Iron(II) Spin Crossover (SCO) Materials Based on Dipyridyl-N-Alkylamine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Crystal Structure Descriptions
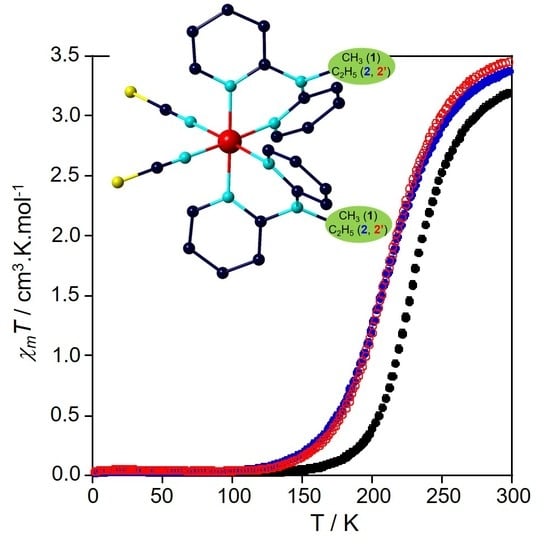
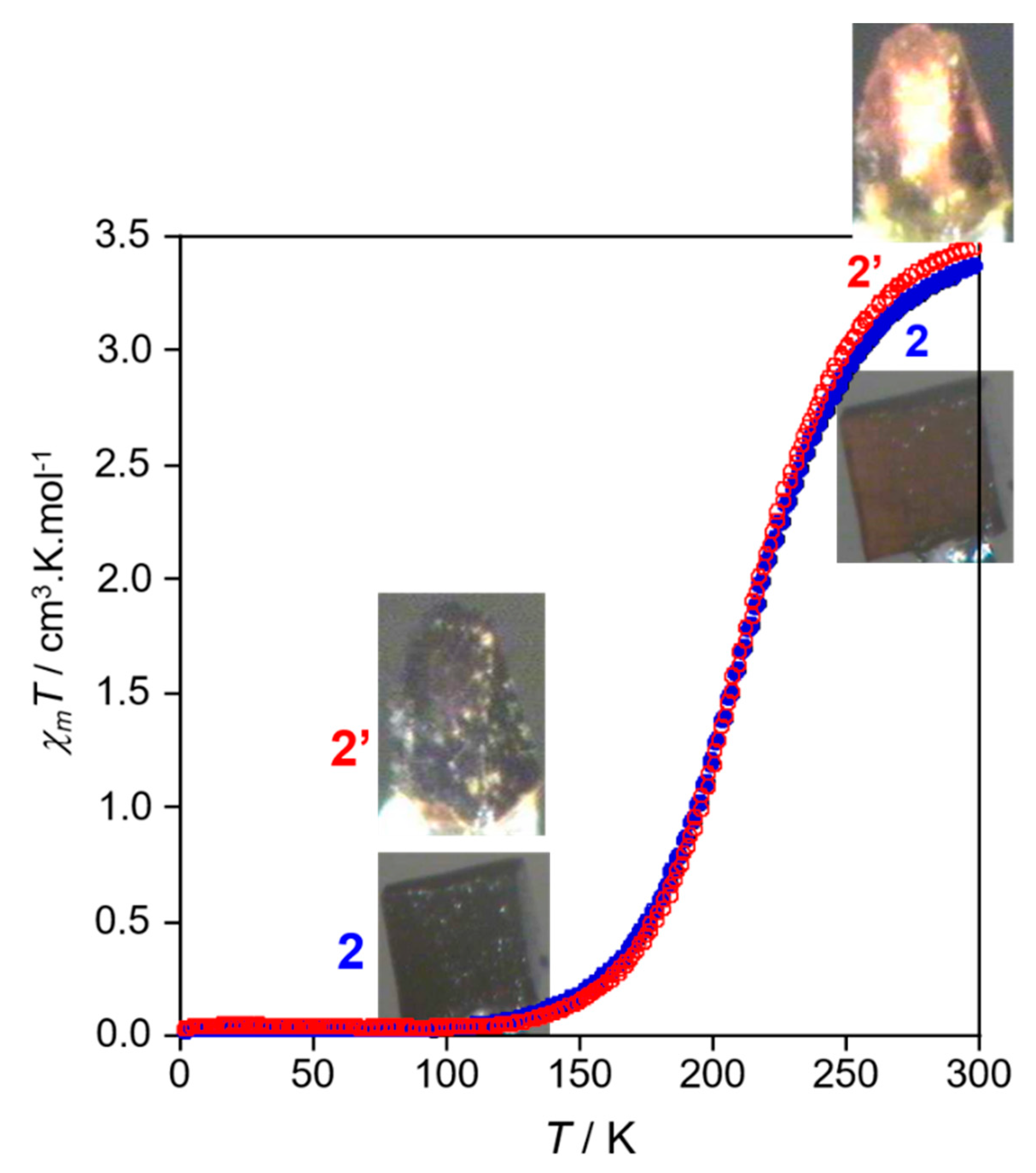
2.3. Magnetic Properties
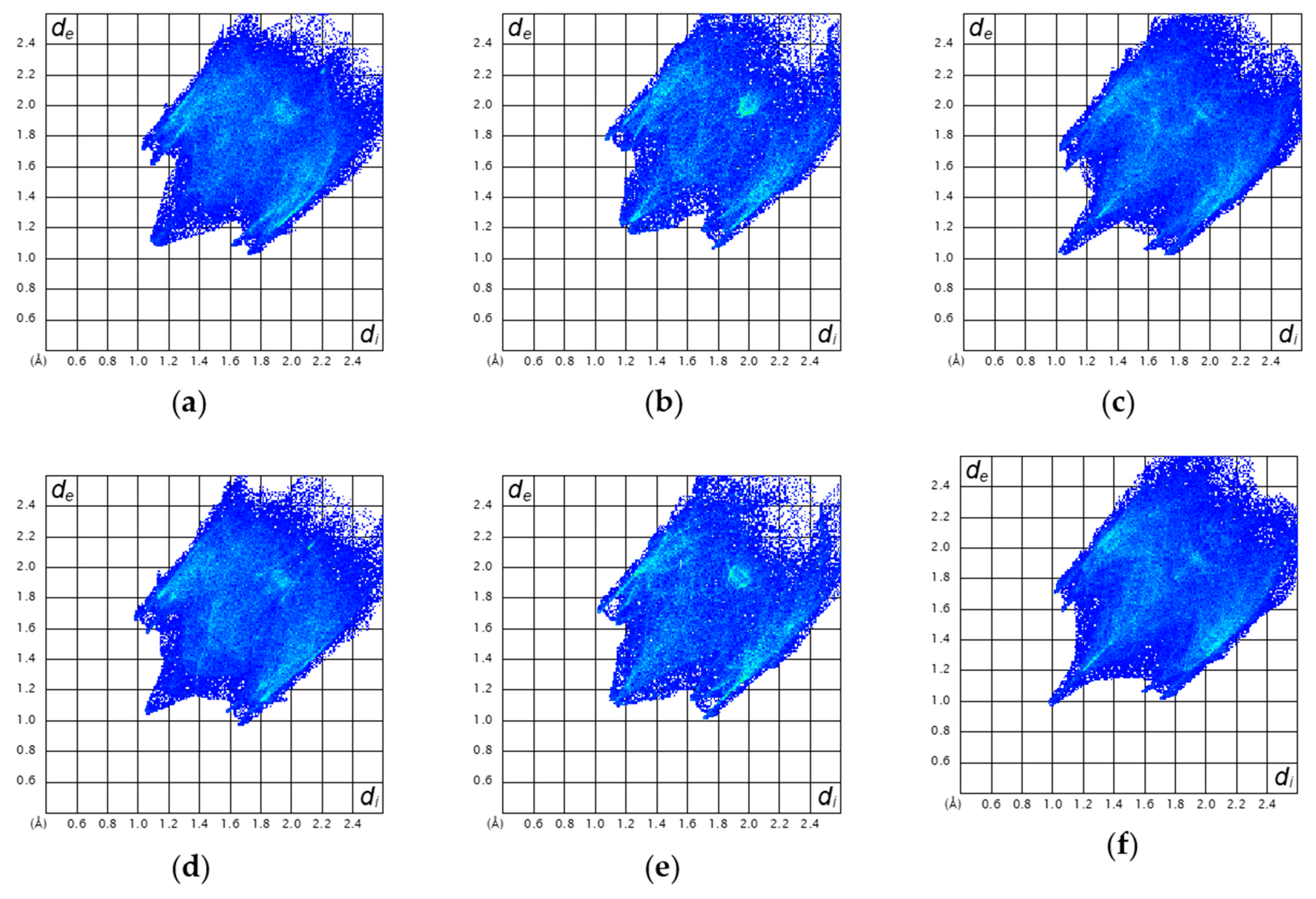
2.4. Magneto-Structural Relationships
3. Experimental Section
3.1. Materials and Instrumentation
3.2. Syntheses of the 2,2’-Dipyridyl-N-Alkylamine Ligands
3.3. Preparation of the Fe(II) Complexes [Fe(dpea)2(NCS)2] (1) and [Fe(dppa)2(NCS)2] Polymorphs (2 and 2’)
3.4. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halcrow, M.A. (Ed.) Spin-Crossover Materials, Properties and Applications; John Wiley & Sons Ltd.: Oxford, UK, 2013. [Google Scholar]
- Gütlich, P.; Goodwin, H.A. (Eds.) Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2004; pp. 233–235. [Google Scholar]
- Pittala, N.; Thétiot, F.; Triki, S.; Boukheddaden, K.; Chastanet, G.; Marchivie, M. Cooperative 1D Triazole-Based Spin Crossover FeII Material with Exceptional Mechanical Resilience. Chem. Mater. 2017, 29, 490–494. [Google Scholar] [CrossRef]
- Pittala, N.; Thétiot, T.; Charles, C.; Triki, S.; Boukheddaden, K.; Chastanet, G.; Marchivie, M. An unprecedented trinuclear FeII triazole-based complex exhibiting a concerted and complete sharp spin transition above room temperature. Chem. Commun. 2017, 53, 8356–8359. [Google Scholar] [CrossRef] [PubMed]
- Milin, E.; Patinec, V.; Triki, S.; Bendeif, E.-E.; Pillet, S.; Marchivie, M.; Chastanet, G.; Boukheddaden, K. Elastic Frustration Triggering Photoinduced Hidden Hysteresis and Multistability in a Two-Dimensional Photoswitchable Hofmann-Like Spin-Crossover Metal–Organic Framework. Inorg. Chem. 2016, 55, 11652–11661. [Google Scholar] [CrossRef] [PubMed]
- Shatruk, M.; Phan, H.; Chrisostomo, B.A.; Suleimenova, A. Symmetry-breaking structural phase transitions in spin crossover complexes. Coord. Chem. Rev. 2015, 289–290, 62–73. [Google Scholar] [CrossRef]
- Atmani, C.; El Hajj, F.; Benmansour, S.; Marchivie, M.; Triki, S.; Conan, F.; Patinec, V.; Handel, H.; Dupouy, G.; Gómez-García, C.J. Guidelines to design new spin crossover materials. Coord. Chem. Rev. 2010, 254, 1559–1569. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Monrabal-Capilla, M.; García-Martínez, J.; Pardo-Ibáñez, P. Bistable Spin-Crossover Nanoparticles Showing Magnetic Thermal Hysteresis near Room Temperature. Adv. Mater. 2007, 19, 1359–1361. [Google Scholar] [CrossRef]
