Ferroelectricity in Simple Binary Crystals
Abstract
:1. Introduction
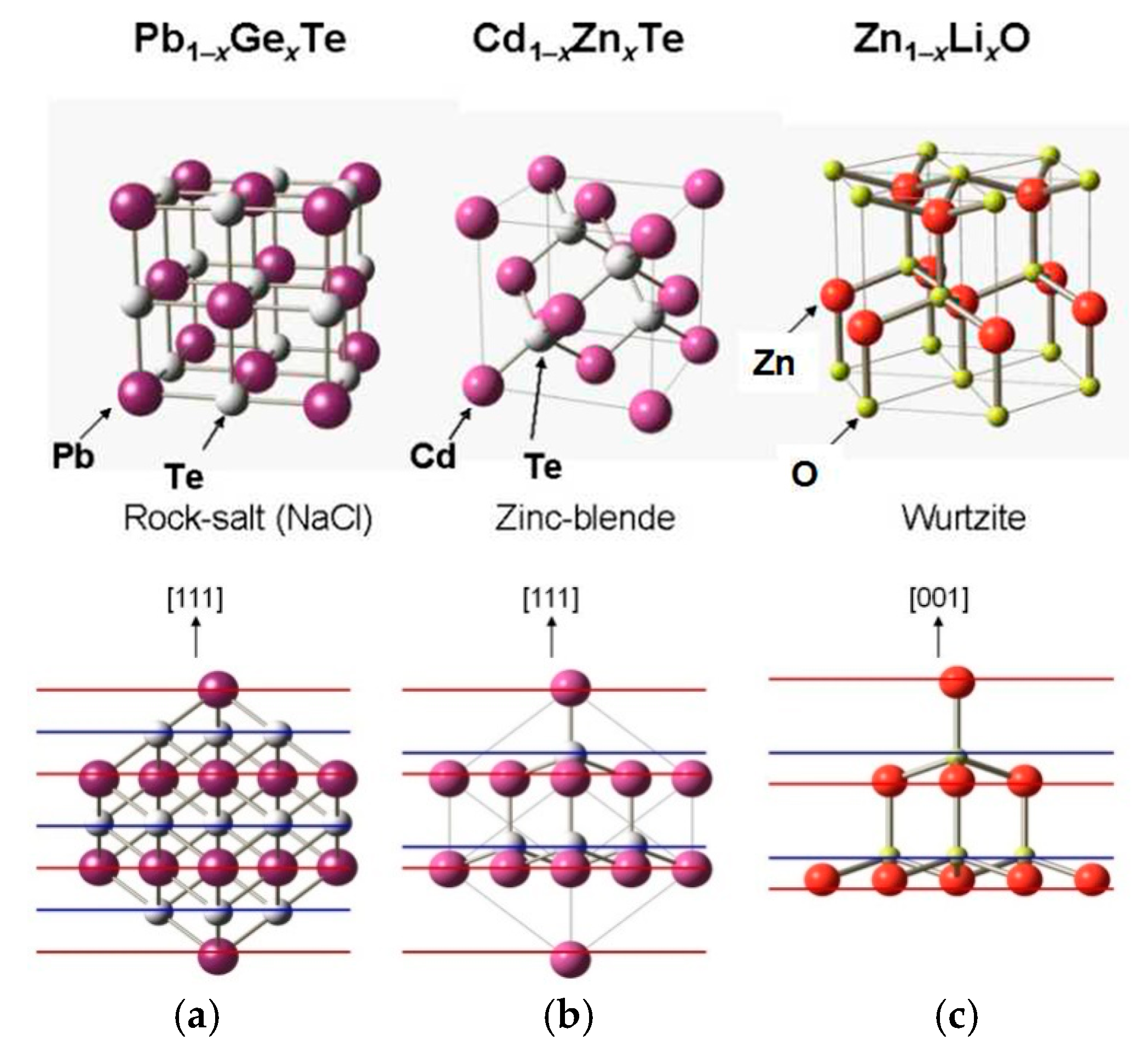
2. Ferroelectricity in Binary Semiconductors
2.1. Narrow-Gap Ferroelectric Semiconductor PbTe
2.2. Wide-Gap Ferroelectric Semiconductor CdTe
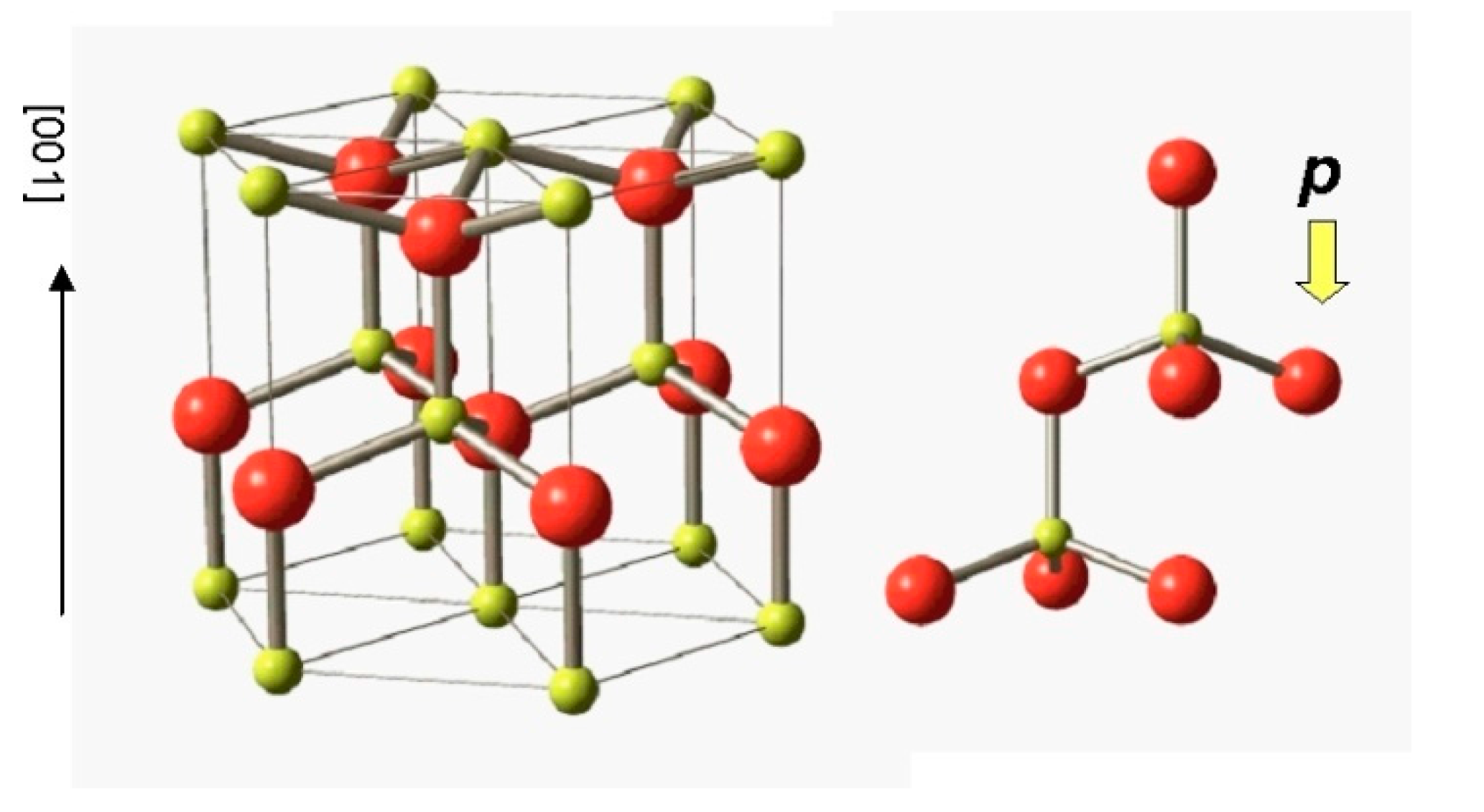
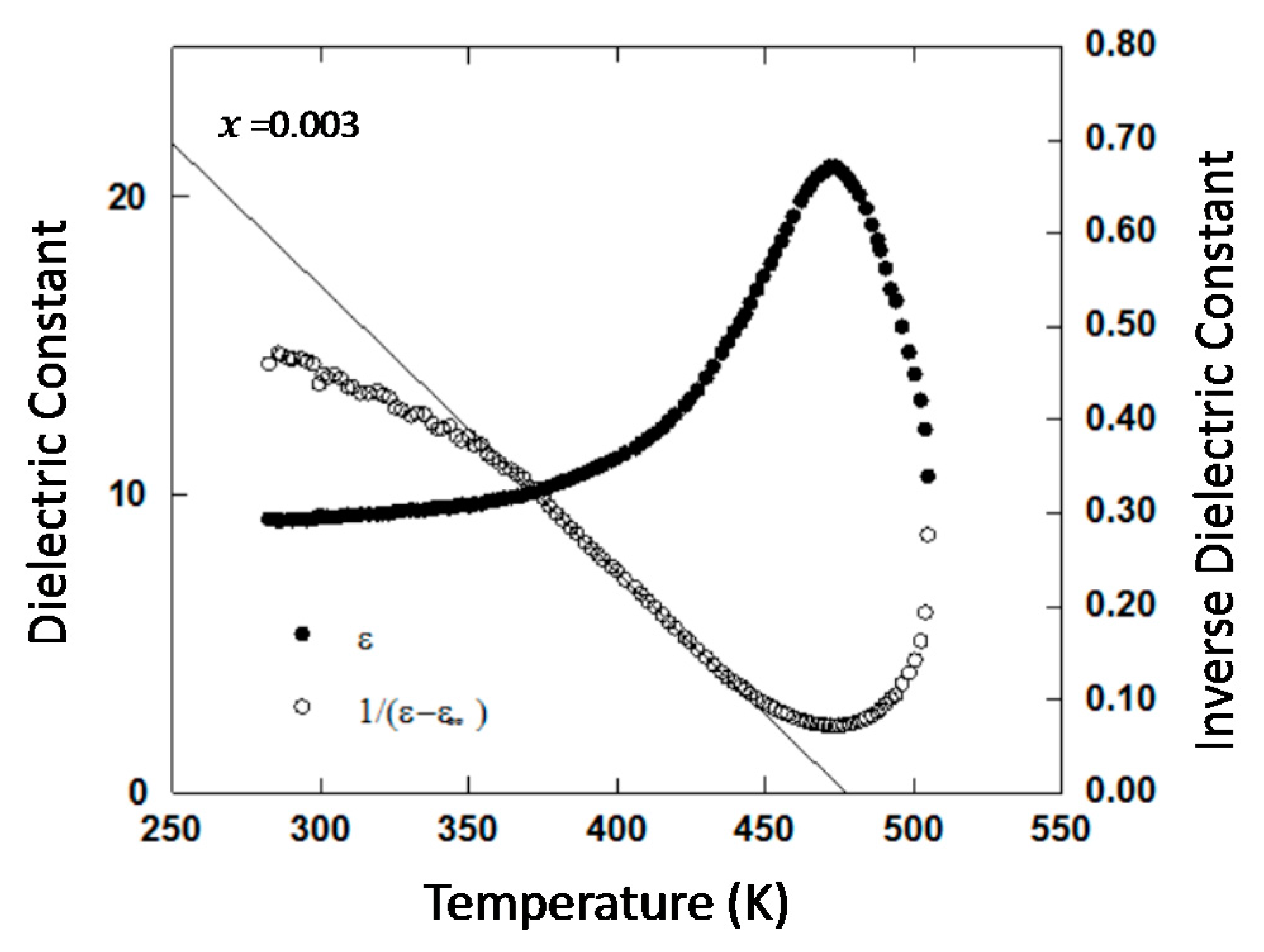
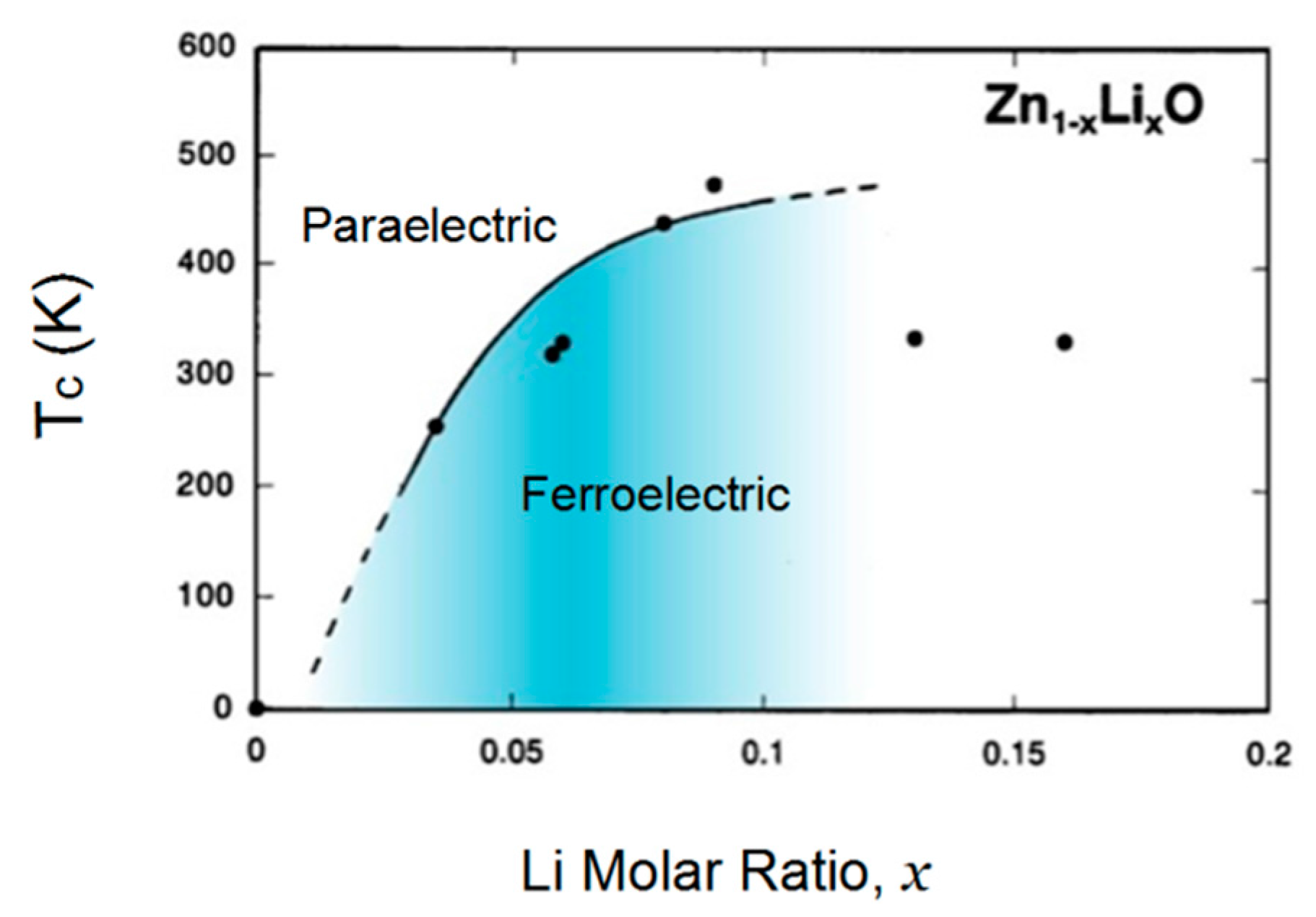
2.3. II-VI Wide-Gap Semiconductor ZnO
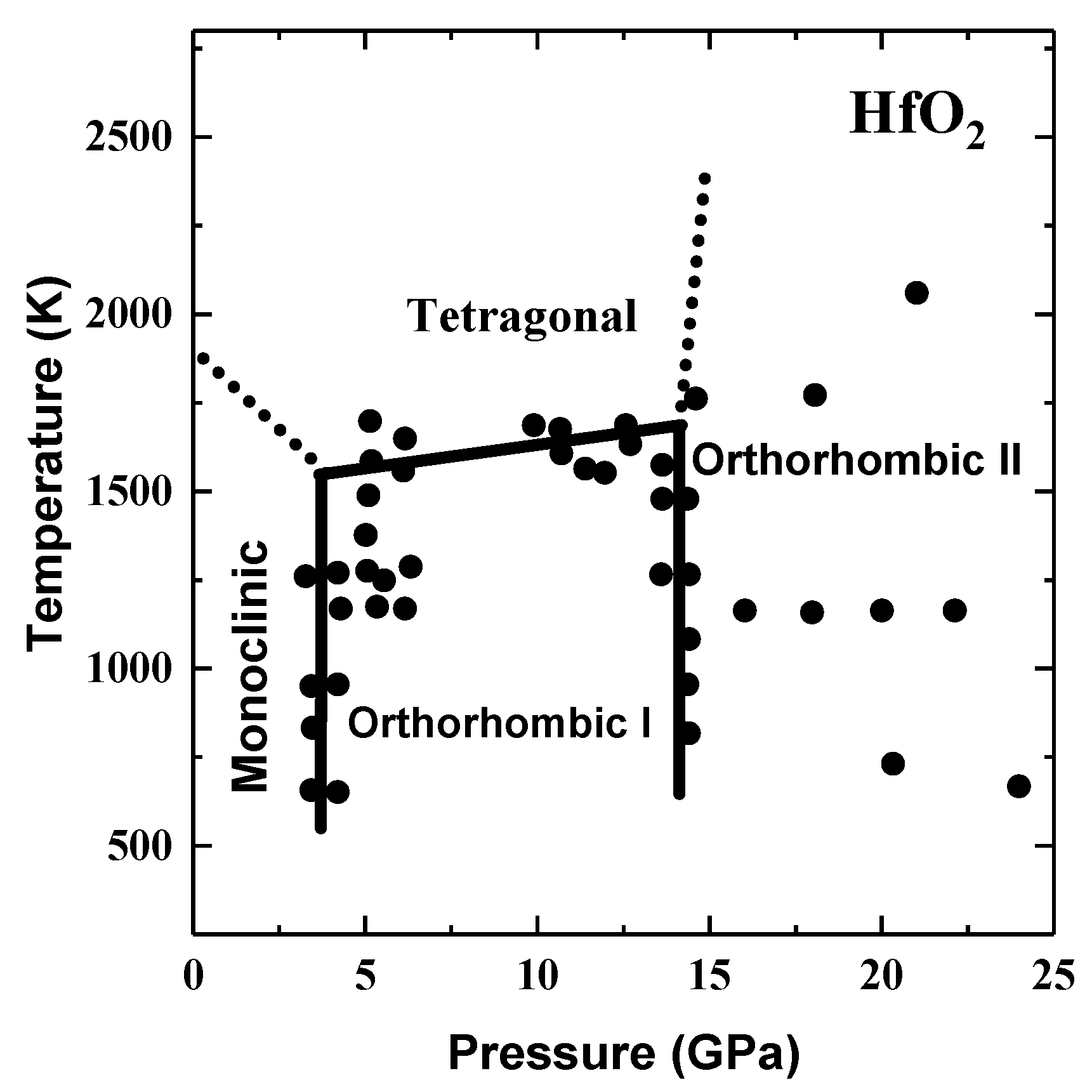
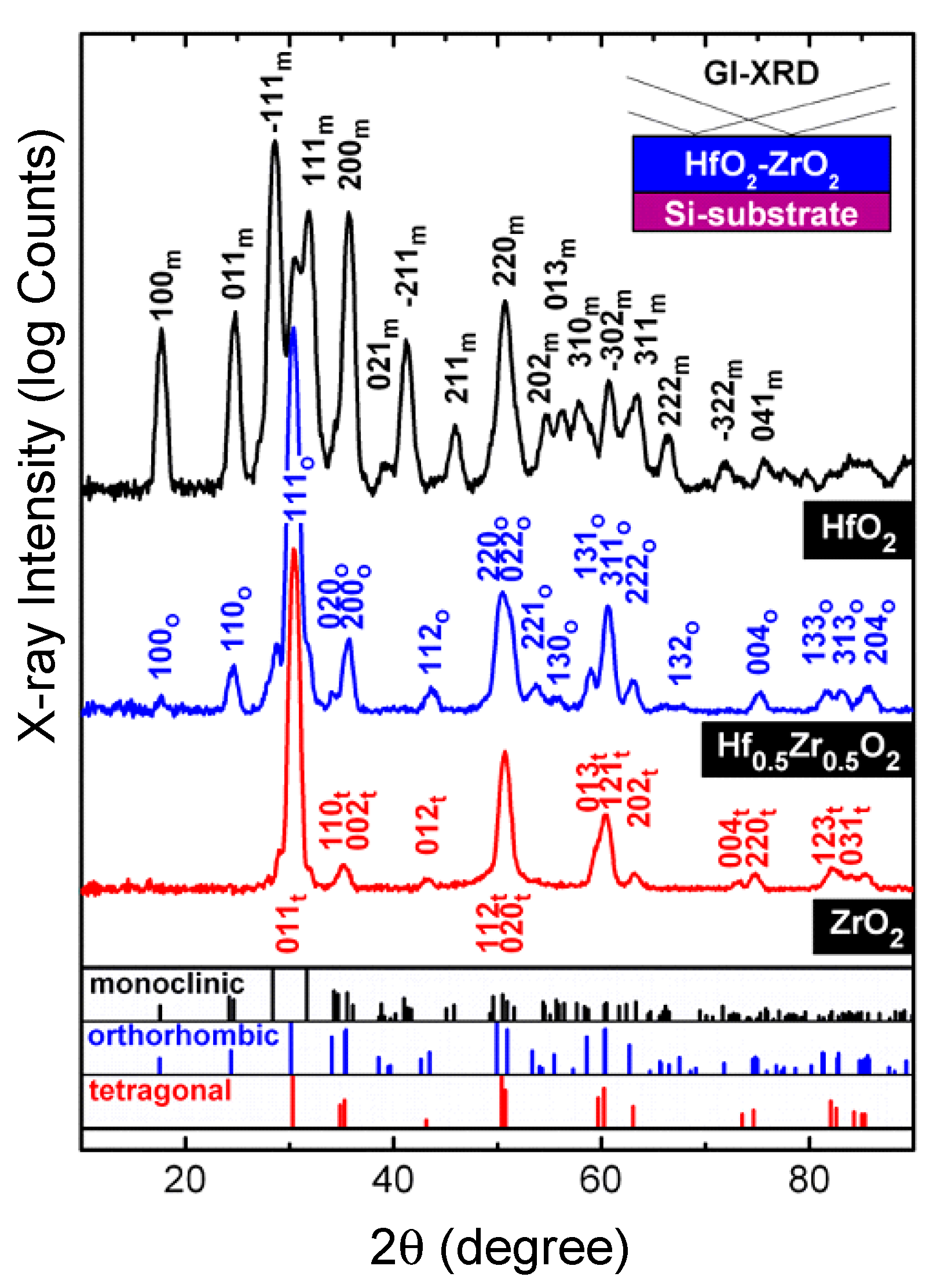
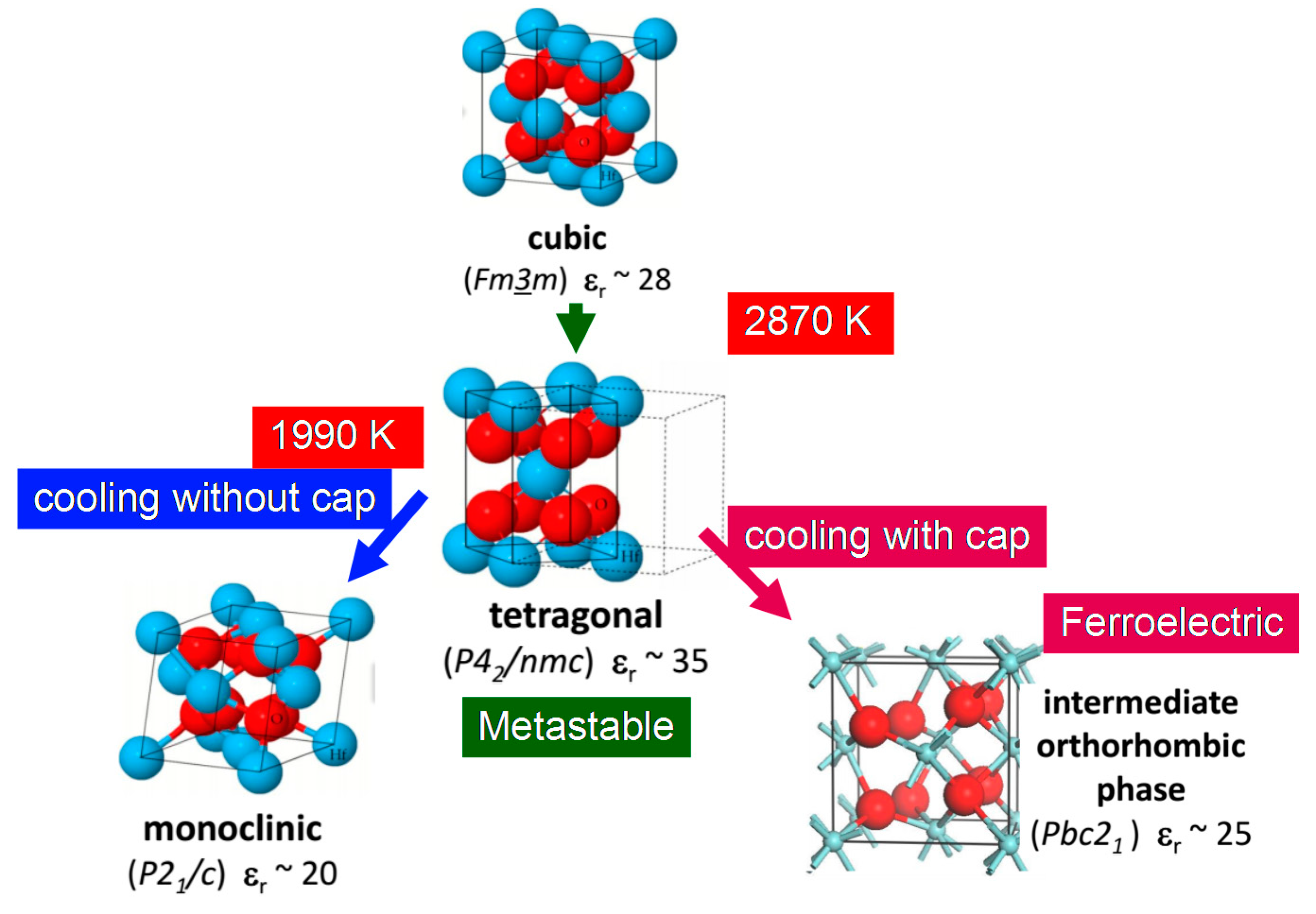
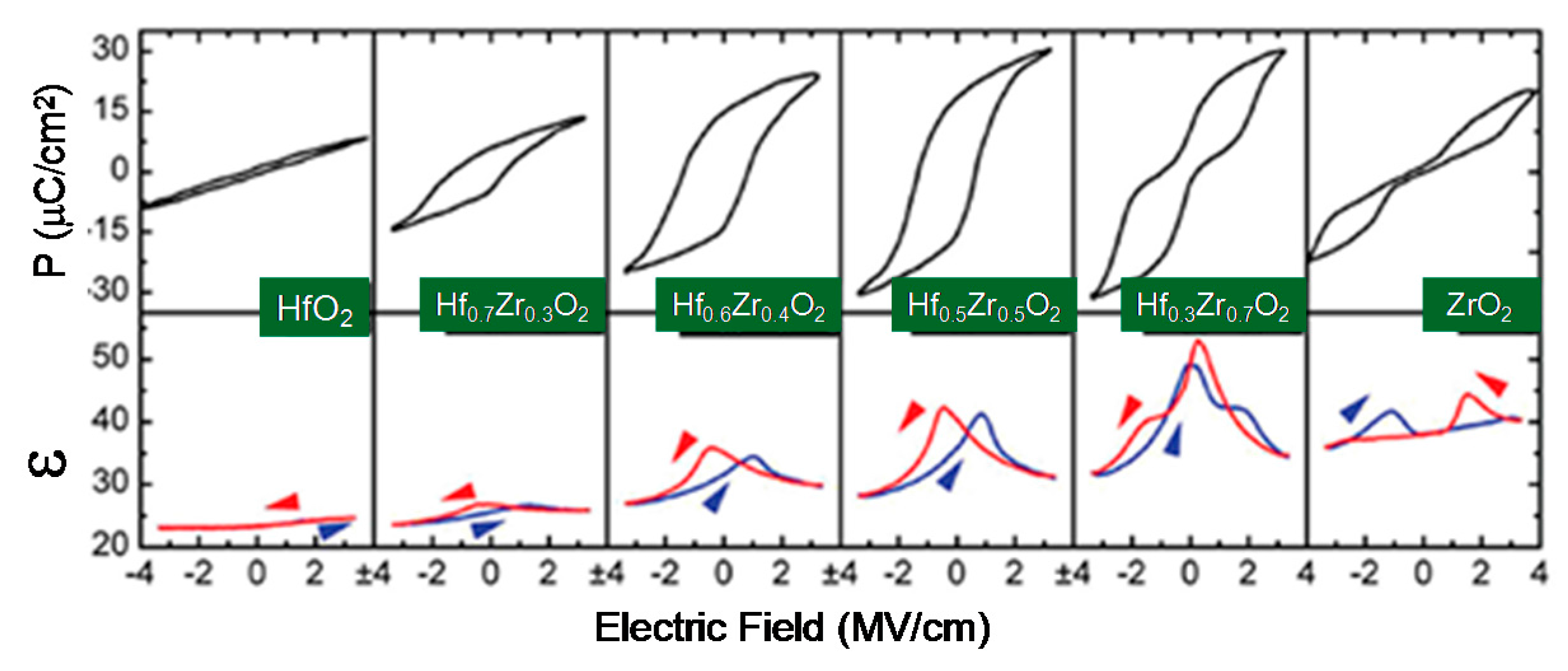
2.4. High-k Materials HfO2 and ZrO2
3. Phenomenological Treatment for the Appearance of Ferroelectricity
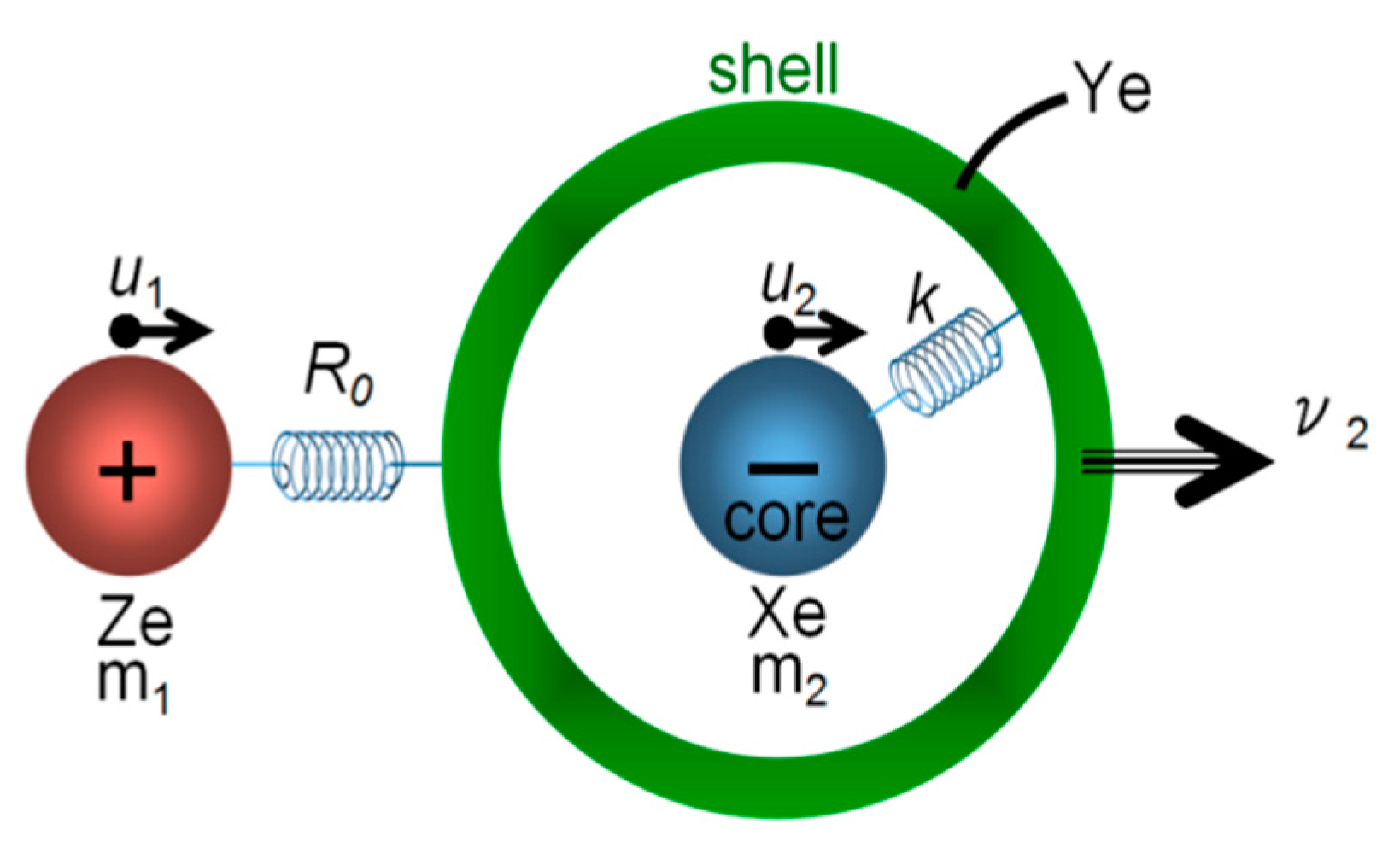
4. Microscopic Consideration after Cochran’s Lattice Dynamical Theory
5. Discussion
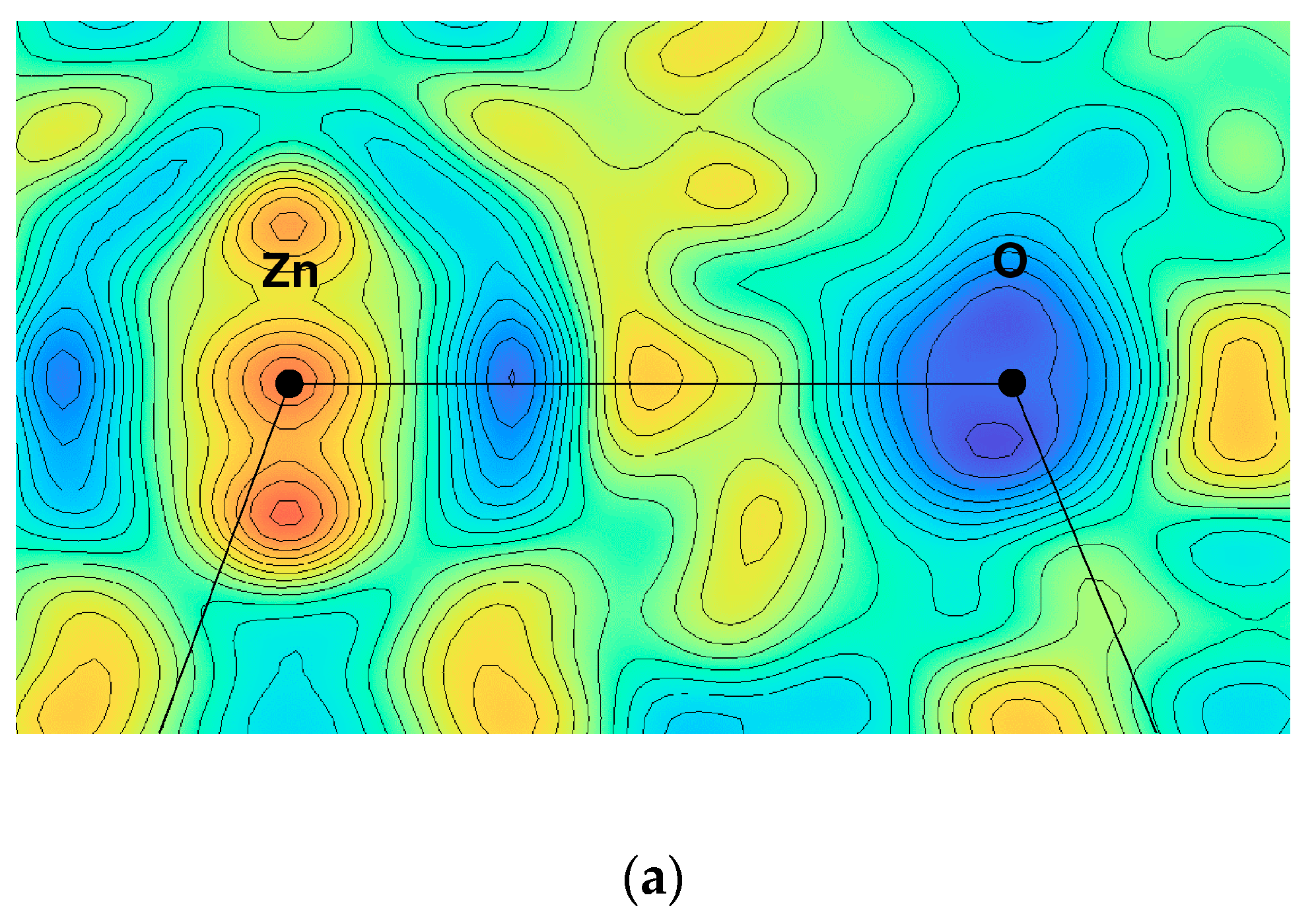
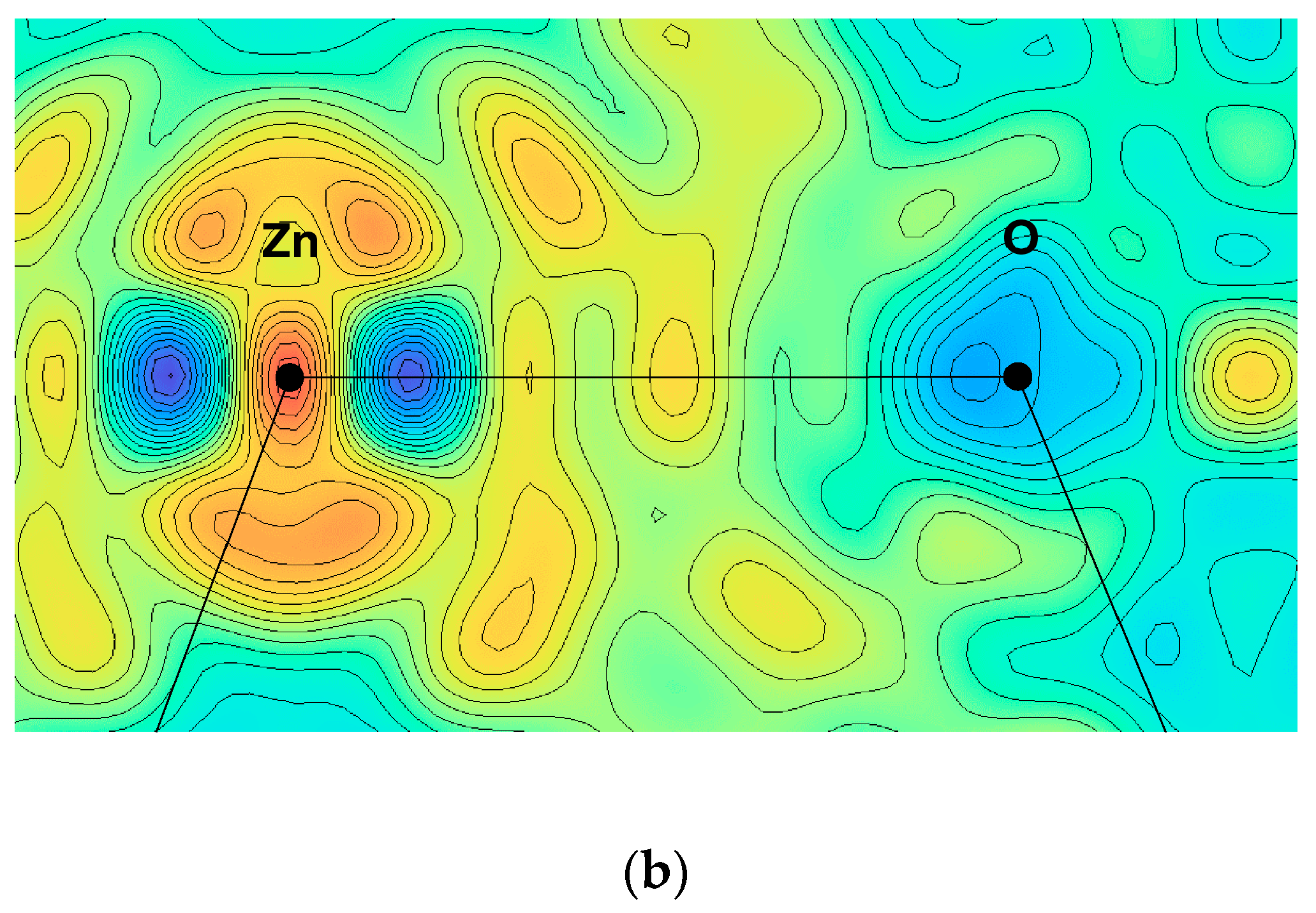
5.1. Electronic Ferroelectricity in ZnO; Effect of Dopants
5.2. Structural Modification by Dopants
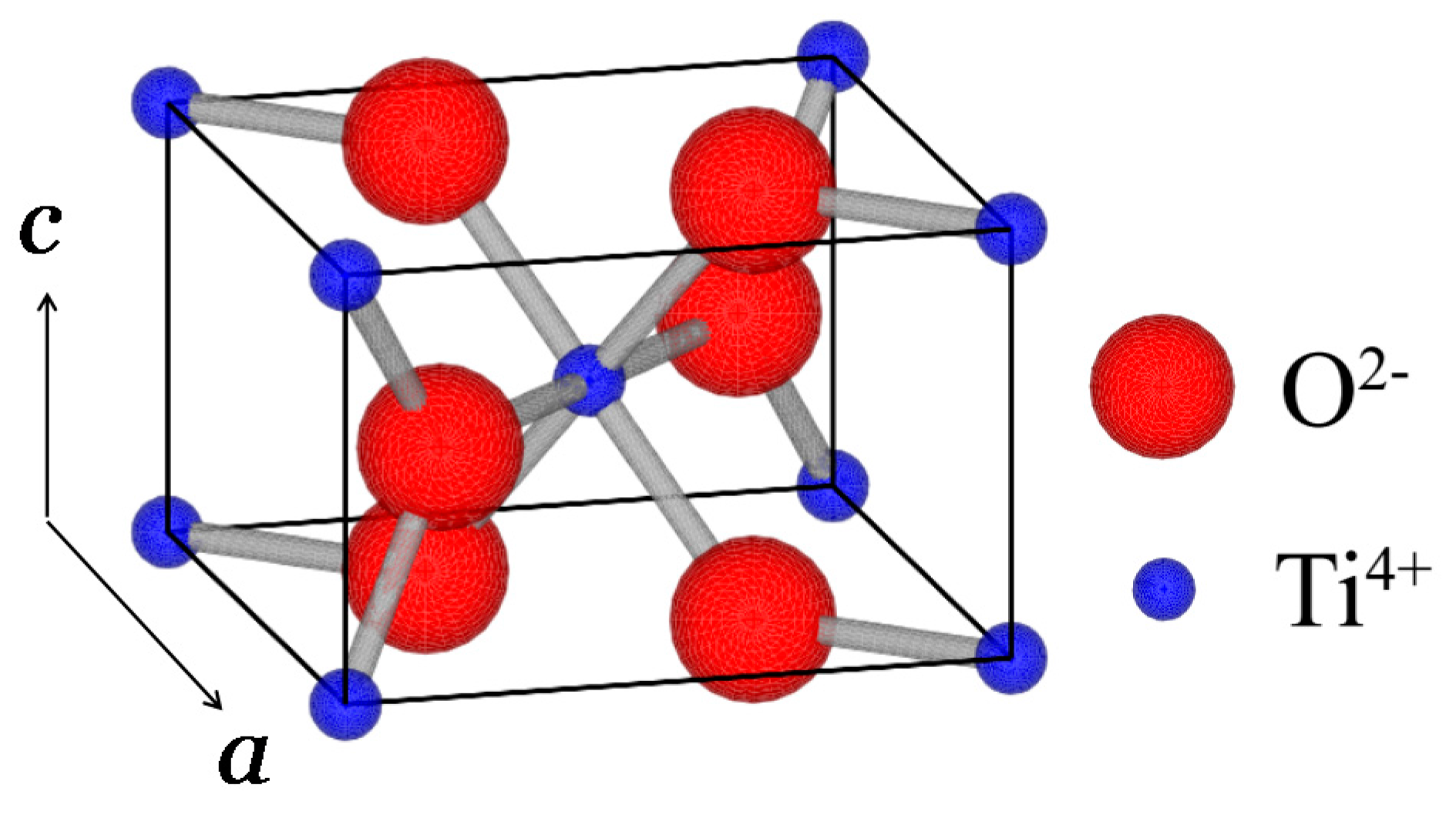
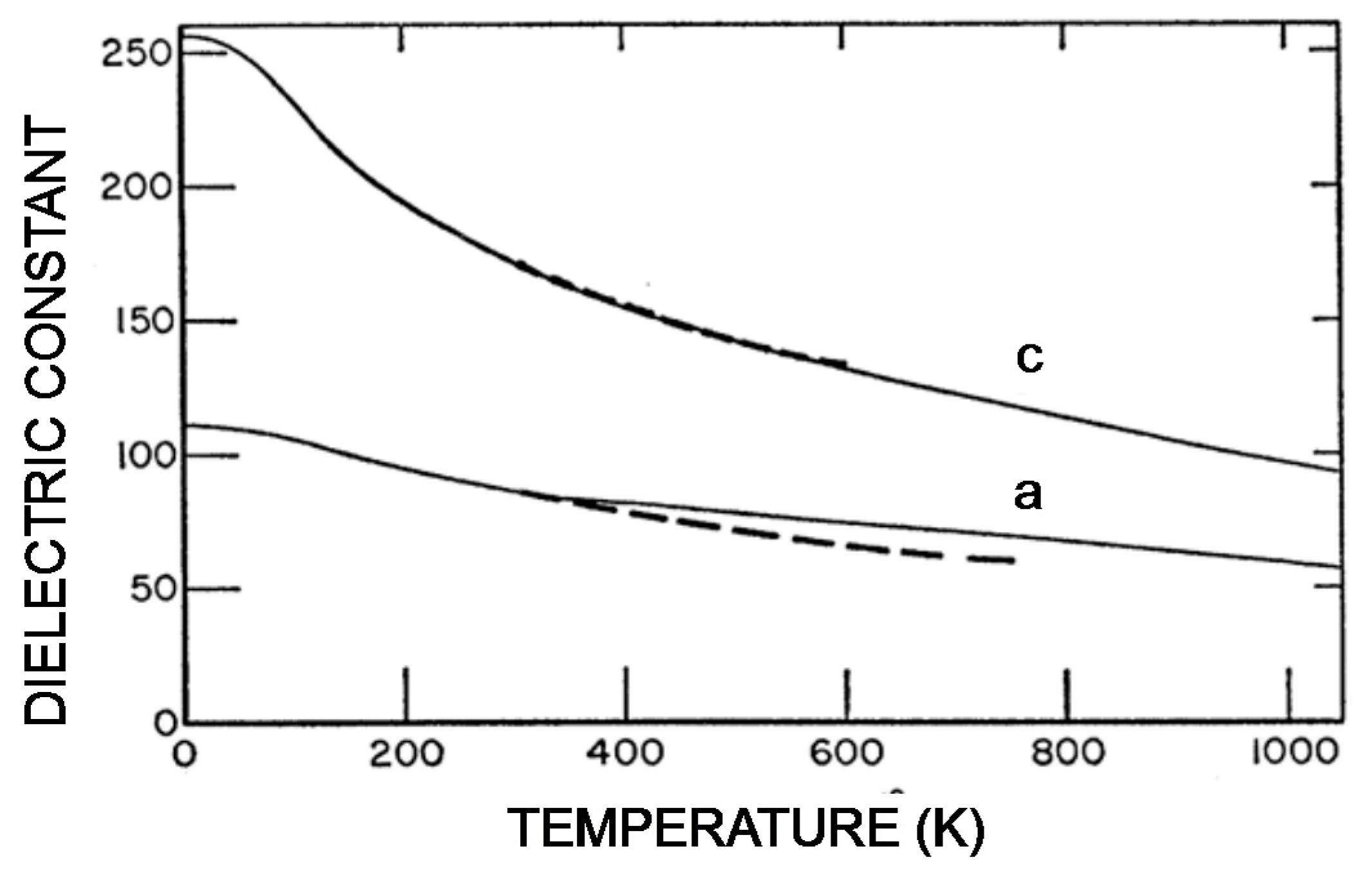
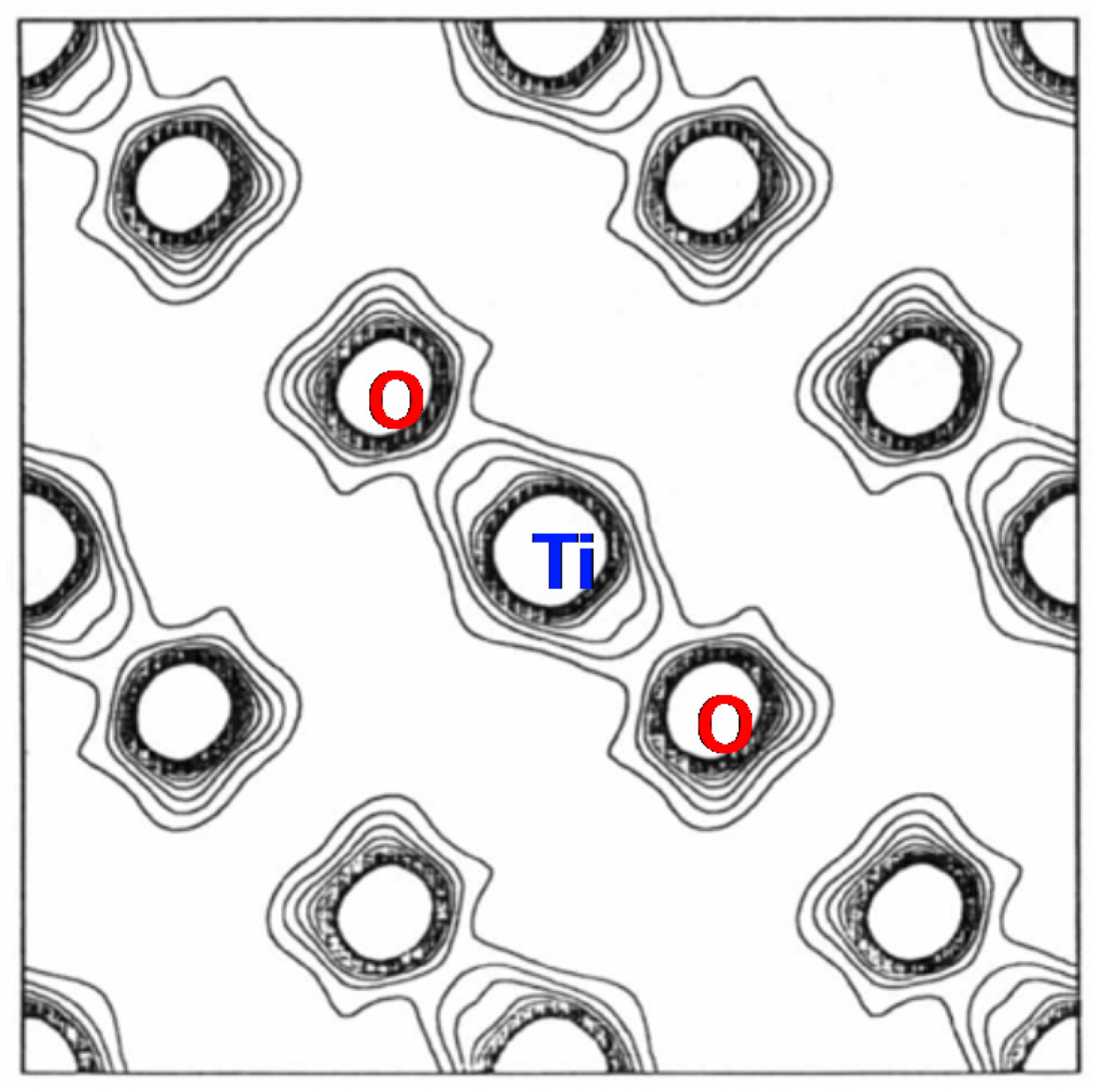
6. Another Possible Ferroelectric TiO2
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Auciello, O.; Scott, J.F.; Ramesh, R. Ultrahigh-Intensity Lasers: Physics of the Extreme on a Tabletop. Phys. Today 1998, 51, 22. [Google Scholar] [CrossRef]
- Scott, J.F. The physics of ferroelectric ceramic thin films for memory applications. Ferroelectr. Rev. 1998, 1, 1. [Google Scholar] [CrossRef]
