Abstract
The crystal structures of salts 6–9 prepared from (R)-2-methoxy-2-(1-naphthyl)propanoic acid [(R)-MαNP acid, (R)-1] and (R)-1-arylethylamines [salt 6, (R)-1-(4-methoxyphenyl)ethylamine∙(R)-1; salt 7, (R)-1-(4-fluorophenyl)ethylamine∙(R)-1; salt 8, (R)-1-(4-chlorophenyl)ethylamine∙(R)-1; and salt 9, (R)-1-(3-chlorophenyl)ethylamine∙(R)-1] were elucidated by X-ray crystallography. The solid-state associations and conformations of the MαNP salts were defined using the concepts of supramolecular- and planar chirality, respectively, and the crystal structures of salts 6–9 were interpreted as a three-step hierarchical assembly. The para-substituents of the (R)-1-arylethylammonium cations were found on sheet structures consisting of 21 columns. Thus, salts possessing smaller para-substituents, that is, salt 7 (p-F) and salt 9 (p-H), and larger para-substituents, that is, salt 6 (p-OMe) and salt 8 (p-Cl), crystallized in the space groups P21 and C2, respectively. Additionally, weak intermolecular interactions, that is, aromatic C–H···π, C–H···F, and C–H···O interactions, were examined in crystalline salts 6–9.
1. Introduction
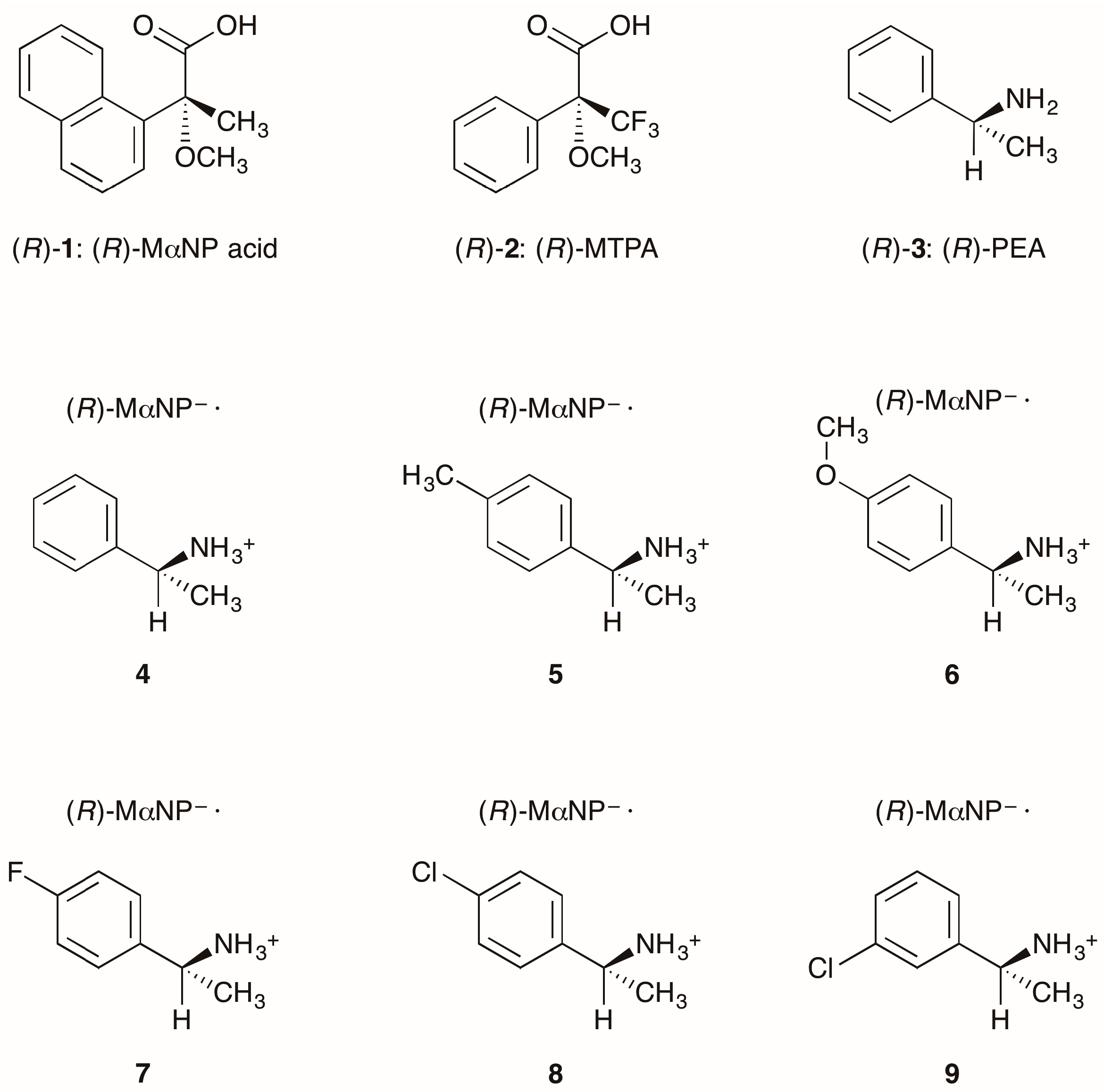
Stereochemistry is important in biofunctional molecules [1]. Therefore, methods that facilitate the elucidation of absolute configurations and the preparation of single enantiomers are highly desired [2]. Based on stereochemical studies of biofunctional molecules, we synthesized a chiral resolving agent, MαNP acid (acid 1, Figure 1) [3,4]. Acid 1 is superior to Mosher’s 3,3,3-trifluoro-2-methoxy-2-phenylpropanoic acid (MTPA, 2) [5] for the enantioresolution of secondary alcohols [2].
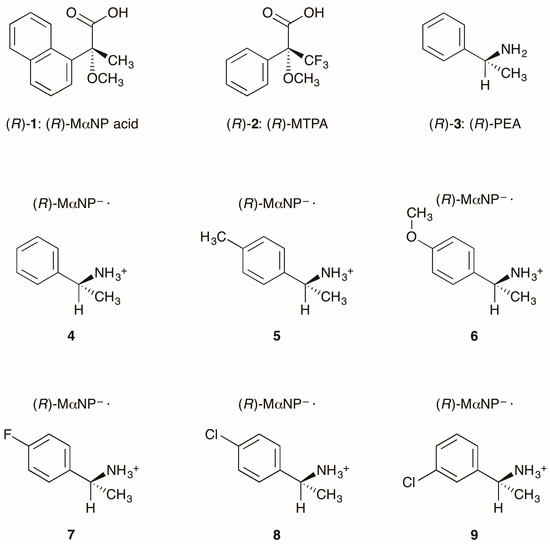
Figure 1.
Structures of chiral resolving agents and MαNP salts. See refs. [6] and [11] for salt 4 (CCDC 801461) and salt 5 (CCDC 871216), respectively. (R)-MαNP– represents the (R)-2-methoxy-2-(1-naphthyl)propanoate anion.
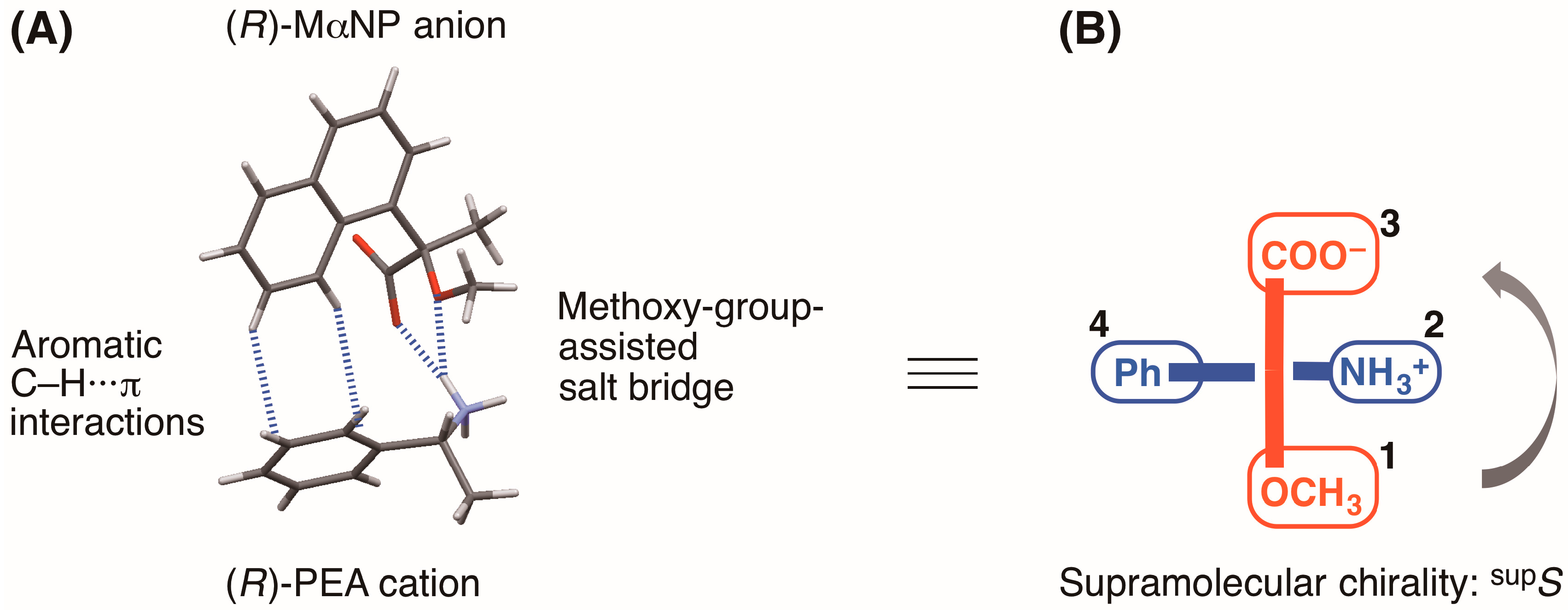
Goto et al. reported the enantioresolution of rac-1 via diastereomeric salt formation with (R)-3 [3]. In 2011, we examined the crystal structures of the less-soluble salt 4 [(R)-3∙(R)-1] and the more-soluble diastereomeric salt (R)-3∙(S)-1 by X-ray crystallography [6]. Those crystal structures revealed a chiral recognition mechanism during the enantioresolution process. With the less-soluble salt 4, the (R)-MαNP anion and the (R)-PEA cation form a close ion-pair via a methoxy-group-assisted salt bridge and aromatic C–H∙∙∙π interactions (Figure 2A). The close ion-pairs then join with the salt bridges to form 21 columns. Additionally, results have shown that intercolumnar aromatic C–H∙∙∙π interactions [7,8,9] are more effective with the less-soluble salt 4.
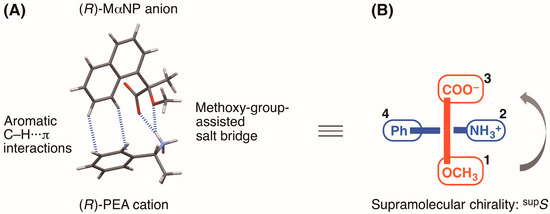
Figure 2.