- Shalabaeva, V.; Ridier, K.; Rat, S.; Manrique-Juarez, M.D.; Salmon, L.; Séguy, I.; Rotaru, A.; Molnár, G.; Bousseksou, A. Room temperature current modulation in large area electronic junctions of spin crossover thin films. Appl. Phys. Lett. 2018, 112, 013301. [Google Scholar] [CrossRef]
- Senthil Kumar, K.; Ruben, M. Emerging trends in spin crossover (SCO) based functional materials and devices. Coord. Chem. Rev. 2017, 346, 176–205. [Google Scholar] [CrossRef]
- Dugay, J.; Giménez-Marqués, M.; Kozlova, T.; Zandbergen, H.W.; Coronado, E.; van der Zant, H.S.J. Spin switching in electronic devices based on 2D assemblies of spin-crossover nanoparticles. Adv. Mater. 2015, 27, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, F.; Sebki, G.; Patinec, V.; Marchivie, M.; Triki, S.; Handel, H.; Yefsah, S.; Tripier, R.; Gomez-García, C.-J.; Coronado, E. Macrocycle-Based Spin-Crossover Materials. Inorg. Chem. 2009, 48, 10416–10423. [Google Scholar] [CrossRef] [PubMed]
- Baadji, N.; Sanvito, S. Giant resistance change across the phase transition in spin-crossover molecules. Phys. Rev. Lett. 2012, 108, 217201. [Google Scholar] [CrossRef] [PubMed]
- Prins, F.; Monrabal-Capilla, M.; Osorio, E.A.; Coronado, E.; van der Zant, H.S.J. Room-temperature electrical addressing of a bistable spin-crossover molecular system. Adv. Mater. 2011, 23, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Setifi, F.; Benmansour, S.; Marchivie, M.; Dupouy, G.; Triki, S.; Sala-Pala, J.; Salaün, J.-Y.; Gómez-García, C.J.; Pillet, S.; Lecomte, C.; et al. Magnetic bistability and thermochromism in molecular CuII chain. Inorg. Chem. 2009, 48, 1269–1271. [Google Scholar] [CrossRef] [PubMed]
- Arroyave, A.; Lennartson, A.; Dragulescu-Andrasi, A.; Pedersen, K.S.; Piligkos, S.; Stoian, S.A.; Greer, S.M.; Pak, C.; Hietsoi, O.; Phan, H.; et al. Spin Crossover in Fe(II) Complexes with N4S2 Coordination. Inorg. Chem. 2016, 55, 5904–5913. [Google Scholar] [CrossRef] [PubMed]