- Haertling, G.H. Ferroelectric Ceramics: History and Technology. J. Am. Ceram. Soc. 1999, 82, 797–818. [Google Scholar] [CrossRef]
- Scott, J.F.; Paz de Araujo, C.A. Ferroelectric memories. Science 1989, 246, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Uchino, K. Ceramic actuators: Principles and applications. MRS Bull. 1993, 18, 42–48. [Google Scholar] [CrossRef]
- Wemple, S.H.; DiDomenico, M., Jr. Oxygen-Octahedra Ferroelectrics. II. Electro-optical and Nonlinear-Optical Device Applications. J. Appl. Phys. 1969, 40, 735. [Google Scholar] [CrossRef]
- Hoshino, S.; Shimaoka, S.; Niimura, K. Ferroelectricity in Solid Hydrogen Halides. Phys. Rev. Lett. 1967, 19, 1286. [Google Scholar] [CrossRef]
- Cochran, W. Crystal Stability and the Theory of Ferroelectricity. Phys. Rev. Lett. 1959, 3, 412. [Google Scholar] [CrossRef]
- Cochran, W. Crystal stability and the theory of ferroelectricity. Adv. Phys. 1960, 9, 387. [Google Scholar] [CrossRef]
- Cochran, W. Crystal stability and the theory of ferroelectricity part II. Piezoelectric crystals. Adv. Phys. 1961, 10, 401. [Google Scholar] [CrossRef]
- Corso, D.A.; Posternak, M.; Resta, R.; Baldereshi, A. Ab initio study of piezoelectricity and spontaneous polarization in ZnO. Phys. Rev. 1994, B50, 10715. [Google Scholar] [CrossRef]
- Tamaki, N.; Onodera, A.; Sawada, T.; Yamashita, H. Measurements of D-E Hysteresis Loop and Ferroelectric Activity in Piezoelectric Li-doped ZnO. J. Korean Phys. 1996, 29, 668. [Google Scholar]
