Breathing 3D Frameworks with T-Shaped Connecting Ligand Exhibiting Solvent Induction, Metal Ions Effect and Luminescent Properties
Abstract
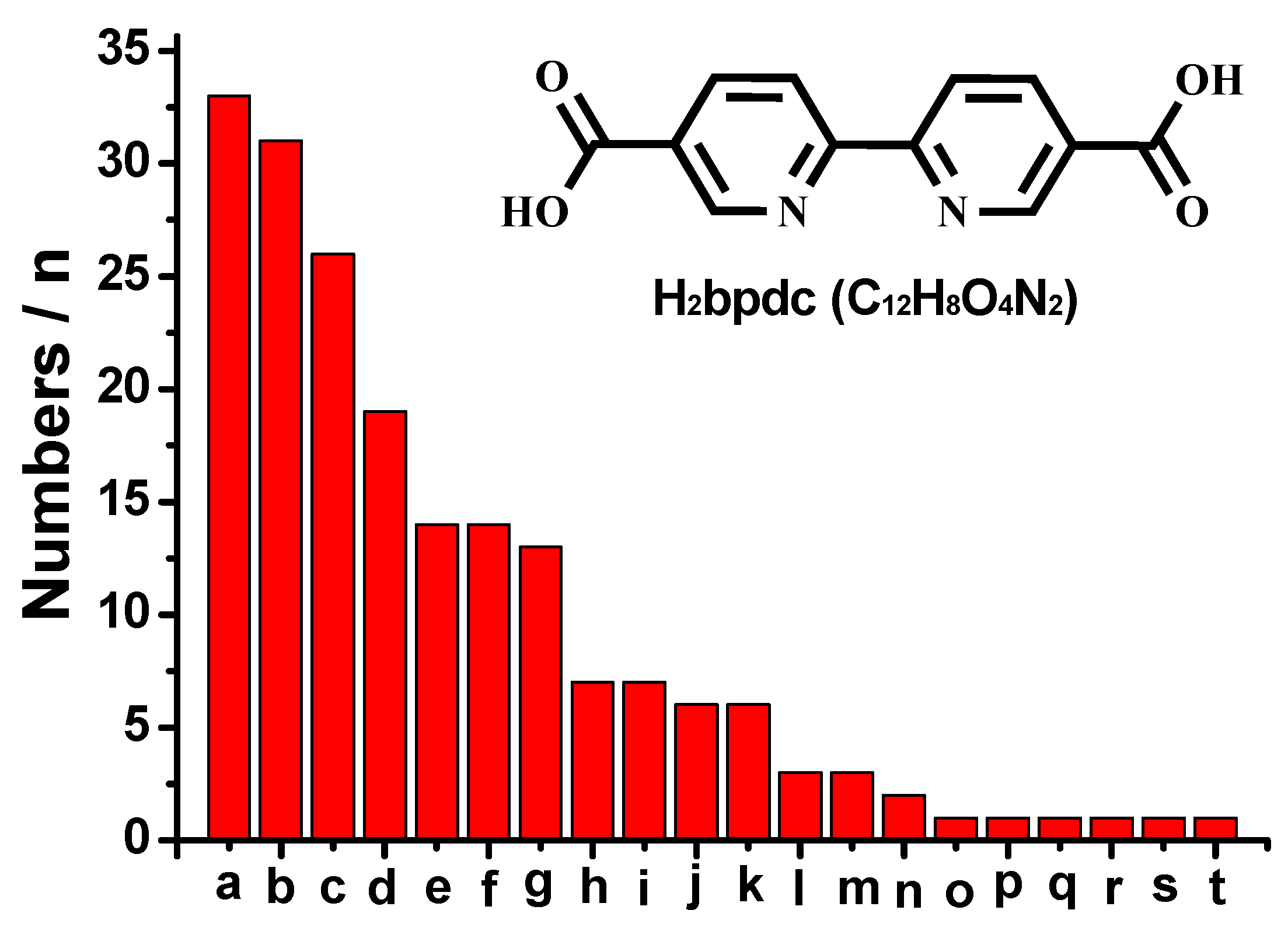
1. Introduction
2. Experimental
2.1. Materials and Physical Measurements
2.2. [Cd2(bpdc)2](DMF)3(H2O)
2.3. [Cd(bpdc)](DMF)(H2O)
2.4. [Zn(bpdc)](DMF)(H2O)
2.5. X-ray Crystallography
3. Results and Discussion
3.1. Synthesis Conditions
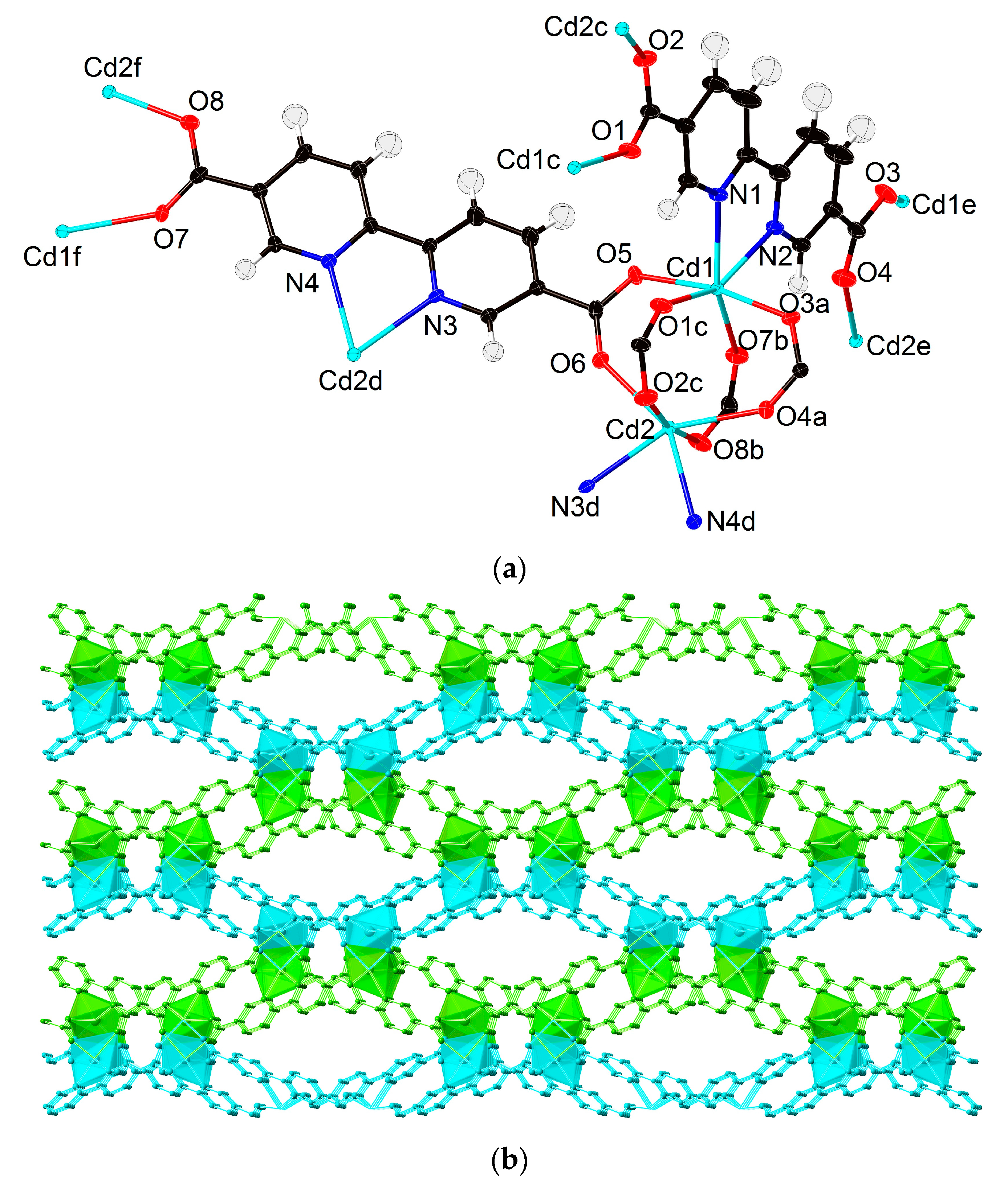
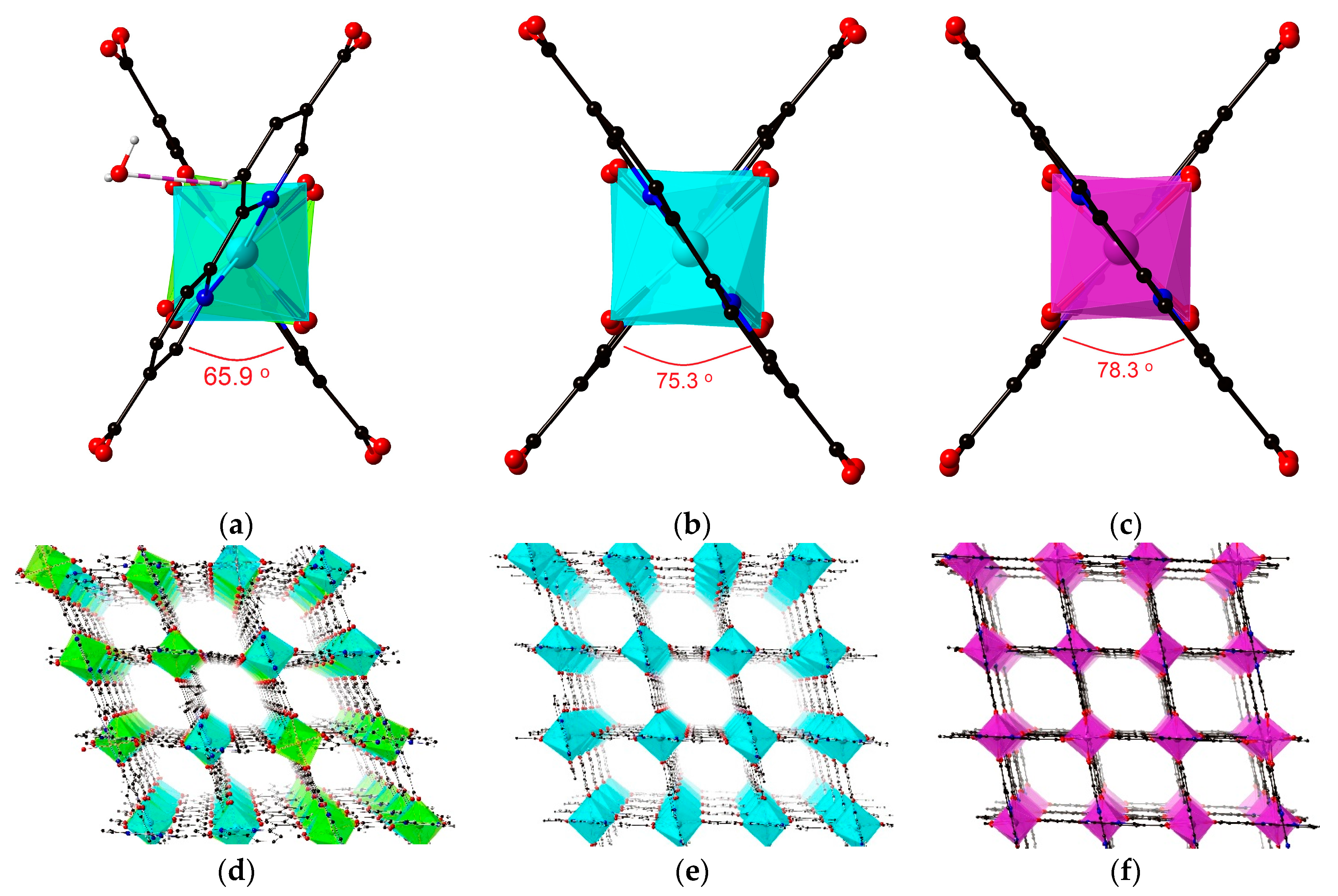
3.2. Crystal Structures of 1–3
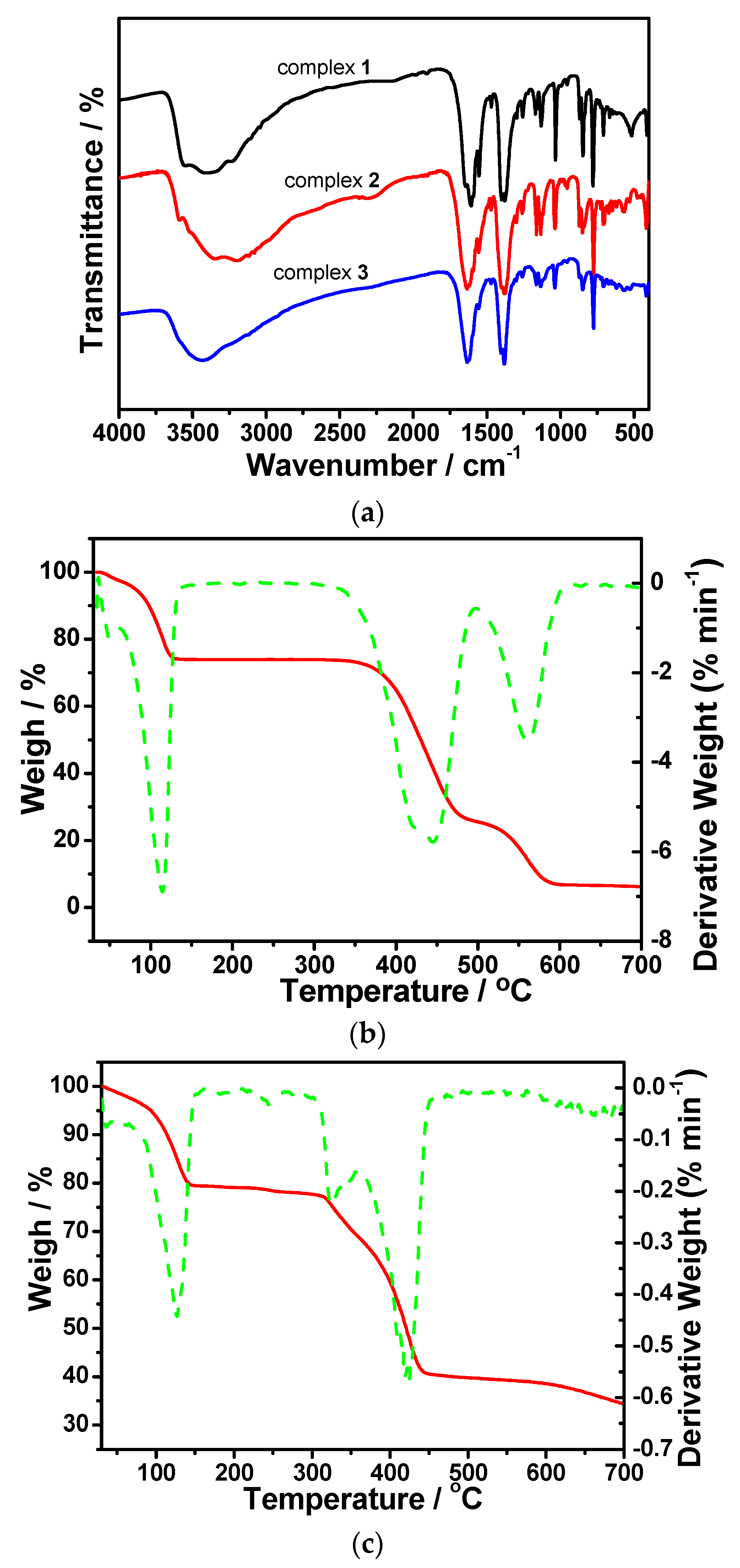
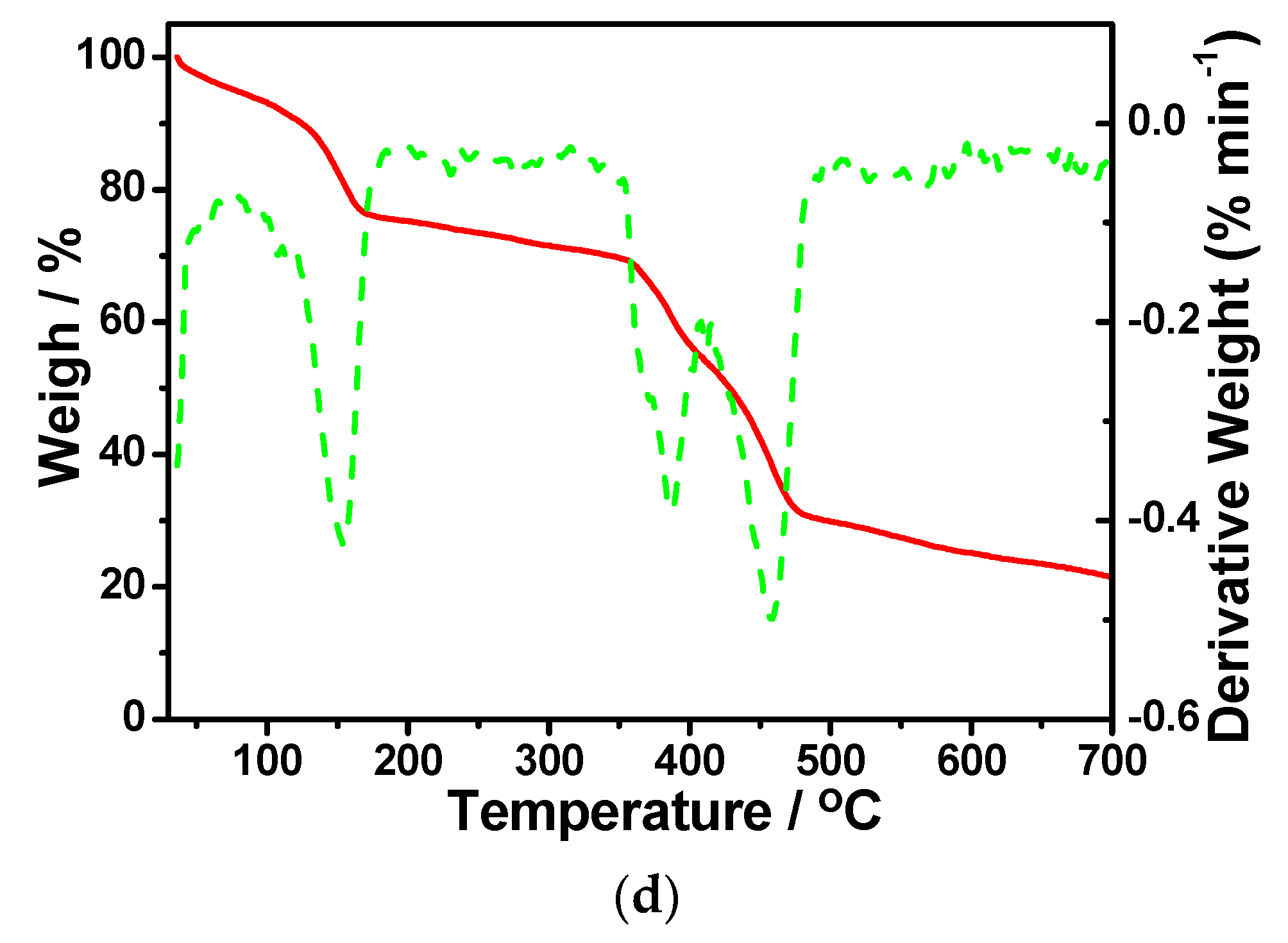
3.3. IR Spectra and TG Measurements
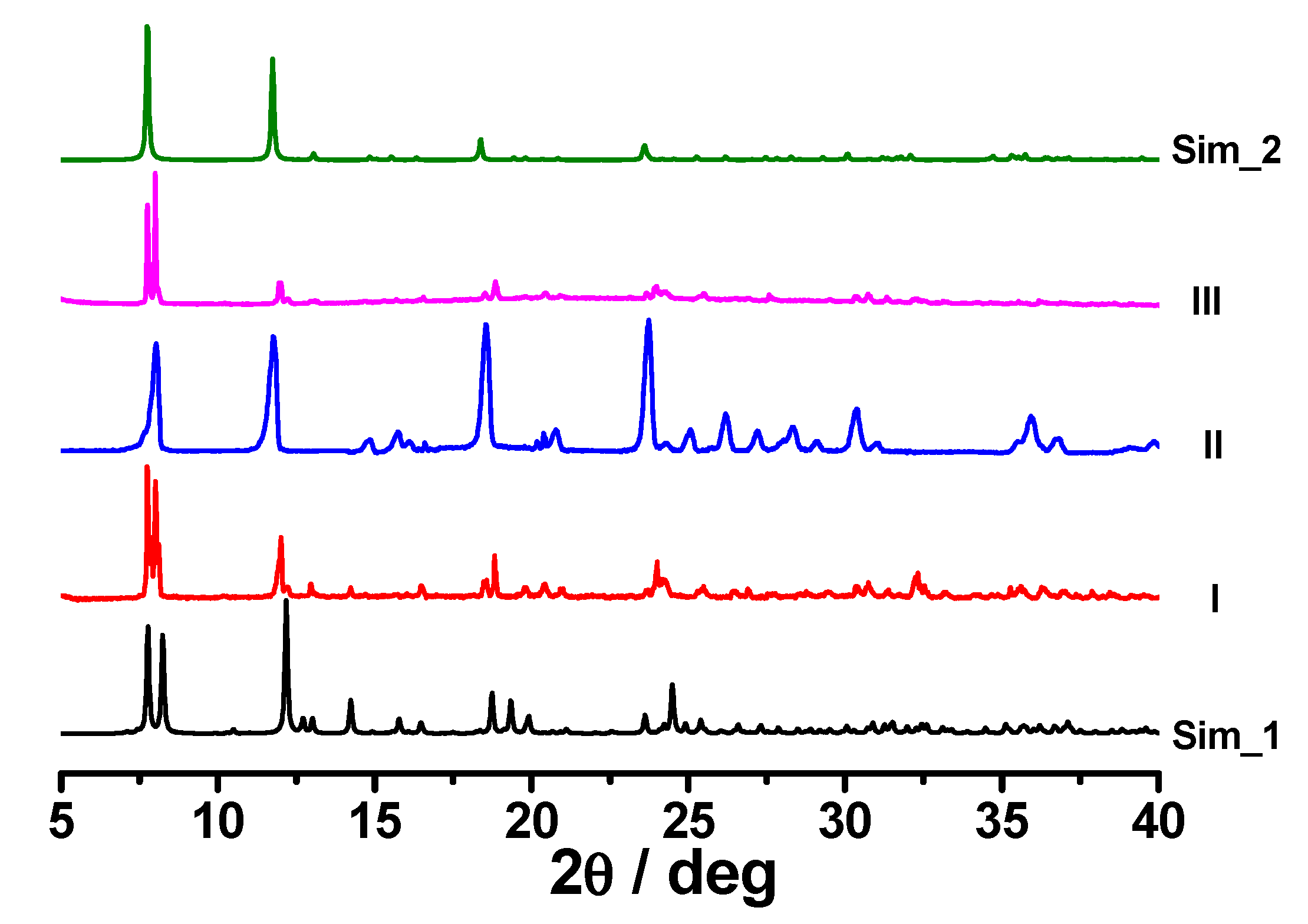
3.4. Stabilities and Structure Transformation
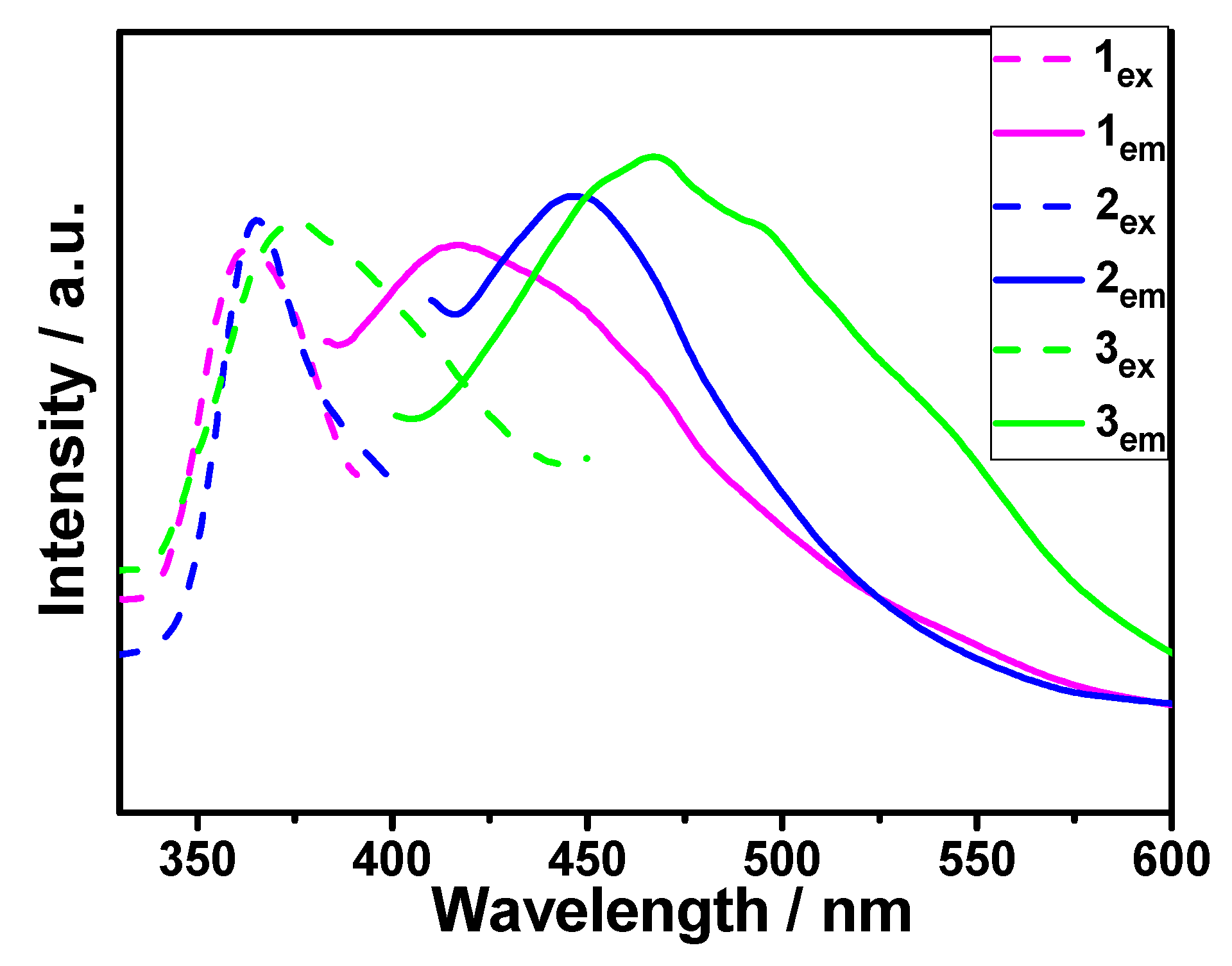
3.5. Luminescent Properties
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, H.; Yang, S.; Rao, S.R.; Narayanan, S.; Kapustin, E.A.; Furukawa, H.; Umans, A.S.; Yaghi, O.M.; Wang, E.N. Water harvesting from air with metal-organic frameworks powered by natural sunlight. Science 2017, 356, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wen, H.-M.; Cui, Y.; Zhou, W.; Qian, G.; Chen, B. Emerging multifunctional metal–organic framework materials. Adv. Mater. 2016, 28, 8819–8860. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.B.; Gong, L.L.; Huang, L.; Luo, F.; Krishna, R.; Yi, X.F.; Zheng, A.M.; Zhang, L.; Pu, S.Z.; Feng, X.F.; et al. Significant enhancement of C2H2/C2H4 separation by a photochromic diarylethene unit: A temperature- and light-responsive separation switch. Angew. Chem. Int. Ed. 2017, 56, 7900–7906. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-N.; Song, X.-Z.; Song, S.-Y.; Zhang, H.-J. Highly efficient heterogeneous catalytic materials derived from metalorganic framework supports/precursors. Coord. Chem. Rev. 2017, 337, 80–96. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ou, S.; Wu, C.-D. Porous metal-organic frameworks for heterogeneous biomimetic catalysis. Acc. Chem. Res. 2014, 47, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal–organic frameworks: Opportunities for catalysis. Angew. Chem. Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.-P.; Liu, J.-L.; Hoque, M.N.; Liu, W.; Li, J.-Y.; Chen, Y.-C.; Tong, M.-L. Recent advances in guest effects on spin-crossover behavior in Hofmann-type metal–organic frameworks. Coord. Chem. Rev. 2017, 335, 28–43. [Google Scholar] [CrossRef]
- Coronado, E.; Espallargas, G.M. Dynamic magnetic MOFs. Chem. Soc. Rev. 2013, 42, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.-B.; Liu, S.-Y.; Ye, J.-W.; Li, X.-Y.; Zhang, J.-P. Photoluminescent metal–organic frameworks for gas sensing. Adv. Sci. 2016, 3, 1500434–1500453. [Google Scholar] [CrossRef] [PubMed]
- Wales, D.J.; Grand, J.; Ting, V.P.; Burke, R.D.; Edler, K.J.; Bowen, C.R.; Mintova, S.; Burrows, A.D. Gas sensing using porous materials for automotive applications. Chem. Soc. Rev. 2015, 44, 4290–4321. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Deiberta, B.J.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed]
