Enhanced High Voltage Performance of Chlorine/Bromine Co-Doped Lithium Nickel Manganese Cobalt Oxide
Abstract
1. Introduction
2. Materials and Methods
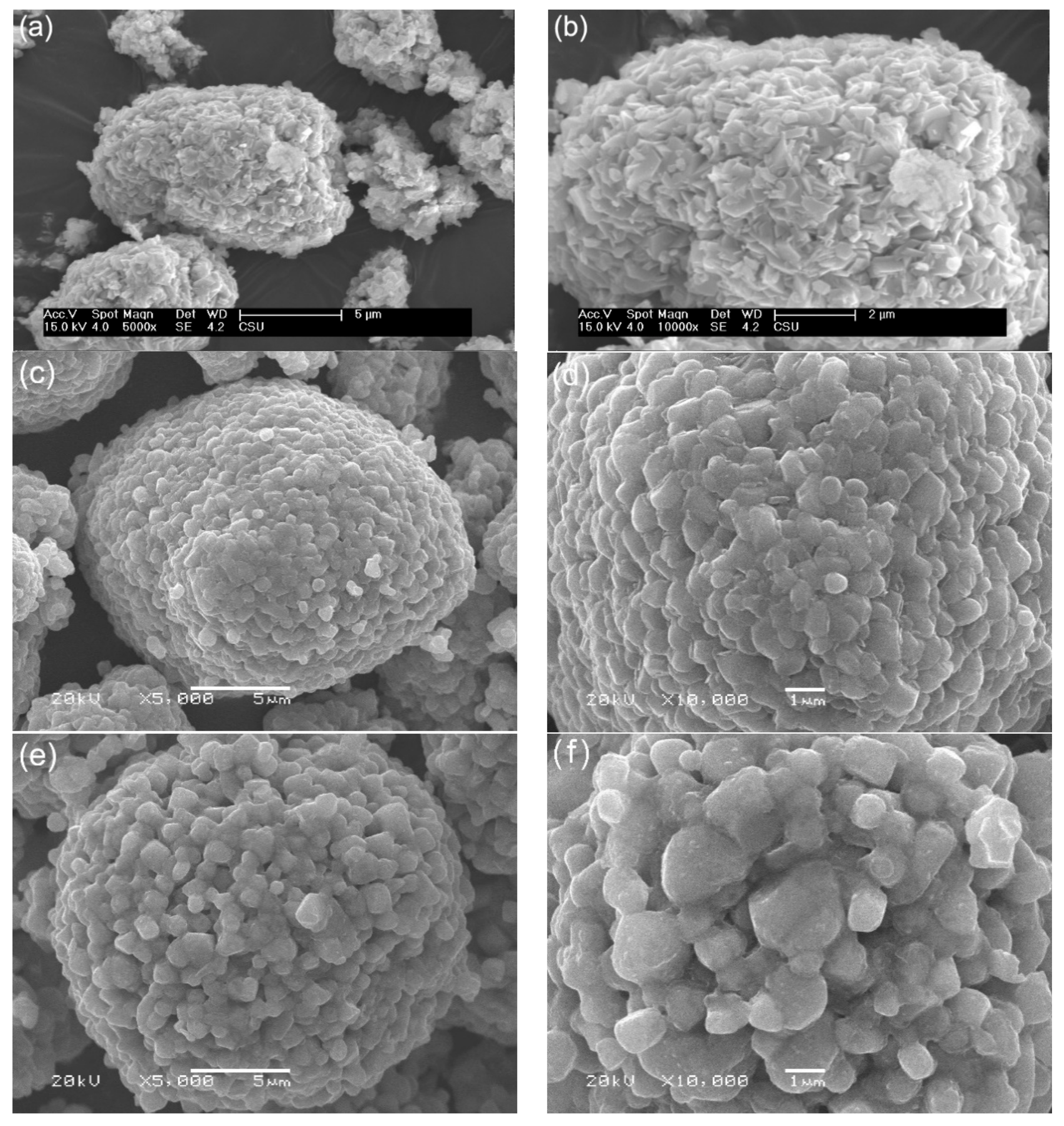
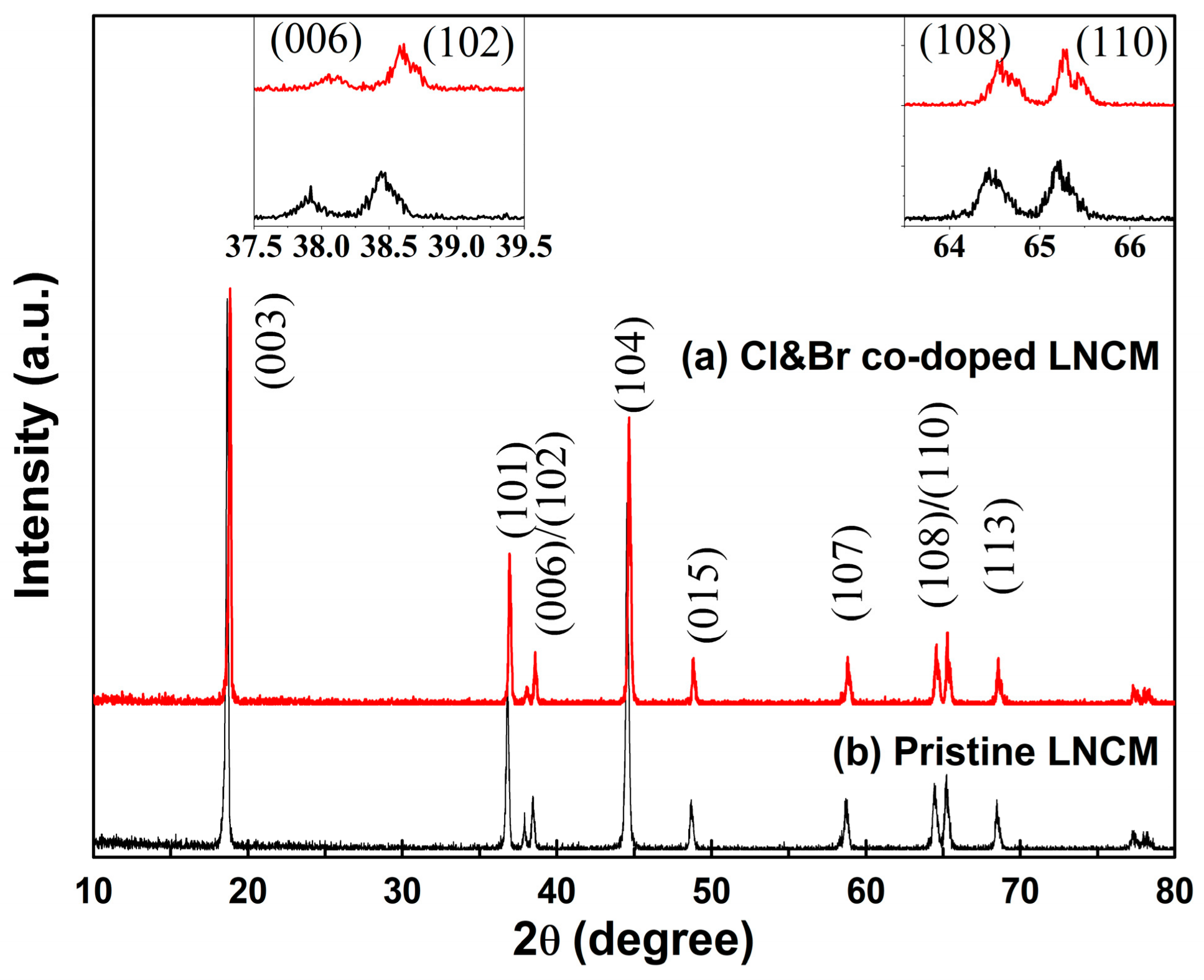
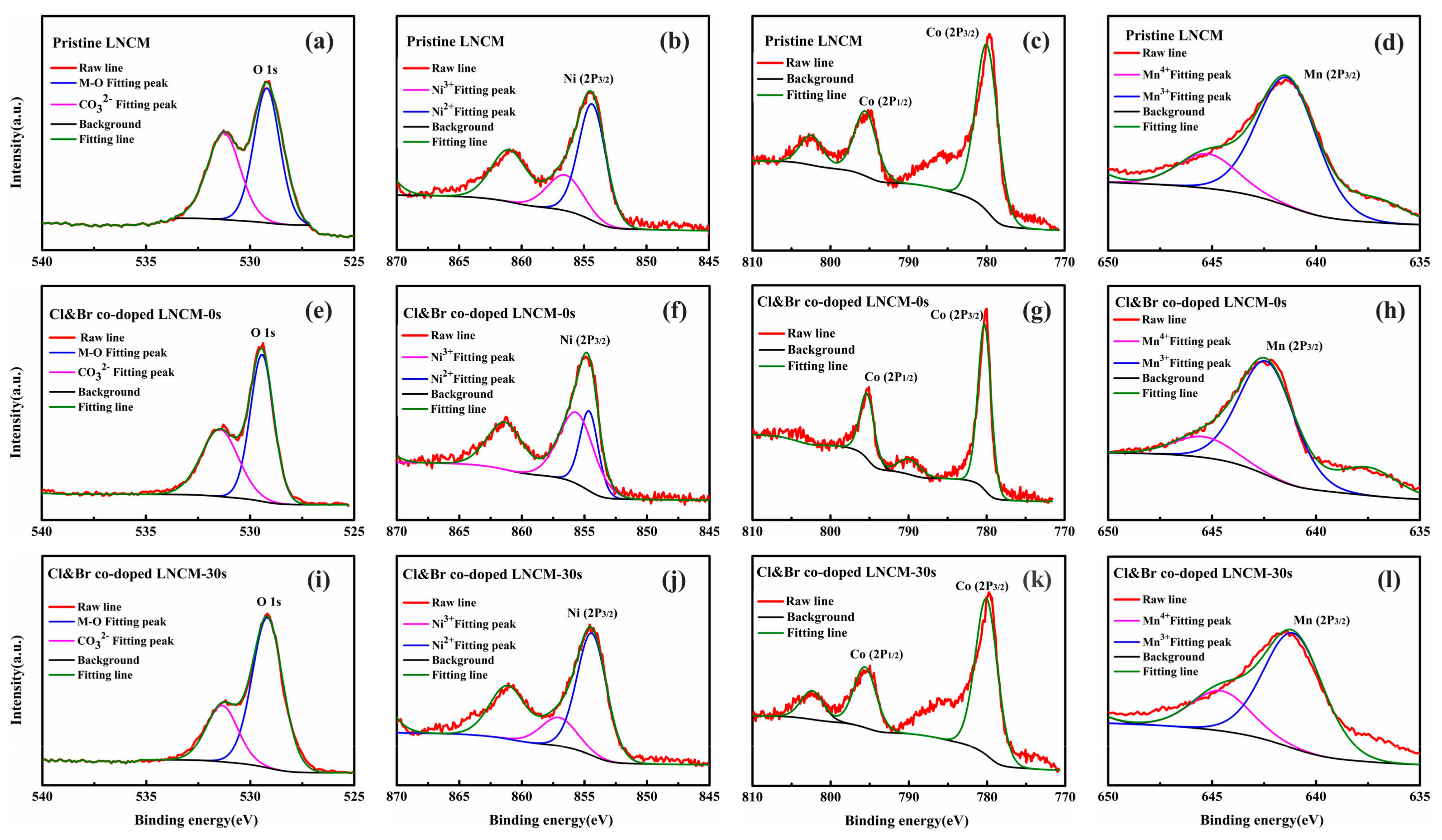
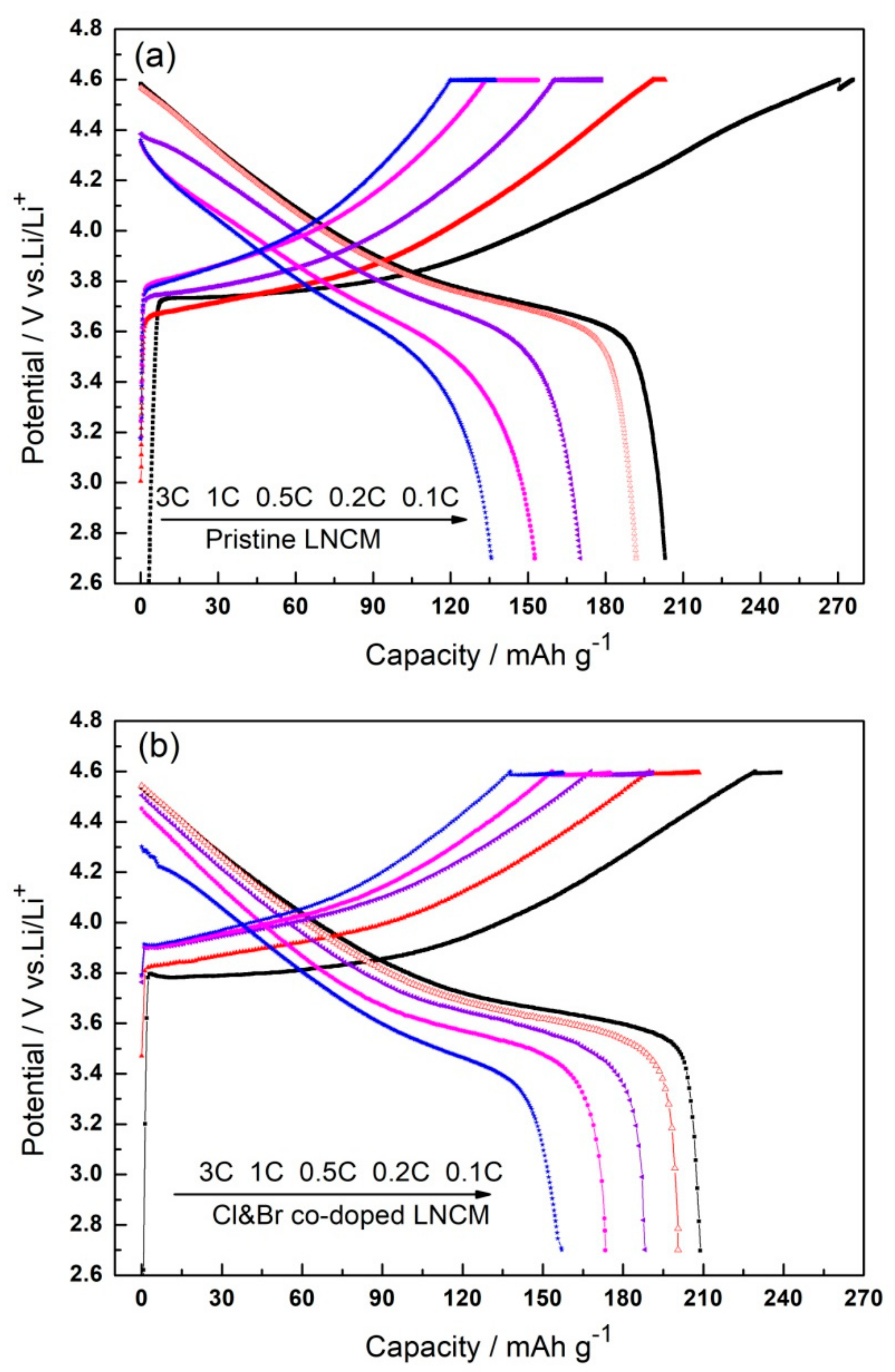
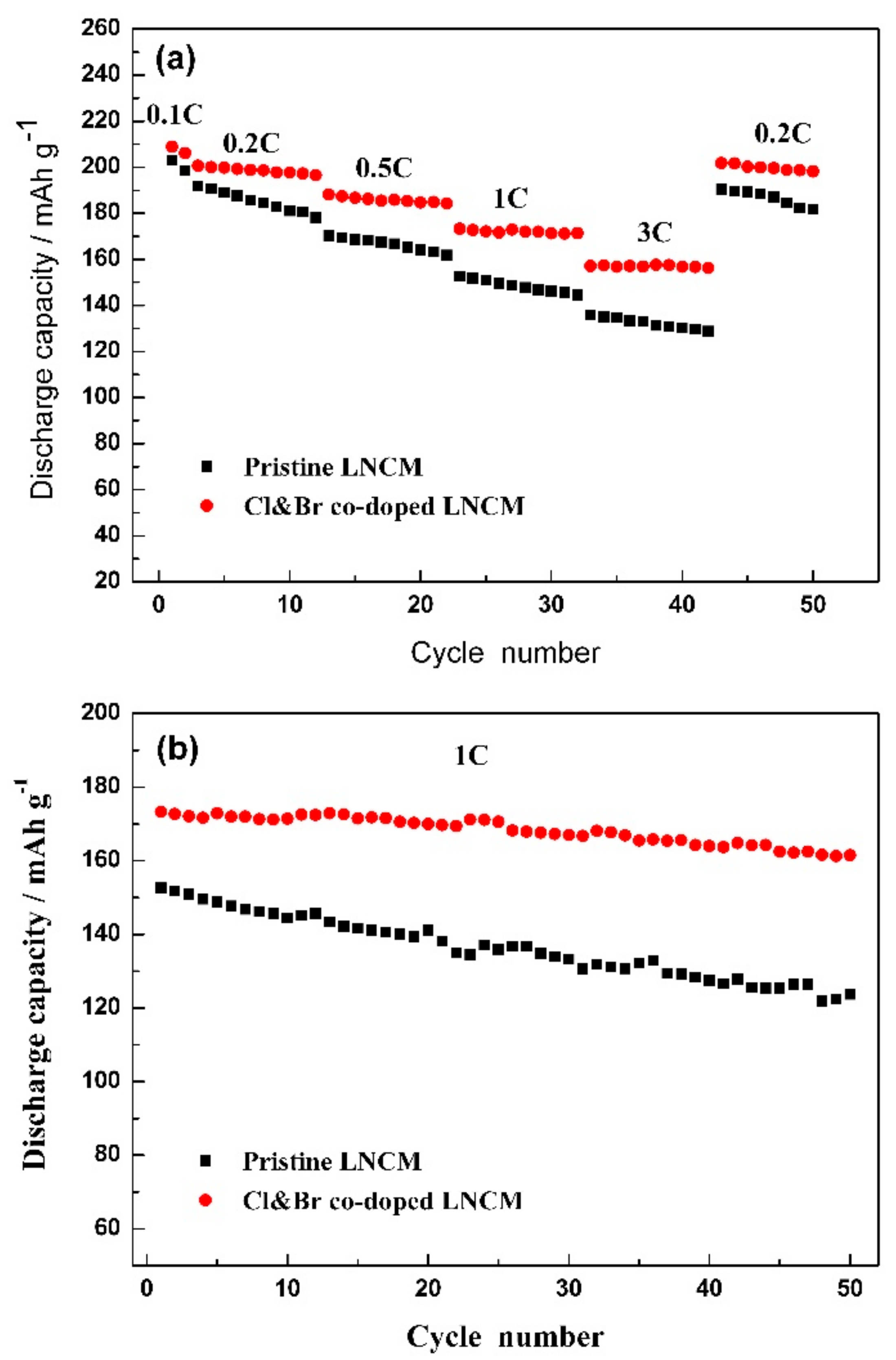
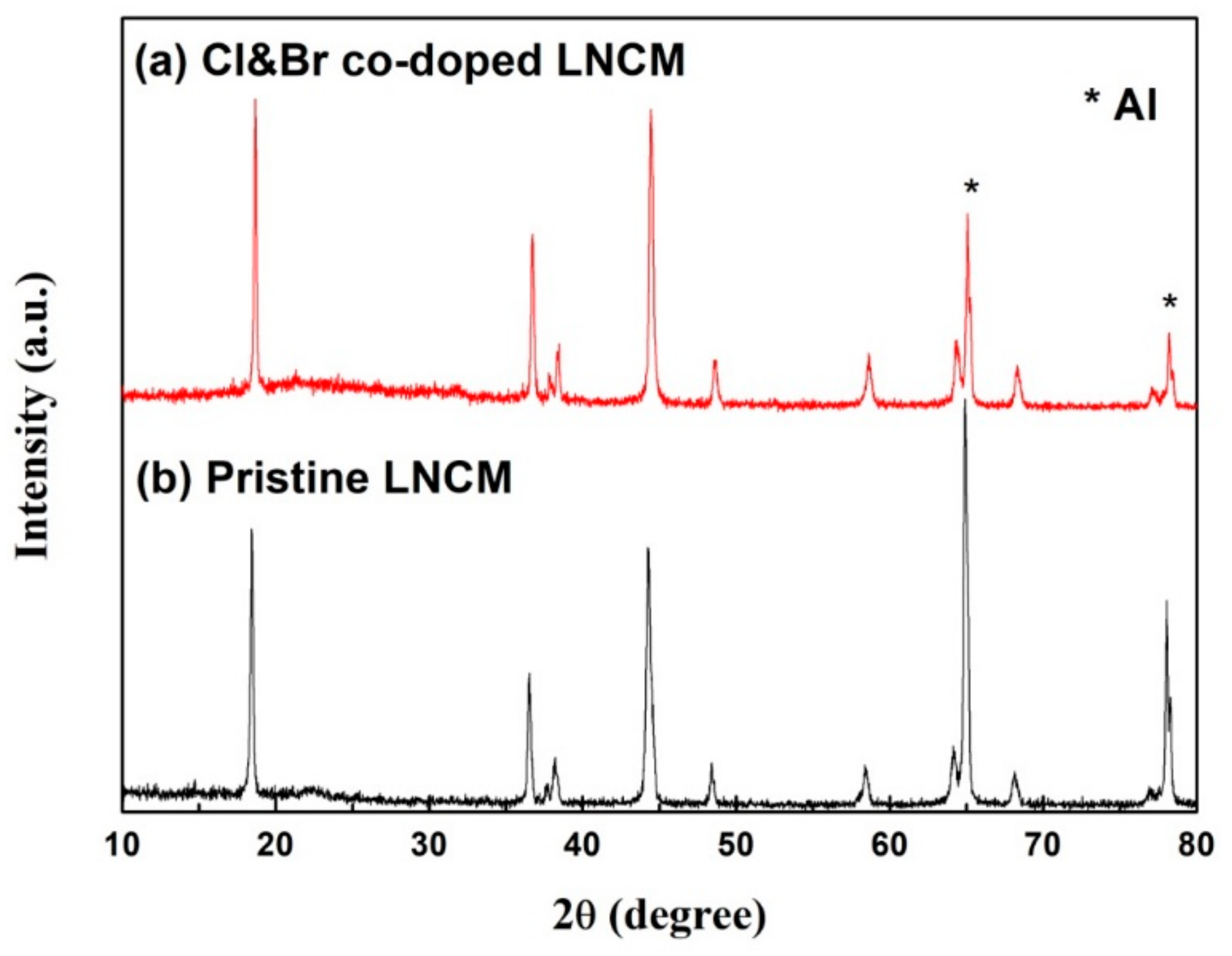
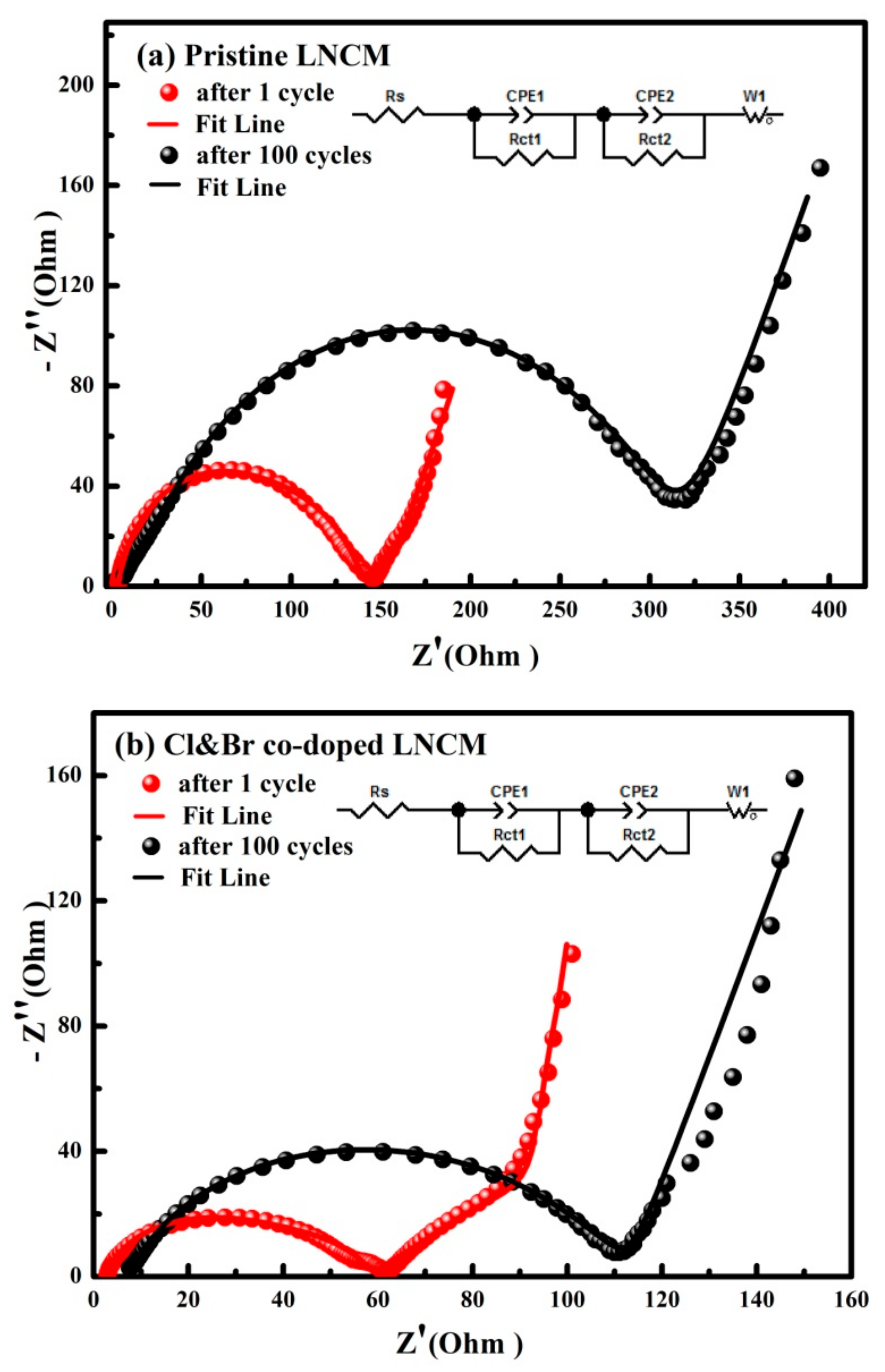
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable batteries. J. Power Sources 2011, 196, 6688–6694. [Google Scholar] [CrossRef]
- Chen, Z.; Qin, Y.; Amine, K.; Sun, Y.-K. Role of surface coating on cathode materials for lithium-ion batteries. J. Mater. Chem. 2010, 20, 7606–7612. [Google Scholar] [CrossRef]
- Li, H.; Zhou, H. Enhancing the performances of Li-ion batteries by carbon-coating: Present and future. Chem. Commun. 2012, 48, 1201–1217. [Google Scholar] [CrossRef] [PubMed]
- Ohzuku, T.; Makimura, Y. Layered Lithium insertion material of LiNi1/2Mn1/2O2: A possible alternative to LiCoO2 for advanced lithium-ion batteries. Chem. Lett. 2001, 30, 744–745. [Google Scholar] [CrossRef]
