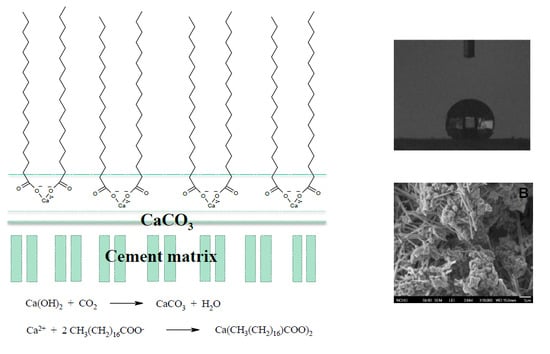
Hydrophobic Calcium Carbonate for Cement Surface
Abstract
:1. Introduction
2. Experimental Section
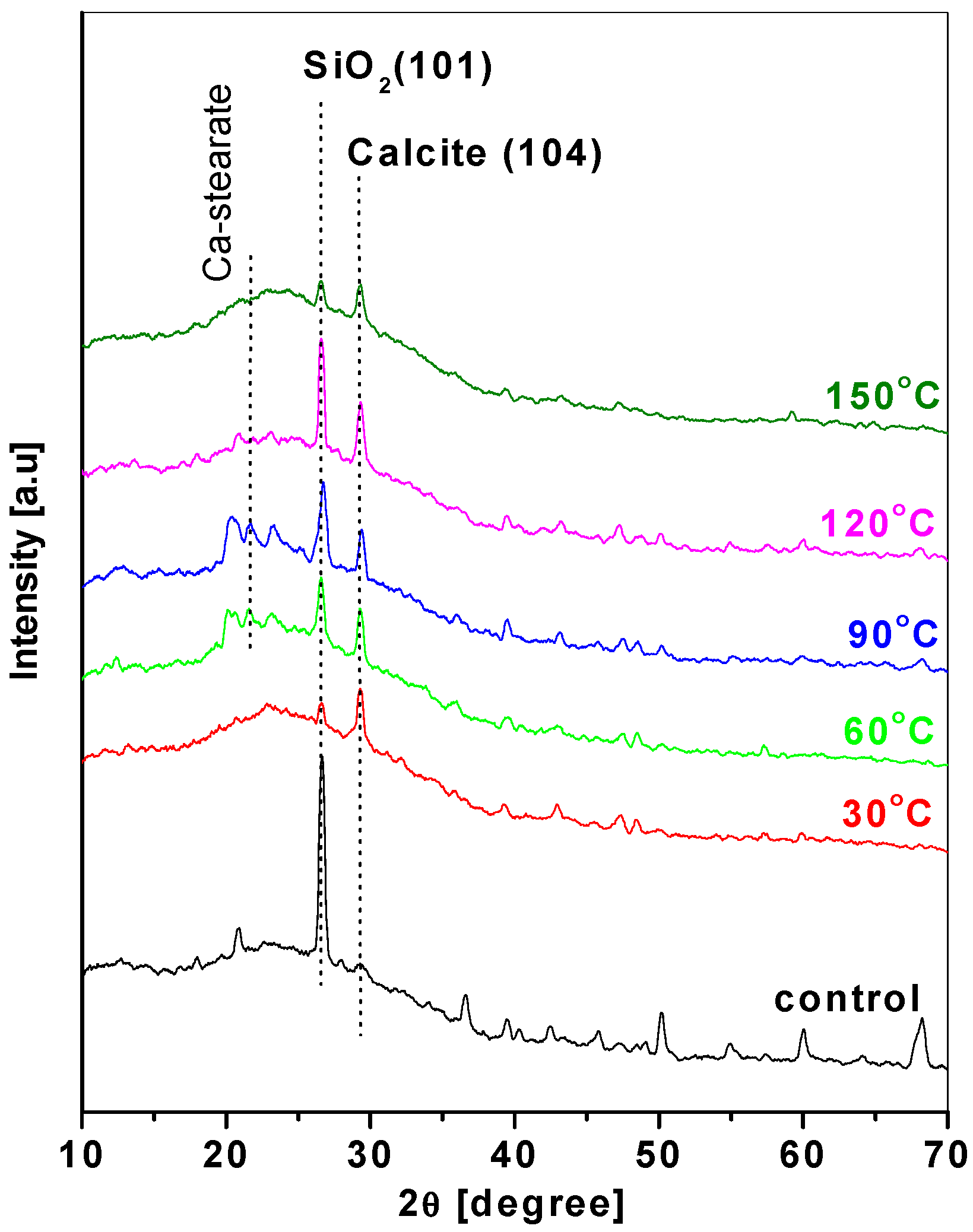
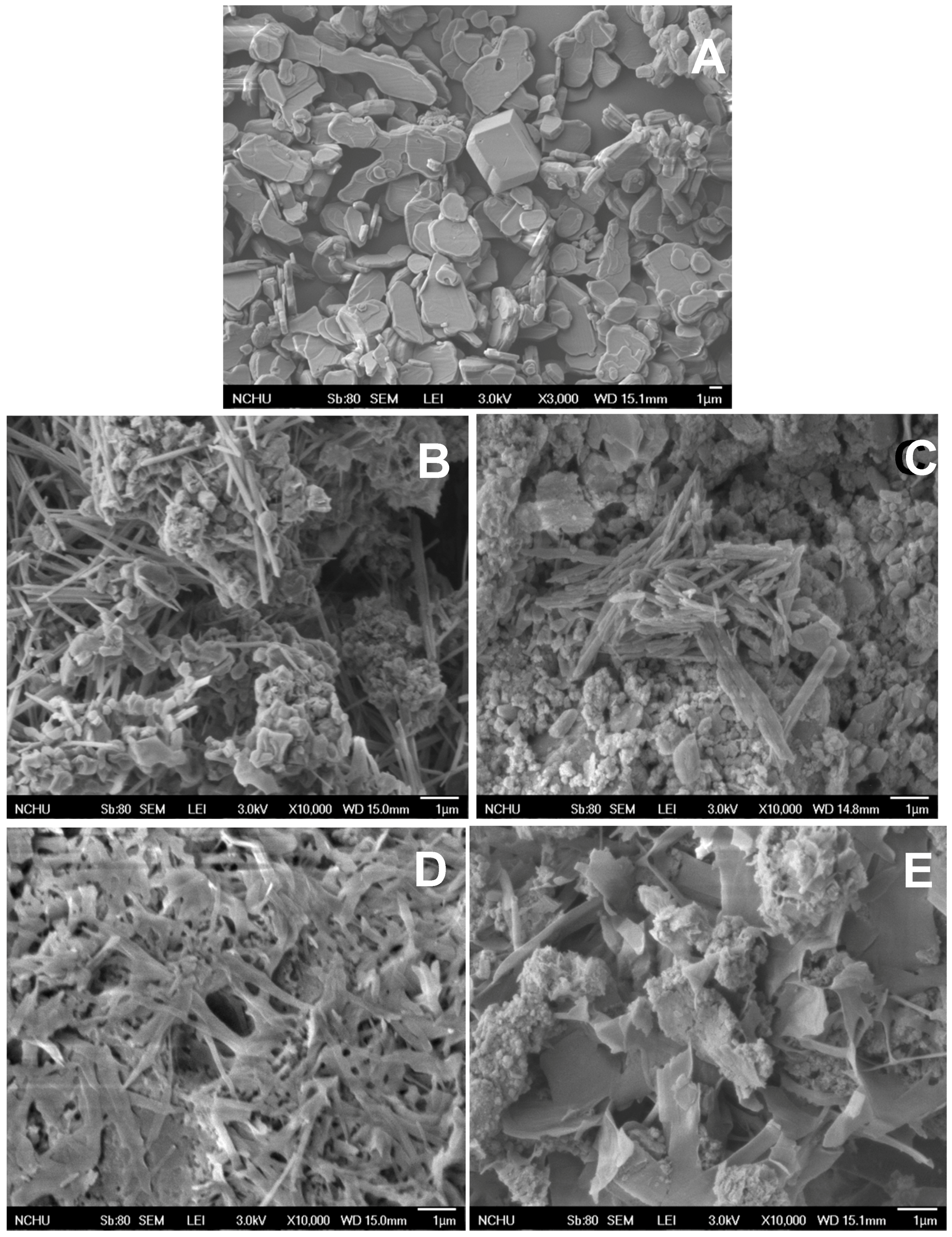
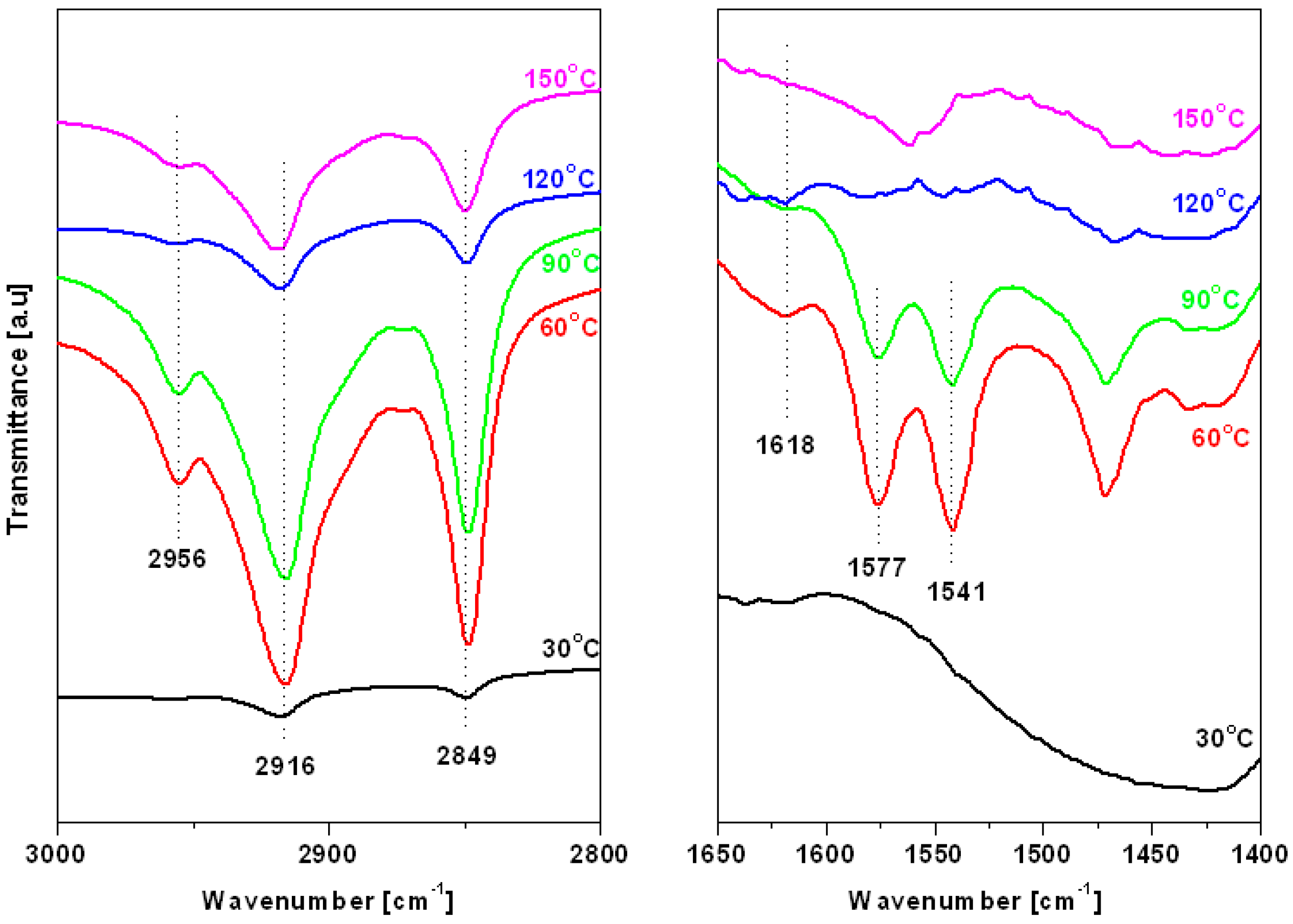
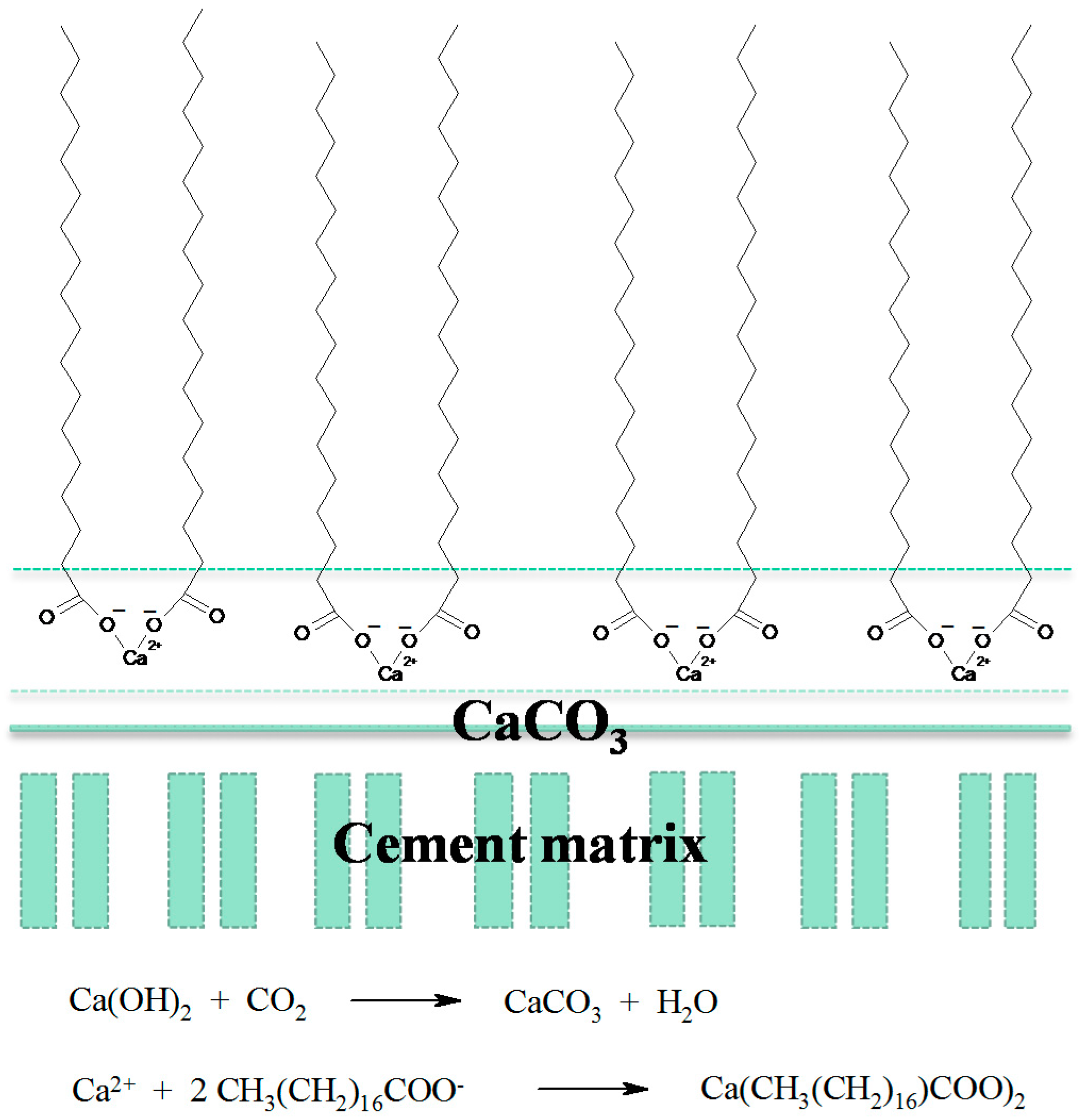
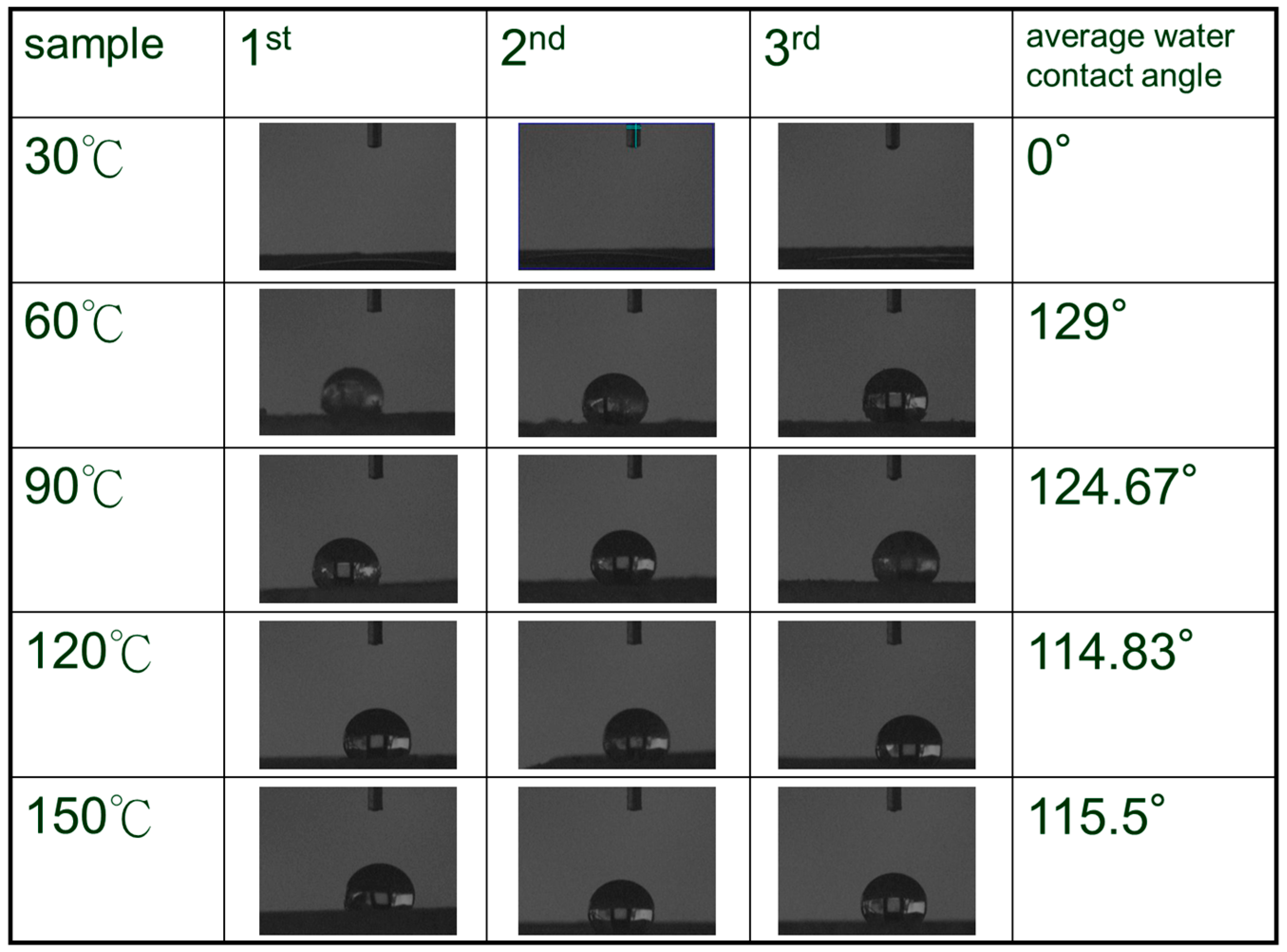
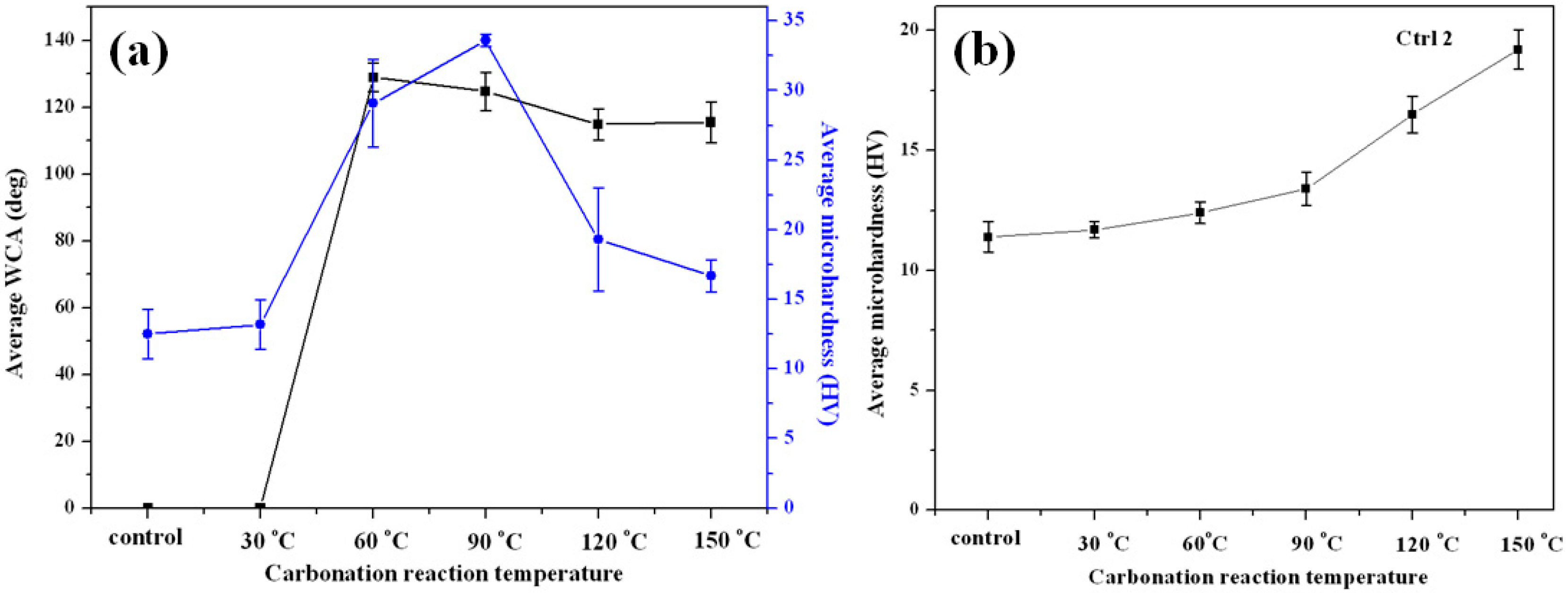
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Naka, K.; Chujo, Y. Control of Crystal Nucleation and Growth of Calcium Carbonate by Synthetic Substrates. Chem. Mater. 2001, 13, 3245–3259. [Google Scholar] [CrossRef]
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of advanced hybrid organic-inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef] [PubMed]
- Mindess, S.; Young, J.F.; Darwin, D. Concrete; Prentice Hall: Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- Monteiro, P.J.M.; Mehta, P.K. Concrete: Structure, Properties, and Materials; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 1986. [Google Scholar]
