Abstract
A new 1D chained Ca(II) coordination polymer—namely, [CaL2(H2O)2]n (HL = 3,5-bis(4-pyridylmethoxy)benzoic acid)—was synthesized though the reaction of Ca(ClO4)2·4H2O, 3,5-bis(4-pyridylmethoxy)benzoic acid and NaOH in H2O/CH3CH2OH (v:v = 1:2) solution. Its structure was determined by elemental analysis, infrared spectrum, and single-crystal XRD. Structural analyses show that each Ca(II) ion is eight-coordinated by six oxygen atoms of four 3,5-bis(4-pyridylmethoxy)benzoate ligands and two oxygen atoms of two coordinated H2O molecules to form a square-antiprismatic CaO8 polyhedron. The Ca(II) complex displays a 1D chained structure constructed by the bridging effect of the bidentate carboxyl group of 3,5-bis(4-pyridylmethoxy)benzoate ligand. The catalytic activity of the Ca-complex was tested for the preparation of propargylamine in the A3 coupling reaction.
1. Introduction
Coordination polymers (CPs) materials have attracted great interest during the past two decades [1,2,3,4,5,6] because they show intriguing structures and excellent properties in many aspects, such as gas adsorption [7], fluorescence [8], magnetic [9], catalysis [10,11], biological activity [12], and so on. In previous studies, N-heterocyclic and polycarboxylate ligands have often been used to build coordination polymer materials as multidentate ligands [13,14,15,16]. Recently, our group synthesized and structurally characterized some new coordination polymer materials constructed from Ca(II), Cd(II), Zn(II), and Mg(II) with multidentate ligands containing –COO− or –SO3− groups [17,18,19,20]. To continue our study on coordination polymer materials, in this paper, a new 1D chained Ca(II) coordination polymer—[CaL2(H2O)2]n—has been synthesized though the reaction of Ca(ClO4)2·4H2O, 3,5-bis(4-pyridylmethoxy)benzoic acid, and NaOH in H2O/CH3CH2OH (v:v = 1:2) solution. Its structure has been determined by EA, infrared spectrum, and single-crystal XRD. The catalytic activity of the Ca-complex was tested for the preparation of propargylamine in the A3 coupling reaction.
2. Results and Discussion
2.1. Structural Description of [CaL2(H2O)2]n
The X-ray single-crystal structural analysis revealed that [CaL2(H2O)2]n crystallizes in monoclinic C2/c space group, and that the Ca(II) ion is surrounded by six O atoms from four 3,5-bis(4-pyridylmethoxy)benzoate ligands and two O atoms from two coordinated H2O molecules (Figure 1). The Figure 1 displays that the eight-coordination Ca(II) ion forms a distorted square-antiprismatic CaO8 polyhedron. The 3,5-bis(4-pyridylmethoxy)benzoate ligand only uses its O atoms for coordinating, and its pyridyl nitrogens do not coordinate to Ca(II) ion, which is different from the Zn(II), Ni(II), and Cd(II) complex with 3,5-bis(4-pyridylmethoxy)benzoate ligand [21,22,23,24,25]. The pyridyl rings have dihedral angles of 24.0° (C9–C10–C11–N1–C12–C13) and 19.2° (C15–C16–C17–N2–C18–C19) with phenyl ring (C2–C3–C4–C5–C6–C7) in an L ligand. [CaL2(H2O)2]n forms a 2D layered structure by O–H···N hydrogen bonds (Figure 2). Meanwhile, a looped structure and 3D supramolecular network structure have formed by O–H···N hydrogen bonds (Figure 3 and Figure 4). The selected bond lengths and bond angles for [CaL2(H2O)2]n are listed in Table 1.
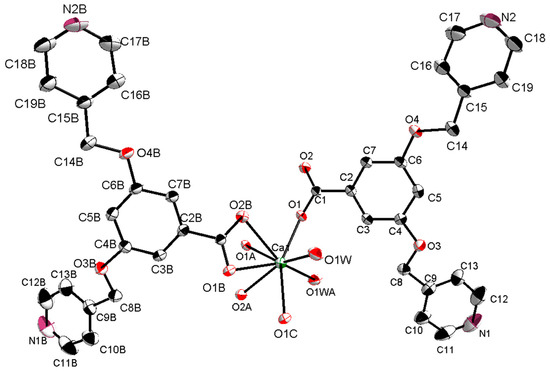
Figure 1.