- Onodera, A.; Tamaki, N.; Kawamura, Y.; Sawada, T.; Yamashita, H. Dielectric Activity and Ferroelectricity in Piezoelectric Semiconductor Li-Doped ZnO. Jpn. J. Appl. Phys. 1996, 35, 5160. [Google Scholar] [CrossRef]
- Onodera, A.; Tamaki, N.; Jin, K.; Yamashita, H. Ferroelectric Properties in Piezoelectric Semiconductor Zn 1-xMxO (M=Li, Mg). Jpn. J. Appl. Phys. 1997, 36, 6008. [Google Scholar] [CrossRef]
- Onodera, A.; Takesada, M. Electronic Ferroelectricity in II-VI Semiconductor ZnO. In Advances in Ferroelectrics; INTECH: Rijeka, Croatia, 2012. [Google Scholar]
- Bilz, H.; Bussmann-Holder, A.; Jantsch, W.; Vogel, P. Dynamical Properties of IV-VI Compounds; Springer: Berlin, Germany, 1983. [Google Scholar]
- Weil, R.; Nkum, R.; Muranevich, E.; Benguigui, L. Ferroelectricity in zinc cadmium telluride. Phys. Rev. Lett. 1989, 62, 2744. [Google Scholar] [CrossRef] [PubMed]
- Böscke, T.S.; Müller, J.; Brauhaus, D.; Schröder, U.; Böttger, U. Ferroelectricity in hafnium oxide thin films. Appl. Phys. Lett. 2011, 99, 102903. [Google Scholar] [CrossRef]
- Böscke, T.S.; Teichert, S.; Brauhaus, D.; Müller, J.; Schröder, U.; Böttger, U.; Mikolajick, T. Phase transitions in ferroelectric silicon doped hafnium oxide. Appl. Phys. Lett. 2011, 99, 112904. [Google Scholar] [CrossRef]
- Mitsui, T.; Tatsuzaki, I.; Nakamura, E. An Introduction to the Physics of Ferroelectrics; Gordon and Breach: New York, NY, USA, 1976; p. 202. [Google Scholar]