- Matar, S.F.; Guionneau, P.; Guillaume Chastanet, G. Multiscale Experimental and Theoretical Investigations of Spin Crossover FeII Complexes: Examples of [Fe(phen)2(NCS)2] and [Fe(PM-BiA)2(NCS)2]. Int. J. Mol. Sci. 2015, 16, 4007–4027. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.B.; Agustí, G.; Martínez, V.; Muñoz, M.C.; Levchenko, G.; Real, J.A. Spin crossover behaviour in the iron(II)-2,2-dipyridilamine system: Synthesis, X-ray structure and magnetic studies. Inorg. Chim. Acta 2005, 358, 4089–4094. [Google Scholar] [CrossRef]
- Gaspar, A.B.; Ksenofontov, V.; Real, J.A.; Gütlich, P. Coexistence of spin-crossover and antiferromagnetic coupling phenomena in the novel dinuclear Fe(II) complex [Fe(dpa)(NCS)2]2bpym. Chem. Phys. Lett. 2003, 373, 385–391. [Google Scholar] [CrossRef]
- Nebbali, K.; Mekuimemba, C.D.; Charles, C.; Yefsah, S.; Chastanet, G.; Mota, A.J.; Colacio, E.; Triki, S. One-dimensional Thiocyanato-Bridged Fe(II) Spin Crossover Cooperative Polymer With Unusual FeN5S Coordination Sphere. Inorg. Chem. 2018, 57, 12338–12346. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, H.; Grünsteudel, H.; Meyer-Klaucke, W.; Gerdan, M.; Grünsteudel, H.F.; Chumakov, A.I.; Rüffer, R.; Winkler, H.; Toftlund, H.; Trautwein, A.X. The spin-crossover complex [Fe(tpa)(NCS)2]. Eur. Phys. J. 2001, B23, 463–472. [Google Scholar] [CrossRef]
- Bonnet, S.; Siegler, M.A.; Sanchez Costa, J.; Molnar, G.; Bousseksou, A.; Spek, A.L.; Gamez, P.; Reedijk, J. A two-step spin crossover mononuclear iron(II) complex with a [HS–LS–LS] intermediate phase. Chem. Commun. 2008, 5619–5621. [Google Scholar] [CrossRef] [PubMed]
- Milin, E.; Belaïd, S.; Patinec, V.; Triki, S.; Chastanet, G.; Marchivie, M. Dinuclear Spin-Crossover Complexes Based on Tetradentate and Bridging Cyanocarbanion Ligands. Inorg. Chem. 2016, 55, 9038–9046. [Google Scholar] [CrossRef] [PubMed]
- Dupouy, G.; Marchivie, M.; Triki, S.; Sala-Pala, J.; Salaün, C.J.Y.; Gómez-García, C.J.; Guionneau, P. The Key Role of the Intermolecular π-π Interactions on the Presence of Spin Crossover in Neutral [Fe(abpt)2A2] Complexes (A = terminal monoanion N-ligand). Inorg. Chem. 2008, 47, 8921–8931. [Google Scholar] [CrossRef] [PubMed]
- Dupouy, G.; Marchivie, M.; Triki, S.; Sala-Pala, J.; Gomez-Garcia, C.J.; Pillet, S.; Lecomte, C.; Létard, J.-F. Photoinduced HS state in the first spin-crossover chain containing a cyanocarbanion as bridging ligand. Chem. Commun. 2009, 23, 3404–3406. [Google Scholar] [CrossRef] [PubMed]