- Roberts, M.; Owen, J. High-throughput method to study the effect of precursors and temperature, applied to the synthesis of LiNi1/3Co1/3Mn1/3O2 for lithium batteries. ACS Comb. Sci. 2011, 13, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, X.; Huang, M.; Huang, J.; Fang, Z. Preparation and Rate Capability of Carbon Coated LiNi1/3Co1/3Mn1/3O2 as Cathode Material in Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 12408–12415. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Li, Y.; Liu, H.; Pinto, J.; Jiang, X.; Yang, G. Improved high rate capacity and lithium diffusion ability of LiNi1/3Co1/3Mn1/3O2 with ordered crystal structure. J. Electrochem. Soc. 2012, 159, A506–A513. [Google Scholar] [CrossRef]
- Jiang, K.-C.; Xin, S.; Lee, J.-S.; Kim, J.; Xiao, X.-L.; Guo, Y.-G. Improved kinetics of LiNi1/3Mn1/3Co1/3O2 cathode material through reduced graphene oxide networks. Phys. Chem. Chem. Phys. 2012, 14, 2934–2939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jia, D.; Tang, Y.; Huang, Y.; Pang, W.; Guo, Z.; Zhou, Z. In situ chelating synthesis of hierarchical LiNi1/3Co1/3Mn1/3O2 polyhedron assemblies with ultralong cycle life for Li-ion batteries. Small 2018, 14, 1704354. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, J.; Chen, Z.; Li, Q.; Xie, T.; Li, L.; Lai, Y. Molten salt synthesis and electrochemical properties of LiNi1/3Co1/3Mn1/3O2 cathode materials. Synth. Met. 2014, 187, 123–129. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Xie, T.; Li, L.J.; Xu, M.; Zhu, H.L.; Wang, W.H. Characterization of Na substituted LiNi1/3Co1/3Mn1/3O2 cathode materials for lithium ion battery. Ionics 2014, 20, 629–634. [Google Scholar] [CrossRef]
- Shaju, K.M.; Rao, G.V.S.; Chowdari, B.V.R. Performance of layered Li(Ni1/3Co1/3Mn1/3)O2 as cathode for Li-ion batteries. Electrochim. Acta 2002, 48, 145–151. [Google Scholar] [CrossRef]
- Periasamy, P.; Kalaiselvi, N.; Kim, H.S. High voltage and high capacity characteristics of LiNi1/3Co1/3Mn1/3O2 cathode for lithium battery applications. Int. J. Electrochem. Sci. 2007, 2, 689–699. [Google Scholar]
- Kim, G.-H.; Kim, J.-H.; Myung, S.-T.; Yoon, C.S.; Sun, Y.-K. Improvement of high-voltage cycling behavior of surface-modified Li[Ni1/3Co1/3Mn1/3]O2 cathodes by fluorine substitution for li-ion batteries. J. Electrochem. Soc. 2005, 152, A1707–A1713. [Google Scholar] [CrossRef]