(A) Chiral recognition and (B) supramolecular chirality in the close ion-pair of the less soluble MαNP salt 4 [6,10]. Assigned numbers indicate priority based on the Cahn–Ingold–Prelog rules. The symbol “supS” represents the left-handed supramolecular chirality.
Recently, we introduced the concept of supramolecular chirality as a means of defining the association of close ion-pairs in the solid state [10]. Considering the virtual chiral center of the carboxylate and methoxy groups of the (R)-MαNP anion and the phenyl and ammonium groups of the (R)-PEA cation, the supramolecular chirality of salt 4 was assigned as supS (Figure 2B) [10].
Salts 4 and 5 [(R)-1-(p-tolyl)ethylamine∙(R)-1] yielded space groups P21 and C2, respectively [6,11]. This implied an effect of para-substitution of the PEA cation on the latter stage of hierarchical assembly [12,13].
This report describes substituent effects on the crystal structures of salts 6–9. The 4-methoxyphenyl- and 3-chlrorophenyl groups were fixed in the crystal lattice. Thus, the planar chirality [14] of the (R)-1-arylethylammonium cations was assigned in salts 6 and 9. The molecular packing of salts 6–9 was interpreted as the three-step hierarchical assembly [12,13].
A large number of agrochemicals and pharmaceuticals are halogenated compounds. In 2007, Müller et al. reported that ca. 20% of all pharmaceuticals, and even more agrochemicals (up to 30%), contained fluorine atoms [15]. The ratio of chlorinated drugs was next to the fluorinated drugs in all halogenated drugs [16]. However, the effects of halogen atoms, especially fluorine, are ambivalent. Organic crystals are now considered a type of supermolecule [7]. Therefore, the elucidation of substitution effects in organic crystals will contribute to an overall understanding of weak intermolecular interactions. The current study explores aromatic C–H···π, C–H···F, and C–H···O interactions in crystalline salts 6–9.
Crystal engineering of organic salts is important as a means of enantioresolution [17,18,19]. Investigations of crystal structures provide information on weak intermolecular interactions that are useful in the design of biofunctional molecules.
2. Results and Discussion
2.1. Preparation of Crystalline MαNP Salts
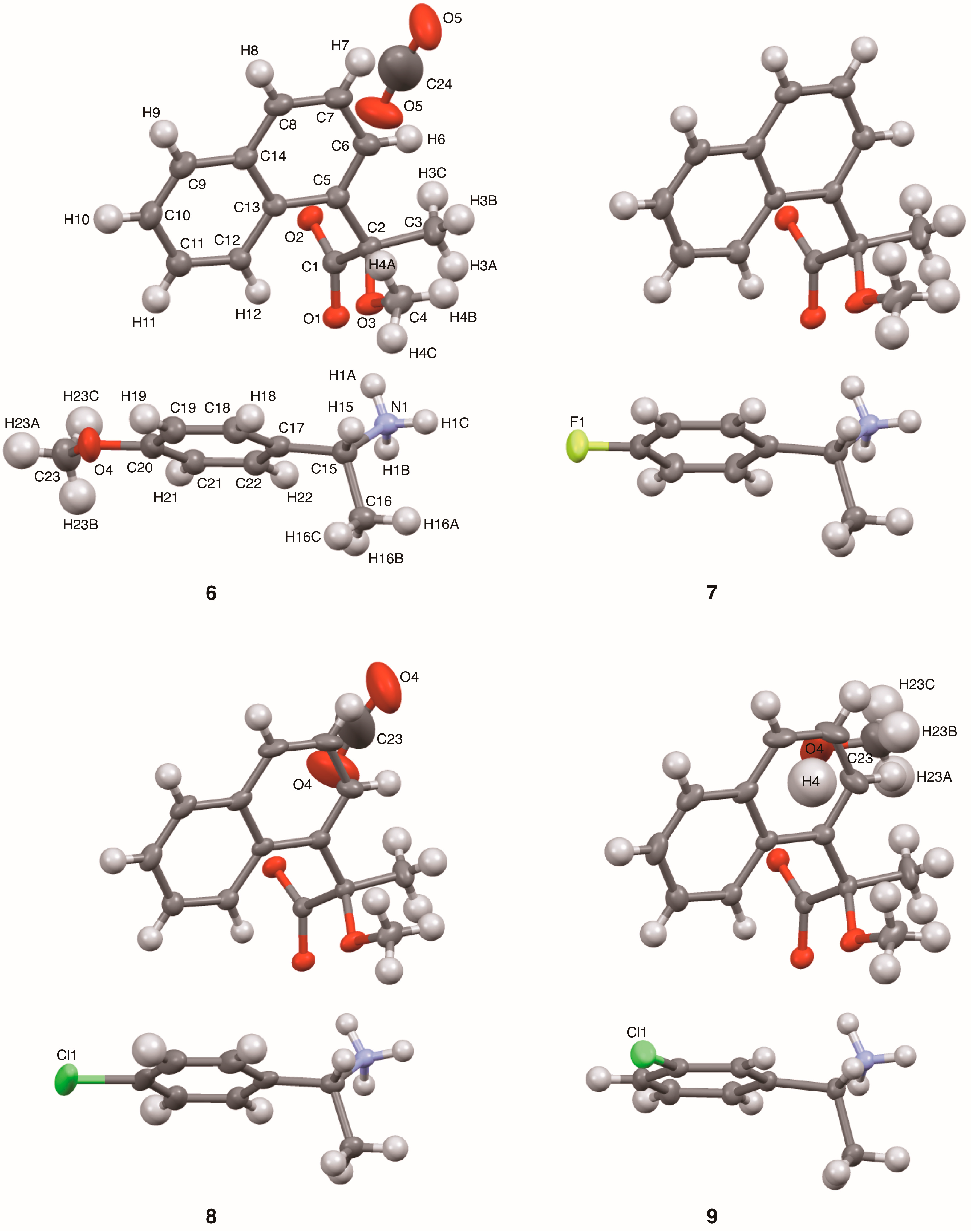
Single crystals of salts 6–9 were prepared from (R)-1 and (R)-1-arylethylamines with MeOH/CHCl3 and were analyzed using X-ray crystallography (Figure 1). Table 1 and Figure 3 show their crystallographic data and ORTEP diagrams, respectively. Salts 6 and 8 crystallized in the monoclinic space group C2 with four ion-pairs per unit cell, while salts 7 and 9 belonged to the space group P21 with two ion-pairs per unit cell. For salts possessing larger substituents, that is, salt 6 (p-OMe), salt 8 (p-Cl), and salt 9 (m-Cl), methanol molecules functioned as space-fillers [19], stabilizing the crystal lattice.

Table 1.
X-ray crystallographic data for salts 6–9.
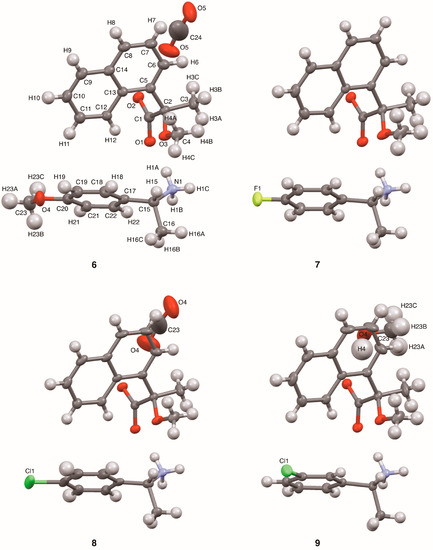
Figure 3.
ORTEP diagrams of salts 6–9 with ellipsoids set at 50% probability. Crystals of salts 6, 8, and 9 were methanol solvates. The methanol molecules in salts 6 and 8 showed positional disorder. The atom labels for salt 6 were also used for the other salts except for the substituents and methanol molecules.
2.2. Crystal Conformations of MαNP Salts
The conformations of the MαNP anions in salts 6–9 were similar to the major conformations of MαNP esters (Table 2) [2,6]: (1) The carboxylate oxygen atom O1 was synperiplanar to the methoxy oxygen atom O3; (2) The methoxy carbon atom C4 was antiperiplanar to the carboxylate carbon atom C1; (3) The methyl carbon atom C3 was in the naphthyl plane. The 1-arylethylammonium cations of salts 6–9 also exhibited the conformations similar to those of PEA cations [20]; that is, the benzylic hydrogen atom H15 was almost in the phenyl plane.

Table 2.
Conformational parameters of salts 6–9.
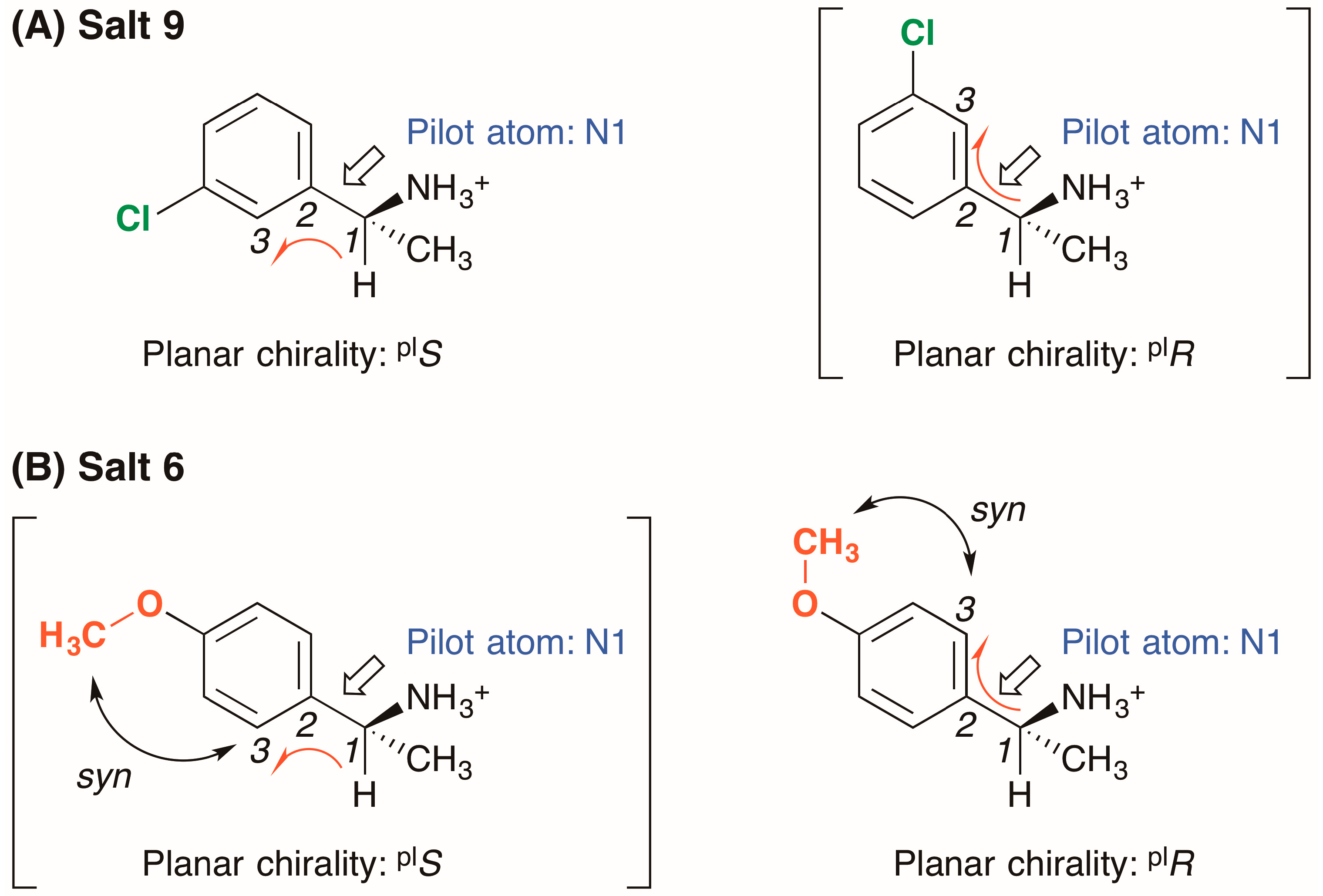
The 3-chlorophenyl group of salt 9 was fixed in the solid state; thus, the conformation of the (R)-1-(3-chlorophenyl)ethylammonium cation was elucidated using the concept of planar chirality [14] (Figure 4A): (1) The nitrogen atom N1, selected as the pilot atom, was not in the phenyl plane itself but was attached to the benzylic carbon atom at the end of the phenyl plane; (2) Considering the Cahn–Ingold–Prelog rules on the priority of ortho-carbon atoms, the three adjacent, in-plane carbon atoms 1–3 were selected; (3) Viewed from the pilot atom N1, the carbon atoms 1–3 were positioned in an anticlockwise direction in crystalline salt 9. Therefore, the planar chirality of the (R)-1-(3-chlorophenyl)ethylammonium cation was assigned to plS.
