Synthesis and Fluorescence Properties of a Structurally Characterized Hetero-Hexanuclear Zn(II)-La(III) Salamo-Like Coordination Compound Containing Auxiliary Ligands
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Instrumentation
2.2. Preparation of Ligand H2L
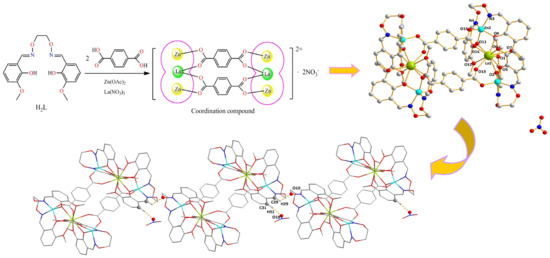
2.3. Preparation of the Zn(II)-La(III) Coordination Compound
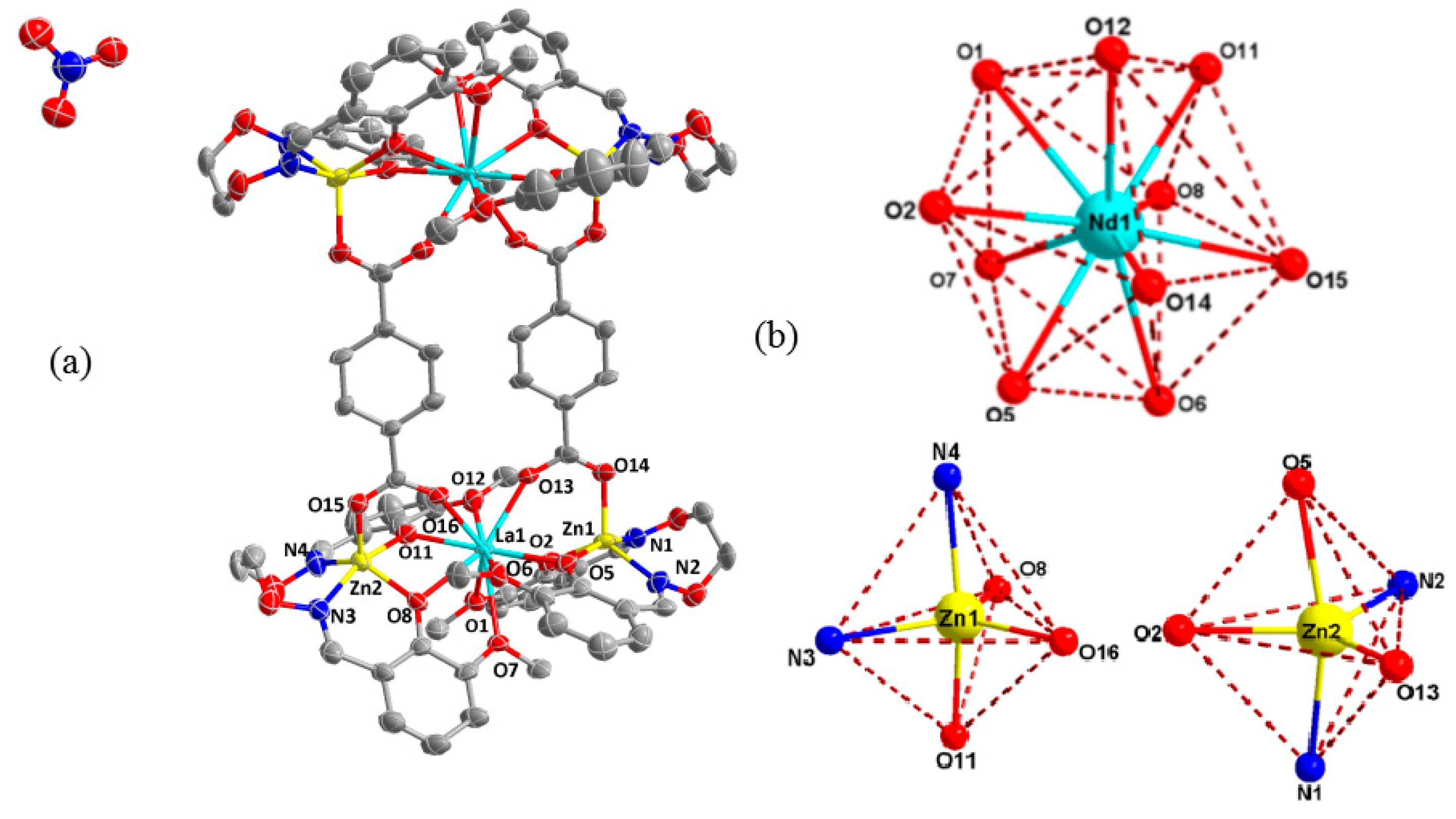
2.4. Structure Description of the Zn(II)-La(III) Coordination Compound
3. Results and Discussion
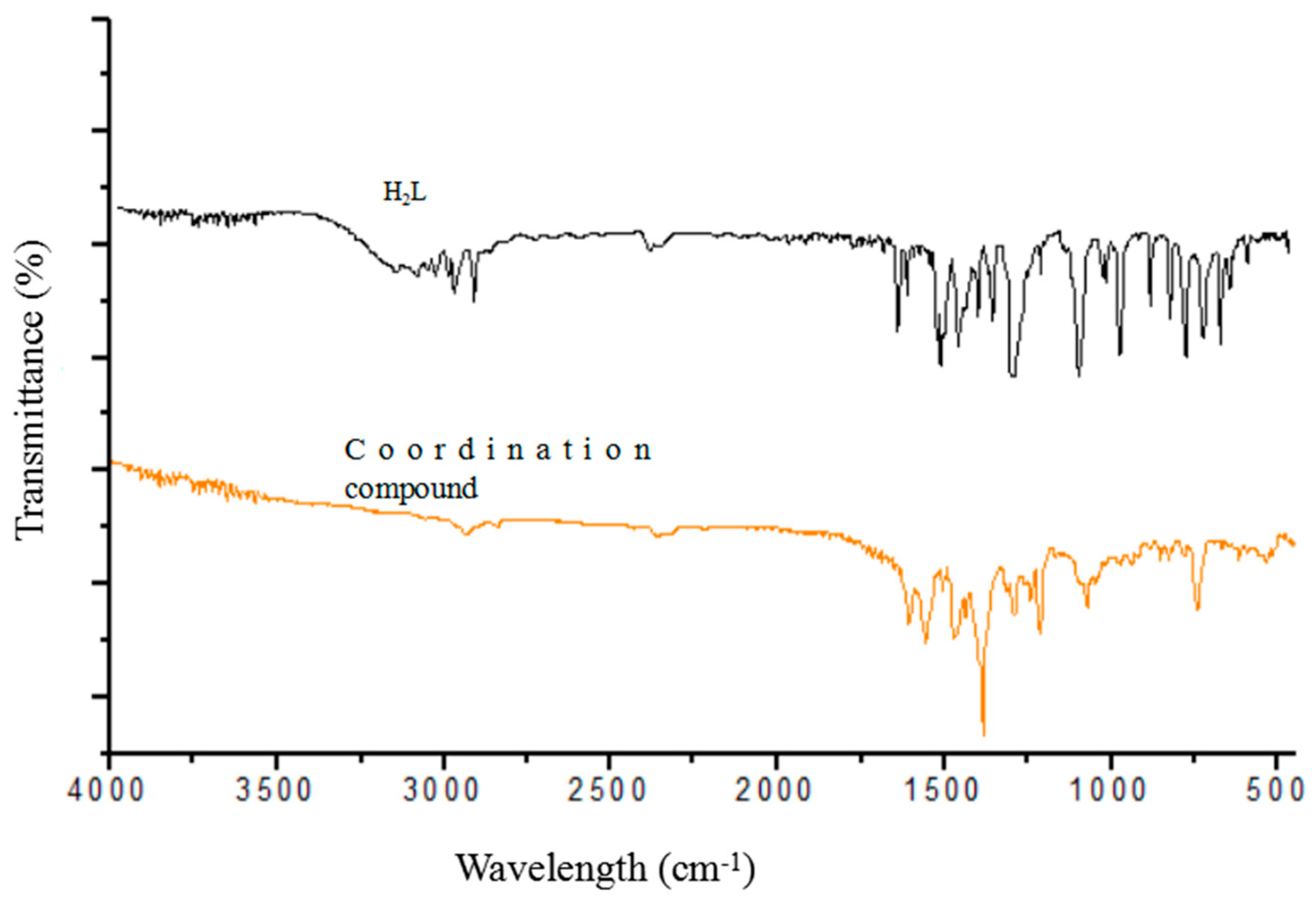
3.1. Infrared Spectra
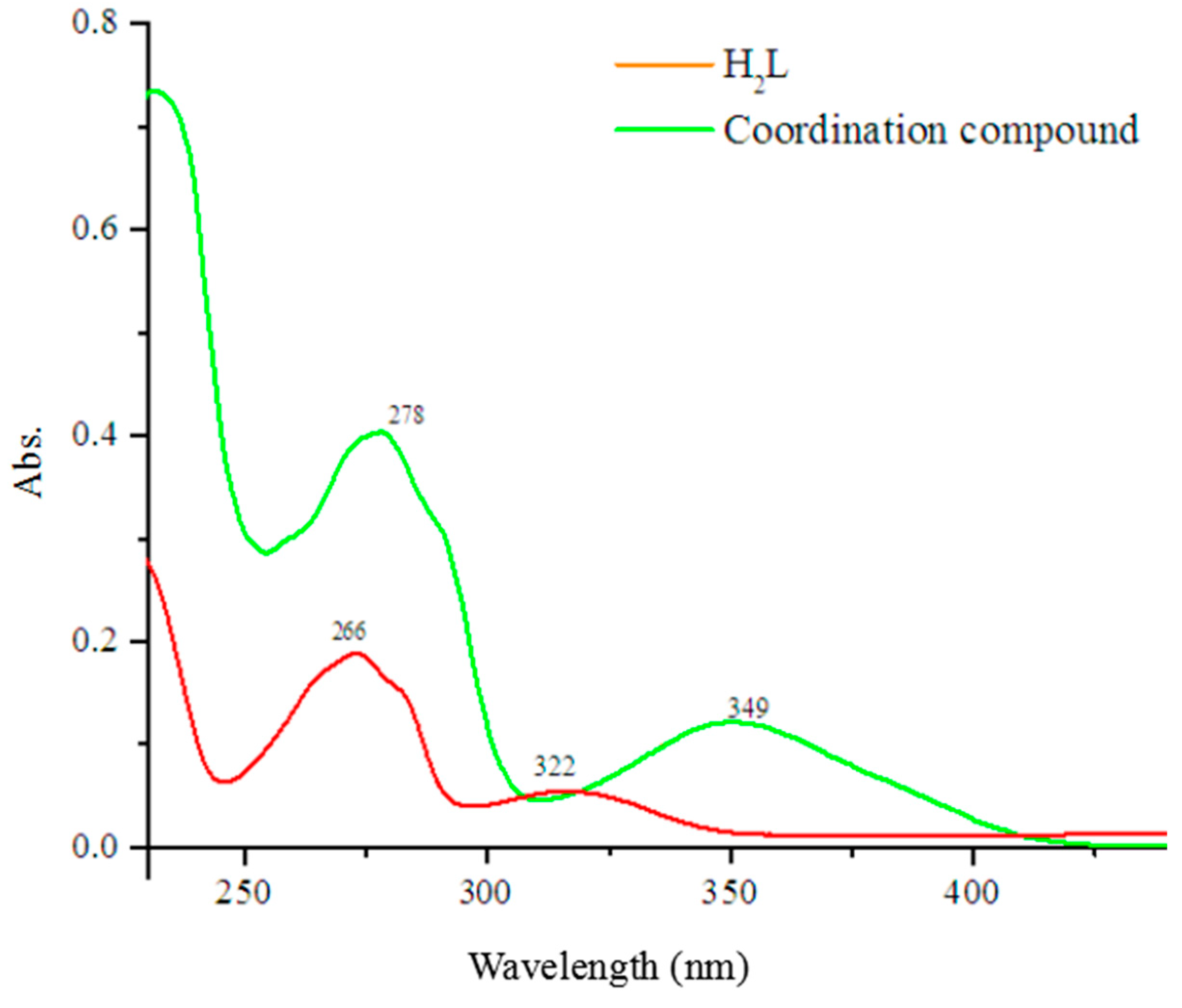
3.2. UV-Vis Spectra
3.3. Crystal Structure of Zn(II)-La(III) Coordination Compound
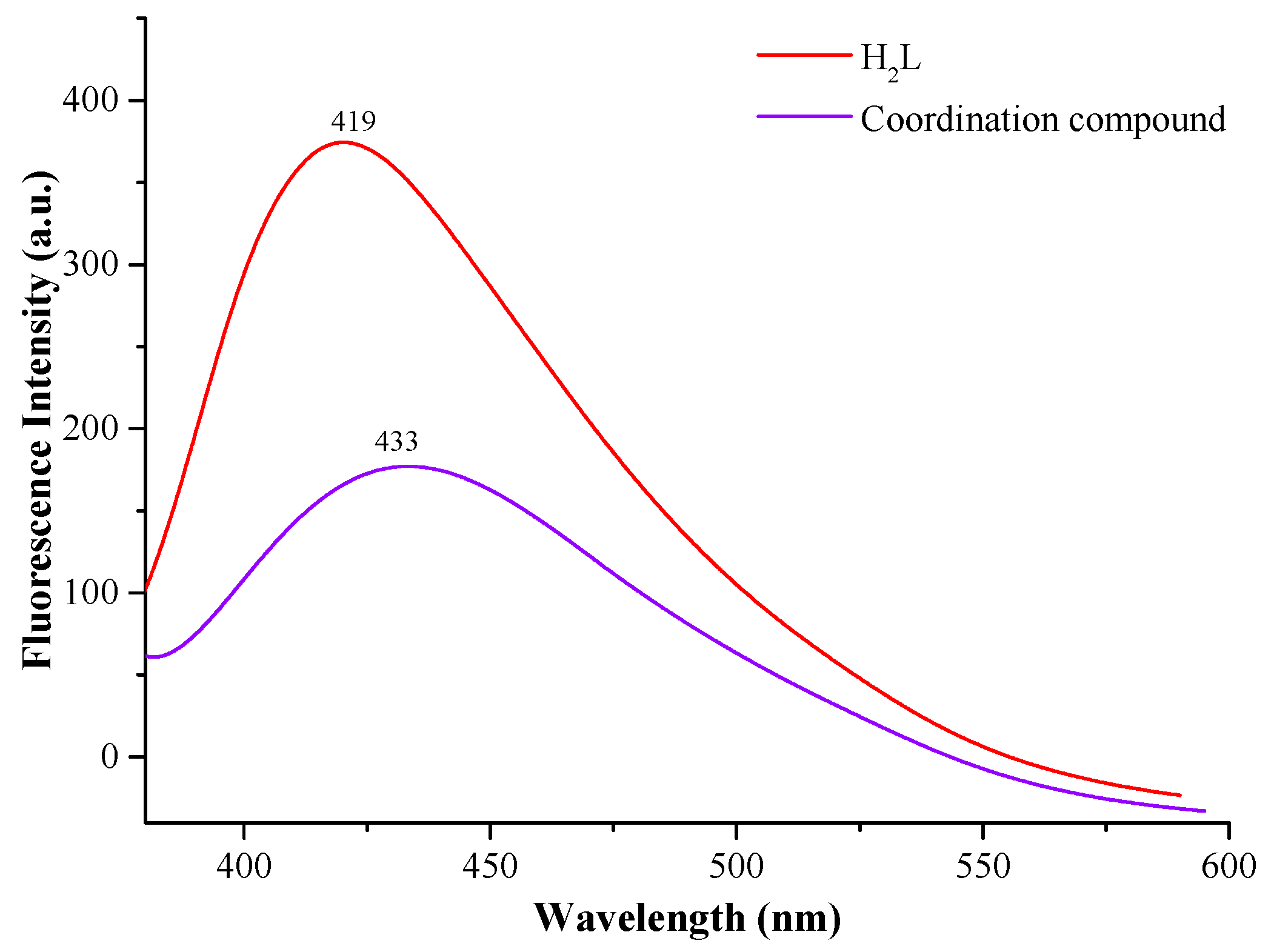
3.4. Fluorescence Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, J.J.; Song, X.Q.; Liu, Y.A.; Wang, X.L. Substituent-tuned structure and luminescence sensitizing towards Al3+ based on phenoxy bridged dinuclear EuIII complexes. RSC Adv. 2017, 7, 25549–25559. [Google Scholar] [CrossRef]
- Akine, S. Novel ion recognition systems based on cyclic and acyclic oligo(salen)-type ligands. J. Incl. Phenom. Macrocycl. Chem. 2012, 72, 25–54. [Google Scholar] [CrossRef]
- Akine, S.; Varadi, Z.; Nabeshima, T. Synthesis of planar metal complexes and the ctacking abilities of naphthalenediol-based acyclic and macrocyclic Salen-type ligands. Eur. J. Inorg. Chem. 2013, 5987–5998. [Google Scholar] [CrossRef]
- Che, C.M.; Huang, J.S. Metal complexes of chiral binaphthyl Schiff-base ligands and their application in stereoselective organic transformations. Coord. Chem. Rev. 2003, 242, 97–113. [Google Scholar] [CrossRef]
- Song, X.Q.; Cheng, G.Q.; Wang, X.R.; Xu, W.Y.; Liu, P.P. Structure-based description of a step-by-step synthesis of heterodinuclear ZnIILnIII complexes and their luminescence properties. Inorg. Chim. Acta 2015, 425, 145–153. [Google Scholar] [CrossRef]
- Chai, L.Q.; Huang, J.J.; Zhang, H.S. An unexpected cobalt (III) complex containing a schiff base ligand: Synthesis, crystal structure, spectroscopic behavior, electrochemical property and SOD-like activity. Spectrochim. Acta Part A 2014, 131, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.Q.; Huang, J.J.; Zhang, J.Y.; Li, Y.X. Two 1-D and 2-D cobalt(II) complexes: Synthesis, crystal structures, spectroscopic and electrochemical properties. J. Coord. Chem. 2015, 68, 1224–1237. [Google Scholar] [CrossRef]
- Chai, L.Q.; Tang, L.J.; Chen, L.C.; Huang, J.J. Structural, spectral, electrochemical and DFT studies of two mononuclear manganese(II) and zinc(II) complexes. Polyhedron 2017, 122, 228–240. [Google Scholar] [CrossRef]