- Zeng, Y.W. Investigation of LiNi1/3Co1/3Mn1/3O2 cathode particles after 300 discharge/charge cycling in a lithium-ion battery by analytical TEM. J. Power Sources 2008, 183, 316–324. [Google Scholar] [CrossRef]
- Li, X.; Wei, Y.J.; Ehrenberg, H.; Du, F.; Wang, C.Z.; Chen, G. Characterizations on the structural and electrochemical properties of LiNi1/3Mn1/3Co1/3O2 prepared by a wet-chemical process. Solid State Ionics 2008, 178, 1969–1974. [Google Scholar] [CrossRef]
- Lv, D.; Wang, L.; Hu, P.; Sun, Z.; Chen, Z.; Zhang, Q.; Cheng, W.; Ren, W.; Bian, L.; Xu, J.; et al. Li2O-B2O3-Li2SO4 modified LiNi1/3Co1/3Mn1/3O2 cathode material for enhanced electrochemical performance. Electrochim. Acta 2017, 247, 803–811. [Google Scholar] [CrossRef]
- Li, X.; Peng, H.; Wang, M.-S.; Zhao, X.; Huang, P.-X.; Yang, W.; Xu, J.; Wang, Z.-Q.; Qu, M.-Z.; Yu, Z.-L. Enhanced electrochemical performance of Zr-modified layered LiNi1/3Co1/3Mn1/3O2 cathode material for lithium-ion batteries. ChemElectroChem 2015, 3, 130–137. [Google Scholar] [CrossRef]
- Wu, F.; Wang, M.; Su, Y.; Bao, L.; Chen, S. Surface of LiCo1/3Ni1/3Mn1/3O2 modified by CeO2-coating. Electrochim. Acta 2009, 54, 6803–6807. [Google Scholar] [CrossRef]