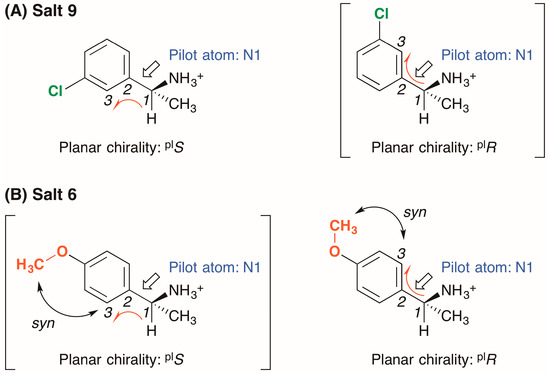
Figure 4.
Planar chirality of (R)-1-arylethylammonium cations in crystalline (A) salt 9 and (B) salt 6. The symbols “plS” and “plR” represent left- and right-handed planar chirality, respectively. In salt 6, higher priority was given to the ortho-carbon atom that is syn to the methoxy group.
In salt 6, the methoxy group of the (R)-1-(4-methoxyphenyl)ethylammonium cation is in the phenyl plane [15] (Figure 3). Considering the orientation of the methoxy group, the ortho-carbon atom 3 was selected so as to be syn to the methoxy group (Figure 4B). The three adjacent carbon atoms 1–3 were positioned in a clockwise direction in crystalline salt 6; thus, the planar chirality of the (R)-1-(4-methoxyphenyl)ethylammonium cation was assigned as plR. It should be noted that the planar chirality of the (R)-1-arylethylammonium cations in salts 6 and 9 is not genuine due to the unrestricted rotation of the C15–C17 bonds.
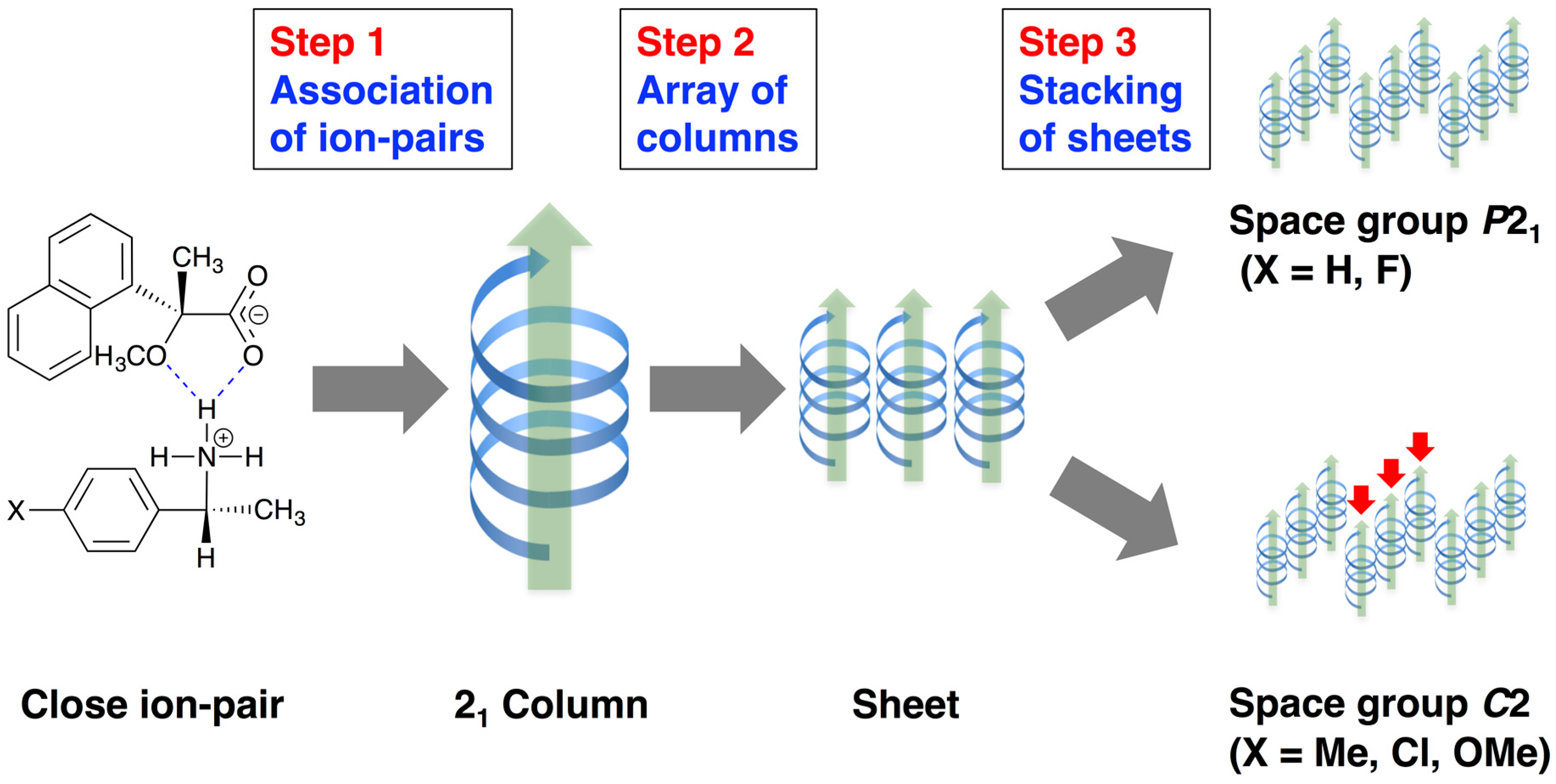
2.3. Three-Step Hierarchical Assembly
The molecular packing of salts 6–9 was interpreted as a three-step hierarchical assembly [12,13] (Figure 5):
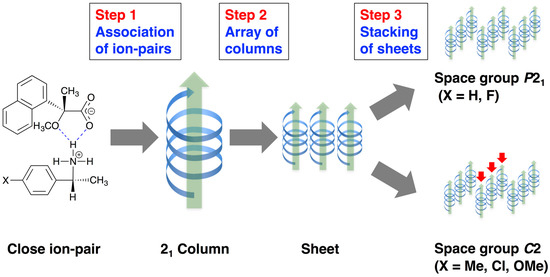
Figure 5.
Three-step hierarchical assemblies of MαNP salts.
- Close ion-pairs joined with salt bridges to form 21 columns.
- The 21 columns formed a sheet structure via homo-aromatic C–H∙∙∙π interactions between 1-naphthyl groups.
- These sheet structures stacked to form the whole crystal.
The para-substituents of 1-arylethylammonium cations were positioned on the surface of sheet structures. Therefore, they were important in the latter stage of the hierarchical assembly. With salts possessing smaller para-substituents, such as salt 7 (p-F) and salt 9 (p-H), the sheet structures stacked in the same manner, yielding a space group P21. With larger para-substituents, such as those of salt 6 (p-OMe) and salt 8 (p-Cl), the sheet structures stacked in an offset manner, yielding a space group C2. In addition, the space between columns formed in salts possessing the larger substituents, that is, salts 6, 8, and 9, were filled with methanol molecules [19].