- Benmansour, S.; Atmani, C.; Setifi, F.; Triki, S.; Marchivie, M.; Gómez-García, C.J. Polynitrile anions as ligands: From magnetic polymeric architectures to spin crossover materials. Coord. Chem. Rev. 2010, 254, 1468–1478. [Google Scholar] [CrossRef]
- Dupouy, G.; Triki, S.; Marchivie, M.; Cosquer, N.; Gómez-García, C.J.; Pillet, S.; Bendeif, E.-E.; Lecomte, C.; Asthana, S.; Létard, J.-F. Cyanocarbanion-based spin crossover materials: Photocrystallographic and photomagnetic studies of a new iron(II) neutral chain. Inorg. Chem. 2010, 49, 9358–9368. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Ishida, T. First Iron(II) Spin-crossover Complex with an N5S Coordination Sphere. Chem. Lett. 2015, 44, 920–921. [Google Scholar] [CrossRef]
- Mekuimemba, C.D.; Conan, F.; Mota, A.J.; Palacios, M.A.; Colacio, E.; Triki, S. On the Magnetic Coupling and Spin Crossover Behavior in Complexes Containing the Head-to-Tail [FeII2(μ-SCN)2] Bridging Unit: A Magnetostructural Experimental and Theoretical Study. Inorg. Chem. 2018, 57, 2184–2192. [Google Scholar] [CrossRef] [PubMed]
- Park, J.G.; Jeon, I.-R.; Harris, T.D. Electronic Effects of Ligand Substitution on Spin Crossover in a Series of Diiminoquinonoid-Bridged FeII2 Complexes. Inorg. Chem. 2015, 54, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.-J.; Li, B.; Tao, J.; Huang, R.-B.; Zheng, L.-S.; Zheng, Z. Making Spin-Crossover Crystals by Successive Polymorphic Transformations. Inorg. Chem. 2011, 50, 1170–1172. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.-J.; Huo, Q.; Tao, J.; Huang, R.-B.; Zheng, L.-S. Spin-Crossover FeII4 Squares: Two-Step Complete Spin Transition and Reversible Single-Crystal-to-Single-Crystal Transformation. Angew. Chem. Int. Ed. 2011, 50, 8940–8943. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wei, R.-J.; Tao, J.; Huang, R.-B.; Zheng, L.-S.; Zheng, Z. Solvent-Induced Transformation of Single Crystals of a Spin-Crossover (SCO) Compound to Single Crystals with Two Distinct SCO Centers. J. Am. Chem. Soc. 2010, 132, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.S.; Ross, T.M.; Phonsri, W.; Moubaraki, B.; Chastanet, G.; Létard, J.-F.; Batten, S.R.; Murray, K.S. Discrete FeII Spin-Crossover Complexes of 2,2′-Dipyridylamino-Substituted s-Triazine Ligands with Phenoxo, Cyanophenoxo and Dibenzylamino Functionalities. Eur. J. Inorg. Chem. 2015, 763–777. [Google Scholar] [CrossRef]