- Wyszogrodzka, G.; Marszałek, B.; Gil, B.; Dorożyński, P. Metal-organic frameworks: Mechanisms of antibacterial action and potential applications. Drug Discov. Today 2016, 21, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Rocca, J.D.; Liu, D.; Lin, W. Nanoscale metal–organic frameworks for biomedical imaging and drug delivery. Acc. Chem. Res. 2011, 44, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Horike, S.; Umeyama, D.; Kitagawa, S. Ion conductivity and transport by porous coordination polymers and metal-organic frameworks. Acc. Chem. Res. 2013, 46, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Schneemann, A.; Bon, V.; Schwedler, I.; Senkovska, I.; Kaskel, S.; Fischer, R.A. Flexible metal–organic rameworks. Chem. Soc. Rev. 2014, 43, 6062–6069. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-J.; Lv, J.; Hong, M.; Cao, R. Metal–organic frameworks based on flexible ligands (FL-MOFs): Structures and applications. Chem. Soc. Rev. 2014, 43, 5867–5895. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.-C.; Gao, X.; Zhou, Y.; Chen, Y.-C.; Wang, L.-F.; Wu, J.-Z.; Tong, M.-L. Magnetic properties and photoluminescence of lanthanide coordination polymers constructed with conformation-flexible cyclohexane-tetracarboxylate ligands. Cryst. Growth Des. 2016, 16, 946–952. [Google Scholar] [CrossRef]