- Basheer, P.A.M.; Basheer, L.; Cleland, D.J.; Long, A.E. Surface treatments for concrete: Assessmentmethods and reported performance. Constr. Build. Mater. 1997, 11, 413–429. [Google Scholar] [CrossRef]
- Tkach, E.V.; Semenov, V.S.; Tkach, S.A.; Rozovskaya, T.A. Highly Effective Water-repellent Concrete with Improved Physical and Technical Properties. Procedia Eng. 2015, 111, 763–769. [Google Scholar] [CrossRef]
- Zhang, T.; Shang, S.; Yin, F.; Aishah, A.; Salmiah, A.; Ooi, T.L. Adsorptive behavior of surfactants on surface of Portland cement. Cem. Concr. Res. 2001, 31, 1009–1015. [Google Scholar] [CrossRef]
- Maryoto, A. Resistance of Concrete with Calcium Stearate Due to Chloride Attack Tested by Accelerated Corrosion. Procedia Eng. 2017, 171, 511–516. [Google Scholar] [CrossRef]
- Medeiros, M.H.F.; Helene, P. Surface treatment of reinforced concrete in marine environment: Influence on chloride diffusion coefficient and capillary water absorption. Constr. Build. Mater. 2009, 23, 1476–1484. [Google Scholar] [CrossRef]
- Park, D.C. Carbonation of concrete in relation to CO2 permeability and degradation of coatings. Constr. Build. Mater. 2008, 22, 2260–2268. [Google Scholar] [CrossRef]
- Izaguirre, A.; Lanas, J.; Álvarez, J.I. Effect of water-repellent admixtures on the behaviour of aerial lime-based mortars. Cem. Concr. Res. 2009, 39, 1095–1104. [Google Scholar] [CrossRef]
- Maryoto, A. Improving Microstructures of Concrete Using Ca(C18H35O2)2. Procedia Eng. 2015, 125, 631–637. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, X.; Zhao, G.; Wang, X. Facile preparation of cubic calcium carbonate nanoparticles with hydrophobic properties via a carbonation route. Powder Technol. 2010, 200, 144–148. [Google Scholar] [CrossRef]
