Modulating Nucleation by Kosmotropes and Chaotropes: Testing the Waters
Abstract
1. Introduction
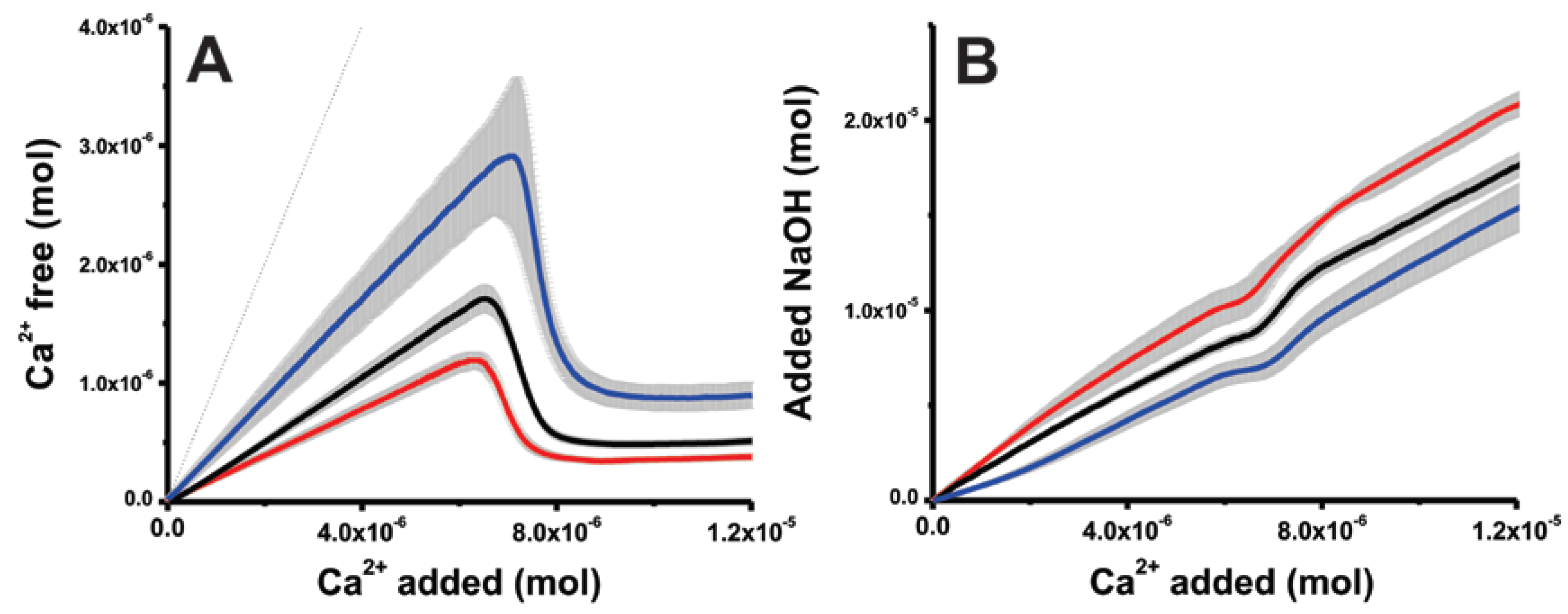
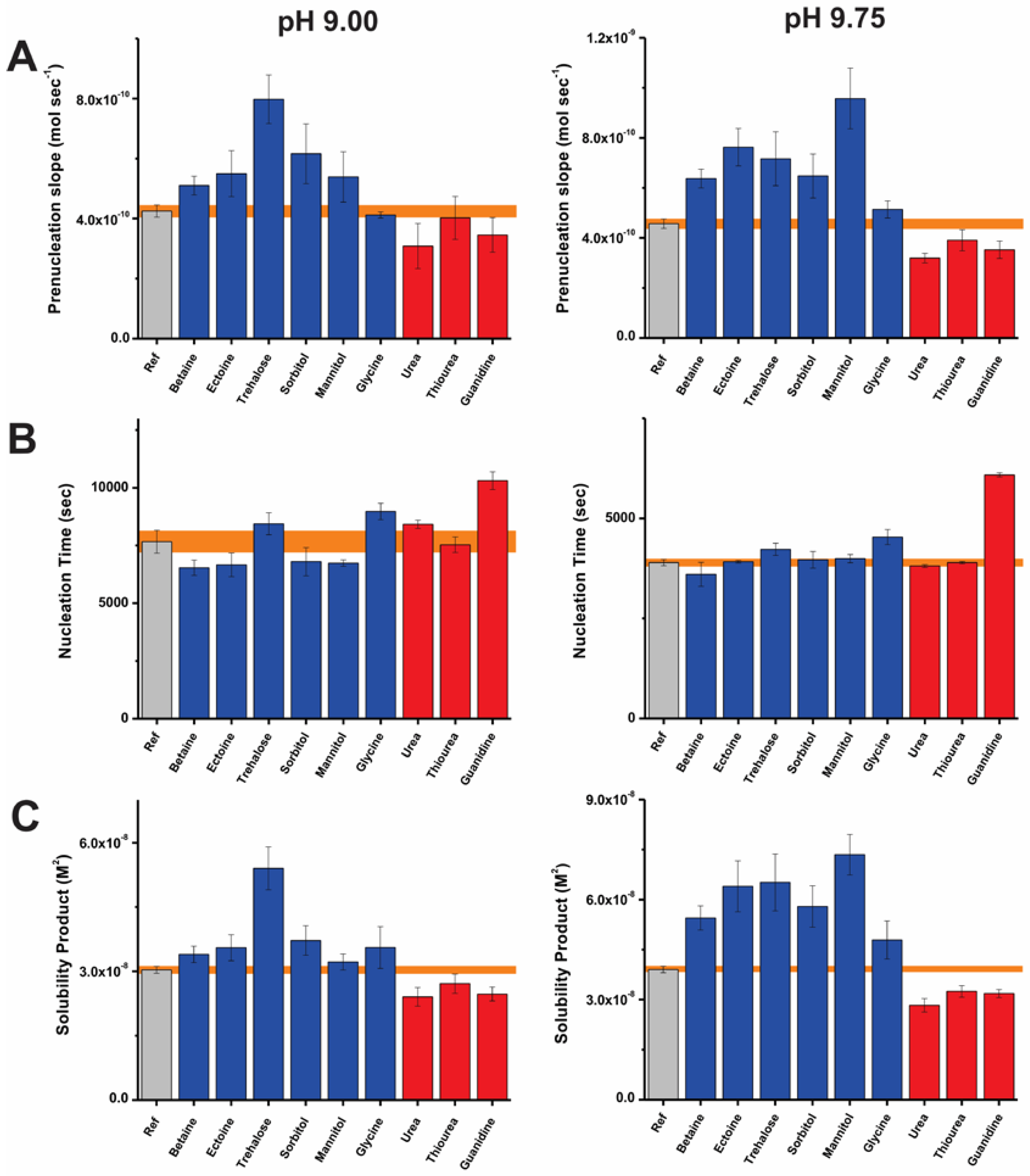
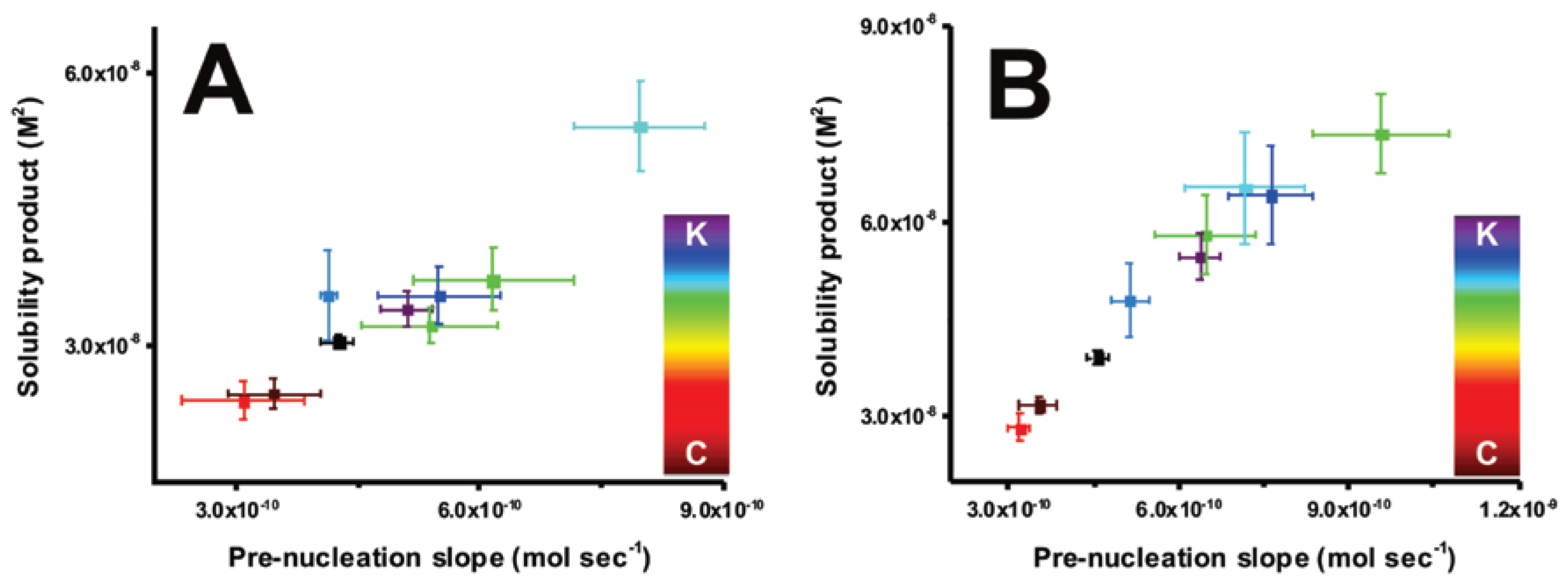
2. Results
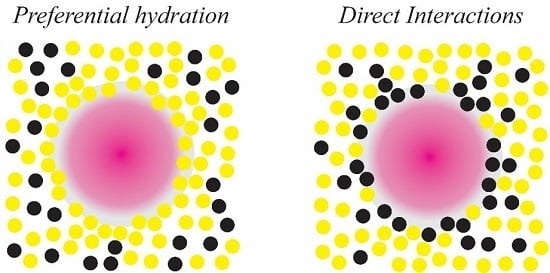
2.1. On Kosmotropes and Chaotropes
2.2. On Sugar Stereoisomers
3. Discussion
4. Materials and Methods
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Encrenaza, T.; Spohnb, T. Water in the solar system. In Encyclopedia of Astrobiology; Springer Berlin Heidelberg: Heidelberg, Germany, 2014. [Google Scholar]
- Ball, P. Water as an active constituent in cell biology. Chem. Rev. 2008, 108, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Ball, P.; Hallsworth, J.E. Water structure and chaotropicity: Their uses, abuses and biological implications. Phys. Chem. Chem. Phys. 2015, 17, 8297–8305. [Google Scholar] [CrossRef] [PubMed]
- Kunz, W.; Henle, J.; Ninham, B.W. ‘Zur lehre von der wirkung der salze’ (about the science of the effect of salts): Franz hofmeister's historical papers. Curr. Opin. Colloid Inter. 2004, 9, 19–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Furyk, S.; Bergbreiter, D.E.; Cremer, P.S. Specific ion effects on the water solubility of macromolecules: Pnipam and the hofmeister series. J. Am. Chem. Soc. 2005, 127, 14505–14510. [Google Scholar] [CrossRef] [PubMed]
- Mason, P.E.; Dempsey, C.E.; Vrbka, L.; Heyda, J.; Brady, J.W.; Jungwirth, P. Specificity of ion-protein interactions: Complementary and competitive effects of tetrapropylammonium, guanidinium, sulfate, and chloride ions. J. Phys. Chem. B 2009, 113, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- McCammick, E.M.; Gomase, V.S.; McGenity, T.J.; Timson, D.J.; Hallsworth, J.E. Water-hydrophobic compound interactions with the microbial cell. In Handbook of Hydrocarbon and Lipid Microbiology; Springer Berlin Heidelberg: Heidelberg, Germany, 2010. [Google Scholar]
- Kunz, W. Specific ion effects in colloidal and biological systems. Curr. Opin. Colloid Inter. 2010, 15, 34–39. [Google Scholar] [CrossRef]
- Vrbka, L.; Jungwirth, P.; Bauduin, P.; Touraud, D.; Kunz, W. Specific ion effects at protein surfaces: A molecular dynamics study of bovine pancreatic trypsin inhibitor and horseradish peroxidase in selected salt solutions. J. Phys. Chem. B 2006, 110, 7036–7043. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.H.; Clark, M.E.; Hand, S.C.; Bowlus, R.D.; Somero, G.N. Living with water stress: Evolution of osmolyte systems. Science 1982, 217, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Lever, M.; Blunt, J.W.; Maclagan, R.G.A.R. Some ways of looking at compensatory kosmotropes and different water environments. Comp. Biochem. Physiol. A 2001, 130, 471–486. [Google Scholar] [CrossRef]