- Liu, G.; Zeller, M.; Su, K.; Pang, J.; Ju, Z.; Yuan, D.; Hong, M. Controlled orthogonal self-assembly of heterometal-decorated coordination cages. Chem. Eur. J. 2016, 22, 17345–17350. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.I.; Bloch, E.D.; Mason, J.A.; Teat, S.J.; Long, J.R. Single-crystal-to-single-crystal metalation of a metal–organic framework: A route toward structurally well-defined catalysts. Inorg. Chem. 2015, 54, 2995–3005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, L.; Zhao, S.; Sun, Z.; Luo, J. Construction of interpenetrated ruthenium metal–organic frameworks as stable photocatalysts for CO2 reduction. Inorg. Chem. 2015, 54, 8375–8379. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.J.; Champness, N.R.; Easun, T.L.; Allan, D.R.; Nowell, H.; George, M.W.; Jia, J.; Sun, X.-Z. Photoreactivity examined through incorporation in metal-organic frameworks. Nat. Chem. 2010, 2, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Bon, V.; Kavoosi, N.; Senkovska, I.; Müller, P.; Schaber, J.; Wallacher, D.; Többens, D.M.; Mueller, U.; Kaskel, S. Tuning the flexibility in MOFs by SBU functionalization. Dalton Trans. 2016, 45, 4407–4415. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jing, X.; Cao, Y.; Li, G.; Huo, Q.; Liu, Y. Structural diversity and magnetic properties of three metal–organic frameworks assembled from a T-shaped linker. CrystEngComm 2015, 17, 604–611. [Google Scholar] [CrossRef]