The coordination environment of Ca(II) ion.
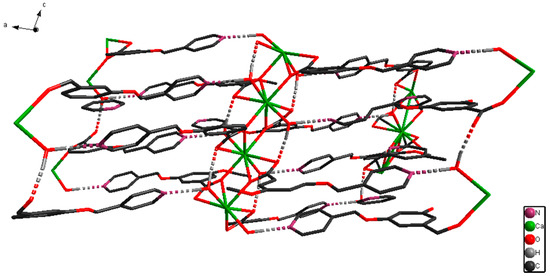
Figure 2.
2D layered structure of [CaL2(H2O)2]n.
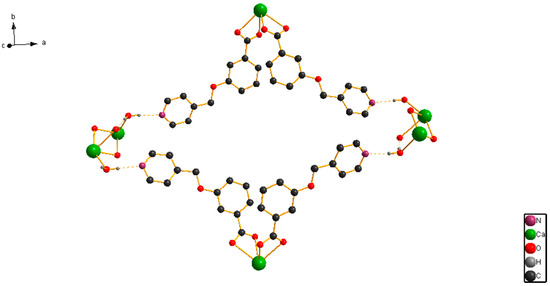
Figure 3.
The looped structure by hydrogen bonds in [CaL2(H2O)2]n.
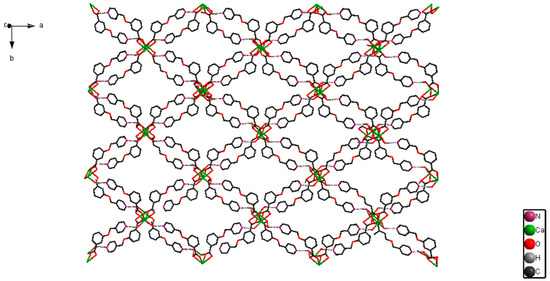
Figure 4.
3D supramolecular network structure of [CaL2(H2O)2]n.

Table 1.
Selected bond lengths d (Å) and bond angles (°) for [CaL2(H2O)2]n.
2.2. UV-Vis Spectra
The UV-Vis spectra of 3,5-bis(4-pyridylmethoxy)benzoic acid ligand and its Ca(II) coordination polymer are shown in Figure 5. The 3,5-bis(4-pyridylmethoxy)benzoic acid ligand displays one absorption peak at 302 nm (ε = 1.59 × 105 L·mol−1·cm−1), and the Ca(II) coordination polymer shows an absorption peak at 299 nm (ε = 1.99 × 105 L·mol−1·cm−1), respectively, which may be due to the π–π* transition of 3,5-bis(4-pyridylmethoxy)benzoate.
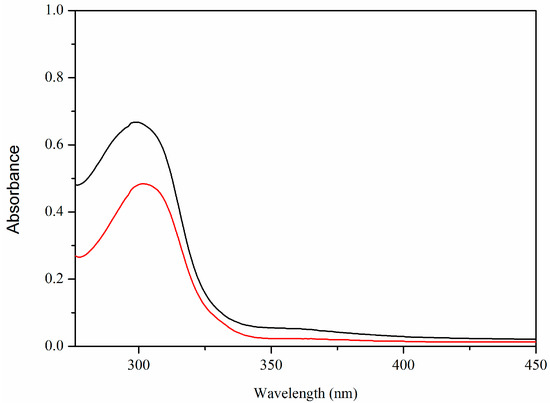
Figure 5.
The UV-Vis spectra of 3,5-bis(4-pyridylmethoxy)benzoic acid (HL) and [CaL2(H2O)2]n·HL ligand (red) and Ca(II) complex (black).