- Crowe, J.; Crowe, L. Membrane integrity in anhydrobiotic organisms: Toward a mechanism for stabilizing dry cells. In Water and Life; Springer: Berlin, Germany, 1992; pp. 87–103. [Google Scholar]
- Albertyn, J.; Hohmann, S.; Thevelein, J.M.; Prior, B.A. GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol. Cell. Biol. 1994, 14, 4135–4144. [Google Scholar] [CrossRef] [PubMed]
- Gekko, K.; Timasheff, S.N. Mechanism of protein stabilization by glycerol: Preferential hydration in glycerol-water mixtures. Biochemistry 1981, 20, 4667–4676. [Google Scholar] [CrossRef] [PubMed]
- Courtenay, E.S.; Capp, M.W.; Anderson, C.F.; Record, M.T. Vapor pressure osmometry studies of osmolyte-protein interactions: Implications for the action of osmoprotectants in vivo and for the interpretation of “osmotic stress” experiments in vitro. Biochemistry 2000, 39, 4455–4471. [Google Scholar] [CrossRef] [PubMed]
- Moelbert, S.; Normand, B.; De Los Rios, P. Kosmotropes and chaotropes: Modelling preferential exclusion, binding and aggregate stability. Biophy. Chem. 2004, 112, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Bolen, D.W.; Baskakov, I.V. The osmophobic effect: Natural selection of a thermodynamic force in protein folding. J. Mol. Biol. 2001, 310, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, P.M. High and low density intracellular water. Cell. Mol. Biol. 2001, 47, 735–744. [Google Scholar] [PubMed]
- Nozaki, Y.; Tanford, C. The solubility of amino acids, diglycine, and triglycine in aqueous guanidine hydrochloride solutions. J. Biol. Chem. 1970, 245, 1648–1652. [Google Scholar] [PubMed]
- De Xammar Oro, J. Role of co-solute in biomolecular stability: Glucose, urea and the water structure. J. Biol. Phys. 2001, 27, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Timasheff, S.N. Protein-solvent preferential interactions, protein hydration, and the modulation of biochemical reactions by solvent components. Proc. Natl. Acad. Sci. USA 2002, 99, 9721–9726. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.P.; Megaw, J.; Magill, C.L.; Nowotarski, K.; Williams, J.P.; Bhaganna, P.; Linton, K.; Patterson, M.F.; Underwood, G.J.C.; Mswaka, A.Y.; et al. Solutes determine the temperature windows for microbial survival and growth. Proc. Natl. Acad. Sci. USA 2010, 107, 7835–7840. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, J.D.; Olteanu, A.; Tripathy, A.; Pielak, G.J. Impact of protein denaturants and stabilizers on water structure. J. Am. Chem. Soc. 2004, 126, 1958–1961. [Google Scholar] [CrossRef] [PubMed]