- Wang, J.; Luo, J.; Zhao, J.; Li, D.-S.; Li, G.; Huo, Q.; Liu, Y. Assembly of two flexible metal–organic frameworks with stepwise gas adsorption and highly selective CO2 adsorption. Cryst. Growth Des. 2014, 14, 2375–2380. [Google Scholar] [CrossRef]
- Shen, L.; Gray, D.; Masel, R.I.; Girolami, G.S. Synthesis and characterization of a zinc metal–organic framework with chiral nano-pores. CrystEngComm 2012, 14, 5145–5147. [Google Scholar] [CrossRef]
- Gustafsson, M.; Su, J.; Yue, H.; Yao, Q.; Zou, X. A family of flexible lanthanide bipyridinedicarboxylate metal–organic frameworks showing reversible single-crystal to single-crystal transformations. Cryst. Growth Des. 2012, 12, 3243–3249. [Google Scholar] [CrossRef]
- Huh, S.; Jung, S.; Kim, Y.; Kim, S.-J.; Park, S. Two-dimensional metal–organic frameworks with blue luminescence. Dalton Trans. 2010, 39, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luo, J.; Zhi, B.; Li, G.; Huo, Q.; Liu, Y. Anion-templated assembly of three indium–organic frameworks with diverse topologies. CrystEngComm 2014, 16, 9810–9816. [Google Scholar] [CrossRef]
- Zhao, B.; Fang, M.; Shi, P.-F.; Jiang, D.-X.; Chang, P.; Shi, W. A series of 3d–4f heterometallic three-dimensional coordination polymers: Syntheses, structures and magnetic properties. Dalton Trans. 2012, 41, 6820–6826. [Google Scholar]
- Liu, D.; Huxford, R.C.; Lin, W. Phosphorescent nanoscale coordination polymers as contrast agents for optical imaging. Angew. Chem. Int. Ed. 2011, 50, 3696–3700. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.-X.; Wang, J.-F.; Wu, X.-Q.; Pan, L.-Q.; Li, D.-S. One novel near-infrared ytterbium metal–organic framework based on an unprecedented [Yb6(μ2-OH)2(μ3-OH)6]10+ cluster. Inorg. Chem. Commun. 2016, 70, 111–114. [Google Scholar] [CrossRef]
