Mesocrystals: Past, Presence, Future
Abstract
:1. Introduction and Brief Historical Remarks
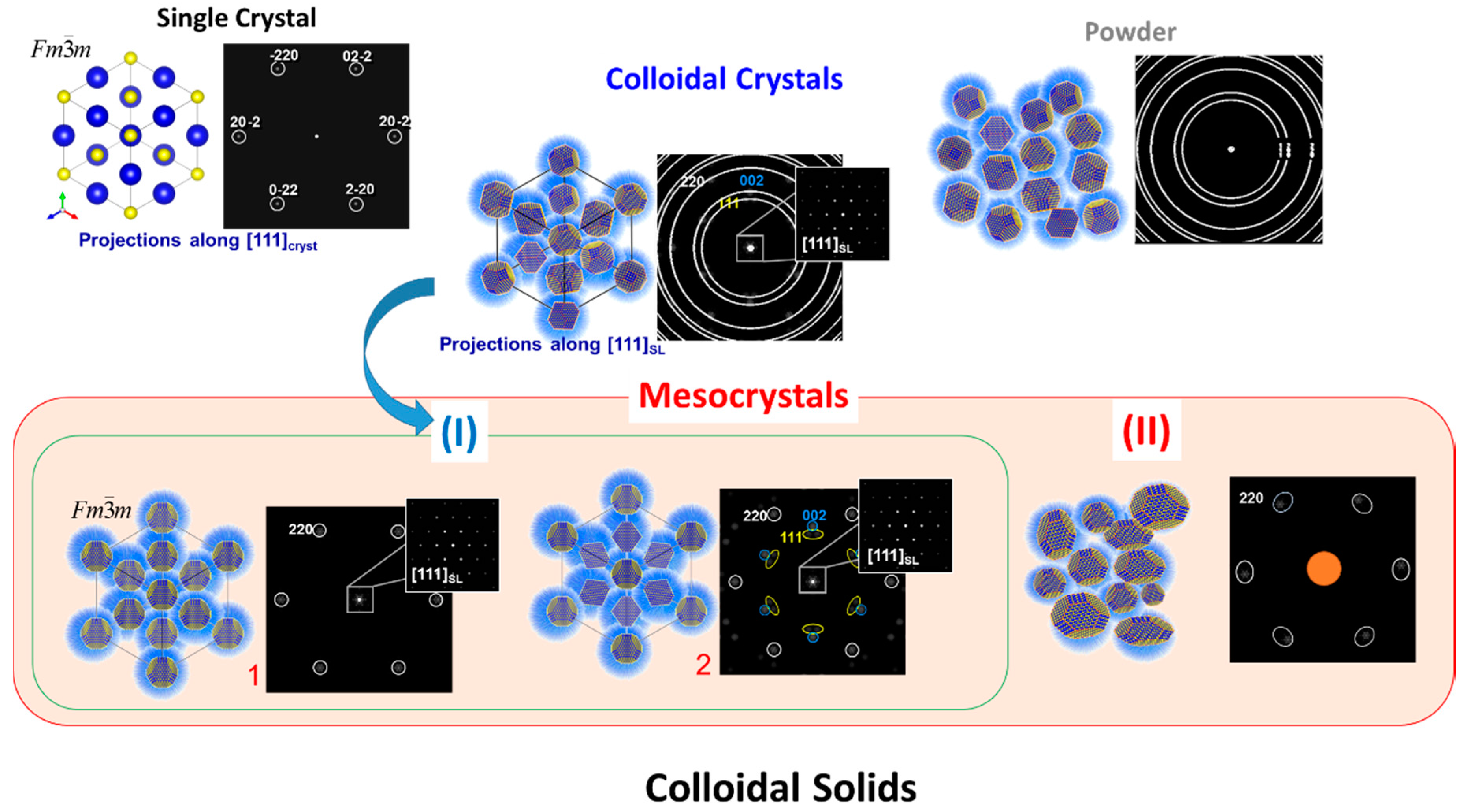
2. Current Status: What Is Known about Mesocrystals?
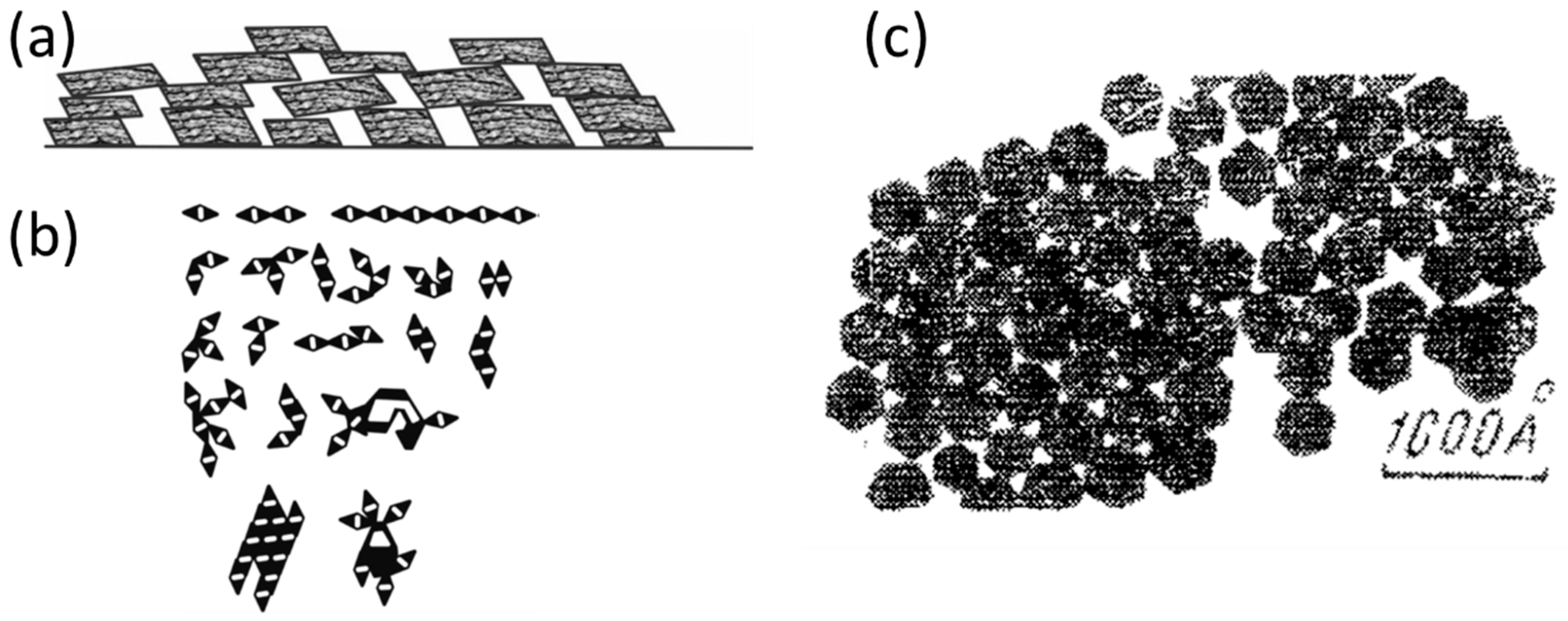
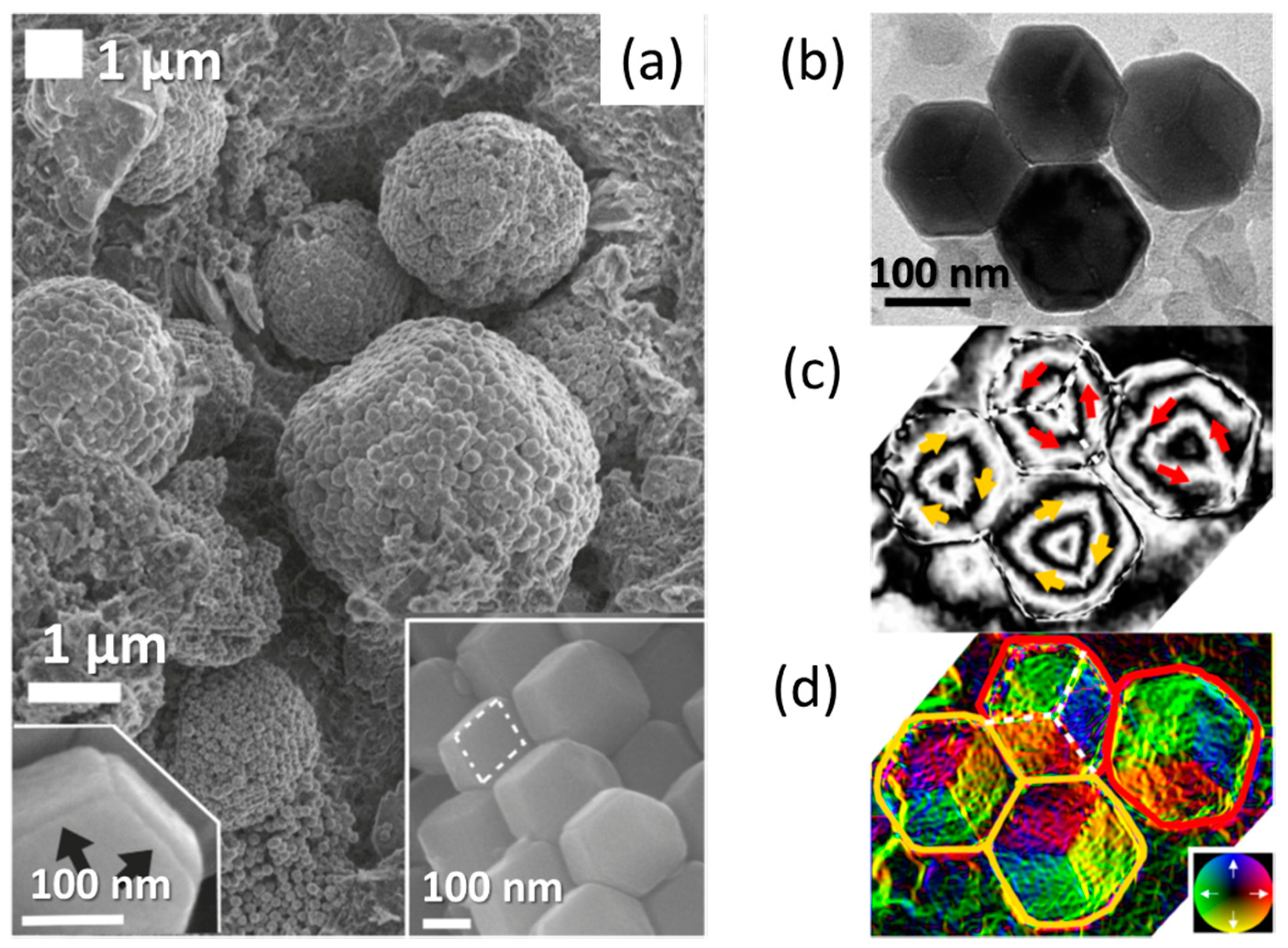
2.1. Structural Principles
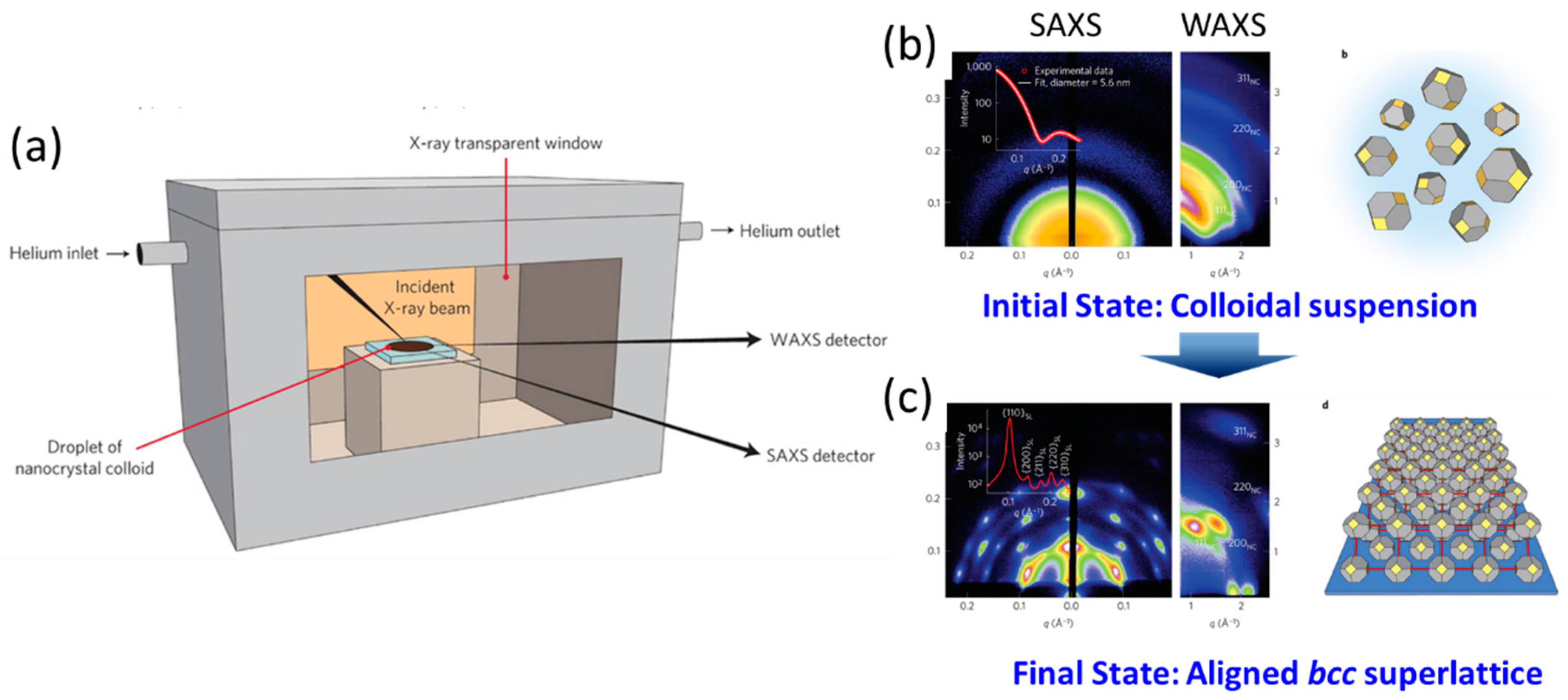
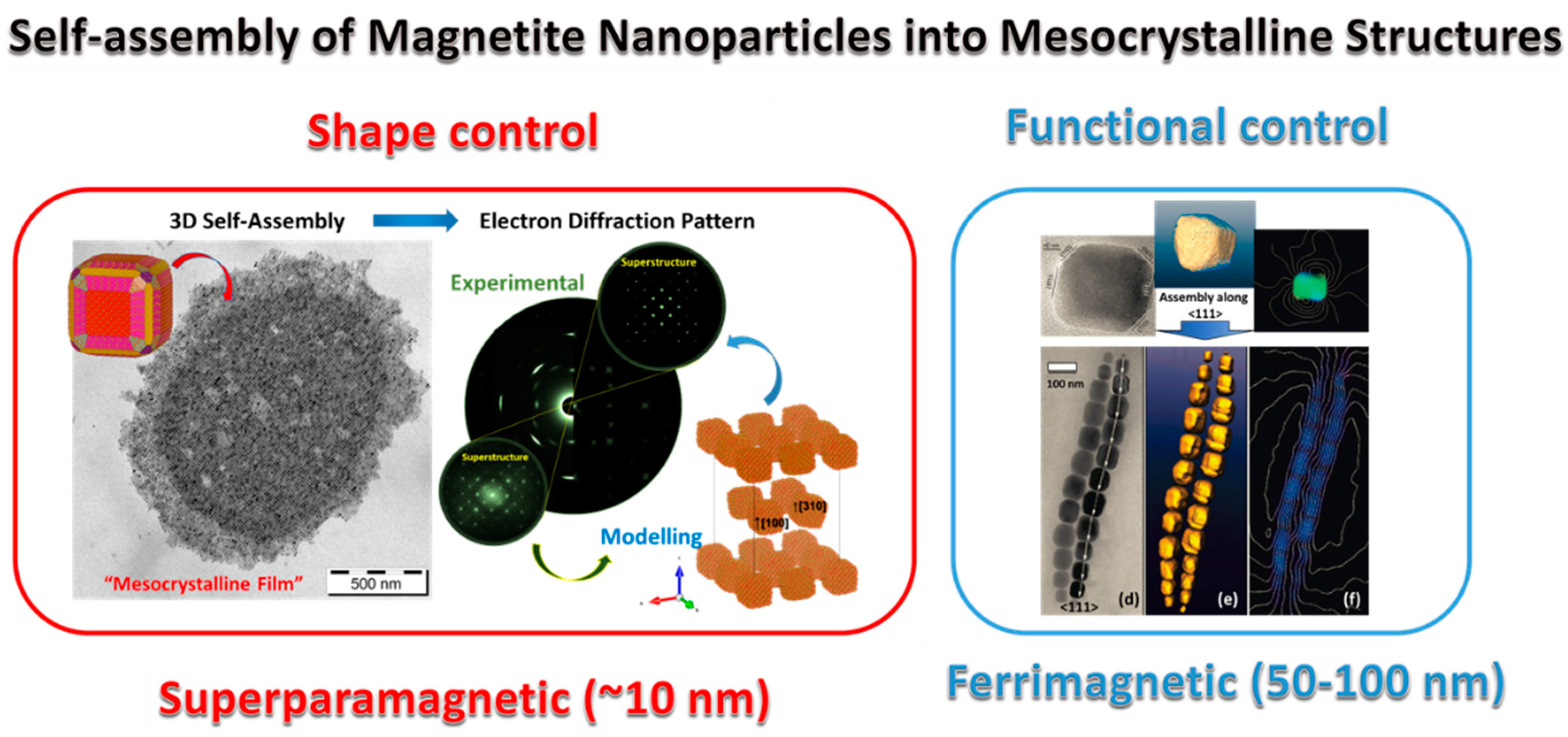
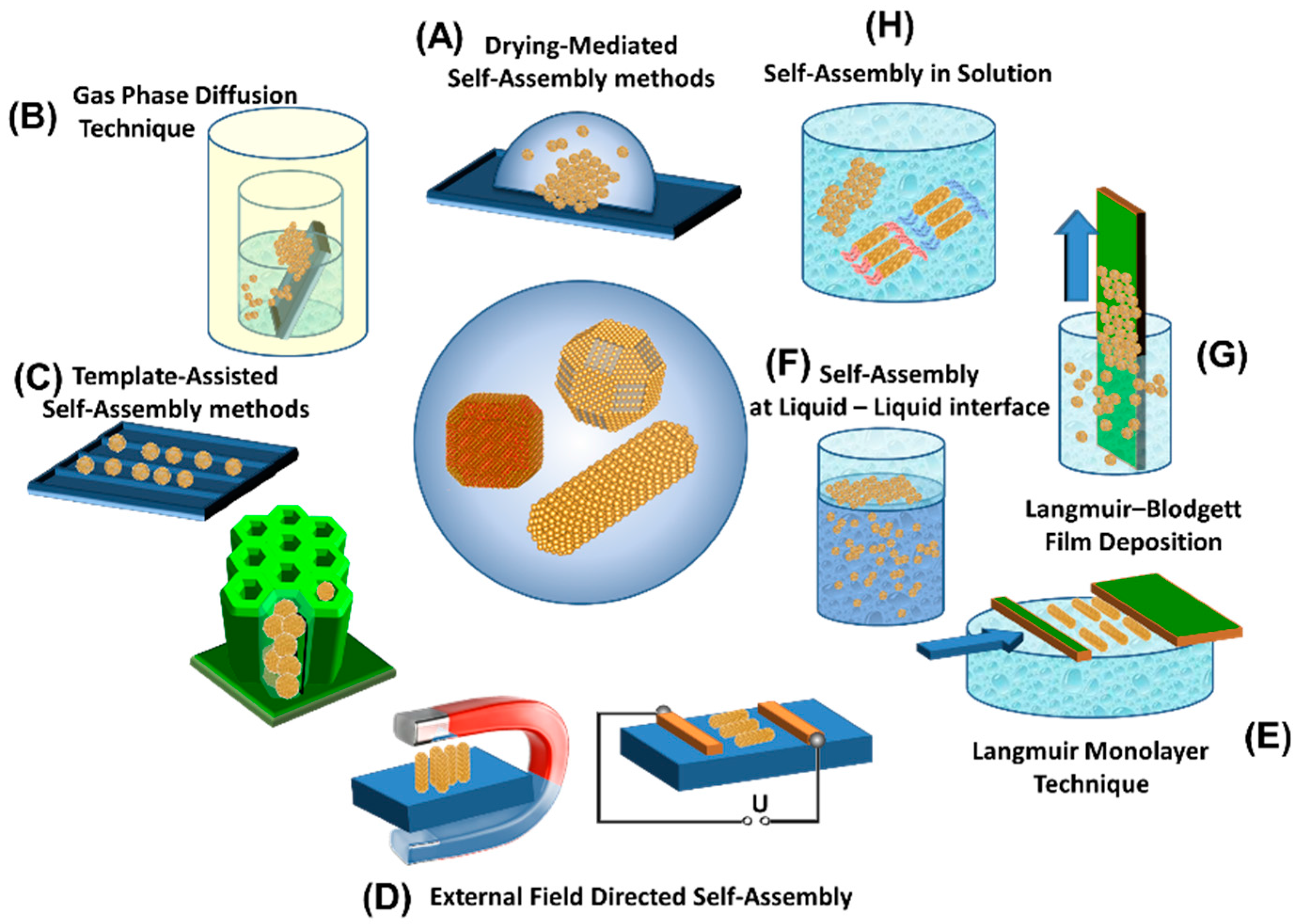
2.2. Formation Principles and Fabrication Routes
3. Future Outlook in Mesocrystal Research and Developments
Acknowledgments
Conflicts of Interest
References
- Cölfen, H.; Mann, S. Higher-order organization by mesoscale self-assembly and transformation of hybrid nanostructures. Angew. Chem. Int. Edit. 2003, 42, 2350–2365. [Google Scholar] [CrossRef] [PubMed]
- Cölfen, H.; Antonietti, M. Mesocrystals: Inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew. Chem. Int. Edit. 2005, 44, 5576–5591. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, M.; Cölfen, H. Oriented attachment and mesocrystals: Non-classical crystallization mechanisms based on nanoparticle assembly. Phys. Chem. Chem. Phys. 2006, 8, 3271–3287. [Google Scholar] [CrossRef] [PubMed]
- Cölfen, H.; Antonietti, M. Mesocrystals and Nonclassical Crystallization; John Wiley & Sons: Chichester, UK, 2008. [Google Scholar]
- Canaveras, J.C.; SanchezMoral, S.; Calvo, J.P.; Hoyos, M.; Ordonez, S. Dedolomites associated with karstification. An example of early dedolomitization in lacustrine sequences from the tertiary madrid basin, central spain. Carbonates Evaporites 1996, 11, 85–103. [Google Scholar] [CrossRef]
- Depmeier, W. Mesocrystallography. Ann. Chim. Sci. Mat. 1998, 23, 43–48. [Google Scholar] [CrossRef]
- Hartley, A.J.; May, G. Miocene gypcretes from the calama basin, northern chile. Sedimentology 1998, 45, 351–364. [Google Scholar] [CrossRef]
- Ko, C.H.; Kim, J.M.; Ryoo, R. Mesocrystal engineering using non-bonded interaction to obtain optically transparent mesoporous silica films and plates with uniform orientation. Microporous Mesoporous Mat. 1998, 21, 235–243. [Google Scholar] [CrossRef]
- Yamada, K.; Kohiki, S. Dielectric and optical properties of batio3 mesocrystals. Physica E 1999, 4, 228–230. [Google Scholar] [CrossRef]
- Schaskolsky, M.; Schubnikow, A. On the synthesis of regular crystal intergrowths of potassium alum. Zeitschrift Fur Kristallographie 1933, 85, 1–16. [Google Scholar]
- Chernov, A.A. Modern Crystallography: Crystal Growth, 1st ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 1984; Volume 3. [Google Scholar]