2.3. Catalytic Studies of Three Component Coupling Reaction
The catalytic activity of Ca-complex was tested for the preparation of propargylamine in the A3 coupling reaction according to the literature method [26]. The benzaldehyde conversion of 38.3% was obtained over Ca-complex for the coupling reaction of benzaldehyde, phenylacetylene, and piperidine with 1,4-dioxane as solvent at 120 °C for 12 h. Recovery and reusability of catalysts was an important theme in catalysis. This allows the catalysts to be used in many catalytic cycles, and thereby more commercial for industrial catalysis. The reusability of the Ca-complex was investigated in the A3 coupling reaction of benzaldehyde, phenylacetylene, and piperidine in 1,4-dioxane at 120 °C. The recovered catalyst worked well up to four catalytic runs. In four successive cycles, the conversion of benzaldehyde was 38.3%, 35.4%, 32.5%, and 29.8% at 120 °C for 12 h, respectively.
3. Experimental Section
3.1. Materials and Instrumentation
3,5-Bis(4-pyridylmethoxy)benzoic acid, Ca(ClO4)2·4H2O, and solvent used in this work were purchased from Jinan Henghua Chemical Reagent Company and used without purification. Element analyses (C, H, and N) were determined on an Elementar Vario III EL elemental analyzer (Hanau, Germany). IR spectra (KBr discs, range 4000 cm−1–400 cm−1) were recorded on a Nicolet AVATAR 360 Fourier transform infrared (FTIR) spectrophotometer (Nicolet Instrument Inc., Madison, WI, USA). UV-Vis spectra (190–700 nm) were recorded in CH3CH2OH on a TU-1901 spectrophotometer (Persee Instrument Inc., Beijing, China). Single crystal X-ray diffraction was carried out by a Bruker Smart CCD diffractometer (Bruker, Billerica, MA, USA).
3.2. Synthesis of [CaL2(H2O)2]n
A mixture of 3,5-bis(4-pyridylmethoxy)benzoic acid (0.2 mmol) and NaOH (0.2 mmol) was dissolved in 15 mL H2O/CH3CH2OH (v:v = 1:2). After 0.5 h, 0.1 mmol of solid Ca(ClO4)2·4H2O was added to the above solution. The reactant mixture was heated to 60 °C and kept for 5 h. Then, the reactant mixture was cooled and filtered. The block crystals were obtained by evaporation after 15 days at room temperature. Yield 38%. Analytical calculate for C38H34CaN4O10: C, 61.06; H, 4.55; N, 7.50. Found: C, 61.32; H, 4.87; N, 7.11. Most IR bands (Ca(II) complex): 3398 cm−1·(H2O), 1569 cm−1 (νas (COOH)), and 1415 cm−1 (νs (COOH)).
3.3. Crystal Structure Determination
The single crystal data of [CaL2(H2O)2]n were collected on a Bruker Smart APEX CCD diffractometer with graphite-monochromated MoKα radiation (λ = 0.71073 Å) at 293(2) K. The structure was solved by direct method and refined by full-matrix least squares on F2 using the SHELX-97 program (University of Göttingen, Göttingen, Germany) [27]. The crystallographic data and structural refinement are listed in Table 2.

Table 2.
Crystallographic data and structure refinement for [CaL2(H2O)2]n.
3.4. General Procedure for the Three Component Coupling Reaction (A3)
A mixture of catalyst (40 mg), aldehyde (0.13 mmol), amine (0.15 mmol), alkyne (0.17 mmol), and 1,4-dioxane (1.5 g) was stirred for 12 h at 120 °C. After completion of the reaction, the mixture was cooled to room temperature and the product was obtained by centrifugation. The catalyst was dried at 60 °C under vacuum for 3 h and stored in a desiccator for its use in subsequent catalytic runs. The conversion of aldehyde was determined by GC analysis (GC-1100, capillary column, SE-54).
4. Conclusions
In summary, we obtained a new [CaL2(H2O)2]n coordination polymer by the reaction of Ca(ClO4)2·4H2O, 3,5-bis(4-pyridylmethoxy)benzoic acid and NaOH in H2O/CH3CH2OH (v:v = 1:2) solution. Structural analyses show that the Ca(II) complex forms a 1D chained structure constructed by the bridging effect of bidentate carboxyl group of 3,5-bis(4-pyridylmethoxy)benzoate ligand. The catalytic activity of Ca-complex was tested for the preparation of propargylamine in the A3 coupling reaction.
Acknowledgments
This project was supported by Science Foundation of Weifang.