- Chai, L.Q.; Zhang, K.Y.; Tang, L.J.; Zhang, J.Y.; Zhang, H.S. Two mono- and dinuclear Ni(II) complexes constructed from quinazoline-type ligands: Synthesis, x-ray structures, spectroscopic, electrochemical, thermal, and antimicrobial studies. Polyhedron 2017, 130, 100–107. [Google Scholar] [CrossRef]
- Chen, C.Y.; Zhang, J.W.; Zhang, Y.H.; Yang, Z.H.; Wu, H.L. Gadolinium(III) and dysprosium(III) complexes with a schiff base bis(N-salicylidene)-3-oxapentane-1,5-diamine: Synthesis, characterization, antioxidation, and DNA-binding studies. J. Coord. Chem. 2015, 68, 1054–1071. [Google Scholar] [CrossRef]
- Wu, H.L.; Pan, G.L.; Bai, Y.C.; Wang, H.; Kong, J. Synthesis, structure, antioxidation, and DNA-bindingstudies of a binuclear ytterbium(III) complex with bis(N-salicylidene)-3-oxapentane-1,5-diamine. Res. Chem. Intermed. 2015, 41, 3375–3388. [Google Scholar] [CrossRef]
- Wu, H.L.; Bai, Y.C.; Zhang, Y.H.; Pan, G.L.; Kong, J.; Shi, F.; Wang, X.L. Two lanthanide(III) complexes based on the schiff base N,N-Bis(salicylidene)-1,5-diamino-3-oxapentane: Synthesis, characterization, DNA-binding properties, and antioxidation. Z. Anorg. Allg. Chem. 2014, 640, 2062–2071. [Google Scholar] [CrossRef]
- Wu, H.L.; Bai, Y.C.; Zhang, Y.H.; Li, Z.; Wu, M.C.; Chen, C.Y.; Zhang, J.W. Synthesis, crystal structure, antioxidation and DNA-binding properties of a dinuclear copper(II) complex with bis(N-salicylidene)-3-oxapentane-1,5-diamine. J. Coord. Chem. 2014, 67, 3054–3066. [Google Scholar] [CrossRef]
- Wu, H.L.; Pan, G.L.; Bai, Y.C.; Zhang, Y.H.; Wang, H.; Shi, F.R.; Wang, X.L.; Kong, J. Study on synthesis, crystal structure, antioxidant and DNA-binding of mono-, di-and poly-nuclear lanthanides complexes with bis (N-salicylidene)-3-oxapentane-1, 5-diamine. J. Photochem. Photobiol. B 2014, 135, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.L.; Pan, G.L.; Bai, Y.C.; Wang, H.; Kong, J.; Shi, F.R.; Zhang, Y.H.; Wang, X.L. Preparation, structure, DNA-binding properties, and antioxidant activities of a homodinuclear erbium(III) complex with a pentadentate schiff base ligand. J. Chem. Res. 2014, 38, 211–217. [Google Scholar] [CrossRef]
- Wu, H.L.; Wang, C.P.; Wang, F.; Peng, H.P.; Zhang, H.; Bai, Y.C. A new manganese(III) complex from bis(5-methylsalicylaldehyde)-3-oxapentane-1,5-diamine: Synthesis, characterization, antioxidant activity and luminescence. J. Chin. Chem. Soc. 2015, 62, 1028–1034. [Google Scholar] [CrossRef]