- Scott, H.S.; Moubaraki, B.; Paradis, N.; Chastanet, G.; Létard, J.-F.; Batten, S.R.; Murray, K.S. 2,2′-Dipyridylamino-based ligands with substituted alkyl chain groups and their mononuclear-M(II) spin crossover complexes. J. Mater. Chem. C 2015, 3, 7845–7857. [Google Scholar] [CrossRef]
- Nassirinia, N.; Amani, S.; Teat, S.J.; Roubeau, O.; Gamez, P. Enhancement of spin-crossover cooperativity mediated by lone pair–p interactions and halogen bonding. Chem. Commun. 2014, 50, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Wannarit, N.; Roubeau, O.; Youngme, S.; Teatd, S.J.; Gamez, P. Influence of supramolecular bonding contacts on the spin crossover behaviour of iron(II) complexes from 2,2′-dipyridylamino/s-triazine ligands. Dalton Trans. 2013, 42, 7120–7130. [Google Scholar] [CrossRef] [PubMed]
- Wannarit, N.; Roubeau, O.; Youngme, S.; Gamez, P. Subtlety of the Spin-Crossover Phenomenon Observed with Dipyridylamino-Substituted Triazine Ligands. Eur. J. Inorg. Chem. 2013, 730–737. [Google Scholar] [CrossRef]
- Scott, H.S.; Ross, T.M.; Batten, S.R.; Gass, I.A.; Moubaraki, B.; Neville, S.M.; Murray, K.S. Iron(II) Mononuclear Materials Containing Functionalised Dipyridylamino-Substituted Triazine Ligands: Structure, Magnetism and Spin Crossover. Aust. J. Chem. 2012, 65, 874–882. [Google Scholar] [CrossRef]
- Ross, T.M.; Moubaraki, B.; Neville, S.M.; Batten, S.R.; Murray, K.S. Polymorphism and spin crossover in mononuclear FeII species containing new dipyridylamino-substituted s-triazine ligands. Dalton Trans. 2012, 41, 1512–1523. [Google Scholar] [CrossRef] [PubMed]
- Ross, T.M.; Moubaraki, B.; Wallwork, K.S.; Batten, S.R.; Murray, K.S. A temperature-dependent order-disorder and crystallographic phase transition in a 0D FeII spin crossover compound and its non-spin crossover CoII isomorph. Dalton Trans. 2011, 40, 10147–10155. [Google Scholar] [CrossRef] [PubMed]
- Quesada, M.; Monrabal, M.; Aromí, G.; de la Peña-O’Shea, V.A.; Gich, M.; Molins, E.; Roubeau, O.; Teat, S.J.; MacLean, E.J.; Gamez, P.; et al. Spin transition in a triazine-based Fe(II) complex: Variable-temperature structural, thermal, magnetic and spectroscopic studies. J. Mater. Chem. 2006, 16, 2669–2676. [Google Scholar] [CrossRef]