Author Contributions
Xi-Shi Tai designed the method and wrote the manuscript; Li-Hua Wang synthesized the Cd(II) coordination polymer; Xin Wang analyzed the crystal data of Cd(II) coordination polymer.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Crystallographic data for the structure reported in this paper has been deposited with the Cambridge Crystallographic Data Centre as supplementary publication No. CCDC 1524859. Copy of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (Fax: +44-1223-336-033; E-Mail: deposit@ccdc.cam.ac.uk).
References
- Yaghi, O.M. Reticular chemistry-construction, properties, and precision reactions of frameworks. J. Am. Chem. Soc. 2016, 138, 15507–15509. [Google Scholar] [CrossRef] [PubMed]
- Meundaeng, N.; Rujiwatra, A.; Prior, T.J. Copper coordination polymers constructed from thiazole-5-carboxylic acid: Synthesis, crystal structures, and structural transformation. J. Solid State Chem. 2017, 245, 138–145. [Google Scholar] [CrossRef]
- Chakraborty, P.; Mohanta, S. Syntheses, crystal structures, lone pair functionality and electrospray ionization mass spectral properties of trinuclear, dimer of trinuclear and trinuclear-based one-dimensional systems of copper(II) and lead(II). Inorg. Chim. Acta 2017, 455, 70–80. [Google Scholar] [CrossRef]
- Mukherjee, G.; Biradha, K. Topological Equivalences between Coordination Polymer and Cocrystal: A Tecton Approach in Crystal Engineering. Cryst. Growth Des. 2014, 14, 419–422. [Google Scholar] [CrossRef]
- Wang, X.P.; Han, L.L.; Lin, S.J.; Li, X.Y.; Mei, K.; Sun, D. Synthesis, structure and photoluminescence of three 2D Cd(II) coordination polymers based on varied dicarboxylate ligand. J. Coord. Chem. 2016, 69, 286–294. [Google Scholar] [CrossRef]
- Tai, X.S.; Wang, X. Synthesis and crystal structure of a 1D chained coordination polymers constructed from Ca2+ and 2-[(E)-(2-furoylhydrazono)methyl]benzenesulfonate. Crystals 2015, 5, 458–465. [Google Scholar] [CrossRef]
- Shen, J.J.; Li, M.X.; Wang, Z.X.; Duan, C.Y.; Zhu, S.R.; He, X.S. Unexpected 4-fold [2 + 2] interpenetration and polycatenation behaviors in porous luminescent zinc metal-organic frameworks constructed from flexible 3,5-bis(4-pyridylmethoxy) benzoate ligand. Cryst. Growth Des. 2014, 14, 2818–2830. [Google Scholar] [CrossRef]
- Li, T.; Huang, X.H.; Zhao, Y.F.; Li, H.H.; Wu, S.T.; Huang, C.C. An unusual double T5(2) water tape trapped in silver(I) coordination polymer hosts: Influence of the solvent on the assembly of Ag(I)-4,4′-bipyridine chains with trans-cyclohexanedicarboxylate and their luminescent properties. Dalton Trans. 2012, 41, 12872–12881. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.Y.; Yang, D.D.; Li, N.; Huang, R.D. Ligands effect on the structures of a series of coordination polymers: Syntheses, structures, luminescence and magnetism. Inorg. Chim. Acta 2015, 427, 285–292. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Ashouri, F.; Đaković, M. Synthesis, structural characterization and application of a 2D coordination polymer of Mn-terephthalate as a heterogeneous catalyst for olefin oxidation. Polyhedron 2014, 69, 167–173. [Google Scholar] [CrossRef]
- Yan, K.K.; Fujita, M. A speedy marriage in supramolecular catalysis. Science 2015, 350, 1165–1166. [Google Scholar] [CrossRef] [PubMed]
- Tai, X.S.; Zhao, W.H. Synthesis, crystal structure and antitumor activity of Ca(II) coordination polymer based on 1,5-naphthalenedisulfonate. J. Inorg. Organomet. Polym. Mater. 2013, 23, 1354–1357. [Google Scholar] [CrossRef]