- Song, X.Q.; Liu, P.P.; Wang, C.Y.; Liu, Y.A.; Liu, W.S.; Zhang, M. Three sandwich-type zinc(II)–lanthanide(III) clusters: Structures, luminescence and magnetic properties. RSC Adv. 2017, 7, 22692–22698. [Google Scholar] [CrossRef]
- Sun, Y.X.; Wang, L.; Dong, X.Y.; Ren, Z.L.; Meng, W.S. Synthesis, characterization, and crystal structure of a supramolecular CoII complex containing Salen-type bisoxime. Synth. React. Inorg. Metal-Org. Nano-Metal Chem. 2013, 43, 599–603. [Google Scholar] [CrossRef]
- Sun, Y.X.; Xu, L.; Zhao, T.H.; Liu, S.H.; Dong, X.T. Synthesis and crystal structure of a 3D supramolecular copper(II) complex with 1-(3-{[(E)-3-bromo-5-chloro-2-hydroxybenzylidene]amino}phenyl) ethanone oxime. Synth. React. Inorg. Metal-Org. Nano-Metal Chem. 2013, 43, 509–513. [Google Scholar] [CrossRef]
- Sun, Y.X.; Zhang, S.T.; Ren, Z.L.; Dong, X.Y.; Wang, L. Synthesis, characterization, and crystal structure of a new supramolecular CdII complex with halogen-substituted Salen-type bisoxime. Synth. React. Inorg. Metal-Org. Nano-Metal Chem. 2013, 43, 995–1000. [Google Scholar] [CrossRef]
- Sun, Y.X.; Gao, X.H. Synthesis, characterization, and crystal structure of a new CuII complex with Salen-type ligand. Synth. React. Inorg. Metal-Org. Nano-Metal Chem. 2011, 41, 973–978. [Google Scholar] [CrossRef]
- Akine, S.; Taniguchi, T.; Nabeshima, T. Synthesis and characterization of novel ligands 1,2-bis(salicylideneaminooxy)ethanes. Chem. Lett. 2001, 30, 682–683. [Google Scholar] [CrossRef]
- Akine, S.; Utsuno, F.; Taniguchi, T.; Nabeshima, T. Dinuclear complexes of the N2O2 oxime chelate ligand with zinc(II)–lanthanide(III) as a selective sensitization system for Sm3+. Eur. J. Inorg. Chem. 2010, 49, 3143–3152. [Google Scholar] [CrossRef]
- Dong, Y.J.; Li, X.L.; Zhang, Y.; Dong, W.K. A highly selective visual and fluorescent sensor for Pb2+ and Zn2+ and crystal structure of Cu2+ complex based-on a novel single-armed Salamo-type bisoxime. Supramol. Chem. 2017, 29, 518–527. [Google Scholar] [CrossRef]
- Chen, L.; Dong, W.K.; Zhang, H.; Zhang, Y.; Sun, Y.X. Structural variation and luminescence properties of triand dinuclear CuII and ZnII complexes constructed from a naphthalenediol-based bis(Salamo)-type ligand. Cryst. Growth Des. 2017, 17, 3636–3648. [Google Scholar] [CrossRef]
- Sun, Y.X.; Liu, L.Z.; Wang, F.; Shang, X.Y.; Chen, L.; Dong, W.K. Structural and hirshfeld surface analyses of a novel hetero-tetranuclear CuII-NaI bis(Salamo)-based coordination compound. Crystals 2018, 8, 227. [Google Scholar] [CrossRef]