- Rothon, R.; Paynter, C. Calcium carbonate fillers. In Encyclopedia of Polymers and Composites; Palsule, S., Ed.; Springer Berlin Heidelberg: Heidelberg, Germany, 2015; pp. 1–9. [Google Scholar]
- Verch, A.; Gebauer, D.; Antonietti, M.; Cölfen, H. How to control the scaling of CaCO3: A “fingerprinting technique” to classify additives. Phys. Chem. Chem. Phys. 2011, 13, 16811–16820. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Cölfen, H.; Verch, A.; Antonietti, M. The multiple roles of additives in CaCO3 crystallization: A quantitative case study. Adv. Mat. 2009, 21, 435–439. [Google Scholar] [CrossRef]
- Demichelis, R.; Raiteri, P.; Gale, J.D.; Quigley, D.; Gebauer, D. Stable prenucleation mineral clusters are liquid-like ionic polymers. Nat. Comm. 2011, 2, 590. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Völkel, A.; Cölfen, H. Stable prenucleation calcium carbonate clusters. Science 2008, 322, 1819–1822. [Google Scholar] [CrossRef] [PubMed]
- Kellermeier, M.; Raiteri, P.; Berg, J.K.; Kempter, A.; Gale, J.D.; Gebauer, D. Entropy drives calcium carbonate ion association. Chemphyschem 2015, 17, 3535–3541. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, N.; Cölfen, H.; Pinna, N.; Antonietti, M.; Nassif, N. Superstructures of calcium carbonate crystals by oriented attachment. Cryst. Growth Des. 2005, 5, 1317–1319. [Google Scholar] [CrossRef]
- Penn, R.L.; Banfield, J.F. Imperfect oriented attachment: Dislocation generation in defect-free nanocrystals. Science 1998, 281, 969–971. [Google Scholar] [CrossRef] [PubMed]
- De Yoreo, J.J.; Gilbert, P.U.; Sommerdijk, N.A.; Penn, R.L.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349, aaa6760. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, F.; Wolf, S.L.P.; Born, B.; Luong, T.Q.; Cölfen, H.; Gebauer, D.; Havenith, M. Water dynamics from THz Spectroscopy reveal the locus of a liquid-liquid binodal limit in aqueous CaCO3 solutions. Angew. Chem. Int. Ed. 2017, 56, 490–495. [Google Scholar]
- Gower, L.B. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem. Rev. 2008, 108, 4551–4627. [Google Scholar] [CrossRef]
- Wallace, A.F.; Hedges, L.O.; Fernandez-Martinez, A.; Raiteri, P.; Gale, J.D.; Waychunas, G.A.; Whitelam, S.; Banfield, J.F.; De Yoreo, J.J. Microscopic evidence for liquid-liquid separation in supersaturated CaCO3 solutions. Science 2013, 341, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Cara, A.; Putnis, C.V.; Rodriguez-Navarro, C.; Ruiz-Agudo, E. Hydration effects on the stability of calcium carbonate pre-nucleation species. Minerals 2017, 7, 126. [Google Scholar] [CrossRef]
- Gebauer, D.; Cölfen, H. Prenucleation clusters and non-classical nucleation. Nano. Today 2011, 6, 564–584. [Google Scholar] [CrossRef]