2.4. Associations of Close Ion-Pairs
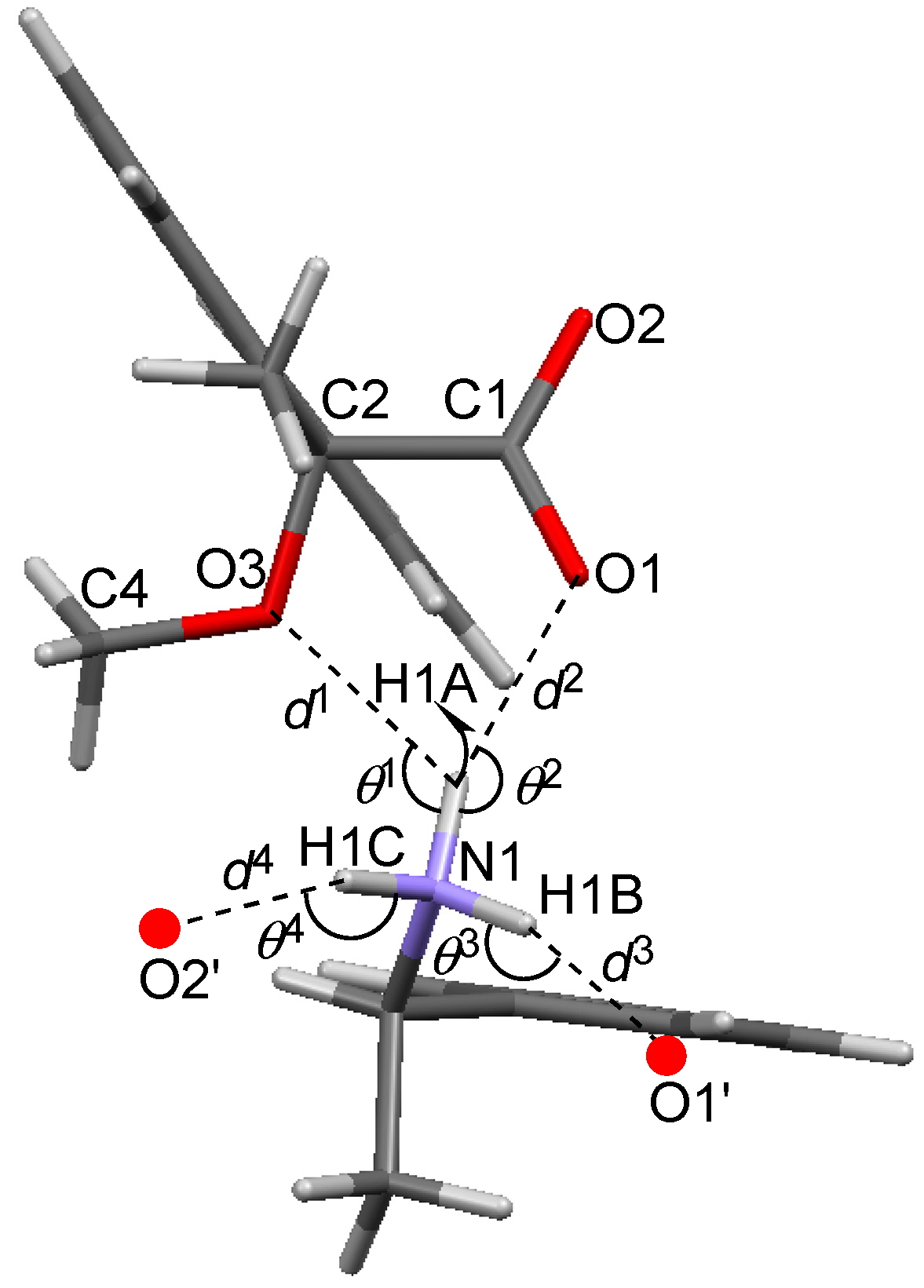
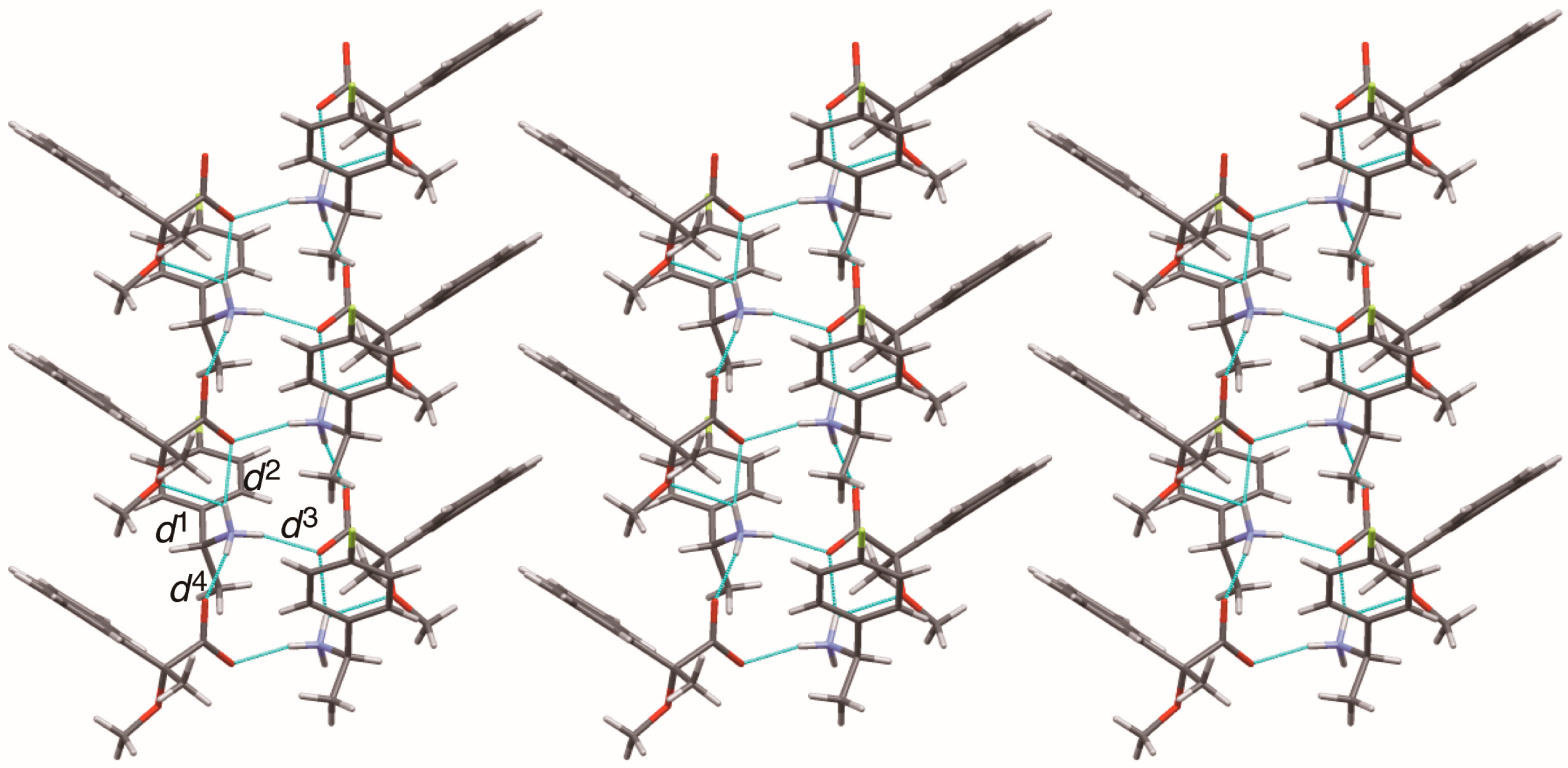
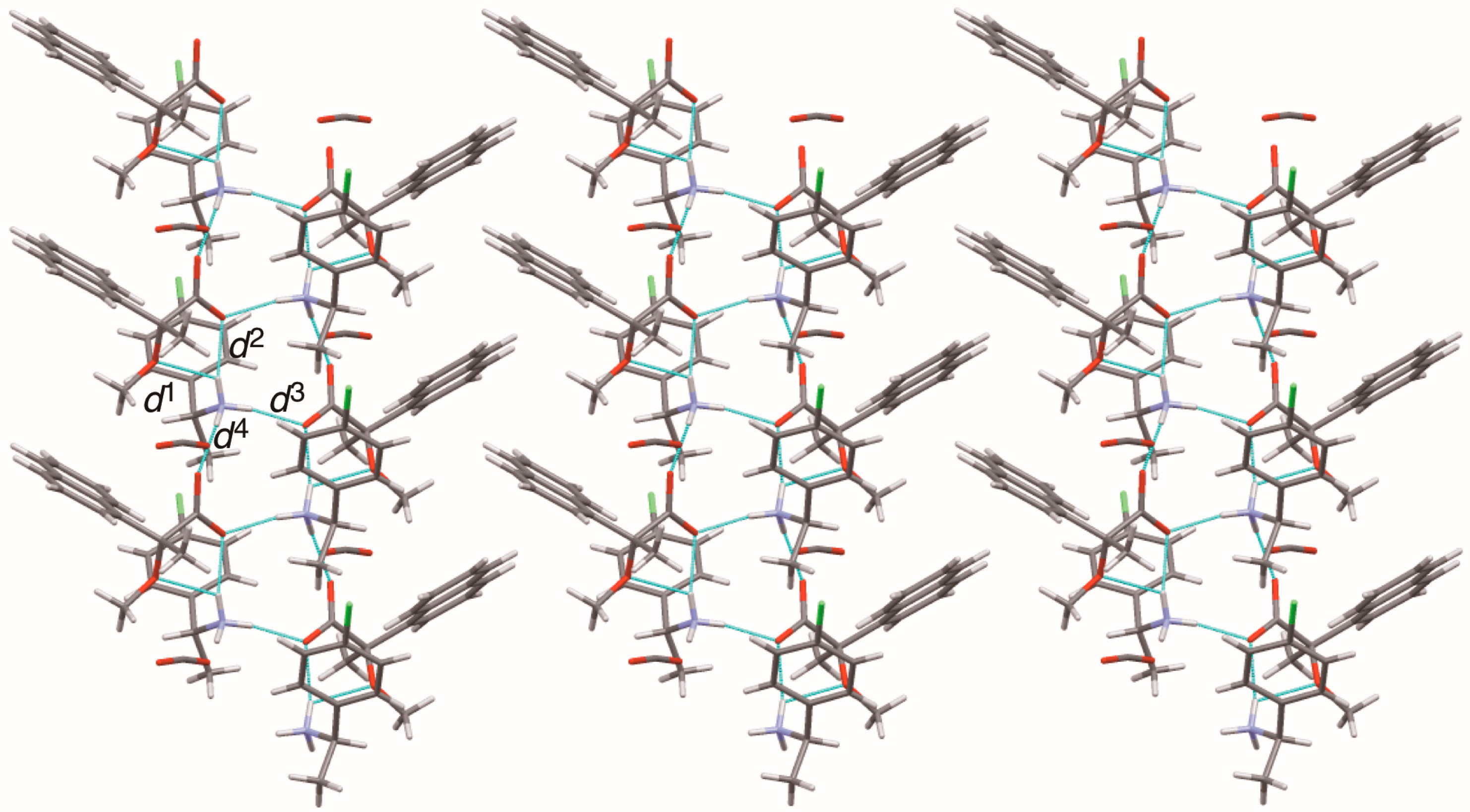
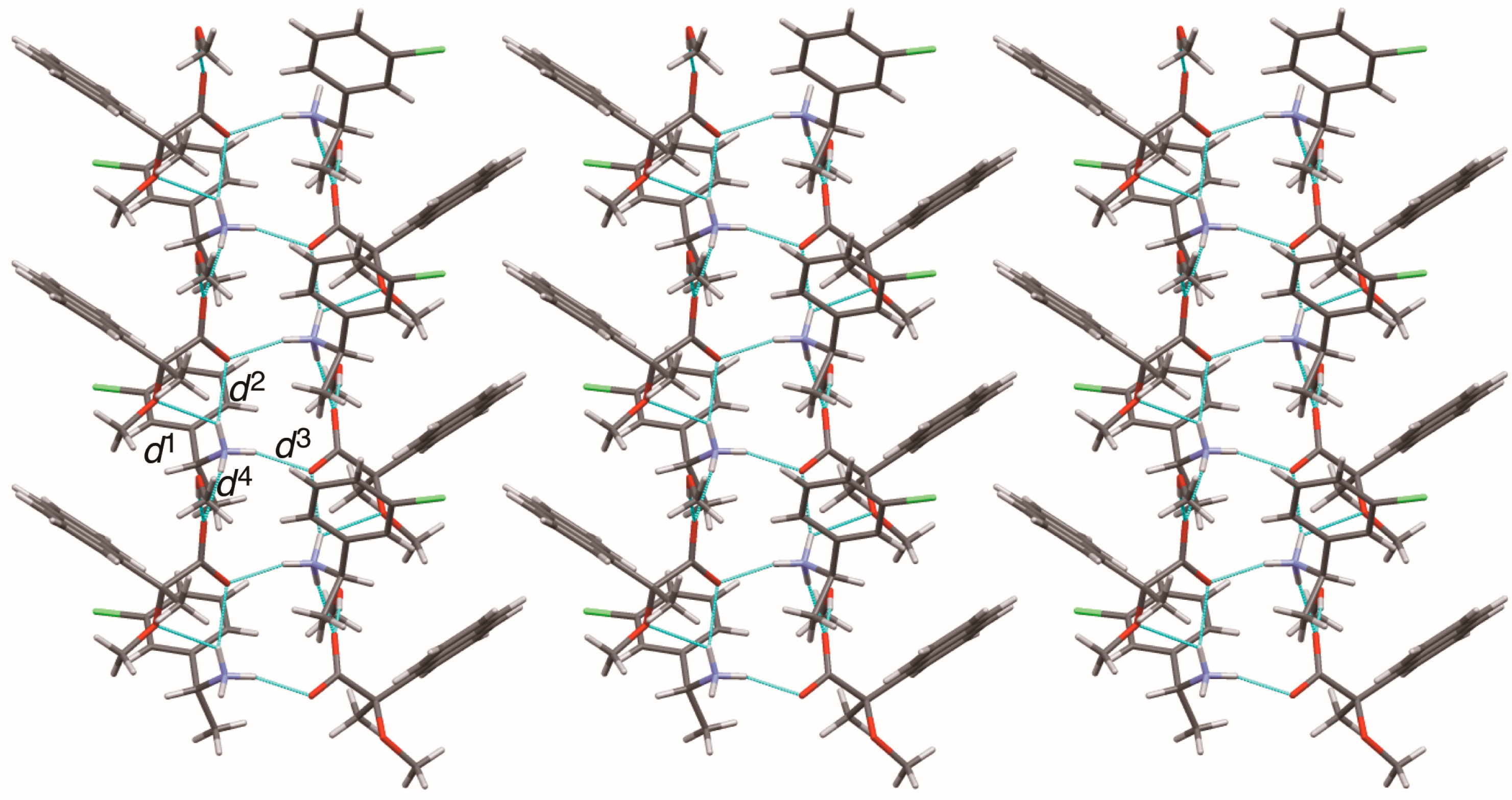
The methoxy-group-assisted salt bridge and the aromatic C–H∙∙∙π interactions joined the close ion-pairs of salts 6–9 as supramolecular synthons (Figure 2A and Figure 3) [6]. Table 3 and Figure 6 show the geometrical parameters of salt bridges in close ion-pairs of salts 6–9.