- Amoore, J.J.M.; Kepert, C.J.; Cashion, J.D.; Moubaraki, B.; Neville, S.M.; Murray, K.S. Structural and Magnetic Resolution of a Two-Step Full Spin-Crossover Transition in a Dinuclear Iron(II) Pyridyl-Bridged Compound. Chem. Eur. J. 2006, 12, 8220–8227. [Google Scholar] [CrossRef] [PubMed]
- Quesada, M.; de Hoog, P.; Gamez, P.; Roubeau, O.; Aromí, G.; Donnadieu, B.; Massera, C.; Lutz, M.; Spek, A.L.; Reedijk, J. Coordination Dependence of Magnetic Properties within a Family of Related[FeII2] Complexes of a Triazine-Based Ligand. Eur. J. Inorg. Chem. 2006, 2006, 1353–1361. [Google Scholar] [CrossRef]
- Neville, S.M.; Leita, B.A.; Offermann, D.A.; Duriska, M.B.; Moubaraki, B.; Chapman, K.W.; Halder, G.J.; Murray, K.S. Spin-Crossover Studies on a Series of 1D Chain and Dinuclear Iron(II) Triazine-Dipyridylamine Compounds. Eur. J. Inorg. Chem. 2007, 1073–1085. [Google Scholar] [CrossRef]
- Scott, H.S.; Ross, T.M.; Chilton, N.F.; Gass, I.A.; Moubaraki, B.; Chastanet, G.; Paradis, N.; Létard, J.-F.; Vignesh, K.R.; Rajaraman, G.; et al. Crown-linked dipyridylamino-triazine ligands and their spin-crossover iron(II) derivatives: Magnetism, photomagnetism and cooperativity. Dalton Trans. 2013, 42, 16494–16509. [Google Scholar] [CrossRef] [PubMed]
- Ross, T.M.; Moubaraki, B.; Batten, S.R.; Murray, K.S. Spin crossover in polymeric and heterometallic FeII species containing polytopic dipyridylamino-substituted-triazine ligands. Dalton Trans. 2012, 41, 2571–2581. [Google Scholar] [CrossRef] [PubMed]
- Ross, T.M.; Moubaraki, B.; Turner, D.R.; Halder, G.J.; Chastanet, G.; Neville, S.M.; Cashion, J.D.; Létard, J.-F.; Batten, S.R.; Murray, K.S. Spin Crossover and Solvate Effects in 1D FeII Chain Compounds Containing Bis(dipyridylamine)-Linked Triazine Ligands. Eur. J. Inorg. Chem. 2011, 1395–1417. [Google Scholar] [CrossRef]
- Neville, S.M.; Leita, B.A.; Halder, G.J.; Kepert, C.J.; Moubaraki, B.; Létard, J.-F.; Murray, K.S. Understanding the Two-Step Spin-Transition Phenomenon in Iron(II) 1D Chain Materials. Chem. Eur. J. 2008, 14, 10123–10133. [Google Scholar] [CrossRef] [PubMed]
- Quesada, M.; de la Peña-O’Shea, V.A.; Aromí, G.; Geremia, S.; Massera, C.; Roubeau, O.; Gamez, P.; Reedijk, J. A Molecule-Based Nanoporous Material Showing Tuneable Spin-Crossover Behavior near Room Temperature. Adv. Mater. 2007, 19, 1397–1402. [Google Scholar] [CrossRef]