- Ivanov, V.K.; Fedorov, P.P.; Baranchikov, A.Y.; Osiko, V.V. Oriented attachment of particles: 100 Years of investigations of non-classical crystal growth. Russ. Chem. Rev. 2014, 83, 1204–1222. [Google Scholar] [CrossRef]
- Shubnikov, A.V. Kak rastut kristally; Academy of Sciences of the USSR: Moscow, Russia, 1935. [Google Scholar]
- Yishkin, N.P. Teoriya Mikroblochnogo Rosta Kristallov v Priridnykh Geotermicheskikh Rastvorakh; Institute of Geology, Komi Branch of Academy of Science of the USSR: Syktyvkar, Russia, 1971. [Google Scholar]
- Melikhov, I.V.; Mikheeva, I.E.; Rudin, V.N. Directional aggregation in highly dispersed suspensions. Colloid J. USSR 1988, 50, 755–760. [Google Scholar]
- Melikhov, I.V.; Mikheeva, I.E.; Rudin, V.N. On the possibility of block crystal-growth of calcium-sulfate semihydrate from high supersaturation solutions. Kristallografiya 1989, 34, 1272–1278. [Google Scholar]
- Kuleshova, O.V. Elementarnye Akty Kristallizacii Polugidrata Sulfata Kalciya iz Rastvorov; Lomonosov Moscow State University, Rotoprint PKB CT MPS: Moscow, Russia, 1991; p. 128. [Google Scholar]
- Vainshtein, B.K. Modern crystallography. Vol. 1. Fundamentals of crystals. Symmetry, and methods of structural crystallography. Acta Cryst. 1995, 51, 234–235. [Google Scholar]
- Melikhov, I.V.; Kitova, E.N.; Kamenskaya, A.N.; Kozlovskaya, E.D.; Mikheev, N.B.; Kulyukhin, S.A. Monocrystallomimicry in the aerosols of ammonium and cesium halogenides. Kolloidnyj Zhurnal 1997, 59, 723–728. [Google Scholar]
- Uyeda, N. Substructures of Colloidal Silver Particles; Institute for Chemical Research, Kyoto University: Kyoto, Japan, 1969; Volume 47, pp. 426–436. [Google Scholar]
- Efremov, I.F. Periodicheskie Kolloidnye Struktury; Khimia: Leninigrad, Russia, 1971; p. 192. [Google Scholar]
- Quan, Z.; Fang, J. Superlattices with non-spherical building blocks. Nano Today 2010, 5, 390–411. [Google Scholar] [CrossRef]
- Vanmaekelbergh, D. Self-assembly of colloidal nanocrystals as route to novel classes of nanostructured materials. Nano Today 2011, 6, 419–437. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Manna, L.; Cabot, A.; Hens, Z.; Talapin, D.V.; Kagan, C.R.; Klimov, V.I.; Rogach, A.L.; Reiss, P.; Milliron, D.J.; et al. Prospects of nanoscience with nanocrystals. ACS Nano 2015, 9, 1012–1057. [Google Scholar] [CrossRef] [PubMed]
- Boles, M.A.; Engel, M.; Talapin, D.V. Self-assembly of colloidal nanocrystals: From intricate structures to functional materials. Chem. Rev. 2016, 116, 11220–11289. [Google Scholar] [CrossRef] [PubMed]