- Riley, L.A.; Atta, S.V.; Cavanagh, A.S.; Yan, Y.; George, S.M.; Liu, P.; Dillon, A.C.; Lee, S.-H. Electrochemical effects of ALD surface modification on combustion synthesized LiNi1/3Mn1/3Co1/3O2 as a layered-cathode material. J. Power Sources 2011, 196, 3317–3324. [Google Scholar] [CrossRef]
- Li, J.; Fan, M.; He, X.; Zhao, R.; Jiang, C.; Wan, C. TiO2 coating of LiNi1/3Co1/3Mn1/3O2 cathode materials for Li-ion batteries. Ionics 2006, 12, 215–218. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Yoo, E.; Ishida, M.; Zhou, H. Fabrication of FePO4 layer coated LiNi1/3Co1/3Mn1/3O2: Towards high-performance cathode materials for lithium ion batteries. Electrochim. Acta 2012, 83, 253–258. [Google Scholar] [CrossRef]
- Son, M.Y.; Hong, Y.J.; Choi, S.H.; Kang, Y.C. Effects of ratios of Li2MnO3 and Li(Ni1/3Mn1/3Co1/3)O2 phases on the properties of composite cathode powders in spray pyrolysis. Electrochim. Acta 2013, 103, 110–118. [Google Scholar] [CrossRef]
- Li, D.; Sasaki, Y.; Kobayakawa, K.; Noguchi, H.; Sato, Y. Preparation, morphology and electrochemical characteristics of LiNi1/3Mn1/3Co1/3O2 with LiF addition. Electrochim. Acta 2006, 52, 643–648. [Google Scholar] [CrossRef]