- Huang, S.-L.; Jia, A.-Q.; Jin, G.-X. Pd(diimine)Cl2 embedded heterometallic compounds with porous structures as efficient heterogeneous catalysts. Chem. Commun. 2013, 49, 2403–2405. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, O.; Malaestean, I.L.; Ellern, A.; van Leusen, J.; Baca, S.G.; Kogerler, P. Assembly of cerium(III) 2,2′-bipyridine-5,5′-dicarboxylate-based metal–organic frameworks by solvent tuning. Cryst. Growth Des. 2014, 14, 3541–3548. [Google Scholar] [CrossRef]
- Lin, X.; Hong, Y.; Zhang, C.; Huang, R.; Wang, C.; Lin, W. Pre-concentration and energy transfer enable the efficient luminescence sensing of transition metal ions by metal–organic frameworks. Chem. Commun. 2015, 51, 16996–16999. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Mei, L.; Wang, L.; Chai, Z.-F.; Shi, W.-Q. Copper/zinc-directed heterometallic uranyl-organic polycatenating frameworks: Synthesis, characterization, and anion-dependent structural regulation. Inorg. Chem. 2016, 55, 10125–10134. [Google Scholar] [CrossRef] [PubMed]
- Allen, F.H.; Taylor, R. Research applications of the Cambridge Structural Database (CSD). Chem. Soc. Rev. 2004, 33, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Van Der Sluis, P.; Spek, A.L. BYPASS: An effective method for the refinement of crystal structures containing disordered solvent regions. Acta Cryst. 1990, A46, 194–201. [Google Scholar] [CrossRef]