- Blüh, O. Einige bei der Untersuchung von Kolloiden im Wechselfeld auftretende Erscheinungen. Kolloid-Zeitschrift 1925, 37, 267–270. [Google Scholar] [CrossRef]
- Zocher, H. On independent structure formation in brine. Z. Anorg. Allg. Chem. 1925, 147, 91–110. [Google Scholar] [CrossRef]
- Zocher, H.; Heller, W. Schiller layers as reaction products of slow iron chloride-hydrolysis. Z. Anorg. Allg. Chem. 1929, 186, 75–96. [Google Scholar] [CrossRef]
- Heller, W.; Zocher, H. Cross-line magneto-optical anisotropy of some colloidal solutions. II. Iron oxide. (a summarising overview.). Zeitschrift für Physikalische Chemie-Abteilung a-Chemische Thermodynamik Kinetik Elektrochemie Eigenschaftslehre 1933, 166, 365–381. [Google Scholar]
- Heller, W. Physical chemistry—Distances between the colloidal particles in the bright layers of certain iron oxide soils. Comptes Rendus Hebdomadaires Des Seances De L Academie Des Sciences 1935, 201, 831–833. [Google Scholar]
- Watson, J.H.L.; Heller, W.; Cardell, R.R. Internal structure of colloidal crystals of beta-feooh and remarks on their assemblies in schiller layers. J. Phys. Chem. 1962, 66, 1757–1763. [Google Scholar] [CrossRef]
- Distler, G.I.; Borisova, N.M. Elektronno-mikroskopicheskie issledovaniya aktivnyh zentrov poverhnostey tverdyh tel. Kinetika i Kataliz 1967, 8, 944. [Google Scholar]
- Bergstrom, L.; Sturm, E.V.; Salazar-Alvarez, G.; Cölfen, H. Mesocrystals in biominerals and colloidal arrays. Acc. Chem. Res. 2015, 48, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Sturm, E.V.; Cölfen, H. Mesocrystals: Structural and morphogenetic aspects. Chem. Soc. Rev. 2016, 45, 5821–5833. [Google Scholar] [CrossRef] [PubMed]
- Seto, J.; Ma, Y.R.; Davis, S.A.; Meldrum, F.; Gourrier, A.; Kim, Y.Y.; Schilde, U.; Sztucki, M.; Burghammer, M.; Maltsev, S.; et al. Structure-property relationships of a biological mesocrystal in the adult sea urchin spine. Proc. Natl. Acad. Sci. USA 2012, 109, 3699–3704. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Xu, Z.H.; Wang, R.Z. In situ observation of nanograin rotation and deformation in nacre. Nano Lett. 2006, 6, 2301–2304. [Google Scholar] [CrossRef] [PubMed]
- Perrin, J.; Vielzeuf, D.; Ricolleau, A.; Dallaporta, H.; Valton, S.; Floquet, N. Block-by-block and layer-by-layer growth modes in coral skeletons. Am. Miner. 2015, 100, 681–695. [Google Scholar] [CrossRef]
- Vielzeuf, D.; Floquet, N.; Chatain, D.; Bonneté, F.; Ferry, D.; Garrabou, J.; Stolper, E.M. Multilevel modular mesocrystalline organization in red coral. Am. Miner. 2010, 95, 242–248. [Google Scholar] [CrossRef]