- Rauterkus, M.J.; Fakih, S.; Mock, C.; Puscasu, I.; Krebs, B. Cisplatin analogues with 2,2-dipyridylamine ligands and their reactions with DNA model nucleobases. Inorg. Chim. Acta 2003, 350, 355–365. [Google Scholar] [CrossRef]
- Guionneau, P.; Marchivie, M.; Bravic, G.; Létard, J.-F.; Chasseau, D. Structural Aspects of Spin Crossover. Example of the [FeIILn(NCS)2] Complexes. Top. Curr. Chem. 2004, 234, 97–128. [Google Scholar]
- Farrugia, L.J. ORTEP-3 for Windows—A version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Cryst. 1997, 30, 565. [Google Scholar] [CrossRef]
- Spackman, M.A.; Byrom, P.G. A novel definition of a molecule in a crystal. Chem. Phys. Lett. 1997, 267, 215–220. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Crawley, Australia, 2017. [Google Scholar]
- Marchivie, M.; Guionneau, P.; Létard, J.-F.; Chasseau, D. Towards direct correlation between spin crossover and structural properties in iron II complexes. Acta Cryst. B 2003, 59, 479–486. [Google Scholar] [CrossRef]
- Oxford Diffraction. Xcalibur CCD/RED CrysAlis Software System; Oxford Diffraction Ltd.: Abingdon, UK, 2006. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, C.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
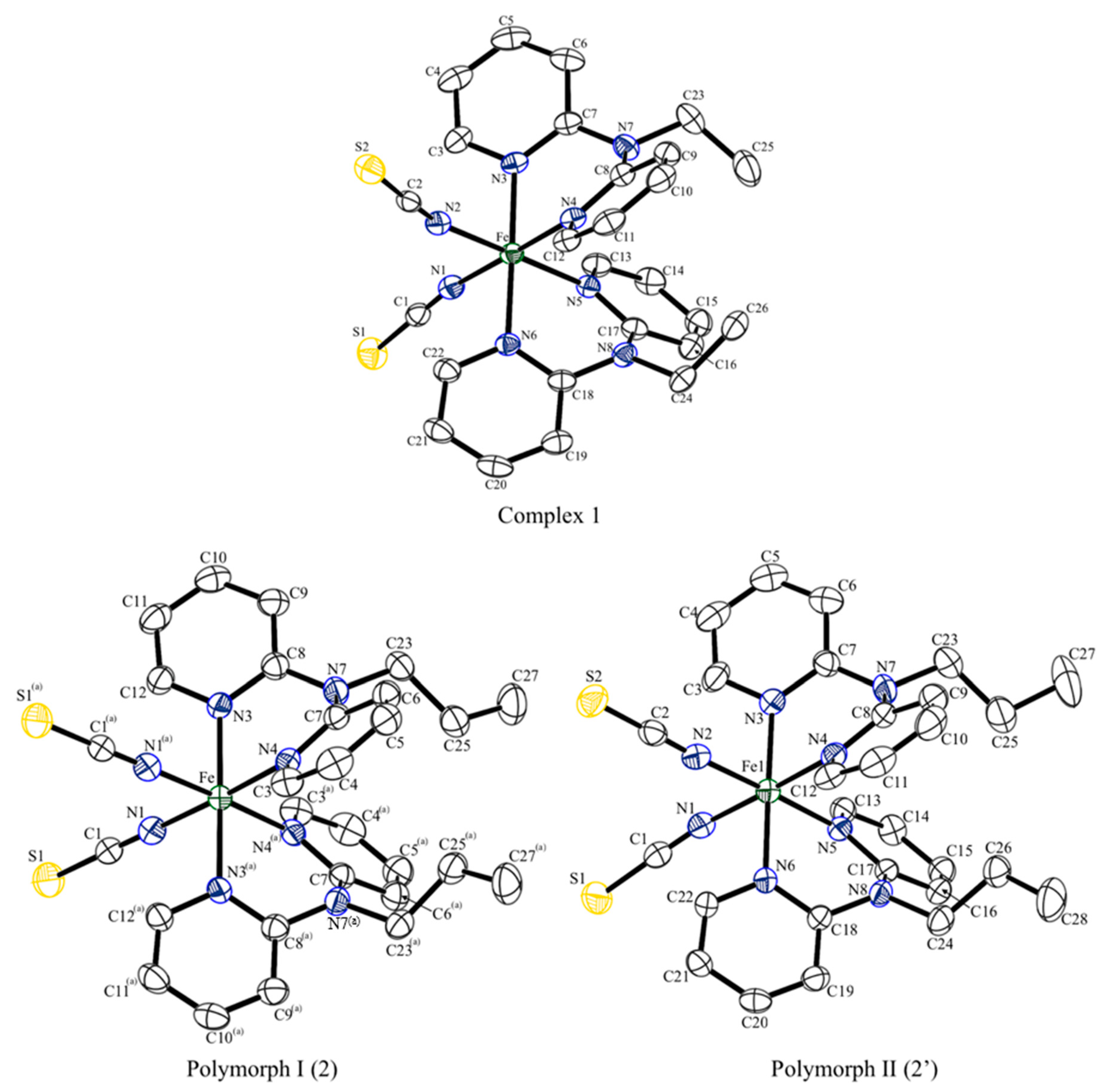

| Complex | 1 | 2’ | 2 | ||||
|---|---|---|---|---|---|---|---|
| T/K | 296 | 170 | 296 | 170 | 296 | 170 | |
| Fe–N1 | 2.150(5) | 1.971 (3) | 2.123(4) | 2.005(3) | Fe–N1 | 2.137(3) | 1.968(2) |
| Fe–N2 | 2.102(4) | 1.968(3) | 2.111(4) | 2.004(3) | Fe–N1 (a) | 2.137(3) | 1.968(2) |
| Fe–N3 | 2.151(5) | 1.983(3) | 2.175(3) | 2.032(3) | Fe–N3 | 2.184(3) | 1.990(2) |
| Fe–N4 | 2.198(4) | 1.986(3) | 2.184(3) | 2.017(3) | Fe–N4 | 2.204(2) | 1.989(2) |
| Fe–N5 | 2.179(4) | 1.976(2) | 2.174(3) | 2.019(3) | Fe–N4 (a) | 2.204(2) | 1.989(2) |
| Fe–N6 | 2.162(4) | 1.978(3) | 2.163(3) | 2.034(3) | Fe–N3 (a) | 2.184(3) | 1.990(2) |
| <d(Fe-N)> | 2.157(5) | 1.977(3) | 2.155(4) | 2.018(3) | <d(Fe–N)> | 2.175(3) | 1.982(2) |
| Fe–N1–C1 | 164.7(5) | 171.5(3) | 171.6(3) | 162.2(3) | Fe–N1–C1 | 174.8(3) | 174.9(2) |
| Fe–N2–C2 | 150.7(4) | 161.6(2) | 155.6(4) | 174.2(3) | Fe–N1 (a)–C1 (a) | 174.8(3) | 174.9(2) |
| N1–Fe–N2 | 94.10(17) | 93.36(11) | 90.98(15) | 89.70(12) | N1–Fe–N1 (a) | 91.21(17) | 90.15(12) |
| N1–Fe–N3 | 94.29(18) | 92.50(11) | 93.75(12) | 93.52(11) | N1–Fe–N3 | 92.75(11) | 91.65(8) |