- Benguigui, L.; Weil, R.; Muranevich, E.; Chack, A.; Fredj, E. Ferroelectric properties of Cd1−xZnxTe solid solutions. J. Appl. Phys. 1993, 74, 513. [Google Scholar] [CrossRef]
- Terauchi, H.; Yoneda, Y.; Kasatani, H.; Sakaue, K.; Koshiba, T.; Murakami, S.; Kuroiwa, Y.; Noda, Y.; Sugai, S.; Nakashima, S.; et al. Ferroelectric behaviors in semiconductive Cd1−xZnxTe crystals. Jpn. J. Appl. Phys. 1993, 32, 728. [Google Scholar] [CrossRef]
- Klingshirn, C.F.; Meyer, B.K.; Waag, A.; Hoffmann, A.; Geurts, J. Zinc Oxide From Fundamental Properties Towards Novel Applications; Springer: Berlin, Germany, 2010. [Google Scholar]
- Yao, T. (Ed.) ZnO Its Most Up-to-Date Technology and Application, Perspectives; CMC Books: Tokyo, Japan, 2007. (In Japanese) [Google Scholar]
- Heiland, G.; Mollwo, E.; Stockmann, F. Electronic Processes in Zinc Oxide. Solid State Phys. 1959, 8, 191–323. [Google Scholar] [CrossRef]
- Campbell, C. Surface Acoustic Wave Devices and Their Signal Processing Application; Academic Press: San Diego, CA, USA, 1989. [Google Scholar]
- Hirshwald, W.; Bonasewicz, P.; Ernst, L.; Grade, M.; Hofmann, D.; Krebs, S.; Littbarski, R.; Neumann, G.; Grunze, M.; Kolb, D.; et al. Current Topics in Materials Science; Kaldis, E., Ed.; North-Holland: Amsterdam, The Netherlands, 1981; Volume 7, p. 148. [Google Scholar]
- Tsukazaki, A.; Ohtomo, A.; Onuma, T.; Ohtani, M.; Makino, T.; Sumiya, M.; Ohtani, K.; Chichibu, S.F.; Fuke, S.; Segawa, Y.; et al. Repeated temperature modulation epitaxy for p-type doping and light-emitting diode based on ZnO. Nat. Mater. 2005, 4, 42–46. [Google Scholar] [CrossRef]