- Chen, Y.; Jiao, Q.; Wang, L.; Hu, Y.; Sun, N.; Shen, Y.; Wang, Y. Synthesis and characterization of Li1.05Co1/3Ni1/3Mn1/3O1.95X0.05 (X = Cl, Br) cathode materials for lithium-ion battery. C. R. Chim. 2013, 16, 845–849. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Zhu, H.L.; Hu, G.R.; Xiao, J.; Peng, Z.D.; Liu, Y.X. Electrochemical performances and structure characteristic of LiMn2O4−xYx(Y = F, Cl, Br) compounds. Trans. Nonferrous Met. Soc. China 2004, 14, 1151–1155. [Google Scholar]
- Chen, Z.-Y.; Gao, L.Z.; Liu, X.Q.; Yu, Z.L. Properties and structure of spinel Li-Mn-O-F compounds for cathode materials of secondary lithium-ion battery. Chin. J. Chem. 2011, 19, 347–351. [Google Scholar] [CrossRef]
- Gao, Y.; Yakovleva, M.V.; Ebner, W.B. Novel LiNi1−xTix/2Mgx/2O2 compounds as cathode materials for safer lithium-ion batteries. Electrochem. Solid-State Lett. 1998, 1, 117–119. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, W.J.; Mauger, A.; Lu, Q.; Gendrond, F.; Julien, C.M. Minimization of the cation mixing in Li1+x(NMC)1-xO2 as cathode material. J. Power Sources 2010, 195, 1292–1301. [Google Scholar] [CrossRef]