- Keum, D.-K.; Kim, K.-M.; Naka, K.; Chujo, Y. Preparation of hydrophobic CaCO3 composite particles by mineralization with sodium trisilanolate in a methanol solution. J. Mater. Chem. 2002, 12, 2449–2452. [Google Scholar] [CrossRef]
- Wang, C.; Sheng, Y.; Hari, B.; Zhao, X.; Zhao, J.; Ma, X.; Wang, Z. A novel aqueous-phase route to synthesize hydrophobic CaCO3 particles in situ. Mater. Sci. Eng. C 2007, 27, 42–45. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, P.; Zhao, J.; Zhao, X.; Liu, Y.; Wang, Z. Biomimetic synthesis of hydrophobic calcium carbonate nanoparticles via a carbonation route. Powder Technol. 2006, 170, 31–35. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhou, B.; Wang, C.; Zhao, X.; Deng, Y.; Wang, Z. In situ preparation of hydrophobic CaCO3 in the presence of sodium oleate. Appl. Surf. Sci. 2006, 253, 1983–1987. [Google Scholar] [CrossRef]
- Wang, C.; Sheng, Y.; Zhao, X.; Pan, Y.; Hari, B.; Wang, Z. Synthesis of hydrophobic CaCO3 nanoparticles. Mater. Lett. 2006, 60, 854–857. [Google Scholar] [CrossRef]