- Joseph, M.; Tabata, H.; Kawai, T. p-Type Electrical Conduction in ZnO Thin Films by Ga and N Codoping. Jpn. J. Appl. Phys. 1999, 38, L1205. [Google Scholar] [CrossRef]
- Onodera, A.; Yoshio, K.; Satoh, H.; Yamashita, H.; Sakagami, N. Li-Substitution Effect and Ferroelectric Properties in Piezoelectric Semiconductor ZnO. Jpn. J. Appl. Phys. 1998, 37, 5315. [Google Scholar] [CrossRef]
- Onodera, A.; Tamaki, N.; Satoh, H.; Yamashita, H.; Sakai, A. Novel ferroelectricity in piezoelectric ZnO by Li-substitution. In Dielectric Ceramic Materials: Ceramic Transactions; Nair, K.M., Bhalla, A.S., Eds.; American Ceramic Society: Westerville, OH, USA, 1999; Volume 100, pp. 77–94. [Google Scholar]
- Islam, E.; Sakai, A.; Onodera, A. Optical Phonons in Ferroelectric-Semiconductor Zn 0.8Li 0.2O Single Crystal Studied by Micro-Raman Scattering. J. Phys. Soc. Jpn. 2001, 70, 576. [Google Scholar] [CrossRef]
- Kagami, D.; Takesada, M.; Onodera, A.; Satoh, H. Photoinduced Effect in Li-doped ZnO studied by Raman Scattering. J. Korean Phys. 2011, 59, 2532. [Google Scholar] [CrossRef]
- Ohtaka, O.; Fukui, H.; Kunisada, T.; Fujisawa, T.; Funakoshi, K.; Utsumi, W.; Irifune, T.; Kuroda, K.; Kikegawa, T. Phase relations and volume changes of hafnia under high pressure and high temperature. J. Am. Ceram. Soc. 2001, 84, 1369–1373. [Google Scholar] [CrossRef]
