Very Strong Parallel Interactions Between Two Saturated Acyclic Groups Closed with Intramolecular Hydrogen Bonds Forming Hydrogen-Bridged Rings
Abstract
:1. Introduction
2. Results
2.1. Search and Analysis of Crystal Structures from the CSD
2.2. Quantum Chemical Calculations
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CSD | Cambridge Structural Database |
| CCSD(T) | Coupled-Cluster with Single and Duble and Perturbative Triple excitations |
| CBS | Complete Basis Set |
| DFT | Density Functional Theory |
| ESI | Electronic Supporting Information |
| ESP | Electrostatic Potential |
| MP2 | Møller-Plesset Perturbation Theory of second order |
| cc-pVTZ | Correlation-Consistent Polarized Valence-only Triple-zeta basis set |
| BSSE | Basis Set Superposition Error |
| WFA-SAS | Wavefunction Analysis-Surface Analysis Suite |
References
- Salonen, L.M.; Ellermann, M.; Diederich, F. Aromatic rings in chemical and biological recognition: Energetics and structures. Angew. Chem. Int. Ed. 2011, 50, 4808–4842. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M.C.; Durham, T.B.; Valdes, H.E.; Dauer, K.L.; Karney, N.J.; Forrestel, A.C.; Bacchi, C.J.; Baker, J.F. Dibenzosuberyl substituted polyamines and analogs of clomipramine as effective inhibitors of trypanothione reductase; molecular docking, and assessment of trypanocidal activities. Bioorgan. Med. Chem. 2015, 23, 996–1010. [Google Scholar] [CrossRef] [PubMed]
- Woziwodzka, A.; Gołuński, G.; Wyrzykowski, D.; Kaźmierkiewicz, R.; Piosik, J. Caffeine and other methylxanthines as interceptors of food-borne aromatic mutagens: Inhibition of Trp-P-1 and Trp-P-2 mutagenic activity. Chem. Res. Toxicol. 2013, 26, 1660–1673. [Google Scholar] [CrossRef] [PubMed]
- Thio, Y.; Toh, S.W.; Xue, F.; Vittal, J.J. Self-assembly of a 15-nickel metallamacrocyclic complex derived from the l-glutamic acid Schiff base ligand. Dalton Trans. 2014, 43, 5998–6001. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Kuang, Y.; Gao, Y.; Zhang, Y.; Gao, P.; Xu, B. Aromatic-aromatic interactions induce the self-assembly of pentapeptidic derivatives in water to form nanofibers and supramolecular hydrogels. J. Am. Chem. Soc. 2010, 132, 2719–2728. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.-J. Binding mechanisms in supramolecular complexes. Angew. Chem. Int. Ed. 2009, 48, 3924–3977. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, W.B.; Dunitz, J.D. Quantum Mechanical Calculations for Benzene Dimer Energies: Present Problems and Future Challenges. J. Chem. Theory Comput. 2006, 2, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, S.; Honda, K.; Uchimaru, T.; Mikami, M.; Tanabe, K. Origin of Attraction and Directionality of the π/π Interaction: Model Chemistry Calculations of Benzene Dimer Interaction. J. Am. Chem. Soc. 2002, 124, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Sinnokrot, M.O.; Valeev, E.F.; Sherrill, C.D. Estimates of the Ab Initio Limit for π−π Interactions: The Benzene Dimer. J. Am. Chem. Soc. 2002, 124, 10887–10893. [Google Scholar] [CrossRef] [PubMed]
- Rezáč, J.; Riley, K.E.; Hobza, P. S66: A Well-balanced Database of Benchmark Interaction Energies Relevant to Biomolecular Structures. J. Chem. Theory Comput. 2011, 7, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Janowski, T.; Pulay, P. High accuracy benchmark calculations on the benzene dimer potential energy surface. Chem. Phys. Lett. 2007, 447, 27–32. [Google Scholar] [CrossRef]