Table 3.
Geometrical parameters of methoxy-group-assisted salt bridges and other salt bridges in salts 6–9.
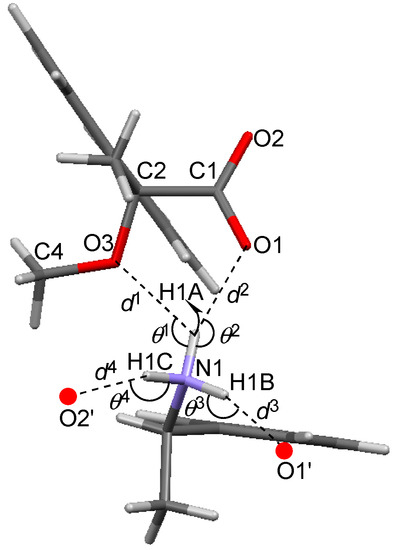
Figure 6.
Geometrical parameters of methoxy-group-assisted salt bridges and other salt bridges in salts 6–9.
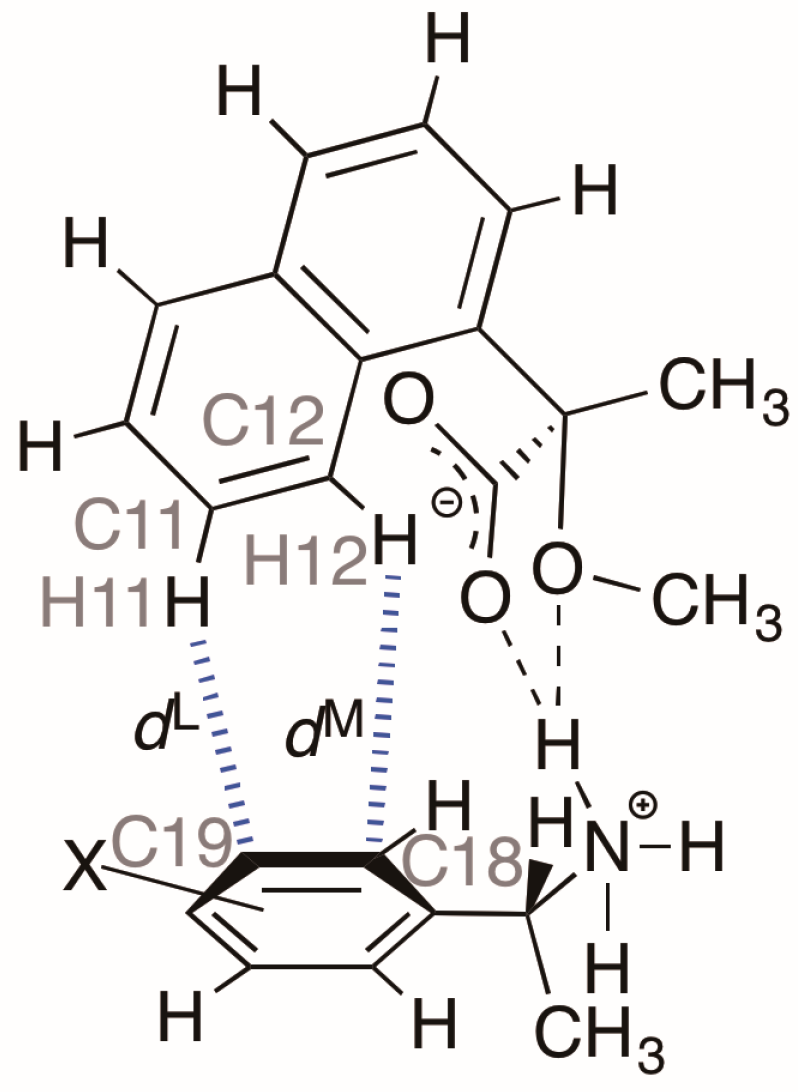
Table 4 and Figure 7 show the interatomic distances H11∙∙∙C19 and H12∙∙∙C18 (dL and dM, respectively) and the interatomic angles C11–H11∙∙∙C19 and C12–H12∙∙∙C18 in the close ion-pairs of salts 6–9. These data suggest the presence of aromatic C–H∙∙∙π interactions [8]. It has been reported that the strength of these C–H∙∙∙π interactions depends on the substituent in the phenyl group [9].

Table 4.
Geometrical parameters of hetero-aromatic C–H···π interactions in the close ion-pairs of salts 6–9.
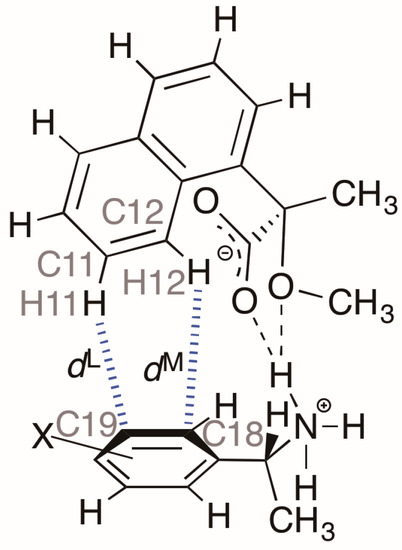
Figure 7.
Geometrical parameters of hetero-aromatic C–H···π interactions in the close ion-pairs of salts 6–9.
Considering the virtual chiral center of the carboxylate and methoxy groups of the (R)-MαNP anion and the phenyl and ammonium groups of the (R)-1-arylethylammonium cation, each of the supramolecular chirality in the close ion-pairs of salts 6–9 was assigned as supS (Figure 2B and Figure 3). This class of organic salts prefers the supS association over the supR association [10].
2.5. Molecular Packing of MαNP Salts (1): Arrays of Columns
The close ion-pairs of salts 6–9 form 21 columns via salt bridges (Figure 8, Figure 9, Figure 10 and Figure 11) [6,11]. Salts 6–9 revealed a similar herringbone motif [7] to form sheet structures. Table 5 and Figure 12 show the geometrical parameters of the homo-aromatic C–H···π interactions between the naphthyl groups of (R)-MαNP anions in salts 6–9. Space-filling models revealed that para-substituents of the 1-arylethylammonium cations are positioned on the sheet structures (Figure S1).
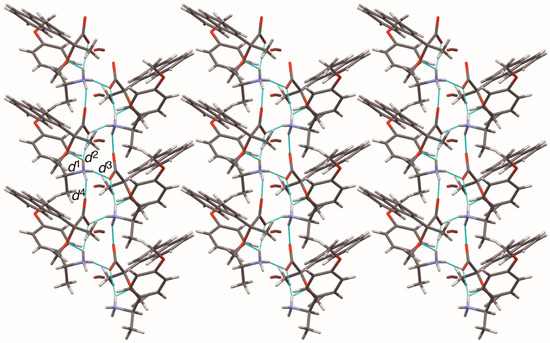
Figure 8.
