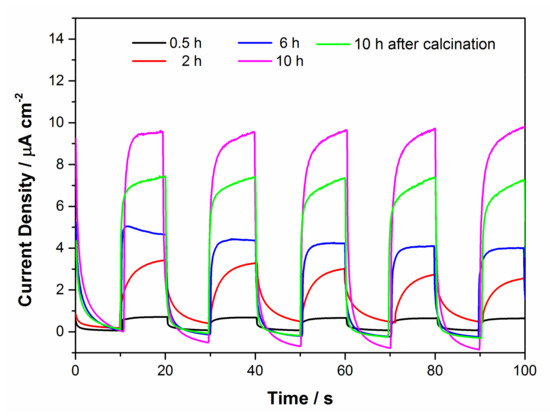
Figure 10.
Transient photocurrent response of the samples with different reaction conditions.
4. Conclusions
In conclusion, we have successfully explored a simple, efficient approach to synthesize flower-like TiO2 nanostructure. We examined the effects of different reaction times on the morphology and photocatalysis of the samples. Subsequently, a reasonable mechanism for the morphological growth of the sample was proposed by means of time-dependent experiments. In addition, flower-like TiO2 nanostructures showed higher photocatalytic activity and photocurrent response than other samples. It can be attributed to such factors as: (I) Special structure of flower-like morphology, (II) high crystallinity and (III) large specific surface area. Based on the above characteristics, the flower-like TiO2 has great potential applications in the field of dye-sensitized solar cells and photocatalysis.
Author Contributions
H.L. provided ideas for the whole experiment and synthesized flower-like sample TiO2. L.Z. carried out a series of characterization tests and wrote the paper. T.L. conducted the photocatalytic experiments.
Funding
This study was funded by the financial supports of the Key Research Project of Shandong Province (No. 2017GGX40121), the financial supports of the National Natural Science Foundation of China (Nos. 51402157, 51602164) and the Scientific Research Innovation Team in Colleges and Universities of Shandong Province.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rolison, D.R.; Long, J.W.; Lytle, J.C.; Fischer, A.E.; Rhodes, C.P.; McEvoy, T.M.; Bourg, M.E.; Lubers, A.M. Multifunctional 3D nanoarchitectures for energy storage and conversion. Chem. Soc. Rev. 2009, 38, 226–252. [Google Scholar] [CrossRef]
- Weng, Z.; Guo, H.; Liu, X.; Wu, S.; Yeung, K.W.K.; Chu, P.K. Nanostructured TiO2 for energy conversion and storage. Rsc Adv. 2013, 3, 24758–24775. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 Photocatalysis and Related Surface Phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; And, O.K.V.; Grimes, C.A. Use of Highly-Ordered TiO2 Nanotube Arrays in Dye-Sensitized Solar Cells. Nano Lett. 2006, 6, 215–218. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Wang, R.; Cai, X.; Shen, F. Preparation of TiO2 hollow microspheres by a novel vesicle template method and their enhanced photocatalytic properties. Ceram. Int. 2013, 39, 9465–9470. [Google Scholar] [CrossRef]
- Li, F.B.; Li, X.Z.; Hou, M.F.; Cheah, K.W.; Choy, W.C.H. Enhanced photocatalytic activity of Ce3+-TiO2 for 2-mercaptobenzothiazole degradation in aqueous suspension for odour control. Appl. Catal. A Gen. 2005, 285, 181–189. [Google Scholar] [CrossRef]
- Yu, J.; Yu, J.C.; Ho, W.; Leung, M.K.P.; Cheng, B.; Zhang, G.; Zhao, X. Effects of alcohol content and calcination temperature on the textural properties of bimodally mesoporous titania. Appl. Catal. A Gen. 2003, 255, 309–320. [Google Scholar] [CrossRef]