- Ou, Y.-C.; Zhong, J.-X.; Song, Y.-Y.; Zhu, L.-L.; Wu, J.-Z. Two 1D chain structures derived from alkaline-earth metal complexes and 2,2′-bipyridine-5,5′-dicarboxylate ligand: Syntheses, crystal structures and luminescent properties. Chin. J. Inorg. Chem. 2016, 32, 738–744. [Google Scholar]
- Guionneau, P.; Marchivie, M.; Bravic, G.; Létard, J.F.; Chasseau, D. Co (II) molecular complexes as a reference for the spin crossover in Fe (II) analogues. Mater. Chem. 2002, 12, 2546–2551. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Klein, N.; Hoffmann, H.C.; Cadiau, A.; Getzschmann, J.; Lohe, M.R.; Paasch, S.; Heydenreich, T.; Adil, K.; Senkovska, I.; Brunner, E.; et al. Structural flexibility and intrinsic dynamics in the M2(2,6-ndc)2(dabco) (M = Ni, Cu, Co, Zn) metal–organic frameworks. J. Mater. Chem. 2012, 22, 10303–10312. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]

| Complex | 1 (293 K) | 2 (293 K) | 3 (296 K) |
|---|---|---|---|
| Empirical formula | C33H35N7O12Cd2 | C15H15N3O6Cd | C15H15N3O6Zn |
| fw | 946.48 | 445.70 | 398.67 |
| Crystal system | Monoclinic | Orthorhombic | Orthorhombic |
| Space group | P21/c | Fddd | Fddd |
| a/Å | 11.8988(2) | 17.4483(5) | 15.930(7) |
| b/Å | 24.8683(4) | 19.4148(6) | 19.280(8) |
| c/Å | 12.8119(2) | 23.8529(7) | 23.109(9) |
| β/° | 93.0640(10) | 90 | 90 |
| V/Å3 | 3785.66(11) | 8080.3(4) | 7097(5) |