- Wang, C.; Piao, C.; Zhai, X.; Hickman, F.N.; Li, J. Synthesis and characterization of hydrophobic calcium carbonate particles via a dodecanoic acid inducing process. Powder Technol. 2010, 198, 131–134. [Google Scholar] [CrossRef]
- Jumate, E.; Manea, D.L.; Moldovan, D.; Fechete, R. The Effects of Hydrophobic Redispersibele Powder Polymer in Portland Cement Based Mortars. Procedia Eng. 2017, 181, 316–323. [Google Scholar] [CrossRef]
- Falchi, L.; Zendri, E.; Müller, U.; Fontana, P. The influence of water-repellent admixtures on the behaviour and the effectiveness of Portland limestone cement mortars. Cem. Concr. Compos. 2015, 59, 107–118. [Google Scholar] [CrossRef]
- Izaguirre, A.; Lanas, J.; Álvarez, J.I. Ageing of lime mortars with admixtures: Durability and strength assessment. Cem. Concr. Res. 2010, 40, 1081–1095. [Google Scholar] [CrossRef]
- Maranhao, F.; John, V.; Loh, K.; Pileggi, R. The influence of silicone based water repellents as admixtures on the rheological properties of cement slurry. In Proceedings of the Hydrophobe V, 5th International Conference on Water Repellent Treatment of Building Materials, Brussels, Belgium, 15–16 April 2008; pp. 255–258. [Google Scholar]
- Klisińska-Kopacz, A.; Tišlova, R. Effect of hydrophobization treatment on the hydration of repair Roman cement mortars. Constr. Build. Mater. 2012, 35, 735–740. [Google Scholar] [CrossRef]
- Falchi, L.; Müller, U.; Fontana, P.; Izzo, F.C.; Zendri, E. Influence and effectiveness of water-repellent admixtures on pozzolana–lime mortars for restoration application. Constr. Build. Mater. 2013, 49, 272–280. [Google Scholar] [CrossRef]
- Xue, X.; Li, Y.; Yang, Z.; He, Z.; Dai, J.-G.; Xu, L.; Zhang, W. A systematic investigation of the waterproofing performance and chloride resistance of a self-developed waterborne silane-based hydrophobic agent for mortar and concrete. Constr. Build. Mater. 2017, 155, 939–946. [Google Scholar] [CrossRef]