- Reimers, J.N.; Rossen, E.; Jones, C.D.; Dahn, J.R. Structure and electrochemistry of LixFeyNi1−yO2. Solid State Ionics 1993, 61, 335–344. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A.; Nagayama, M. Electrochemistry and structural chemistry of LiNiO2 (R3m) for 4 volt secondary lithium cells. J. Electrochem. Soc. 1993, 140, 1862–1870. [Google Scholar] [CrossRef]
- Oswald, S.; Brückner, W. XPS depth profile analysis of non-stoichiometric NiO films. Surf. Interface Anal. 2004, 36, 17–22. [Google Scholar] [CrossRef]
- Kageyama, M.; Li, D.; Kobayakawa, K.; Sato, Y.; Lee, Y.-S. Structural and electrochemical properties of LiNi1/3Mn1/3Co1/3O2−xFx prepared by solid state reaction. J. Power Sources 2006, 157, 494–500. [Google Scholar] [CrossRef]
- Iwanowski, R.J.; Heinonen, M.H.; Janik, E. X-ray photoelectron spectra of zinc-blende MnTe. Chem. Phys. Lett. 2004, 387, 110–115. [Google Scholar] [CrossRef]
- Zhu, H.L.; Xie, T.; Chen, Z.Y.; Li, L.J.; Xu, M.; Wang, W.H.; Lai, Y.Q.; Li, J. The impact of vanadium substitution on the structure and electrochemical performance of LiNi0.5Co0.2Mn0.3O2. Electrochim. Acta 2014, 135, 77–85. [Google Scholar] [CrossRef]

| Sample | Ni (wt%) | Co (wt%) | Mn (wt%) | Molar Ratio of Ni:Co:Mn |
|---|---|---|---|---|
| fresh pristine LNCM | 20.04 | 19.40 | 18.39 | 0.340:0.327:0.333 |