- Müller, J.; Schröder, U.; Böscke, T.S.; Müller, I.; Böttger, U.; Wilde, L.; Sundqvist, J.; Lemberger, M.; Kücher, P.; Mikolajick, T.; et al. Ferroelectricity in yttrium-doped hafnium oxide. J. Appl. Phys. 2011, 110, 114113. [Google Scholar] [CrossRef]
- Müller, S.; Müller, J.; Singh, A.; Riedel, S.; Sundqvist, J.; Schröder, U.; Mikolajick, T. Incipient Ferroelectricity in Al-Doped HfO2 Thin Films. Adv. Funct. Mater. 2012, 22, 2412–2417. [Google Scholar] [CrossRef]
- Müller, J.; Böscke, T.S.; Brauhaus, D.; Schröder, U.; Böttger, U.; Sundqvist, J.; Kücher, P.; Mikolajick, T.; Frey, L. Ferroelectric Zr0.5Hf0.5O2 thin films for nonvolatile memory applications. Appl. Phys. Lett. 2011, 99, 112901. [Google Scholar] [CrossRef]
- Müller, J.; Böscke, T.S.; Schröder, U.; Müller, S.; Brauhaus, D.; Böttger, U.; Frey, L.; Mikolajick, T. Ferroelectricity in Simple Binary ZrO2 and HfO2. Nano Lett. 2012, 12, 4318–4323. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Katayama, K.; Kiguchi, T.; Akama, A.; Konno, J.; Sakata, O.; Funakubo, H. The demonstration of significant ferroelectricity in epitaxial Y-doped HfO2 film. Sci. Rep. 2016, 6, 32931. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Katayama, K.; Kiguchi, T.; Akama, A.; Konno, T.J.; Hiroshi Funakubo, H. Growth of epitaxial orthorhombic YO1.5-substituted HfO2 thin film. Appl. Phys. Lett. 2015, 107, 032910. [Google Scholar] [CrossRef]
- Phillips, J.C. Bonds and Bands in Semiconductors; Academic Press: New York, NY, USA, 1973. [Google Scholar]