- Gönen, M.; Öztürk, S.; Balköse, D.; Okur, S.; Ülkü, S. Preparation and Characterization of Calcium Stearate Powders and Films Prepared by Precipitation and Langmuir–Blodgett Techniques. Ind. Eng. Chem. Res. 2010, 49, 1732–1736. [Google Scholar] [CrossRef]
- Cunningham, I.D.; Courtois, J.-P.; Danks, T.N.; Heyes, D.M.; Moreton, D.J.; Taylor, S.E. Synthesis and characterisation of calixarene-stabilised calcium carbonate overbased detergents. Colloids Surf. A Physicochem. Eng. Asp. 2003, 229, 137–147. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef]
- Corinaldesi, V. Combined effect of expansive, shrinkage reducing and hydrophobic admixtures for durable self compacting concrete. Constr. Build. Mater. 2012, 36, 758–764. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atla, S.B.; Huang, Y.-H.; Yang, J.; Chen, H.-J.; Kuo, Y.-H.; Hsu, C.-M.; Lee, W.-C.; Chen, C.-C.; Hsu, D.-W.; Chen, C.-Y. Hydrophobic Calcium Carbonate for Cement Surface. Crystals 2017, 7, 371. https://doi.org/10.3390/cryst7120371
Atla SB, Huang Y-H, Yang J, Chen H-J, Kuo Y-H, Hsu C-M, Lee W-C, Chen C-C, Hsu D-W, Chen C-Y. Hydrophobic Calcium Carbonate for Cement Surface. Crystals. 2017; 7(12):371. https://doi.org/10.3390/cryst7120371
Chicago/Turabian StyleAtla, Shashi B., Yi-Hsun Huang, James Yang, How-Ji Chen, Yi-Hao Kuo, Chun-Mei Hsu, Wen-Chien Lee, Chien-Cheng Chen, Duen-Wei Hsu, and Chien-Yen Chen. 2017. "Hydrophobic Calcium Carbonate for Cement Surface" Crystals 7, no. 12: 371. https://doi.org/10.3390/cryst7120371
APA StyleAtla, S. B., Huang, Y.-H., Yang, J., Chen, H.-J., Kuo, Y.-H., Hsu, C.-M., Lee, W.-C., Chen, C.-C., Hsu, D.-W., & Chen, C.-Y. (2017). Hydrophobic Calcium Carbonate for Cement Surface. Crystals, 7(12), 371. https://doi.org/10.3390/cryst7120371