- Gebauer, D.; Gunawidjaja, P.N.; Ko, J.; Bacsik, Z.; Aziz, B.; Liu, L.; Hu, Y.; Bergström, L.; Tai, C.W.; Sham, T.K. Proto-calcite and proto-vaterite in amorphous calcium carbonates. Angew. Chem. 2010, 49, 8889–8891. [Google Scholar] [CrossRef] [PubMed]
- Addadi, L.; Raz, S.; Weiner, S. Taking advantage of disorder: Amorphous calcium carbonate and its roles in biomineralization. Adv. Mat. 2003, 15, 959–970. [Google Scholar] [CrossRef]
- Politi, Y.; Arad, T.; Klein, E.; Weiner, S.; Addadi, L. Sea urchin spine calcite forms via a transient amorphous calcium carbonate phase. Science 2004, 306, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, J.H.; Checa, A.G.; Gale, J.D.; Gebauer, D.; Sainz-Díaz, C.I. Calcium carbonate polyamorphism and its role in biomineralization: How many amorphous calcium carbonates are there? Angew. Chem. Inter. Ed. 2012, 51, 11960–11970. [Google Scholar] [CrossRef] [PubMed]
- Radha, A.; Forbes, T.Z.; Killian, C.E.; Gilbert, P.; Navrotsky, A. Transformation and crystallization energetics of synthetic and biogenic amorphous calcium carbonate. Proc. Natl. Acad. Sci. USA 2010, 107, 16438–16443. [Google Scholar] [CrossRef] [PubMed]
- Ihli, J.; Wong, W.C.; Noel, E.H.; Kim, Y.Y.; Kulak, A.N.; Christenson, H.K.; Duer, M.J.; Meldrum, F.C. Dehydration and crystallization of amorphous calcium carbonate in solution and in air. Nat. Comm. 2014, 5, 3169. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-R.; Cai, A.-H.; Liu, R.; Pan, H.-H.; Tang, R.-K.; Cho, K. The roles of water and polyelectrolytes in the phase transformation of amorphous calcium carbonate. J. Cryst. Growth 2008, 310, 3779–3787. [Google Scholar] [CrossRef]
- Luo, Y.; Sonnenberg, L.; Cölfen, H. Novel method for generation of additive free high-energy crystal faces and their reconstruction in solution. Cryst. Growth Des. 2008, 8, 2049–2051. [Google Scholar] [CrossRef]
- Merzel, F.; Smith, J.C. Is the first hydration shell of lysozyme of higher density than bulk water? Proc. Natl. Acad. Sci. USA 2002, 99, 5378–5383. [Google Scholar] [CrossRef] [PubMed]
- Pizzitutti, F.; Marchi, M.; Sterpone, F.; Rossky, P.J. How protein surfaces induce anomalous dynamics of hydration water. J. Phys. Chem. B 2007, 111, 7584–7590. [Google Scholar] [CrossRef] [PubMed]
- Raiteri, P.; Gale, J.D. Water is the key to nonclassical nucleation of amorphous calcium carbonate. J. Am. Chem. Soc. 2010, 132, 17623–17634. [Google Scholar] [CrossRef] [PubMed]
- Tritschler, U.; Kellermeier, M.; Debus, C.; Kempter, A.; Cölfen, H. A simple strategy for the synthesis of well-defined bassanite nanorods. CrystEngComm 2015, 17, 3772–3776. [Google Scholar] [CrossRef]