- Yun, J.; Jin, D.; Lee, Y.; Kim, H. Photocatalytic treatment of acidic waste water by electrospun composite nanofibers of pH-sensitive hydrogel and TiO2. Mater. Lett. 2010, 64, 2431–2434. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Baraton, M.I. Nano-TiO2 for dye-sensitized solar cells. Recent Pat. Nanotechnol. 2012, 6, 10–15. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6781. [Google Scholar] [CrossRef]
- Ho, W.; Yu, J.C.; Lee, S. Synthsis of hierarchical nanoporous F− doped TiO2 spheres with visible light photocatalytic activity. Chem Commun. 2006, 111, 1115–1117. [Google Scholar] [CrossRef]
- Xu, X.; Fang, X.; Zhai, T.; Zeng, H.; Liu, B.; Hu, X.; Bando, Y.; Golberg, D. Tube-in-Tube TiO2 Nanotubes with Porous Walls: Fabrication, Formation Mechanism, and Photocatalytic Properties. Small 2011, 7, 445–449. [Google Scholar] [CrossRef]
- Wu, J.J.; Lu, S.L.; Ge, D.H.; Zhang, L.Z.; Chen, W.; Gu, H.W. Photocatalytic properties of Pd/TiO2 nanosheets for hydrogen evolution from water splitting. RSC Adv. 2016, 6, 67502–67508. [Google Scholar] [CrossRef]
- Kim, C.W.; Choi, M.J.; Lee, S.; Park, H.; Moon, B.; Kang, Y.S.; Kang, Y.S. Crystalline Matrix of Mesoporous TiO2 Framework for Dye-Sensitized Solar Cell Application. J. Phys. Chem. C 2015, 119, 24902–24909. [Google Scholar] [CrossRef]
- Chemseddine, A.; Moritz, T. ChemInform Abstract: Nanostructuring Titania: Control over Nanocrystal Structure, Size, Shape, and Organization. Eur. J. Inorg. Chem. 1999, 30, 235–245. [Google Scholar] [CrossRef]
- Feng, X.; Shankar, K.; Varghese, O.K.; Paulose, M.; Latempa, T.J.; Grimes, C.A. Vertically aligned single crystal TiO2 nanowire arrays grown directly on transparent conducting oxide coated glass: Synthesis details and applications. Nano Lett. 2008, 8, 3781–3786. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Feng, J.; Yin, M.; Wang, Z.; Yan, S.; Wan, L.; Li, Z.; Zou, Z. Facile synthesis of anatase TiO2 mesocrystal sheets with dominant {001} facets based on topochemical conversion. CrystEngComm 2010, 12, 3425–3429. [Google Scholar] [CrossRef]
- Wu, X.; Fang, S.; Zheng, Y.; Sun, J.; Lv, K. Thiourea-Modified TiO2 Nanorods with Enhanced Photocatalytic Activity. Molecules 2016, 21, 181. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.G.; Liu, G.; Qiao, S.Z.; Sun, C.H.; Jin, Y.G.; Smith, S.C.; Zou, J.; Cheng, H.M.; Lu, G.Q. Solvothermal Synthesis and Photoreactivity of Anatase TiO2 Nanosheets with Dominant {001} Facets. J. Am. Chem. Soc. 2009, 131, 4078–4083. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.S.; Chen, Z.; Forman, A.J.; Kim, D.R.; Rao, P.M.; Jaramillo, T.F.; Zheng, X. Branched TiO2 nanorods for photoelectrochemical hydrogen production. Nano Lett. 2011, 11, 4978–4984. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Tan, Y.L.; Li, C.M.; Cheah, Y.L.; Luan, D.; Madhavi, S.; Boey, F.Y.; Archer, L.A.; Lou, X.W. Constructing Hierarchical Spheres from Large Ultrathin Anatase TiO2 Nanosheets with Nearly 100% Exposed {001} Facets for Fast Reversible Lithium Storage. J. Am. Chem. Soc. 2010, 132, 6124–6130. [Google Scholar] [CrossRef]