- Bludský, O.; Rubes, M.; Soldán, P.; Nachtigall, P. Investigation of the benzene-dimer potential energy surface: DFT/CCSD(T) correction scheme. J. Chem. Phys. 2008, 128, 114102. [Google Scholar] [CrossRef] [PubMed]
- Hohenstein, E.G.; Sherrill, C.D. Effects of heteroatoms on aromatic π-π interactions: Benzene-pyridine and pyridine dimer. J. Phys. Chem. A 2009, 113, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Geronimo, I.; Lee, E.C.; Singh, N.J.; Kim, K.S. How Different are Electron-Rich and Electron-Deficient π Interactions? J. Chem. Theory Comput. 2010, 6, 1931–1934. [Google Scholar] [CrossRef] [PubMed]
- Ninković, D.B.; Janjić, G.V.; Veljković, D.Ž.; Sredojević, D.N.; Zarić, S.D. What are the preferred horizontal displacements in parallel aromatic-aromatic interactions? Significant interactions at large displacements. Chemphyschem 2011, 12, 3511–3514. [Google Scholar] [CrossRef] [PubMed]
- Ninković, D.B.; Andrić, J.M.; Malkov, S.N.; Zarić, S.D. What are the preferred horizontal displacements of aromatic-aromatic interactions in proteins? Comparison with the calculated benzene-benzene potential energy surface. Phys. Chem. Chem. Phys. 2014, 16, 11173–11177. [Google Scholar] [CrossRef] [PubMed]
- Sinnokrot, M.O.; Sherrill, C.D. High-accuracy quantum mechanical studies of π-π interactions in benzene dimers. J. Phys. Chem. A 2006, 110, 10656–10668. [Google Scholar] [CrossRef] [PubMed]
- Podeszwa, R.; Bukowski, R.; Szalewicz, K. Potential energy surface for the benzene dimer and perturbational analysis of π-π interactions. J. Phys. Chem. A 2006, 110, 10345–10354. [Google Scholar] [CrossRef] [PubMed]
- Pitoňák, M.; Neogrády, P.; Řezáč, J.; Jurečka, P.; Urban, M.; Hobza, P. Benzene Dimer: High-Level Wave Function and Density Functional Theory Calculations. J. Chem. Theory Comput. 2008, 4, 1829–1834. [Google Scholar]
- Ninković, D.B.; Janjić, G.V.; Zarić, S.D. Crystallographic and ab Initio Study of Pyridine Stacking Interactions. Local Nature of Hydrogen Bond Effect in Stacking Interactions. Cryst. Growth Des. 2012, 12, 1060–1063. [Google Scholar] [CrossRef]
- Ninković, D.B.; Andrić, J.M.; Zarić, S.D. Parallel interactions at large horizontal displacement in pyridine-pyridine and benzene-pyridine dimers. Chemphyschem 2013, 14, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Craven, E.; Zhang, C.; Janiak, C.; Rheinwald, G.; Lang, H. Synthesis, Structure and Solution Chemistry of (5, 5′-Dimethyl-2, 2′-bipyridine)(IDA)copper(II) and Structural Comparison With Aqua(IDA)(1, 10-phenanthroline)copper(II) (IDA = iminodiacetato). Z. Anorg. Allg. Chem. 2003, 629, 2282–2290. [Google Scholar] [CrossRef]
- Janjić, G.V.; Veljković, D.Z.; Zarić, S.D. Water/Aromatic Parallel Alignment Interactions. Significant Interactions at Large Horizontal Displacements. Cryst. Growth Des. 2011, 11, 2680–2683. [Google Scholar] [CrossRef]
- Sredojević, D.; Bogdanović, G.A.; Tomić, Z.D.; Zarić, S.D. Stacking vs. CH–π interactions between chelate and aryl rings in crystal structures of square-planar transition metal complexes. CrystEngComm 2007, 9, 793–798. [Google Scholar] [CrossRef]
- Sredojević, D.N.; Tomić, Z.D.; Zarić, S.D. Evidence of Chelate−Chelate Stacking Interactions in Crystal Structures of Transition-Metal Complexes. Cryst. Growth Des. 2010, 10, 3901–3908. [Google Scholar] [CrossRef]