- Khoshkhoo, S.; Anwar, J. Crystallization of polymorphs: The effect of solvent. J. Phys. D 1993, 26, B90. [Google Scholar] [CrossRef]
- Niederberger, M.; Cölfen, H. Oriented attachment and mesocrystals: Non-classical crystallization mechanisms based on nanoparticle assembly. Phys. Chem. Chem. Phys. 2006, 8, 3271–3287. [Google Scholar] [CrossRef] [PubMed]
- Polleux, J.; Pinna, N.; Antonietti, M.; Hess, C.; Wild, U.; Schlögl, R.; Niederberger, M. Ligand functionality as a versatile tool to control the assembly behavior of preformed titania nanocrystals. Chem. Eur. J. 2005, 11, 3541–3551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Banfield, J.F. Interatomic coulombic interactions as the driving force for oriented attachment. CrystEngComm 2014, 16, 1568–1578. [Google Scholar] [CrossRef]
- Fichthorn, K.A. Atomic-scale aspects of oriented attachment. Chem. Eng. Sci. 2015, 121, 10–15. [Google Scholar] [CrossRef]
- Wiggins, P. Life depends upon two kinds of water. PLoS ONE 2008, 3, e1406. [Google Scholar] [CrossRef] [PubMed]
- Picker, A.; Kellermeier, M.; Seto, J.; Gebauer, D.; Cölfen, H. The multiple effects of amino acids on the early stages of calcium carbonate crystallization. Z. Krist. Cryst. Mat. 2012, 227, 744–757. [Google Scholar] [CrossRef]
- Rao, A.; Berg, J.K.; Kellermeier, M.; Gebauer, D. Sweet on biomineralization: Effects of carbohydrates on the early stages of calcium carbonate crystallization. Eur. J. Min. 2014, 26, 537–552. [Google Scholar] [CrossRef]
- Rao, A.; Fernández, M.S.; Cölfen, H.; Arias, J.L. Distinct effects of avian egg derived anionic proteoglycans on the early stages of calcium carbonate mineralization. Cryst. Growth Des. 2015, 15, 2052–2056. [Google Scholar] [CrossRef]
- Rao, A.; Seto, J.; Berg, J.K.; Kreft, S.G.; Scheffner, M.; Cölfen, H. Roles of larval sea urchin spicule SM50 domains in organic matrix self-assembly and calcium carbonate mineralization. J. Struc. Biol. 2013, 183, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Verch, A.; Borner, H.G.; Cölfen, H. Influence of selected artificial peptides on calcium carbonate precipitation—A quantitative study. Cryst. Growth Des. 2009, 9, 2398–2403. [Google Scholar] [CrossRef]
- Kellermeier, M.; Gebauer, D.; Melero-García, E.; Drechsler, M.; Talmon, Y.; Kienle, L.; Cölfen, H.; García-Ruiz, J.M.; Kunz, W. Colloidal stabilization of calcium carbonate prenucleation clusters with silica. Adv. Func. Mat. 2012, 22, 4301–4311. [Google Scholar] [CrossRef]