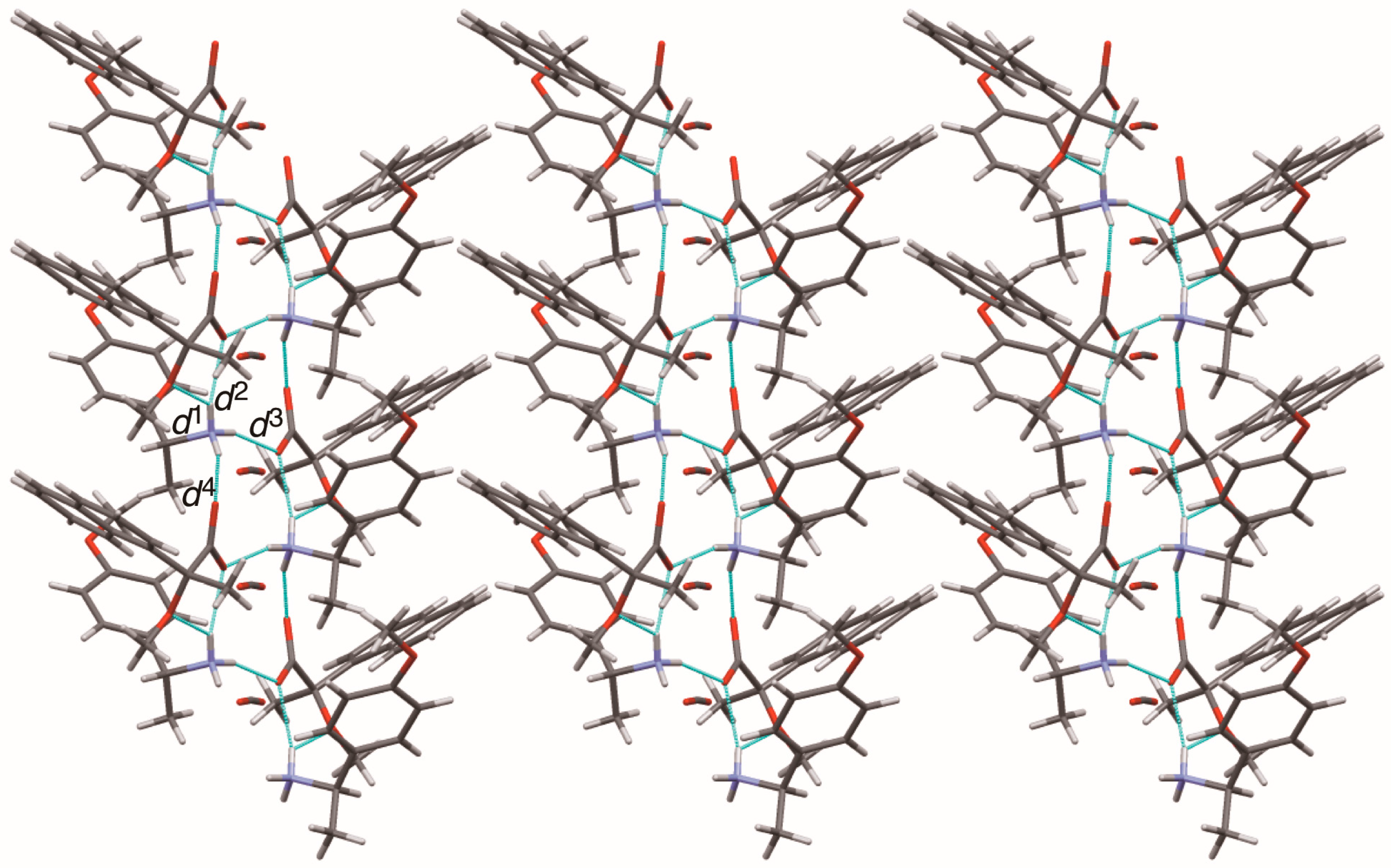
Sheet structure in crystalline salt 6, consisting of 21 columns. The pale-blue lines show salt bridges and hydrogen bonds. See Table 3 for the interatomic distances d1–d4.
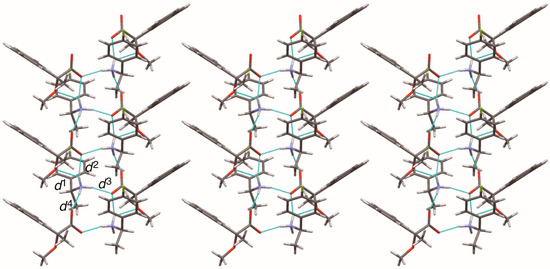
Figure 9.
Sheet structure in crystalline salt 7, consisting of 21 columns. The pale-blue lines show salt bridges and hydrogen bonds. See Table 3 for the interatomic distances d1–d4.
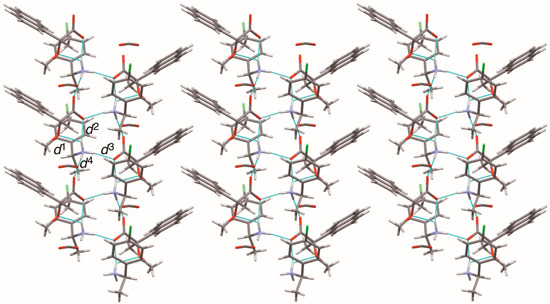
Figure 10.
Sheet structure in crystalline salt 8, consisting of 21 columns. The pale-blue lines show salt bridges and hydrogen bonds. See Table 3 for the interatomic distances d1–d4.
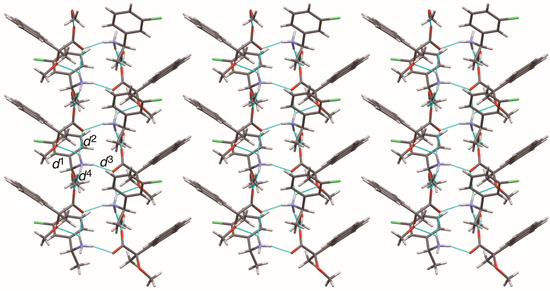
Figure 11.
Sheet structure in crystalline salt 9, consisting of 21 columns. The pale-blue lines show salt bridges and hydrogen bonds. See Table 3 for the interatomic distances d1–d4.

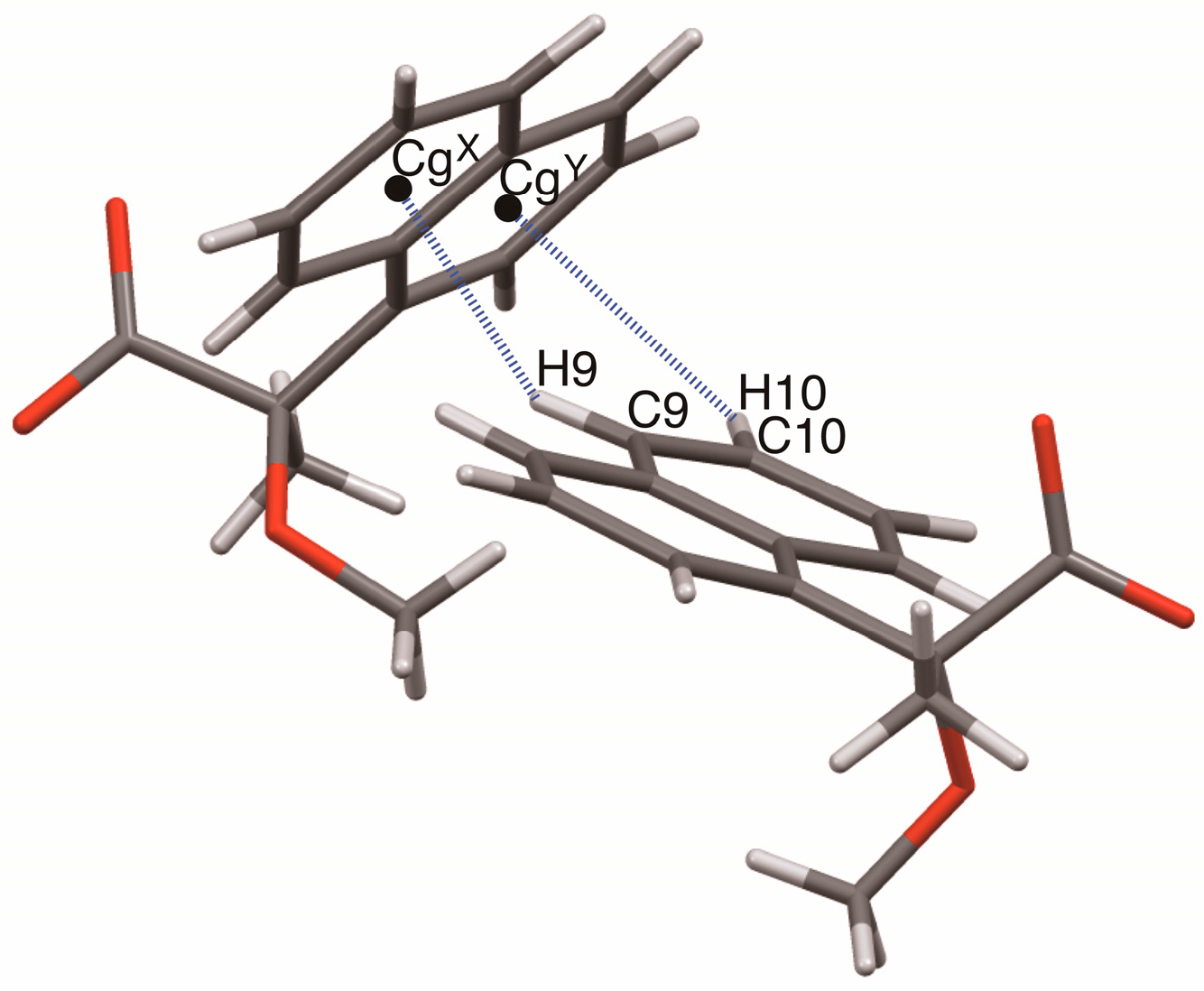
Table 5.
Geometrical parameters of the homo-aromatic C–H···π interactions between the naphthyl groups in salts 6–9 1.
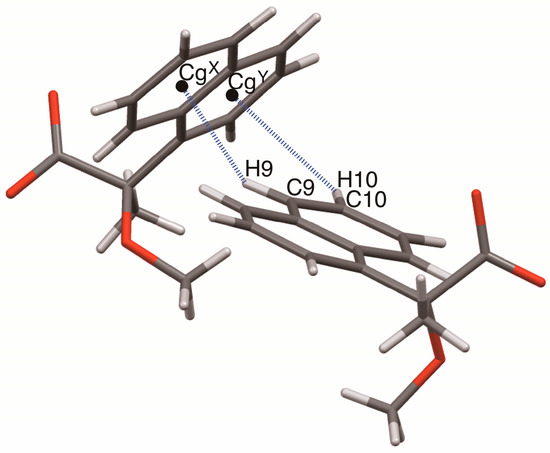
Figure 12.
Geometrical parameters of the homo-aromatic C–H···π interactions between the naphthyl groups in salts 6–9. Centroids of benzene rings: CgX, C9–C14; CgY, C5–C6–C7–C8–C14–C–13.
For smaller para-substituents, that is, salts 4 [6], 9 (p-H) and 7 (p-F), the sheet structures stacked in the same manner to yield a space group P21 (Figure 5). For larger para-substituents, that is, salt 5 (p-Me) [11]; salt 6 (p-MeO), and salt 8 (p-Cl), the sheets stacked in an offset manner to yield a space group C2. These results indicate that the para-substituents defined the space groups of salts 4–9. The following factors are deemed important for the stability of the diastereomeric salts [17]: (1) hydrogen bonding to form 21 columns; (2) van der Waals interactions between the columns; and (3) intra- and intercolumnar C–H∙∙∙π interactions. As noted above, methanol molecules filled the space [19] between the columns in salts 6, 8, and 9.