- Zhang, L.W.; Li, X.Y.; Kang, Q.P.; Liu, L.Z.; Ma, J.C.; Dong, W.K. Structures and fluorescent and magnetic behaviors of newly synthesized NiII and CuII coordination compounds. Crystals 2018, 8, 173. [Google Scholar] [CrossRef]
- Akine, S.; Kagiyama, S.; Nabeshima, T. Modulation of multimetal complexation behavior of tetraoxime ligand by covalent transformation of olefinic functionalities. Inorg. Chem. 2010, 49, 2141–2152. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Dong, W.K.; Zhang, Y.; Akogun, S.F.; Xu, L. Syntheses, structures and catecholase activities of homo- and hetero-trinuclear cobalt(II) complexes constructed from an acyclic naphthalenediol-based bis(Salamo)-type ligand. Appl. Organomet. Chem. 2017, 31, e3818. [Google Scholar] [CrossRef]
- Li, X.Y.; Chen, L.; Gao, L.; Zhang, Y.; Akogun, S.F.; Dong, W.K. Syntheses, crystal structures and catalytic activities of two solvent-induced homotrinuclear Co(II) complexes with a naphthalenediol-based bis(Salamo)-type tetraoxime ligand. RSC Adv. 2017, 7, 35905–35916. [Google Scholar] [CrossRef]
- Wang, L.; Hao, J.; Zhai, L.X.; Zhang, Y.; Dong, W.K. Synthesis, crystal structure, luminescence, electrochemical and antimicrobial properties of bis(Salamo)-based Co(II) complex. Crystals 2017, 7, 277. [Google Scholar] [CrossRef]
- Wang, B.J.; Dong, W.K.; Zhang, Y.; Akogun, S.F. A novel relay-sensor for highly sensitive and selective detection of Zn2+/Pic− and fluorescence on/off switch response of H+/OH−. Sens. Actuators B 2017, 247, 254–264. [Google Scholar] [CrossRef]
- Dong, W.K.; Li, X.L.; Wang, L.; Zhang, Y.; Ding, Y.J. A new application of Salamo-type bisoximes: As a relay-sensor for Zn2+/Cu2+ and its novel complexes for successive sensing of H+/OH−. Sens. Actuators B 2016, 229, 370–378. [Google Scholar] [CrossRef]
- Zheng, S.S.; Dong, W.K.; Zhang, Y.; Chen, L.; Ding, Y.J. Four Salamo-type 3d–4f hetero-bimetallic [ZnIILnIII] complexes: Syntheses, crystal structures, and luminescent and magnetic properties. New J. Chem. 2017, 41, 4966–4973. [Google Scholar] [CrossRef]
- Dong, Y.J.; Dong, X.Y.; Dong, W.K.; Zhang, Y.; Zhang, L.S. Three asymmetric Salamo-type copper(II) and cobalt(II) complexes: Syntheses, structures, fluorescent properties. Polyhedron 2017, 123, 305–315. [Google Scholar] [CrossRef]
- Dong, X.Y.; Li, X.Y.; Liu, L.Z.; Zhang, H.; Ding, Y.J.; Dong, W.K. Tri- and hexanuclear heterometallic Ni(II)–M(II) (M = Ca, Sr and Ba) bis(Salamo)-type complexes: Synthesis, structure and fluorescence properties. RSC Adv. 2017, 7, 48394–48403. [Google Scholar] [CrossRef]