- Vielzeuf, D.; Garrabou, J.; Baronnet, A.; Grauby, O.; Marschal, C. Nano to macroscale biomineral architecture of red coral. Am. Miner. 2008, 93, 1799–1815. [Google Scholar] [CrossRef]
- Floquet, N.; Vielzeuf, D. Ordered misorientations and preferential directions of growth in mesocrystalline red coral sclerites. Cryst. Growth Des. 2012, 12, 4805–4820. [Google Scholar] [CrossRef]
- Oaki, Y.; Kotachi, A.; Miura, T.; Imai, H. Bridged nanocrystals in biominerals and their biomimetics: Classical yet modern crystal growth on the nanoscale. Adv. Funct. Mater. 2006, 16, 1633–1639. [Google Scholar] [CrossRef]
- Cai, J.G.; Qi, L.M. Tio2 mesocrystals: Synthesis, formation mechanisms and applications. Sci. China Chem. 2012, 55, 2318–2326. [Google Scholar] [CrossRef]
- Ma, M.G.; Cölfen, H. Mesocrystals–applications and potential. Curr. Opin. Colloid Interface Sci. 2014, 19, 56–65. [Google Scholar] [CrossRef]
- Tachikawa, T.; Majima, T. Metal oxide mesocrystals with tailored structures and properties for energy conversion and storage applications. NPG Asia Mate. 2014, 6, e100. [Google Scholar] [CrossRef]
- Uchaker, E.; Cao, G.Z. Mesocrystals as electrode materials for lithium-ion batteries. Nano Today 2014, 9, 499–524. [Google Scholar] [CrossRef]
- Haberkorn, H.; Franke, D.; Frechen, T.; Goesele, W.; Rieger, J. Early stages of particle formation in precipitation reactions—Quinacridone and boehmite as generic examples. J. Colloid Interface Sci. 2003, 259, 112–126. [Google Scholar] [CrossRef]
- Pontoni, D.; Bolze, J.; Dingenouts, N.; Narayanan, T.; Ballauff, M. Crystallization of calcium carbonate observed in-situ by combined small- and wide-angle x-ray scattering. J. Phys. Chem. B 2003, 107, 5123–5125. [Google Scholar] [CrossRef]
- Thomas, J.M.; Simpson, E.T.; Kasama, T.; Dunin-Borkowski, R.E. Electron holography for the study of magnetic nanomaterials. Acc. Chem. Res. 2008, 41, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Sato, T.; Nakamura, N.; Nozawa, J.; Nakamura, T.; Tsukamoto, K.; Yamamoto, K. Vortex magnetic structure in framboidal magnetite reveals existence of water droplets in an ancient asteroid. Nat. Commun. 2013, 4, 2649. [Google Scholar] [CrossRef] [PubMed]
- Brunner, J.; Baburin, I.A.; Sturm, S.; Kvashnina, K.; Rossberg, A.; Pietsch, T.; Andreev, S.; Sturm, E.; Cölfen, H. Self-assembled magnetite mesocrystalline films: Toward structural evolution from 2D to 3D superlattices. Adv. Mater. Interfaces 2017, 4, 1600431. [Google Scholar] [CrossRef]