2.6. Molecular Packing of MαNP Salts (2): Stacking of Sheet Structures
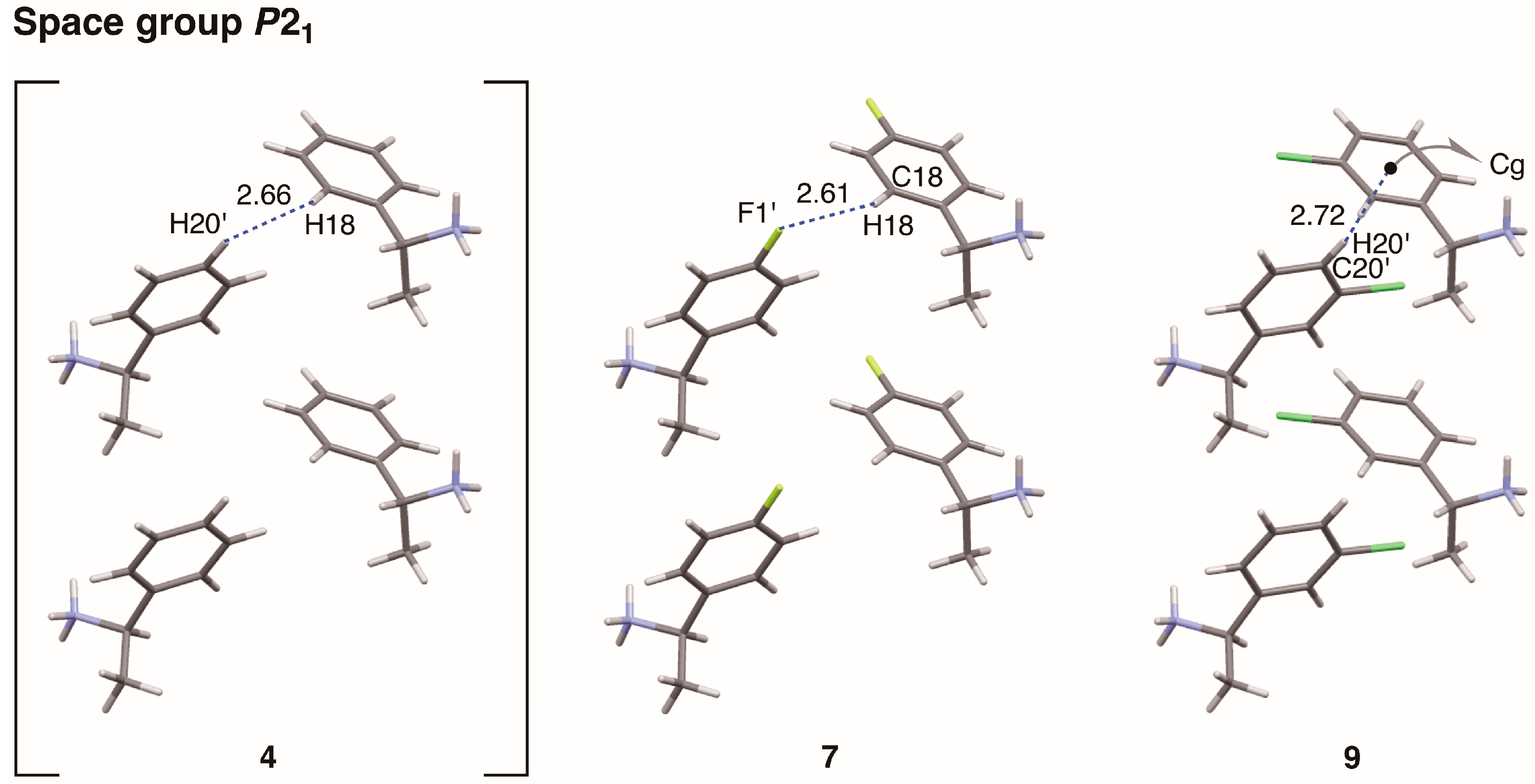
The interactions between sheet structures were examined in crystalline salts 7 and 9 (Figure 13 and Table 6). The sheet structures of salts 7 and 9 (Figure 9 and Figure 11, respectively) stacked in a space group P21. The interactions between p-fluorophenyl groups in crystalline salt 7 were similar to those of phenyl groups in crystalline salt 4 [6]. There were no intermolecular contacts shorter than the sum of the van der Waals radii between the sheet structures of salt 7. The aromatic hydrogen atom H18 of the p-fluorophenyl group was relatively close to the neighboring fluorine atom F1’. The interatomic distance between H18···F1’ was 2.61 Å (the van der Waals radii: aromatic H, 1.00 Å; F, 1.47 Å) while the interatomic angle C18–H18···F1’ was 118°. However, the C–H···F interaction is reportedly very weak [21]. Despite large differences in physical and chemical properties, the substitution of a fluorine atom for a hydrogen atom only marginally affected the molecular packing.
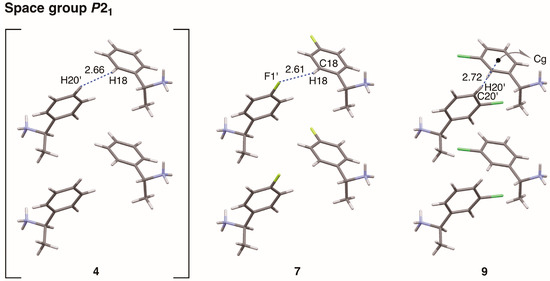
Figure 13.
Molecular packing of salts 4 and 7 viewed along the a-axis, and salt 9 viewed along the c-axis. Numbers without units represent interatomic distances (Å). Cg: centroid of the phenyl ring (C17–C22). Van der Waals radii: aromatic H, 1.00 Å; F, 1.47 Å; half-thickness of the benzene ring, 1.77 Å.

Table 6.
Interatomic distances and angles between the sheet structures of salts 6–9.
A clear homo-aromatic C–H···π interaction was observed between the m-chlorophenyl groups of salt 9 (Figure 13). The interatomic distance between the aromatic hydrogen atom H20’ and the centroid of the neighboring phenyl ring (Cg) was 2.72 Å while the interatomic angle C20’–H20’–Cg was 170°. Kinbara et al. reported that the para-chlorine substituent of the 1-(4-chlorophenyl)ethylammonium cation increases the positive charge of the meta-hydrogen atoms, effectively stabilizing the C–H···π interaction [18]. In contrast, the chlorine atom of salt 9 did not show any short contacts (e.g., Cl···Cl, Cl···O, or Cl···π interactions [22]). The same is true for the chlorine atom of salt 8 (see below).
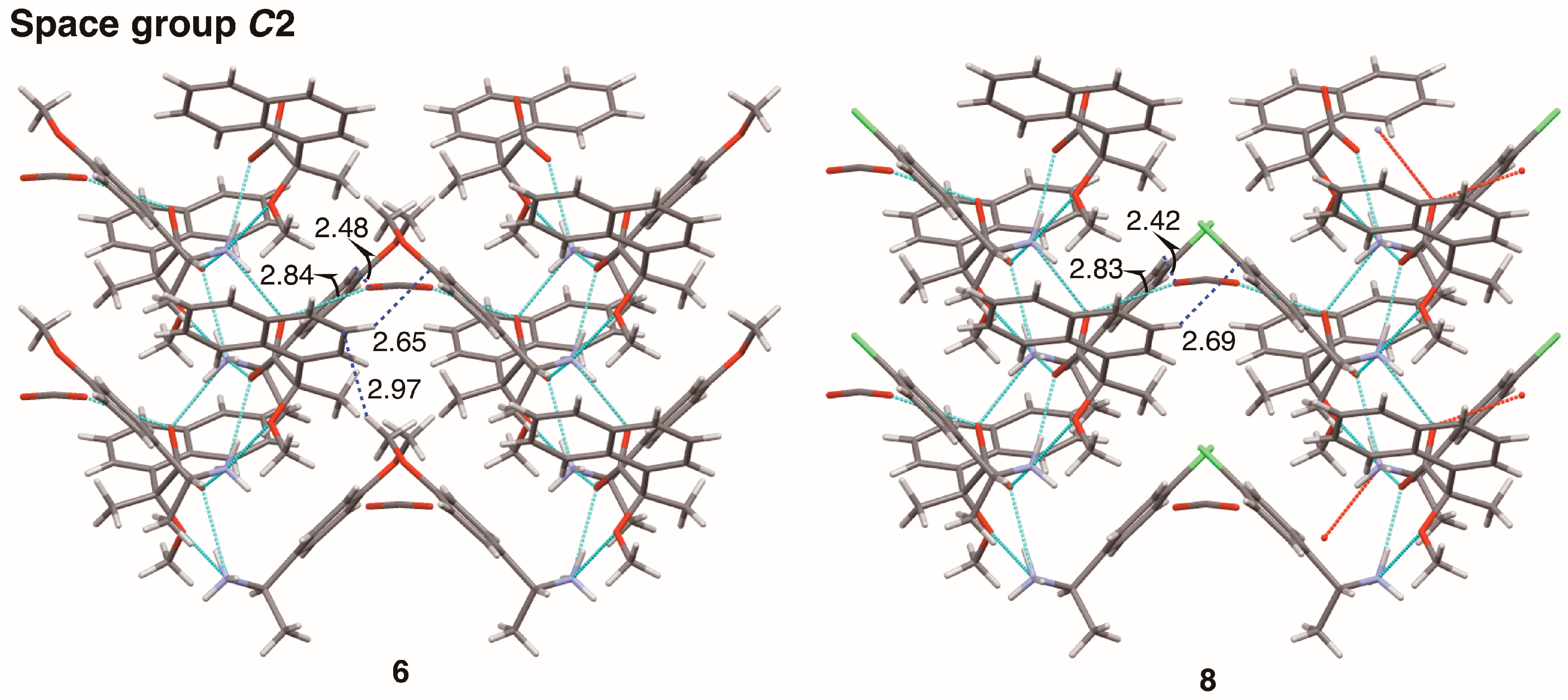
Finally, interactions between sheet structures were examined in crystalline salts 6 and 8. Figure 14 shows the molecular packing of salts 6 and 8 viewed along the c-axis. The sheet structures, shown in Figure 8 and Figure 10, respectively, yielded a space group C2 with hetero-aromatic C–H···π interactions between sheets. The aromatic hydrogen atom H7 of the naphthyl group was positioned nearly on the edge of phenyl group in salts 6 and 8; the distances between H7 and the neighboring phenyl plane were 2.65 Å and 2.69 Å, respectively.
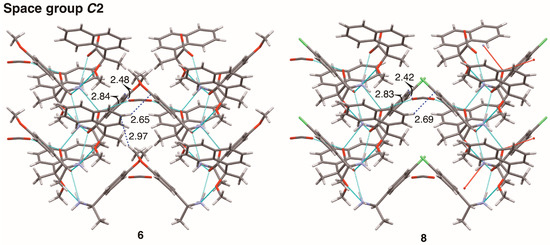
Figure 14.
Molecular packing of salts 6 and 8 viewed along the c-axis. Numbers without units represent interatomic distances (Å). The pale-blue dotted lines indicate hydrogen bonds and salt bridges. Van der Waals radii: aromatic H, 1.00 Å; H, 1.20 Å; O, 1.52 Å; half-thickness of the benzene ring, 1.77 Å.