- Peng, Y.D.; Li, X.Y.; Kang, Q.P.; An, G.X.; Zhang, Y.; Dong, W.K. Synthesis and fluorescence properties of asymmetrical Salamo-type tetranuclear zinc(II) complex. Crystals 2018, 8, 107. [Google Scholar] [CrossRef]
- Li, X.Y.; Kang, Q.P.; Liu, L.Z.; Ma, J.C.; Dong, W.K. Trinuclear Co(II) and mononuclear Ni(II) Salamo-type bisoxime coordination compounds. Crystals 2018, 8, 43. [Google Scholar] [CrossRef]
- Akine, S.; Tadokoro, T.; Nabeshima, T. Oligometallic template strategy for synthesis of a macrocyclic dimer-type octaoxime ligand for its cooperative complexation. Inorg. Chem. 2012, 51, 11478–11486. [Google Scholar] [CrossRef] [PubMed]
- Nabeshima, T.; Akine, S.; Ikeda, C.; Yamamura, M. Metallo-supramolecular systems for synergistic functions based on unique arrangement of ligation sites. Chem. Lett. 2010, 39, 10–16. [Google Scholar] [CrossRef]
- Akine, S.; Nabeshima, T. Cyclic and acyclic oligo(N2O2) ligands for cooperative multi-metal complexation. Dalton Trans. 2009, 47, 10395–10408. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Y.; Gao, L.; Wang, F.; Zhang, Y.; Dong, W.K. Tri- and mono-nuclear zinc(II) complexes based on half- and mono-Salamo chelating ligands. Crystals 2017, 7, 267. [Google Scholar] [CrossRef]
- Dong, W.K.; Ma, J.C.; Zhu, L.C.; Zhang, Y. Nine self-assembled nickel(II)-lanthanide(III) heterometallic complexes constructed from a Salamo-type bisoxime and bearing N- or O-donor auxiliary ligand: Syntheses, structures and magnetic properties. New J. Chem. 2016, 40, 6998–7010. [Google Scholar] [CrossRef]
- Wang, L.; Ma, J.C.; Dong, W.K.; Zhu, L.C.; Zhang, Y. A novel self–assembled nickel(II)–cerium(III) heterotetranuclear dimer constructed from N2O2-type bisoxime and terephthalic acid: Synthesis, structure and photophysical properties. Z. Anorg. Allg. Chem. 2016, 642, 834–839. [Google Scholar] [CrossRef]
- Dong, W.K.; Ma, J.C.; Dong, Y.J.; Zhu, L.C.; Zhang, Y. Di- and tetranuclear heterometallic 3d-4f cobalt(II)-lanthanide(III) complexes derived from a hexadentate bisoxime: Syntheses, structures and magnetic properties. Polyhedron 2016, 115, 228–235. [Google Scholar] [CrossRef]
- Hao, J.; Li, L.H.; Zhang, J.T.; Akogun, S.F.; Wang, L.; Dong, W.K. Four homo- and hetero-bismetallic 3d/3d-2s complexes constructed from a naphthalenediol-based acyclic bis(Salamo)-type tetraoxime ligand. Polyhedron 2017, 134, 1–10. [Google Scholar] [CrossRef]
- Gao, L.; Liu, C.; Wang, F.; Dong, W.K. Tetra-, penta- and hexa-coordinated transition metal complexes constructed from coumarin-containing N2O2 ligand. Crystals 2018, 8, 77. [Google Scholar] [CrossRef]