| Z | 4 | 16 | 16 |
| ρcalcd/g cm−3 | 1.661 | 1.465 | 1.492 |
| μ/mm−1 | 9.599 | 1.112 | 1.419 |
| S | 1.059 | 1.070 | 1.017 |
| R1 [a], wR2 [b] (I > 2σ(I)) | 0.0407, 0.0993 | 0.0313(SQUEEZE), 0.0825(SQUEEZE) | 0.0589(SQUEEZE), 0.1563(SQUEEZE) |
| R1 [a], wR2 [b] (all data) | 0.0561, 0.1104 | 0.0375(SQUEEZE), 0.0873(SQUEEZE) | 0.0900(SQUEEZE), 0.1736(SQUEEZE) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, Y.-C.; Song, Y.-Y.; Du, H.-M.; Hao, M.-M.; Wu, J.-Z. Breathing 3D Frameworks with T-Shaped Connecting Ligand Exhibiting Solvent Induction, Metal Ions Effect and Luminescent Properties. Crystals 2017, 7, 311. https://doi.org/10.3390/cryst7100311
Ou Y-C, Song Y-Y, Du H-M, Hao M-M, Wu J-Z. Breathing 3D Frameworks with T-Shaped Connecting Ligand Exhibiting Solvent Induction, Metal Ions Effect and Luminescent Properties. Crystals. 2017; 7(10):311. https://doi.org/10.3390/cryst7100311
Chicago/Turabian StyleOu, Yong-Cong, Ying-Yi Song, Hui-Ming Du, Meng-Meng Hao, and Jian-Zhong Wu. 2017. "Breathing 3D Frameworks with T-Shaped Connecting Ligand Exhibiting Solvent Induction, Metal Ions Effect and Luminescent Properties" Crystals 7, no. 10: 311. https://doi.org/10.3390/cryst7100311
APA StyleOu, Y.-C., Song, Y.-Y., Du, H.-M., Hao, M.-M., & Wu, J.-Z. (2017). Breathing 3D Frameworks with T-Shaped Connecting Ligand Exhibiting Solvent Induction, Metal Ions Effect and Luminescent Properties. Crystals, 7(10), 311. https://doi.org/10.3390/cryst7100311