In salt 6, the p-methoxy group was located in the phenyl plane [15], giving an extended herringbone motif; the interatomic distance between hydrogen atom H23A’ and aromatic carbon atom C7 was 2.97 Å. The chlorine atom of salt 8 had no short contacts with neighboring molecules. Methanol molecules filled the space between sheet structures in salts 6 and 8 (Figure 14). The oxygen atom of methanol molecule formed an O–H···O hydrogen bond and a C–H···O interaction. In salt 6, the interatomic distances O2···O5 and H21’···O5 were 2.84 Å and 2.48 Å, respectively. In salt 8, the interatomic distances O2···O4 and H21’···O4 were 2.83 Å and 2.42 Å, respectively. In terms of crystal engineering, the p-methoxy group of salt 6 was isosteric with the p-chloro group of salt 8.
3. Conclusions
This study clarified the crystal structures of MαNP salts 6–9 prepared from (R)-1 and (R)-1-arylethylamines. Using the concepts of supramolecular chirality, the solid-state associations of close ion-pairs were elucidated as supS in all salts. In addition, the solid-state planar chirality of the (R)-1-(4-methoxyphenyl)ethylammonium cation of salt 6 and the (R)-1-(3-chlorophenyl)ethylammonium cation of salt 9 were assigned as plR and plS, respectively. It should be noted that these are not genuine planar chirality due to unrestricted rotations. The crystal structures of MαNP salts 6–9 were interpreted as three-step hierarchical assemblies. Para-substituents of phenyl groups were positioned on sheet structures consisting of 21 columns and thereby affected the sheet stacking. Smaller para-substituents, that is, salt 7 (p-F) and salt 9 (p-H), and larger para-substituents, that is, salt 6 (p-OMe) and salt 8 (p-Cl), yielded space groups P21 and C2, respectively. For MαNP salts, p-H and p-OMe groups were isosteric with p-F and p-Cl groups, respectively. The 3-chlorophenyl groups of salt 9 exhibited homo-aromatic C–H···π interactions. For (R)-1-arylethylamines with larger substituents, methanol molecules filled the space in the crystal lattice. These results provide information on supramolecular chemistry for the design and preparation of single-enantiomer biofunctional molecules.
4. Materials and Methods
4.1. X-ray Crystallography
The single crystals (salt 6, 0.300 × 0.100 × 0.060 mm; salt 7, 0.200 × 0.200 × 0.200 mm; salt 8, 0.550 × 0.150 × 0.100 mm; salt 9, 0.600 × 0.600 × 0.500 mm) were covered with paraffin oil and mounted on a glass fiber, respectively. All measurements were made on a Rigaku Mercury70 diffractometer using graphite monochromated Mo-Kα radiation, operating at 50 kV/40 mA. Data were processed on a PC using CrystalClear Software (Rigaku, Tokyo, Japan). Structures were solved using direct methods and refined by full-matrix least-squares methods on F2 (SHELXL-97). CCDC 1442523 (salt 6), CCDC 1442524 (salt 7), CCDC 1442525 (salt 8), and CCDC 1442526 (salt 9) contain the supplementary crystallographic data for this study, which can be obtained from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk. Each crystal was dried in vacuo overnight at 80 °C prior to elemental analysis.
4.2. Preparation of Salt 6
A mixture of (R)-1 (29.2 mg, 127 μmol) and (R)-1-(4-methoxyphenyl)ethylamine (21.7 mg, 144 μmol) was dissolved in CHCl3 and MeOH (0.5 mL and 1.5 mL, respectively). The solution was warmed in a water bath at 45 °C and concentrated to ca. 1 mL in vacuo. Then, the solution was allowed to stand at RT for 5 days to give colorless crystals of salt 6 (MeOH solvate; 13.7 mg, 34 μmol) in 27% yield.
(R)-1-(4-Methoxyphenyl)ethylammonium (R)-2-methoxy-2-(1-naphthyl)propanoate (6). Elemental analysis calculated (%) for C23.5H29NO4.5 (6·0.5MeOH): C 71.01, H 7.35, N 3.52; found: C 71.21, H 7.37, N 3.51.
4.3. Preparation of Salt 7
A mixture of (R)-1 (31.0 mg, 135 μmol) and (R)-1-(4-fluorophenyl)ethylamine (18.7 mg, 134 μmol) was dissolved in CHCl3 and MeOH (2 mL and 1 mL, respectively). Then, the solution was allowed to stand at RT for three days to give colorless crystals of salt 7 (32.6 mg, 88 μmol) in 66% yield.
(R)-1-(4-Fluorophenyl)ethylammonium (R)-2-methoxy-2-(1-naphthyl)propanoate (7). Elemental analysis calculated (%) for C22H24FNO3: C 71.53, H 6.55, N 3.79; found: C 71.52, H 6.22, N 3.72.
4.4. Preparation of Salt 8
A mixture of (R)-1 (31.5 mg, 137 μmol) and (R)-1-(4-chlorophenyl)ethylamine (32.7 mg, 210 μmol) was dissolved in CHCl3 and MeOH (0.5 mL and 1.5 mL, respectively). The solution was warmed in a water bath at 45 °C and allowed to stand at RT for two days to give crude crystals of salt 8. Recrystallization from CHCl3/MeOH (1mL and 0.5 mL, respectively) gave colorless crystals of salt 8 (MeOH solvate; 18.3 mg, 46 μmol) with a total yield of 33%.
(R)-1-(4-Chlorophenyl)ethylammonium (R)-2-methoxy-2-(1-naphthyl)propanoate (8). Elemental analysis calculated (%) for C22.5H26ClNO3.5 (8·0.5MeOH): C 67.24, H 6.52, N 3.49; found: C 67.55, H 6.28, N 3.53.
4.5. Preparation of Salt 9
A mixture of (R)-1 (29.9 mg, 130 μmol) and (R)-1-(3-chlorophenyl)ethylamine (25.9 mg, 166 μmol) was dissolved in CHCl3 and MeOH (1 mL and 2 mL, respectively). The solution was warmed in a water bath at 47 °C and allowed to stand at RT for 10 days to give colorless crystals of salt 9 (MeOH solvate; 33.1 mg, 79 μmol) in 61% yield.
(R)-1-(3-Chlorophenyl)ethylammonium (R)-2-methoxy-2-(1-naphthyl)propanoate (9). Elemental analysis calculated (%) for C22H24ClNO3: C 68.48, H 6.27, N 3.63; found: C 68.50, H 6.24, N 3.56.
Supplementary Materials
The following is available online at www.mdpi.com/2073-4352/7/9/263/s1, Figure S1: Top and side views of sheet structures in salts 6–9 shown in space-filling models.
Author Contributions
Akio Ichikawa designed and performed the experiments; Yuji Mikata obtained and analyzed the data of X-ray crystallography; Akio Ichikawa, Hiroshi Ono, and Yuji Mikata discussed the results.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mori, K. Bioactive natural products and chirality. Chirality 2011, 23, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Harada, N. Determination of absolute configurations by X-ray crystallography and 1H NMR anisotropy. Chirality 2008, 20, 691–723. [Google Scholar] [CrossRef] [PubMed]
- Goto, J.; Hasegawa, M.; Nakamura, S.; Shimada, K.; Nambara, T. New derivatization reagents for the resolution of amino acid enantiomers by high-performance liquid chromatography. J. Chromatogr. 1978, 152, 413–419. [Google Scholar] [CrossRef]
- Ichikawa, A.; Hiradate, S.; Sugio, A.; Kuwahara, S.; Watanabe, M.; Harada, N. Absolute configuration of 2-hydroxy-2-(1-naphthyl)propionic acid as determined by the 1H NMR anisotropy method. Tetrahedron Asymmetry 1999, 10, 4075–4078. [Google Scholar] [CrossRef]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-field FT NMR application of Mosher’s method. The absolute congifurations of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- Ichikawa, A.; Ono, H.; Echigo, T.; Mikata, Y. Crystal structures and chiral recognition of the diastereomeric salts prepared from 2-methoxy-2-(1-naphthyl)propanoic acid. CrystEngComm 2011, 13, 4536–4548. [Google Scholar] [CrossRef]
- Desiraju, G.R. Hydrogen bridges in crystal engineering: Interactions without borders. Acc. Chem. Res. 2002, 35, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Hayashi, N. Solid State Organic Chemistry; Kagakudojin: Kyoto, Japan, 2009; pp. 31–33. [Google Scholar]
- Nishio, M. New Edition: Introduction to Intermolecular Forces in Organic Chemistry; Kodansha Scientific: Tokyo, Japan, 2008; pp. 44–45. [Google Scholar]
- Ichikawa, A.; Ono, H.; Mikata, Y. The crystal structure of the more-soluble Mosher’s salt. Chem. Lett. 2017, 46, 550–553. [Google Scholar] [CrossRef]
- Ichikawa, A.; Ono, H.; Mikata, Y. Naphthyl groups in chiral recognition: Structures of salts and esters of 2-methoxy-2-naphthylpropanoic acids. Chem. Asian J. 2012, 7, 2294–2304. [Google Scholar] [CrossRef] [PubMed]
- Hisaki, I.; Sasaki, T.; Tohnai, N.; Miyata, M. Multipoint approximation method for handedness determination of two-fold helical assemblies and their bundles. J. Synth. Org. Chem. Jpn. 2012, 70, 908–917. [Google Scholar] [CrossRef]
- Miyata, M.; Tohnai, N.; Hisaki, I. Crystalline host–guest assemblies of steroidal and related molecules: Diversity, hierarchy, and supramolecular chirality. Acc. Chem. Res. 2007, 40, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Hidaka, J. Stereochemistry, 2nd ed.; Dainippoon Tosho: Tokyo, Japan, 1994; pp. 129–130. [Google Scholar]
- Müller, K.; Faeh, C.; Diederich, F. Fluorine in pharmaceuticals: Looking beyond intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, M.Z.; Cavalcanti, S.M.T.; Moreira, D.R.M.; Filgueira de Azevedo, W., Jr.; Leite, A.C.L. Halogen atoms in the modern medicinal chemistry: Hints for the drug design. Curr. Drug Targets 2010, 11, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Saigo, K.; Sakai, K. Toward efficient optical resolution by diastereomeric salt formation. J. Synth. Org. Chem. Jpn. 2011, 69, 499–505. [Google Scholar] [CrossRef]
- Kinbara, K.; Oishi, K.; Harada, Y.; Saigo, K. Effect of a substituent on an aromatic group in diastereomeric resolution. Tetrahedron 2000, 56, 6651–6655. [Google Scholar] [CrossRef]
- Sakai, K. Industrial-scale optical resolution with the diastereomeric salt formation method—A novel resolution technology using molecular recognition mechanism. Chem. Chem. Ind. 2004, 57, 507–511. [Google Scholar]
- Karamertzanis, P.G.; Price, S.L. Challenges of crystal structure prediction of diastereomeric salt pairs. J. Phys. Chem. B 2005, 109, 17134–17150. [Google Scholar] [CrossRef] [PubMed]
- Reichenbächer, K.; Süss, H.I.; Hulliger, J. Fluorine in crystal engineering—“The little atom that could”. Chem. Soc. Rev. 2005, 34, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.N.; Inoue, Y.; Nakanishi, I.; Kitaura, K. Cl–π interactions in protein–ligand complexes. Protein Sci. 2008, 17, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).