- Kniep, R.; Simon, P.; Rosseeva, E. Structural complexity of hexagonal prismatic crystal specimens of fluorapatite-gelatine nanocomposites: A case study in biomimetic crystal research. Cryst. Res. Technol. 2014, 49, 4–13. [Google Scholar] [CrossRef]
- Kniep, R.; Simon, P. Fluorapatite-gelatine-nanocomposites: Self-organized morphogenesis, real structure and relations to natural hard materials. In Biomineralization I: Crystallization and Self-Organization Process; Naka, K., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2007; Volume 270, pp. 73–125. [Google Scholar]
- Natalio, F.; Corrales, T.P.; Panthoefer, M.; Schollmeyer, D.; Lieberwirth, I.; Mueller, W.E.G.; Kappl, M.; Butt, H.-J.; Tremel, W. Flexible minerals: Self-assembled calcite spicules with extreme bending strength. Science 2013, 339, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; O’Brien, P. Mesocrystals: A new class of solid materials. Small 2008, 4, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Song, R.Q.; Cölfen, H. Mesocrystals-ordered nanoparticle superstructures. Adv. Mater. 2010, 22, 1301–1330. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.X.; Ding, B.J.; Gleiter, H. Mesocrystals: Syntheses in metals and applications. Chem. Soc. Rev. 2011, 40, 5347–5360. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; O’Brien, P. Mesocrystals—Properties and applications. J. Phys. Chem. Lett. 2012, 3, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Cölfen, H. Polymer-mediated growth of crystals and mesocrystals. In Research Methods in Biomineralization Science, 1st ed.; Yoreo, J.J.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 532, pp. 277–304. [Google Scholar]
- Bahrig, L.; Hickey, S.G.; Eychmuller, A. Mesocrystalline materials and the involvement of oriented attachment—A review. CrystEngComm 2014, 16, 9408–9424. [Google Scholar] [CrossRef]
- Bu, F.X.; Du, C.J.; Jiang, J.S. Synthesis, properties and applications of mesocrystals. Prog. Chem. 2014, 26, 75–86. [Google Scholar]
- Simon, P.; Bahrig, L.; Baburin, I.A.; Formanek, P.; Roeder, F.; Sickmann, J.; Hickey, S.G.; Eychmueller, A.; Lichte, H.; Kniep, R.; et al. Interconnection of nanoparticles within 2D superlattices of PbS/oleic acid thin films. Adv. Mater. 2014, 26, 3042–3049. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Rosseeva, E.; Baburin, I.A.; Liebscher, L.; Hickey, S.G.; Cardoso-Gil, R.; Eychmüller, A.; Kniep, R.; Carrillo-Cabrera, W. PbS–organic mesocrystals: The relationship between nanocrystal orientation and superlattice array. Angew. Chem. Int. Ed. 2012, 51, 10776–10781. [Google Scholar] [CrossRef] [PubMed]