- Kellermeier, M.; Picker, A.; Kempter, A.; Cölfen, H.; Gebauer, D. A straightforward treatment of activity in aqueous CaCO3 solutions and the consequences for nucleation theory. Adv. Mat. 2014, 26, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.L.; Jähme, K.; Gebauer, D. Synergy of mg 2+ and poly (aspartic acid) in additive-controlled calcium carbonate precipitation. CrystEngComm 2015, 17, 6857–6862. [Google Scholar] [CrossRef]
- Verch, A.; Antonietti, M.; Cölfen, H. Mixed calcium-magnesium pre-nucleation clusters enrich calcium. Z. Krist. Cryst. Mat. 2012, 227, 718–722. [Google Scholar] [CrossRef]
- De Lima Alves, F.; Stevenson, A.; Baxter, E.; Gillion, J.L.; Hejazi, F.; Hayes, S.; Morrison, I.E.; Prior, B.A.; McGenity, T.J.; Rangel, D.E.; et al. Concomitant osmotic and chaotropicity-induced stresses in Aspergillus wentii: Compatible solutes determine the biotic window. Curr. Genetics 2015, 61, 457–477. [Google Scholar] [CrossRef] [PubMed]
- Zahn, D. Thermodynamics and kinetics of prenucleation clusters, classical and non-classical nucleation. Chemphyschem 2015, 16, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Kellermeier, M.; Gale, J.D.; Bergstrom, L.; Cölfen, H. Pre-nucleation clusters as solute precursors in crystallisation. Chem. Soc. Rev. 2014, 43, 2348–2371. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Vásquez-Quitral, P.; Fernández, M.S.; Berg, J.K.; Sánchez, M.; Drechsler, M.; Neira-Carrillo, A.; Arias, J.L.; Gebauer, D.; Cölfen, H. Ph-dependent schemes of calcium carbonate formation in the presence of alginates. Cryst. Growth Des. 2016, 16, 1349–1359. [Google Scholar] [CrossRef]
- Cray, J.A.; Russell, J.T.; Timson, D.J.; Singhal, R.S.; Hallsworth, J.E. A universal measure of chaotropicity and kosmotropicity. Env. Microbiol. 2013, 15, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Fox-Powell, M.G.; Hallsworth, J.E.; Cousins, C.R.; Cockell, C.S. Ionic strength is a barrier to the habitability of Mars. Astrobiology 2016, 16, 427–442. [Google Scholar] [CrossRef]
- Rao, A.; Huang, Y.C.; Cölfen, H. Additive speciation and phase behavior modulate mineralization. J. Phys. Chem. C 2017. [Google Scholar] [CrossRef]
- Yu, I.; Jindo, Y.; Nagaoka, M. Microscopic understanding of preferential exclusion of compatible solute ectoine: Direct interaction and hydration alteration. J. Phys. Chem. B 2007, 111, 10231–10238. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, J.K.; Bhat, R. Thermal stability of proteins in aqueous polyol solutions: Role of the surface tension of water in the stabilizing effect of polyols. J. Phys. Chem. B 1998, 102, 7058–7066. [Google Scholar] [CrossRef]
- Kaushik, J.K.; Bhat, R. Why is trehalose an exceptional protein stabilizer? An analysis of the thermal stability of proteins in the presence of the compatible osmolyte trehalose. J. Biol. Chem. 2003, 278, 26458–26465. [Google Scholar] [CrossRef] [PubMed]