| N1–Fe–N5 | 89.94(15) | 89.07(10) | 89.69(13) | 90.02(11) | N1–Fe–N4 (a) | 89.63(11) | 89.44(8) |
| N1–Fe–N6 | 89.42(17) | 86.98(11) | 90.37(12) | 87.23(11) | N1–Fe–N3 (a) | 91.74(11) | 87.92(8) |
| N2–Fe–N3 | 92.31(17) | 88.22(11) | 90.28(13) | 88.16(11) | N1 (a)–Fe–N3 | 91.74(11) | 87.92(8) |
| N2–Fe–N4 | 87.28(16) | 86.98(11) | 90.26(13) | 89.40(11) | N1 (a)–Fe–N4 | 89.63(11) | 89.44(8) |
| N2–Fe–N6 | 93.07(17) | 91.23(11) | 94.85(13) | 92.33(11) | N1 (a)–Fe–N3 (a) | 92.76(11) | 91.65(8) |
| N3–Fe–N4 | 81.24(17) | 86.42(11) | 80.52(11) | 85.91(11) | N3–Fe–N4 | 80.27(9) | 86.21(7) |
| N3–Fe–N5 | 94.29(17) | 93.83(11) | 94.36(12) | 93.73(10) | N3-Fe-N4 (a) | 95.15(9) | 94.22(7) |
| N4–Fe–N5 | 89.23(13) | 90.63(10) | 89.54(12) | 90.90(11) | N4–Fe–N4 (a) | 90.38(13) | 91.05(10) |
| N4–Fe–N6 | 94.90(15) | 94.10(11) | 95.22(12) | 93.34(10) | N3 (a)–Fe–N4 | 95.15(9) | 94.21(7) |
| N5–Fe–N6 | 80.05(18) | 86.74(11) | 80.45(12) | 85.78(10) | N3 (a)–Fe–N4 (a) | 80.27(9) | 86.21(7) |
| bΣ/° | 45.80 | 31.24 | 39.87 | 27.66 | bΣ/° | 41.08 | 25.79 |
| Compound 1 | Compound 2 | Compound 2’ | ||||
|---|---|---|---|---|---|---|
| d(S⋯C) | d(S⋯H) | d(S⋯C) | d(S⋯H) | d(S⋯C) | d(S⋯H) | |
| S1⋯H6-C6 (i) | 3.755 | 2.999 | 3.769 | 3.105 | ||
| S1⋯H9-C9 (ii) | 3.692 | 3.037 | 3.811 | 2.970 | 3.782 | 2.898 |
| S2⋯H6-C6 (iii) | 3.803 | 2.886 | ||||
| S2⋯H10-C10 (iv) | 3.677 | 3.028 | ||||
| S2⋯H19-C19 (V) | 3.702 | 2.873 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houari, T.; Cuza, E.; Pinkowicz, D.; Marchivie, M.; Yefsah, S.; Triki, S. Iron(II) Spin Crossover (SCO) Materials Based on Dipyridyl-N-Alkylamine. Crystals 2018, 8, 401. https://doi.org/10.3390/cryst8110401
Houari T, Cuza E, Pinkowicz D, Marchivie M, Yefsah S, Triki S. Iron(II) Spin Crossover (SCO) Materials Based on Dipyridyl-N-Alkylamine. Crystals. 2018; 8(11):401. https://doi.org/10.3390/cryst8110401
Chicago/Turabian StyleHouari, Taous, Emmelyne Cuza, Dawid Pinkowicz, Mathieu Marchivie, Said Yefsah, and Smail Triki. 2018. "Iron(II) Spin Crossover (SCO) Materials Based on Dipyridyl-N-Alkylamine" Crystals 8, no. 11: 401. https://doi.org/10.3390/cryst8110401
APA StyleHouari, T., Cuza, E., Pinkowicz, D., Marchivie, M., Yefsah, S., & Triki, S. (2018). Iron(II) Spin Crossover (SCO) Materials Based on Dipyridyl-N-Alkylamine. Crystals, 8(11), 401. https://doi.org/10.3390/cryst8110401