- De Yoreo, J.J.; Gilbert, P.; Sommerdijk, N.; Penn, R.L.; Whitelam, S.; Joester, D.; Zhang, H.Z.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F.; et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349, aaa6760. [Google Scholar] [CrossRef] [PubMed]
- Li, D.S.; Nielsen, M.H.; Lee, J.R.I.; Frandsen, C.; Banfield, J.F.; De Yoreo, J.J. Direction-specific interactions control crystal growth by oriented attachment. Science 2012, 336, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- De Yoreo, J.J. In-situ liquid phase tem observations of nucleation and growth processes. Prog. Cryst. Growth Charact. Mater. 2016, 62, 69–88. [Google Scholar] [CrossRef]
- Li, R.; Bian, K.; Hanrath, T.; Bassett, W.A.; Wang, Z. Decoding the superlattice and interface structure of truncate PbS nanocrystal-assembled supercrystal and associated interaction forces. J. Am. Chem. Soc. 2014, 136, 12047–12055. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Bian, K.; Wang, Y.; Xu, H.; Hollingsworth, J.A.; Hanrath, T.; Fang, J.; Wang, Z. An obtuse rhombohedral superlattice assembled by Pt nanocubes. Nano Lett. 2015, 15, 6254–6260. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, J.; Tan, R.; Gerdes, F.; Luo, Z.; Xu, H.; Hollingsworth, J.A.; Klinke, C.; Chen, O.; Wang, Z. Competing interactions between various entropic forces toward assembly of Pt3Ni octahedra into a body-centered cubic superlattice. Nano Lett. 2016, 16, 2792–2799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, J.L.; Li, R.P.; Fang, J.Y.; Wang, Z.W. Entropy-driven Pt3Co nanocube assembles and thermally mediated electrical conductivity with anisotropic variation of the rhombohedral superlattice. Nano Lett. 2017, 17, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Agthe, M.; Hoydalsvik, K.; Mayence, A.; Karvinen, P.; Liebi, M.; Bergstrom, L.; Nygard, K. Controlling orientational and translational order of iron oxide nanocubes by assembly in nanofluidic containers. Langmuir 2015, 31, 12537–12543. [Google Scholar] [CrossRef] [PubMed]
- Agthe, M.; Plivelic, T.S.; Labrador, A.; Bergström, L.; Salazar-Alvarez, G. Following in real time the two-step assembly of nanoparticles into mesocrystals in levitating drops. Nano Lett. 2016, 16, 6838–6843. [Google Scholar] [CrossRef] [PubMed]
- Agthe, M.; Wetterskog, E.; Mouzon, J.; Salazar-Alvarez, G.; Bergstrom, L. Dynamic growth modes of ordered arrays and mesocrystals during drop-casting of iron oxide nanocubes. Crystengcomm 2014, 16, 1443–1450. [Google Scholar] [CrossRef]