- Tomić, Z.D.; Leovac, V.M.; Pokorni, S.V.; Zobel, D.; Zarić, S.D. Crystal Structure of Bis[acetone-1-naphthoylhydrazinato(−1)]copper(II) and Investigations of Intermolecular Interactions. Eur. J. Inorg. Chem. 2003, 6, 1222–1226. [Google Scholar] [CrossRef]
- Tomić, Z.D.; Sredojević, D.; Zarić, S.D. Stacking Interactions between Chelate and Phenyl Rings in Square-Planar Transition Metal Complexes. Cryst. Growth Des. 2006, 6, 29–31. [Google Scholar] [CrossRef]
- Wang, X.-J.; Jian, H.-X.; Liu, Z.-P.; Ni, Q.-L.; Gui, L.-C.; Tang, L.-H. Assembly molecular architectures based on structural variation of metalloligand [Cu(PySal)2] (PySal = 3-pyridylmethylsalicylideneimino). Polyhedron 2008, 27, 2634–2642. [Google Scholar] [CrossRef]
- Granifo, J.; Vargas, M.; Garland, M.T.; Ibáñez, A.; Gaviño, R.; Baggio, R. The novel ligand 4′-phenyl-3,2′:6′,3′′-terpyridine (L) and the supramolecular structure of the dinuclear complex [Zn2(μ-L)(acac)4]·H2O (acac = acetylacetonato). Inorg. Chem. Commun. 2008, 11, 1388–1391. [Google Scholar] [CrossRef]
- Philip, V.; Suni, V.; Prathapachandra Kurup, M.R.; Nethaji, M. Structural and spectral studies of nickel(II) complexes of di-2-pyridyl ketone N4,N4-(butane-1,4-diyl) thiosemicarbazone. Polyhedron 2004, 23, 1225–1233. [Google Scholar] [CrossRef]
- Konidaris, K.F.; Tsipis, A.C.; Kostakis, G.E. Shedding Light on Intermolecular Metal-Organic Ring Interactions by Theoretical Studies. ChemPlusChem 2012, 77, 354–360. [Google Scholar] [CrossRef]
- Konidaris, K.F.; Morrison, C.N.; Servetas, J.G.; Haukka, M.; Lan, Y.; Powell, A.K.; Plakatouras, J.C.; Kostakis, G.E. Supramolecular assemblies involving metal–organic ring interactions: Heterometallic Cu(II)–Ln(III) two-dimensional coordination polymers. CrystEngComm 2012, 14, 1842–1849. [Google Scholar] [CrossRef]
- Konidaris, K.F.; Powell, A.K.; Kostakis, G.E. Peculiar structural findings in coordination chemistry of malonamide–N,N′-diacetic acid. CrystEngComm 2011, 13, 5872–5876. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Molecular crystals by design? Chem. Commun. 2014, 50, 11079–11082. [Google Scholar] [CrossRef] [PubMed]
- Baul, T.S.B.; Kundu, S.; Mitra, S.; Höpfl, H.; Tiekink, E.R.T.; Linden, A. The influence of counter ion and ligand methyl substitution on the solid-state structures and photophysical properties of mercury(II) complexes with (E)-N-(pyridin-2-ylmethylidene)arylamines. Dalton Trans. 2013, 42, 1905–1920. [Google Scholar] [CrossRef] [PubMed]
- Khavasi, H.R.; Sadegh, B.M.M. Influence of N-heteroaromatic π-π stacking on supramolecular assembly and coordination geometry; effect of a single-atom change in the ligand. Dalton Trans. 2015, 44, 5488–5502. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Monfared, H.; Pousaneh, E.; Sadighian, S.; Ng, S.W.; Tiekink, E.R.T. Syntheses, Structures, and Catalytic Activity of Copper(II)-Aroylhydrazone Complexes. Z. Anorg. Allg. Chem. 2013, 639, 435–442. [Google Scholar] [CrossRef]
- Ni, Q.-L.; Jiang, X.-F.; Gui, L.-C.; Wang, X.-J.; Yang, K.-G.; Bi, X.-S. Synthesis, structures and characterization of a series of Cu(I)-diimine complexes with labile N,N′-bis((diphenylphosphino)methyl)naphthalene-1,5-diamine: Diverse structures directed by π–π stacking interactions. New J. Chem. 2011, 35, 2471–2476. [Google Scholar] [CrossRef]
- Molčanov, K.; Jurić, M.; Kojić-Prodić, B. Stacking of metal chelating rings with π-systems in mononuclear complexes of copper(II) with 3,6-dichloro-2,5-dihydroxy-1,4-benzoquinone (chloranilic acid) and 2,2’-bipyridine ligands. Dalton Trans. 2013, 42, 15756–15765. [Google Scholar] [CrossRef] [PubMed]