- Liu, C.B.; Li, Q.; Wang, X.; Che, G.B.; Zhang, X.J. A series of lanthanide(III) coordination polymers derived via in situ hydrothermal decarboxylation of quinoline-2,3-dicarboxylic acid. Inorg. Chem. Commun. 2014, 39, 56–60. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, T.; Zhang, L.; Li, C.C. Structures and properties of coordination polymers based on 5-nitroisophthalic acid and N,N′-bis(4-pyridyl-methyl) piperazine. Z. Anorg. Allg. Chem. 2014, 640, 2503–2507. [Google Scholar] [CrossRef]
- Sharif, S.; Şahin, O.; Khan, B.; Khan, I.U. Hydrothermal synthesis, structural investigation, and magnetic properties of 2-D layered lanthanide (Ln = Pr, Eu, Gd, Tb, and Er) coordination polymers possessing infinite 1-D nanosized cavities. J. Coord. Chem. 2015, 68, 2725–2738. [Google Scholar] [CrossRef]
- Tai, X.S.; Wang, X. Synthesis, characterization and antitumor activity of a Ca(II) coordination polymer based on 3-amino-2-pyrazinecarboxylic acid. Sci. Stud. Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2015, 16, 253–259. [Google Scholar]
- Tai, X.S.; Zhao, W.H. Synthesis, crystal structure, and antibacterial activity of magnesium(II) coordination polymers formed by hydrogen bonding. Res. Chem. Intermed. 2015, 41, 3471–3478. [Google Scholar] [CrossRef]
- Tai, X.S.; Zhao, W.H. Synthesis, structural characterization, and antitumor activity of a Ca(II) coordination polymer based on 1,6-naphthalenedisulfonate and 4,4′-bipyridyl. Materials 2013, 6, 3547–3555. [Google Scholar] [CrossRef]
- Tai, X.S.; Jiang, J.H. Synthesis, crystal structure and luminescent property of Cd(II) complex with N-benzenesulphonyl-l-leucine. Materials 2012, 5, 1626–1634. [Google Scholar] [CrossRef]
- Tai, X.S.; Wang, X.; You, H.Y. Synthesis, crystal structure and antitumor activity of a new Zn(II) complex based on N-acetyl-l-phenylalanine and 1,10-phenanthroline. Chin. J. Struct. Chem. 2016, 35, 586–590. [Google Scholar]
- Ashiry, K.O.; Zhao, Y.H.; Shao, K.Z.; Su, Z.M.; Xu, G.J. Syntheses and characterizations of three coordination polymers based on dipyridylbenzoates and 1,4-bezenedicarboxylate. Polyhedron 2009, 28, 975–979. [Google Scholar] [CrossRef]
- Xu, G.J.; Zhao, Y.H.; Shao, K.Z.; Lan, Y.Q.; Wang, X.L.; Su, Z.M.; Yan, L.K. Secondary ligand-directed assembly of ZnII and CdII coordination architectures: From 1D to 3D compounds based on pyridine carboxylate ligands. J. Mol. Struct. 2010, 983, 93–98. [Google Scholar] [CrossRef]
- Xu, G.J.; Zhao, Y.H.; Shao, K.Z.; Xu, Y.H.; Wang, X.L.; Su, Z.M.; Yan, L.K. Syntheses and characterization of two coordination polymers constructed by the ligand 3,5-bis(pyridin-4-ylmethoxy)benzoic Acid. Z. Anorg. Allg. Chem. 2009, 635, 2671–2675. [Google Scholar]
- Ashiry, K.O.; Zhao, Y.H.; Shao, K.Z.; Su, Z.M.; Fu, Y.M.; Hao, X.R. A metal–organic framework containing meso-helical chains: Synthesis, characterization and luminescent property. Inorg. Chem. Commun. 2008, 11, 1181–1183. [Google Scholar] [CrossRef]
- Xu, G.J.; Zhao, Y.H.; Shao, K.Z.; Yuan, G.; Wang, X.L.; Su, Z.M.; Yan, L.K. A novel luminescent 3D metal–organic framework possessing 4-fold interpenetrating (3,4)-connected net. Inorg. Chem. Commun. 2009, 12, 969–971. [Google Scholar] [CrossRef]
- Tai, X.S.; Liu, L.L.; Yin, J. Synthesis, crystal structure of tetra-nuclear macrocyclic Cu(II) complex material and its application as catalysts for A3 coupling reaction. J. Inorg. Organomet. Polym. 2014, 24, 1014–1020. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).