- Disch, S.; Wetterskog, E.; Hermann, R.P.; Salazar-Alvarez, G.; Busch, P.; Brueckel, T.; Bergstroem, L.; Kamali, S. Shape induced symmetry in self-assembled mesocrystals of iron oxide nanocubes. Nano Lett. 2011, 11, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Faure, B.; Wetterskog, E.; Gunnarsson, K.; Josten, E.; Hermann, R.P.; Brueckel, T.; Andreasen, J.W.; Meneau, F.; Meyer, M.; Lyubartsev, A.; et al. 2D to 3D crossover of the magnetic properties in ordered arrays of iron oxide nanocrystals. Nanoscale 2013, 5, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Wetterskog, E.; Klapper, A.; Disch, S.; Josten, E.; Hermann, R.P.; Rucker, U.; Bruckel, T.; Bergstrom, L.; Salazar-Alvarez, G. Tuning the structure and habit of iron oxide mesocrystals. Nanoscale 2016, 8, 15571–15580. [Google Scholar] [CrossRef] [PubMed]
- Weidman, M.C.; Smilgies, D.M.; Tisdale, W.A. Kinetics of the self-assembly of nanocrystal superlattices measured by real-time in situ x-ray scattering. Nat. Mater. 2016, 15, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Dunin-Borkowski, R.E.; McCartney, M.R.; Frankel, R.B.; Bazylinski, D.A.; Posfai, M.; Buseck, P.R. Magnetic microstructure of magnetotactic bacteria by electron holography. Science 1998, 282, 1868–1870. [Google Scholar] [CrossRef] [PubMed]
- Ohfuji, H.; Boyle, A.P.; Prior, D.J.; Rickard, D. Structure of framboidal pyrite: An electron backscatter diffraction study. Am. Miner. 2005, 90, 1693–1704. [Google Scholar] [CrossRef]
- Ohfuji, H.; Rickard, D. Experimental syntheses of framboids—A review. Earth Sci. Rev. 2005, 71, 147–170. [Google Scholar] [CrossRef]
- McNaught, A.D.; McNaught, A.D. Compendium of Chemical Terminology, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1997; Volume 1669. [Google Scholar]
- Zhou, L.; Boyle, D.S.; O’Brien, P. Uniform NH4TiOF3 mesocrystals prepared by an ambient temperature self-assembly process and their topotaxial conversion to anatase. Chem. Commun. 2007, 2, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Smyth-Boyle, D.; O'Brien, P. A facile synthesis of uniform nh4tiof3 mesocrystals and their conversion to TiO2 mesocrystals. J. Am. Chem. Soc. 2008, 130, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Dang, F.; Hoshino, T.; Oaki, Y.; Hosono, E.; Zhou, H.; Imai, H. Synthesis of Li-Mn-O mesocrystals with controlled crystal phases through topotactic transformation of MnCO3. Nanoscale 2013, 5, 2352–2357. [Google Scholar] [CrossRef] [PubMed]
- Imai, H. Mesostructured crystals: Growth processes and features. Prog. Cryst. Growth Charact. Mater. 2016, 62, 212–226. [Google Scholar] [CrossRef]