- Akine, S.; Varadi, Z.; Nabeshima, T. Synthesis of Planar Metal Complexes and the Stacking Abilities of Naphthalenediol-Based Acyclic and Macrocyclic Salen-Type Ligands. Eur. J. Inorg. Chem. 2013, 35, 5987–5998. [Google Scholar] [CrossRef]
- Melnic, E.; Coropceanu, E.B.; Kulikova, O.V.; Siminel, A.V.; Anderson, D.; Rivera-Jacquez, H.J.; Masunov, A.E.; Fonari, M.S.; Kravtsov, V.C. Robust Packing Patterns and Luminescence Quenching in Mononuclear [Cu(II)(phen)2] Sulfates. J. Phys. Chem. C 2014, 118, 30087–30100. [Google Scholar] [CrossRef]
- Zhao, Y.; Chang, X.-H.; Liu, G.-Z.; Ma, L.-F.; Wang, L.-Y. Five Mn(II) Coordination Polymers Based on 2,3′,5,5′-Biphenyl Tetracarboxylic Acid: Syntheses, Structures, and Magnetic Properties. Cryst. Growth Des. 2015, 15, 966–974. [Google Scholar] [CrossRef]
- Malenov, D.P.; Ninković, D.B.; Sredojević, D.N.; Zarić, S.D. Stacking of benzene with metal chelates: Calculated CCSD(T)/CBS interaction energies and potential-energy curves. Chemphyschem 2014, 15, 2458–2461. [Google Scholar] [CrossRef] [PubMed]
- Malenov, D.P.; Ninković, D.B.; Zarić, S.D. Stacking of metal chelates with benzene: Can dispersion-corrected DFT be used to calculate organic-inorganic stacking? Chemphyschem 2015, 16, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Karabıyık, H.; Karabıyık, H.; Ocak İskeleli, N. Hydrogen-bridged chelate ring-assisted π-stacking interactions. Acta Cryst. B 2012, 68, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Blagojević, J.P.; Zarić, S.D. Stacking interactions of hydrogen-bridged rings—Stronger than the stacking of benzene molecules. Chem. Commun. 2015, 51, 12989–12991. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.I.; Halim, S.N.A.; Ng, S.W.; Tan, S.L.; Zukerman-Schpector, J.; Ferreira, M.A.B.; Tiekink, E.R.T. Investigation of putative arene-C-H···π(quasi-chelate ring) interactions in copper(I) crystal structures. Chem. Commun. 2014, 50, 5984–5986. [Google Scholar] [CrossRef] [PubMed]
- Sobczyk, L.; Grabowski, S.J.; Krygowski, T.M. Interrelation between H-bond and Pi-electron delocalization. Chem. Rev. 2005, 105, 3513–3560. [Google Scholar] [CrossRef] [PubMed]
- Lyssenko, K.A.; Antipin, M.Y. The nature and energy characteristics of intramolecular hydrogen bonds in crystals. Russ. Chem. Bull. 2006, 55, 1–15. [Google Scholar] [CrossRef]
- Sanz, P.; Mó, O.; Yañez, M.; Elguero, J. Resonance-assisted hydrogen bonds: A critical examination. Structure and stability of the enols of beta-diketones and beta-enaminones. J. Phys. Chem. A 2007, 111, 3585–3591. [Google Scholar] [CrossRef] [PubMed]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Cryst. Sect. B—Struct. Sci. 2002, 58, 389–397. [Google Scholar] [CrossRef]
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H., Jr.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Raghavachari, K.; Trucks, G.W.; Pople, J.A.; Head-Gordon, M. A fifth-order perturbation comparison of electron correlation theories. Chem. Phys. Lett. 1989, 157, 479–483. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 (Revision D.01); Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Bulat, F.A.; (Fable Theory & Computation LLC, Washington, DC, USA); Toro-Labbe, A.; (Pontificia Universidad Católica de Chile, Santiago, Chile). Personal communication, 2013.