- Lerbret, A.; Bordat, P.; Affouard, F.; Descamps, M.; Migliardo, F. How homogeneous are the trehalose, maltose, and sucrose water solutions? An insight from molecular dynamics simulations. J. Phys. Chem. B 2005, 109, 11046–11057. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.K.; Roy, I. Effect of trehalose on protein structure. Prot. Sci. 2009, 18, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Gutowski, K.E.; Broker, G.A.; Willauer, H.D.; Huddleston, J.G.; Swatloski, R.P.; Holbrey, J.D.; Rogers, R.D. Controlling the aqueous miscibility of ionic liquids: Aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations. J. Am. Chem. Soc. 2003, 125, 6632–6633. [Google Scholar] [CrossRef] [PubMed]
- Abdolrahimi, S.; Nasernejad, B.; Pazuki, G. Influence of process variables on extraction of cefalexin in a novel biocompatible ionic liquid based-aqueous two phase system. Phys. Chem. Chem. Phys. 2015, 17, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Bewernitz, M.A.; Gebauer, D.; Long, J.; Cölfen, H.; Gower, L.B. A metastable liquid precursor phase of calcium carbonate and its interactions with polyaspartate. Faraday Discuss. 2012, 159, 291–312. [Google Scholar] [CrossRef]
- Colominas, C.; Luque, F.J.; Teixidó, J.; Orozco, M. Cavitation contribution to the free energy of solvation: Comparison of different formalisms in the context of MST calculations. Chem. Phys. 1999, 240, 253–264. [Google Scholar] [CrossRef]
- Bennion, B.J.; Daggett, V. The molecular basis for the chemical denaturation of proteins by urea. Proc. Natl. Acad. Sci. USA 2003, 100, 5142–5147. [Google Scholar] [CrossRef] [PubMed]
- Wallqvist, A.; Covell, D.; Thirumalai, D. Hydrophobic interactions in aqueous urea solutions with implications for the mechanism of protein denaturation. J. Am. Chem. Soc. 1998, 120, 427–428. [Google Scholar] [CrossRef]
- Zangi, R.; Zhou, R.; Berne, B.J. Urea’s action on hydrophobic interactions. J. Am. Chem. Soc. 2009, 131, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- England, J.L.; Pande, V.S.; Haran, G. Chemical denaturants inhibit the onset of dewetting. J. Am. Chem. Soc. 2008, 130, 11854–11855. [Google Scholar] [CrossRef] [PubMed]
- Boiocchi, M.; Del Boca, L.; Gómez, D.E.; Fabbrizzi, L.; Licchelli, M.; Monzani, E. Nature of urea-fluoride interaction: Incipient and definitive proton transfer. J. Am. Chem. Soc. 2004, 126, 16507–16514. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Cölfen, H. On the biophysical regulation of mineral growth: Standing out from the crowd. J. Struc. Biol. 2016, 196, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Yakimov, M.M.; La Cono, V.; Spada, G.L.; Bortoluzzi, G.; Messina, E.; Smedile, F.; Arcadi, E.; Borghini, M.; Ferrer, M.; Schmitt-Kopplin, P.; et al. Microbial community of the deep-sea brine Lake Kryos seawater–brine interface is active below the chaotropicity limit of life as revealed by recovery of mRNA. Env. Microbiol. 2015, 17, 364–382. [Google Scholar] [CrossRef]