| cycled pristine LNCM | 22.75 | 22.40 | 19.60 | 0.345:0.338:0.317 |
| fresh Cl&Br co-doped LNCM | 22.11 | 21.72 | 20.07 | 0.339:0.332:0.329 |
| cycled Cl&Br co-doped LNCM | 21.56 | 20.52 | 19.16 | 0.345:0.327:0.328 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Li, Q.; Gong, X.; Cao, K.; Chen, Z. Enhanced High Voltage Performance of Chlorine/Bromine Co-Doped Lithium Nickel Manganese Cobalt Oxide. Crystals 2018, 8, 425. https://doi.org/10.3390/cryst8110425
Zhu H, Li Q, Gong X, Cao K, Chen Z. Enhanced High Voltage Performance of Chlorine/Bromine Co-Doped Lithium Nickel Manganese Cobalt Oxide. Crystals. 2018; 8(11):425. https://doi.org/10.3390/cryst8110425
Chicago/Turabian StyleZhu, Huali, Qifeng Li, Xiaolong Gong, Kaifeng Cao, and Zhaoyong Chen. 2018. "Enhanced High Voltage Performance of Chlorine/Bromine Co-Doped Lithium Nickel Manganese Cobalt Oxide" Crystals 8, no. 11: 425. https://doi.org/10.3390/cryst8110425
APA StyleZhu, H., Li, Q., Gong, X., Cao, K., & Chen, Z. (2018). Enhanced High Voltage Performance of Chlorine/Bromine Co-Doped Lithium Nickel Manganese Cobalt Oxide. Crystals, 8(11), 425. https://doi.org/10.3390/cryst8110425