- Nakajima, K.; Oaki, Y.; Imai, H. Syntheses of LiCoO2 mesocrystals by topotactic transformation and their electrochemical properties. ChemPlusChem 2013, 78, 1379–1383. [Google Scholar] [CrossRef]
- Zhang, P.; Ochi, T.; Fujitsuka, M.; Kobori, Y.; Majima, T.; Tachikawa, T. Topotactic epitaxy of SrTiO3 mesocrystal superstructures with anisotropic construction for efficient overall water splitting. Angew. Chem. Int. Edit. 2017, 56, 5299–5303. [Google Scholar] [CrossRef] [PubMed]
- Hanrath, T. Colloidal nanocrystal quantum dot assemblies as artificial solids. J. Vac. Sci. Technol. A 2012, 30, 030802. [Google Scholar] [CrossRef]
- Kato, K.; Mimura, K.; Dang, F.; Imai, H.; Wada, S.; Osada, M.; Haneda, H.; Kuwabara, M. BaTiO3 nanocube and assembly to ferroelectric supracrystals. J. Mater. Res. 2013, 28, 2932–2945. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Regulacio, M.D.; Han, M.Y. Self-assembly of colloidal one-dimensional nanocrystals. Chem. Soc. Rev. 2014, 43, 2301–2323. [Google Scholar] [CrossRef] [PubMed]
- Talapin, D.V.; Shevchenko, E.V. Introduction: Nanoparticle chemistry. Chem. Rev. 2016, 116, 10343–10345. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Zhang, C.W. Dissolution enhancement by bio-inspired mesocrystals: The study of racemic (R,S)-(+/−)-sodium ibuprofen dihydrate. Pharm. Res. 2008, 25, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Wohlrab, S.; Pinna, N.; Antonietti, M.; Cölfen, H. Polymer-induced alignment of DL-alanine nanocrystals to crystalline mesostructures. Chem. Eur. J. 2005, 11, 2903–2913. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Gong, H.F.; Grzywa, M.; Volkmer, D.; Gower, L.; Cölfen, H. Microdomain transformations in mosaic mesocrystal thin films. Adv. Funct. Mater. 2013, 23, 1547–1555. [Google Scholar] [CrossRef]
- Huang, M.; Schilde, U.; Kumke, M.; Antonietti, M.; Cölfen, H. Polymer-induced self-assembly of small organic molecules into ultralong microbelts with electronic conductivity. J. Am. Chem. Soc. 2010, 132, 3700–3707. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sturm, E.V.; Cölfen, H. Mesocrystals: Past, Presence, Future. Crystals 2017, 7, 207. https://doi.org/10.3390/cryst7070207
Sturm EV, Cölfen H. Mesocrystals: Past, Presence, Future. Crystals. 2017; 7(7):207. https://doi.org/10.3390/cryst7070207
Chicago/Turabian StyleSturm (née Rosseeva), Elena V., and Helmut Cölfen. 2017. "Mesocrystals: Past, Presence, Future" Crystals 7, no. 7: 207. https://doi.org/10.3390/cryst7070207
APA StyleSturm, E. V., & Cölfen, H. (2017). Mesocrystals: Past, Presence, Future. Crystals, 7(7), 207. https://doi.org/10.3390/cryst7070207