- Addadi, L.; Weiner, S. Biomineralization: Crystals, asymmetry and life. Nature 2001, 411, 753–755. [Google Scholar] [CrossRef] [PubMed]
- Orme, C.; Noy, A.; Wierzbicki, A.; McBride, M.; Grantham, M.; Teng, H.; Dove, P.; DeYoreo, J. Formation of chiral morphologies through selective binding of amino acids to calcite surface steps. Nature 2001, 411, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.E.; Loges, N.; Mathiasch, B.; Panthöfer, M.; Mey, I.; Janshoff, A.; Tremel, W. Phase selection of calcium carbonate through the chirality of adsorbed amino acids. Angew. Chem. Int. Ed. 2007, 46, 5618–5623. [Google Scholar] [CrossRef] [PubMed]
- Oaki, Y.; Imai, H. Amplification of chirality from molecules into morphology of crystals through molecular recognition. J. Am. Chem. Soc. 2004, 126, 9271–9275. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Pacella, M.S.; Athanasiadou, D.; Nelea, V.; Vali, H.; Hazen, R.M.; Gray, J.J.; McKee, M.D. Chiral acidic amino acids induce chiral hierarchical structure in calcium carbonate. Nat. Comm. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Barron, L. From cosmic chirality to protein structure and function: Lord Kelvin’s legacy. QJM 1997, 90, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Heilbronner, E.; Dunitz, J.D. Reflections on Symmetry in Chemistry and Elsewhere; John Wiley & Sons: Hoboken, NJ, USA, 1993. [Google Scholar]
- Kellermeier, M.; Cölfen, H.; Gebauer, D. Investigating the early stages of mineral precipitation by potentiometric titration and analytical ultracentrifugation. Methods Enzymol. 2013, 532, 45–69. [Google Scholar] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, A.; Gebauer, D.; Cölfen, H. Modulating Nucleation by Kosmotropes and Chaotropes: Testing the Waters. Crystals 2017, 7, 302. https://doi.org/10.3390/cryst7100302
Rao A, Gebauer D, Cölfen H. Modulating Nucleation by Kosmotropes and Chaotropes: Testing the Waters. Crystals. 2017; 7(10):302. https://doi.org/10.3390/cryst7100302
Chicago/Turabian StyleRao, Ashit, Denis Gebauer, and Helmut Cölfen. 2017. "Modulating Nucleation by Kosmotropes and Chaotropes: Testing the Waters" Crystals 7, no. 10: 302. https://doi.org/10.3390/cryst7100302
APA StyleRao, A., Gebauer, D., & Cölfen, H. (2017). Modulating Nucleation by Kosmotropes and Chaotropes: Testing the Waters. Crystals, 7(10), 302. https://doi.org/10.3390/cryst7100302