- Percy, G.; Thornton, D. Infrared spectra of N-aryl salicylaldimine complexes substituted in both aryl rings. J. Inorg. Nucl. Chem. 1973, 35, 2319–2327. [Google Scholar] [CrossRef]
- Dong, W.K.; Ma, J.C.; Zhu, L.C.; Zhang, Y. Self-assembled zinc(II)-lanthanide(III) heteromultinuclear complexes constructed from 3-MeO Salamo ligand: Syntheses, structures and luminescent properties. Cryst. Growth Des. 2016, 16, 6903–6914. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; Rijn, J.V.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua [1,7-bis(N-methylbenzimidazol-20-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Liu, Y.A.; Wang, C.Y.; Zhang, M.; Song, X.Q. Structures and magnetic properties of cyclic heterometallic tetranuclear clusters. Polyhedron 2017, 127, 278–286. [Google Scholar] [CrossRef]
- Constable, E.C. Expanded ligands-An assembly principle for supramolecular chemistry. Coord. Chem. Rev. 2008, 252, 842–855. [Google Scholar] [CrossRef]
- Chai, L.Q.; Li, Y.X.; Chen, L.C.; Zhang, J.Y.; Huang, J.J. Synthesis, X-ray structure, spectroscopic, electrochemical properties and DFT calculation of a bridged dinuclear copper(II) complex. Inorg. Chim. Acta 2016, 444, 193–201. [Google Scholar] [CrossRef]
- Sun, Y.X.; Li, C.Y.; Yang, C.J.; Zhao, Y.Y.; Guo, J.Q.; Yu, B. Two Cu(II) complexes with schiff base ligands: Syntheses, crystal structures, spectroscopic properties, and substituent effect. Chin. J. Inorg. Chem. 2016, 32, 327–335. [Google Scholar]
- Li, J.; Zhang, H.X.; Chang, J.; Jia, H.R.; Sun, Y.X.; Huang, Y.Q. Solvent-induced unsymmetric Salamo-like trinuclear NiII complexes: Syntheses, crystal structures, fluorescent and magnetic properties. Crystals 2018, 8, 176. [Google Scholar] [CrossRef]
- Sun, Y.X.; Zhao, Y.Y.; Li, C.Y.; Yu, B.; Guo, J.Q.; Li, J. Supramolecular cobalt(II) and copper(II) complexes with schiff base ligand: Syntheses, characterizations and crystal structures. Chin. J. Inorg. Chem. 2016, 32, 913–920. [Google Scholar]
- Liu, P.P.; Wang, C.Y.; Zhang, M.; Song, X.Q. Pentanuclear sandwich-type ZnII-LnIII clusters based on a new Salen-like salicylamide ligand: Structure, near-infrared emission and magnetic properties. Polyhedron 2017, 129, 133–140. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, S.S.; Shi, X.K.; Shen, K.S.; Wu, H.L. Synthesis, crystal structure, DNA-binding properties and antioxidant activity of a copper(II) complex with naphthalimide Schiff base. Z. Anorg. Allg. Chem. 2017, 643, 1182–1190. [Google Scholar] [CrossRef]