- Liu, M.; Piao, L.; Lu, W.; Ju, S.; Zhao, L.; Zhou, C.; Li, H.; Wang, W. Flower-like TiO2 nanostructures with exposed {001} facets: Facile synthesis and enhanced photocatalysis. Nanoscale 2010, 2, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Chen, W.; Yang, S. Double-Layered Photoanodes from Variable-Size Anatase TiO2 Nanospindles: A Candidate for High-Efficiency Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2010, 49, 3675–3679. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J. Photocatalytic Activity of Hierarchical Flower-Like TiO2 Superstructures with Dominant {001} Facets. Chin. J. Catal. 2011, 32, 525–531. [Google Scholar] [CrossRef]
- Réti, B.; Kiss, G.I.; Gyulavári, T.; Baan, K.; Magyari, K.; Hernadi, K. Carbon sphere templates for TiO2 hollow structures: Preparation, characterization and photocatalytic activity. Catal. Today 2017, 284, 160–168. [Google Scholar] [CrossRef]
- Lin, J.; Liu, X.; Zhu, S.; Liu, Y.; Chen, X. Anatase TiO2 nanotube powder film with high crystallinity for enhanced photocatalytic performance. Nanoscale Res. Lett. 2015, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Yeob, S.J.; Cheng, H.M.; Kang, Y.S. Selectively exposed crystal facet-engineered TiO2 thin film photoanode for the higher performance of the photoelectrochemical water splitting reaction. Energy Environ. Sci. 2015, 8, 3646–3653. [Google Scholar] [CrossRef]
- Gong, X.Q.; Annabella, S.; Andrea, V. Density functional theory study of formic acid adsorption on anatase TiO2 (001): Geometries, energetics, and effects of coverage, hydration, and reconstruction. J. Phys. Chem. B 2006, 110, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, B.; Li, Y.; Yu, S.; Liu, D.; Wang, P. Ethylene glycol-mediated synthesis of metal oxide nanowires. J. Alloys Compd. 2009, 471, 477–480. [Google Scholar] [CrossRef]
- Wei, M.; Zhou, H.; Konishi, Y.; Ichihara, M.; Sugiha, H.; Arakawa, H. Synthesis of tubular titanate via a self-assembly and self-removal process. Inorg. Chem. 2006, 45, 5684–5690. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, J.; Chen, R.; Zhou, D.; Xiang, L. A Review on the Fabrication of Hierarchical ZnO Nanostructures for Photocatalysis Application. Crystals 2016, 6, 148. [Google Scholar] [CrossRef]
- Molla, M.A.I.; Tateishi, I.; Mai, F.; Katsumata, H.; Suzuki, T.; Kaneco, S. Evaluation of Reaction Mechanism for Photocatalytic Degradation of Dye with Self-Sensitized TiO2 under Visible Light Irradiation. Open J. Inorg. Non-Met. Mater. 2017, 7, 1–7. [Google Scholar]
- Hu, C.; Lei, E.; Zhao, D.; Hu, K.; Cui, J.; Xiong, Q.; Liu, Z. Controllable synthesis and formation mechanism of 3D flower-like TiO2 microspheres. J. Mater. Sci. Mater. Electron. 2018, 29, 10277–10283. [Google Scholar] [CrossRef]
- Ma, S.; Xue, J.; Zhou, Y.; Zhang, Z. Photochemical synthesis of ZnO/Ag2O heterostructures with enhanced ultraviolet and visible photocatalytic activity. J. Mater. Chem. A 2014, 2, 7272–7280. [Google Scholar] [CrossRef]
- Yu, J.G.; Su, Y.R.; Cheng, B. Template-Free Fabrication and Enhanced Photocatalytic Activity of Hierarchical Macro-/Mesoporous Titania. Adv. Funct. Mater. 2010, 17, 1984–1990. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, L.; Cheng, B.; Su, Y. Hydrothermal Preparation and Photocatalytic Activity of Hierarchically Sponge-like Macro-/Mesoporous Titania. J. Phys. Chem. C 2007, 111, 10582–10589. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).