- Hagino, S.; Yoshio, K.; Yamazaki, T.; Satoh, H.; Matsuki, K.; Onodera, A. Electronic ferroelectricity in ZnO. Ferroelectrics 2001, 264, 235. [Google Scholar] [CrossRef]
- Grants, F.A. Properties of Rutile (Titanium Dioxide). Rev. Mod. Phys. 1959, 31, 646–674. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Bernstein, J.L. Rutile: Normal Probability Plot Analysis and Accurate Measurement of Crystal Structure. J. Chem. Phys. 1971, 55, 3206–3211. [Google Scholar] [CrossRef]
- Parker, R.A. Static Dielectric Constant of Rutile (TiO2), 1.6–1060°K. Phys. Rev 1961, 124, 1719. [Google Scholar] [CrossRef]
- Von Hippel, A.R. Dielectric Materials and Applications; John Wiley & Sons: New York, NY, USA, 1954. [Google Scholar]
- Gronschorek, W. X-ray charge density study of rutile (TiO2). Z. Kristallogr. 1982, 160, 187–203. [Google Scholar] [CrossRef]
- Sakata, M.; Uno, T.; Takata, M.; Mori, R. Electron Density in Rutile (TiO2) by the Maximum Entropy Method. Acta Crystallogr. 1992, B48, 591–598. [Google Scholar] [CrossRef]
- Montanari, B.; Harrison, N.M. Pressure-induced instabilities in bulk TiO2 rutile. J. Phys. Condens. Matter 2004, 16, 273. [Google Scholar] [CrossRef]
- Bousquet, E.; Spaldin, N.A.; Ghosez, P. Strain-Induced Ferroelectricity in Simple Rocksalt Binary Oxides. Phys. Rev. Lett. 2010, 104, 037601. [Google Scholar] [CrossRef] [PubMed]
- Glinchuk, M.D.; Khist, V.; Eliseev, E.A.; Morozovska, A.N. Ferroic properties of nanosized SnO2. Phase Transit. 2013, 86, 903–909. [Google Scholar] [CrossRef]