- Shi, X.K.; Mao, S.S.; Shen, K.S.; Wu, H.L.; Tang, X. Synthesis, crystal structure, antioxidation and fluorescence of two lanthanide complexes with a noncyclic polyether Schiff base ligand. J. Coord. Chem. 2017, 70, 2015–2028. [Google Scholar] [CrossRef]

| Coordination Compound | Zn(II)-La(III) |
|---|---|
| Empirical formula | C88H80Zn4La2N10O38 |
| Molecular weight, g·mol−1 | 2424.92 |
| Color | Yellow |
| Crystal size, mm3 | 0.15 × 0.12 × 0.06 |
| Habit | Block-shaped |
| Crystal system | Triclinic |
| Space group | P − 1 |
| Unit cell dimension | |
| a (Å) | 12.5918(11) |
| b (Å) | 15.9312(15) |
| c (Å) | 16.1881(17) |
| α (°) | 68.980(12) |
| β (°) | 84.083(8) |
| γ (°) | 86.619(9) |
| V (Å3) | 3014.3(6) |
| Z | 1 |
| Z’ | 0.5 |
| Dc (g·cm−3) | 1.336 |
| μ (mm−1) | 1.550 |
| F(000) | 1216 |
| θ Range (°) | 1.353–26.022 |
| Index ranges | −15 ≤ h ≤ 15, −18 ≤ k ≤ 19, 0 ≤ l ≤ 19 |
| Reflections collected | 11813 |
| Completeness (%) | 99.4 |
| Data/restraints/parameters | 11812/11/644 |
| Final R1/wR2 [I > 2σ(I)] | R1 = 0.0397, wR2 = 0.1053 |
| Final R1/wR2 (all data) | R1 = 0.0521, wR2 = 0.1092 |
| ∆ρmax/min (e·Å−3) | 0.715 and −0.626 |
| Bond | Bond | Bond | |||
|---|---|---|---|---|---|
| La1–O13 | 2.459(3) | La1–O1 | 2.720(3) | O14–Zn1 | 1.998(3) |
| La1–O16 #1 | 2.497(3) | La1–O12 | 2.781(3) | O5–Zn1 | 2.055(3) |
| La1–O8 | 2.506(2) | La1–O6 | 2.813(3) | N4–Zn2 | 1.992(4) |
| La1–O5 | 2.519(3) | La1–Zn1 | 3.5369(7) | N3–Zn2 | 2.122(3) |
| La1–O11 | 2.523(3) | La1–Zn2 | 3.5491(7) | O8–Zn2 | 1.976(3) |
| La1–O2 | 2.536(3) | N1–Zn1 | 2.132(3) | O11–Zn2 | 2.065(3) |
| La1–O7 | 2.690(3) | N2–Zn1 | 2.034(4) | O15–Zn2 #1 | 1.984(3) |
| Angles | Angles | Angles | |||
| O13–La1–O16 #1 | 75.54(9) | O5–La1–O2 | 62.63(9) | O12–La1–O6 | 161.99(9) |
| O13–La1–O8 | 152.43(9) | O11–La1–O2 | 118.54(9) | O2–Zn1–O14 | 113.20(13) |
| O16 #1–La1–O8 | 76.91(9) | O13–La1–O7 | 139.64(9) | O2–Zn1–N2 | 126.73(13) |
| O13–La1–O5 | 70.42(9) | O16 #1–La1–O7 | 123.92(9) | O14–Zn1–N2 | 119.87(14) |
| O16 #1–La1–O5 | 107.80(9) | O8–La1–O7 | 59.53(9) | O2–Zn1–O5 | 81.13(11) |
| O8–La1–O5 | 118.12(9) | O5–La1–O7 | 69.87(9) | O14–Zn1–O5 | 99.14(11) |
| O13–La1–O11 | 108.48(9) | O11–La1–O7 | 111.38(9) | N2–Zn1–O5 | 86.31(13) |
| O16 #1–La1–O11 | 70.54(9) | O2–La1–O7 | 81.02(8) | O2–Zn1–N1 | 86.92(13) |
| O8–La1–O11 | 62.21(9) | O13–La1–O1 | 122.61(9) | O14–Zn1–N1 | 96.36(12) |
| O5–La1–O11 | 178.28(9) | O16 #1–La1–O1 | 142.29(9) | N2–Zn1–N1 | 91.52(14) |
| O13–La1–O2 | 74.77(9) | O8–La1–O1 | 80.96(8) | O5–Zn1–N1 | 163.23(12) |
| O16 #1–La1–O2 | 150.28(9) | O5–La1–O1 | 109.50(9) | O8–Zn2–O15 #1 | 112.61(12) |