| Phase III Ferroelectric | Phase II Paraelectric | Phase I Liquid | |||
|---|---|---|---|---|---|
| HCl | Orthorhombic-Bb21m | Cubic-Fm3m | Liquid | ||
| Below 98 K | 98~159 K | Above 159 K | |||
| HBr | Orthorhombic-Bb21m | Phase IIc Orthorhombic-Bbcm | Phase IIb Cubic | Phase IIa Cubic-Fm3m | Liquid |
| Below 90 K | 90~114 K | 114~117 K | 117~186 K | Above 186 K | |
| Type of Ferroelectrics | C [K] | εmax at Tc | Ps [µC/cm2] | Example |
|---|---|---|---|---|
| Order-disorder | 1~3 × 103 | 103 | 3~5 | TGS § |
| Displacive | 105 | 104 | 10~30 | BaTiO3 |
| Improper | 10 | 10 | Small | ACS # |
| Electronic | - | 21 | 0.9 | ZnO |
| Materials | ε at R.T. | Fractional Degree of Ionicity |
|---|---|---|
| NaCl | 5.6 | 0.94 |
| MgO | 10.0 | 0.84 |
| ZnO | 8.8 | 0.62 |
| CdTe | 7.1 | 0.67 |
| HfO2 | 25 | 0.8 |
| ZrO2 | 30~46 | 0.8 |
| TiO2 | 170 | 0.6 |
| Si | 3.6 | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onodera, A.; Takesada, M. Ferroelectricity in Simple Binary Crystals. Crystals 2017, 7, 232. https://doi.org/10.3390/cryst7080232
Onodera A, Takesada M. Ferroelectricity in Simple Binary Crystals. Crystals. 2017; 7(8):232. https://doi.org/10.3390/cryst7080232
Chicago/Turabian StyleOnodera, Akira, and Masaki Takesada. 2017. "Ferroelectricity in Simple Binary Crystals" Crystals 7, no. 8: 232. https://doi.org/10.3390/cryst7080232
APA StyleOnodera, A., & Takesada, M. (2017). Ferroelectricity in Simple Binary Crystals. Crystals, 7(8), 232. https://doi.org/10.3390/cryst7080232