| O8–La1–O2 | 132.80(9) | O11–La1–O1 | 72.20(9) | O8–Zn2–N4 | 130.16(15) |
| O7–La1–O1 | 66.05(9) | O2–La1–O1 | 58.53(9) | O15 #1–Zn2–N4 | 116.62(15) |
| O11–La1–O12 | 58.19(8) | O13–La1–O12 | 67.16(10) | O8–Zn2–O11 | 79.99(11) |
| O2–La1–O12 | 69.32(9) | O16 #1–La1–O12 | 97.93(9) | O15 #1–Zn2–O11 | 97.48(11) |
| O7–La1–O12 | 132.07(9) | O8–La1–O12 | 117.95(9) | N4–Zn2–O11 | 86.41(14) |
| O1–La1–O12 | 66.44(9) | O5–La1–O12 | 121.99(8) | O8–Zn2–N3 | 87.90(14) |
| O13–La1–O6 | 97.96(9) | O7–La1–O6 | 65.84(8) | O15 #1–Zn2–N3 | 97.41(12) |
| O16 #1–La1–O6 | 67.47(9) | O1–La1–O6 | 131.57(8) | N4–Zn2–N3 | 93.10(15) |
| O8–La1–O6 | 70.46(9) | O11–La1–O6 | 121.87(8) | O11–Zn2–N3 | 163.57(13) |
| O5–La1–O6 | 57.34(8) | O2–La1–O6 | 118.01(9) |
| D‒X···A | d(D‒X) | d(X···A) | d(D···A) | ∠DXA | Symmetry Code |
|---|---|---|---|---|---|
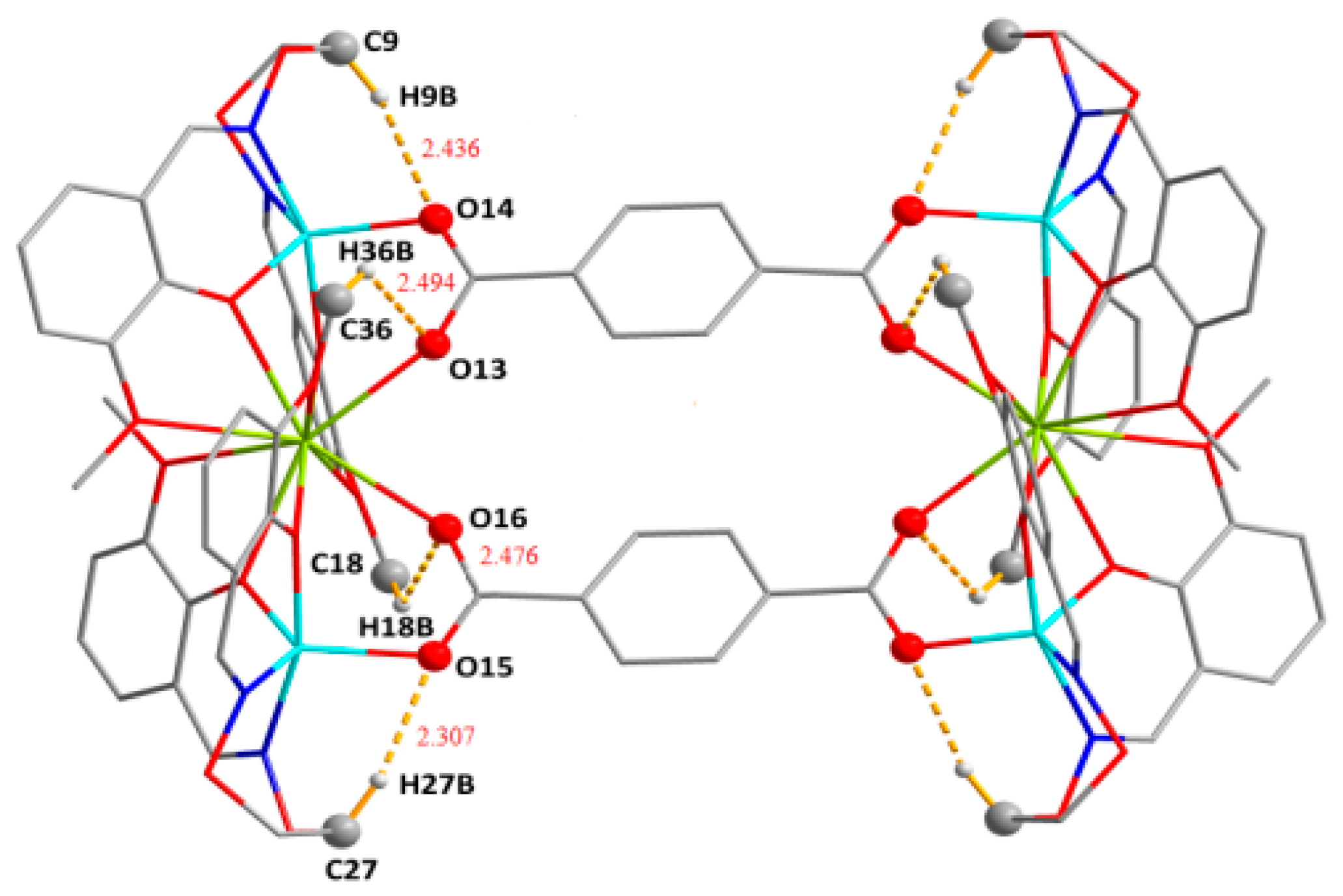
| C9‒H9B···O14 | 0.99 | 2.44 | 3.322(5) | 149 | |
| C18‒H18B···O16 | 0.98 | 2.48 | 3.098(5) | 121 | |
| C27‒H27B···O15 | 0.99 | 2.31 | 3.239(6) | 156 | |
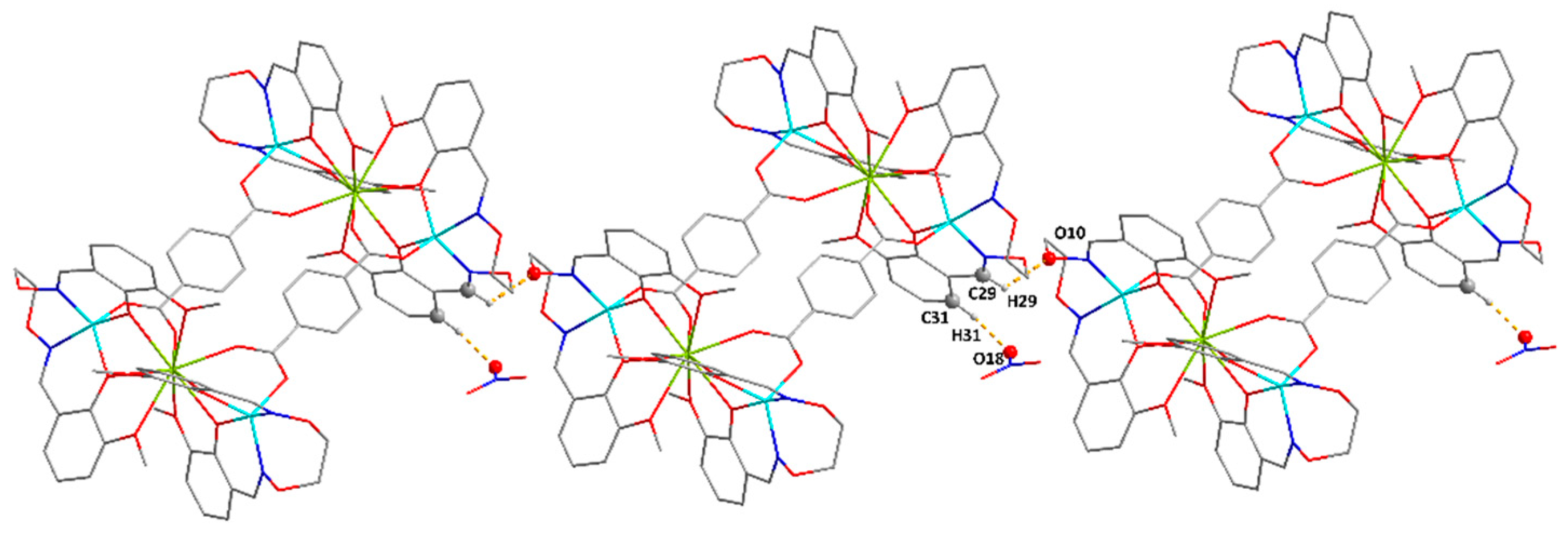
| C29‒H29···O10 | 0.95 | 2.40 | 3.011(7) | 122 | 1 − x, 2 − y, −z |
| C31‒H31···O18 | 0.95 | 1.81 | 2.748(8) | 169 | x, 1 + y, −1 + z |
| C36‒H36B···O13 | 0.98 | 2.49 | 3.122(6) | 122 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.-T.; Liu, L.-Z.; Yu, M.; Wang, F.; Ma, J.-C.; Dong, W.-K. Synthesis and Fluorescence Properties of a Structurally Characterized Hetero-Hexanuclear Zn(II)-La(III) Salamo-Like Coordination Compound Containing Auxiliary Ligands. Crystals 2018, 8, 414. https://doi.org/10.3390/cryst8110414
Guo W-T, Liu L-Z, Yu M, Wang F, Ma J-C, Dong W-K. Synthesis and Fluorescence Properties of a Structurally Characterized Hetero-Hexanuclear Zn(II)-La(III) Salamo-Like Coordination Compound Containing Auxiliary Ligands. Crystals. 2018; 8(11):414. https://doi.org/10.3390/cryst8110414
Chicago/Turabian StyleGuo, Wen-Ting, Ling-Zhi Liu, Meng Yu, Fei Wang, Jian-Chun Ma, and Wen-Kui Dong. 2018. "Synthesis and Fluorescence Properties of a Structurally Characterized Hetero-Hexanuclear Zn(II)-La(III) Salamo-Like Coordination Compound Containing Auxiliary Ligands" Crystals 8, no. 11: 414. https://doi.org/10.3390/cryst8110414
APA StyleGuo, W.-T., Liu, L.-Z., Yu, M., Wang, F., Ma, J.-C., & Dong, W.-K. (2018). Synthesis and Fluorescence Properties of a Structurally Characterized Hetero-Hexanuclear Zn(II)-La(III) Salamo-Like Coordination Compound Containing Auxiliary Ligands. Crystals, 8(11), 414. https://doi.org/10.3390/cryst8110414