- Bulat, F.A.; Toro-Labbé, A.; Brinck, T.; Murray, J.S.; Politzer, P. Quantitative analysis of molecular surfaces: Areas, volumes, electrostatic potentials and average local ionization energies. J. Mol. Model. 2010, 16, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
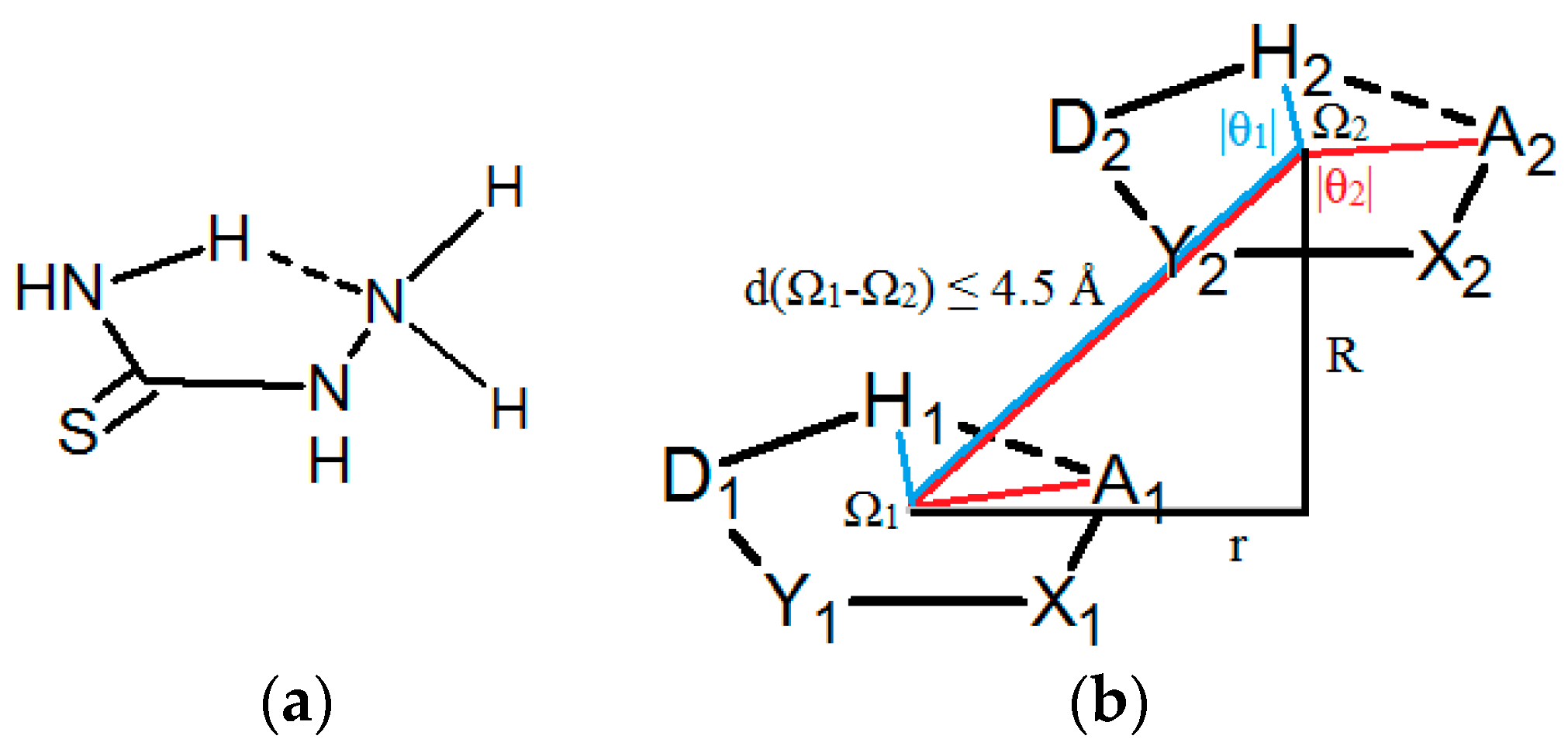
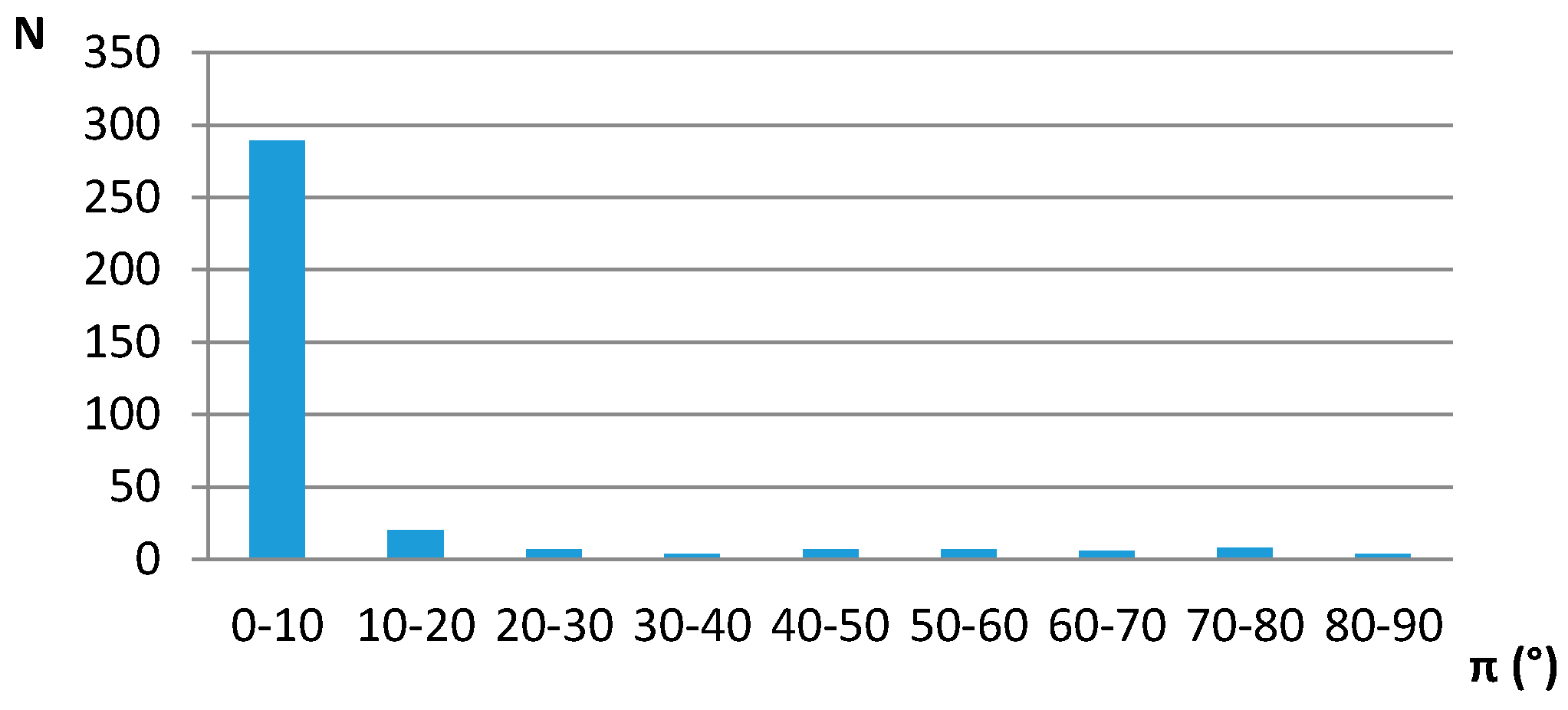
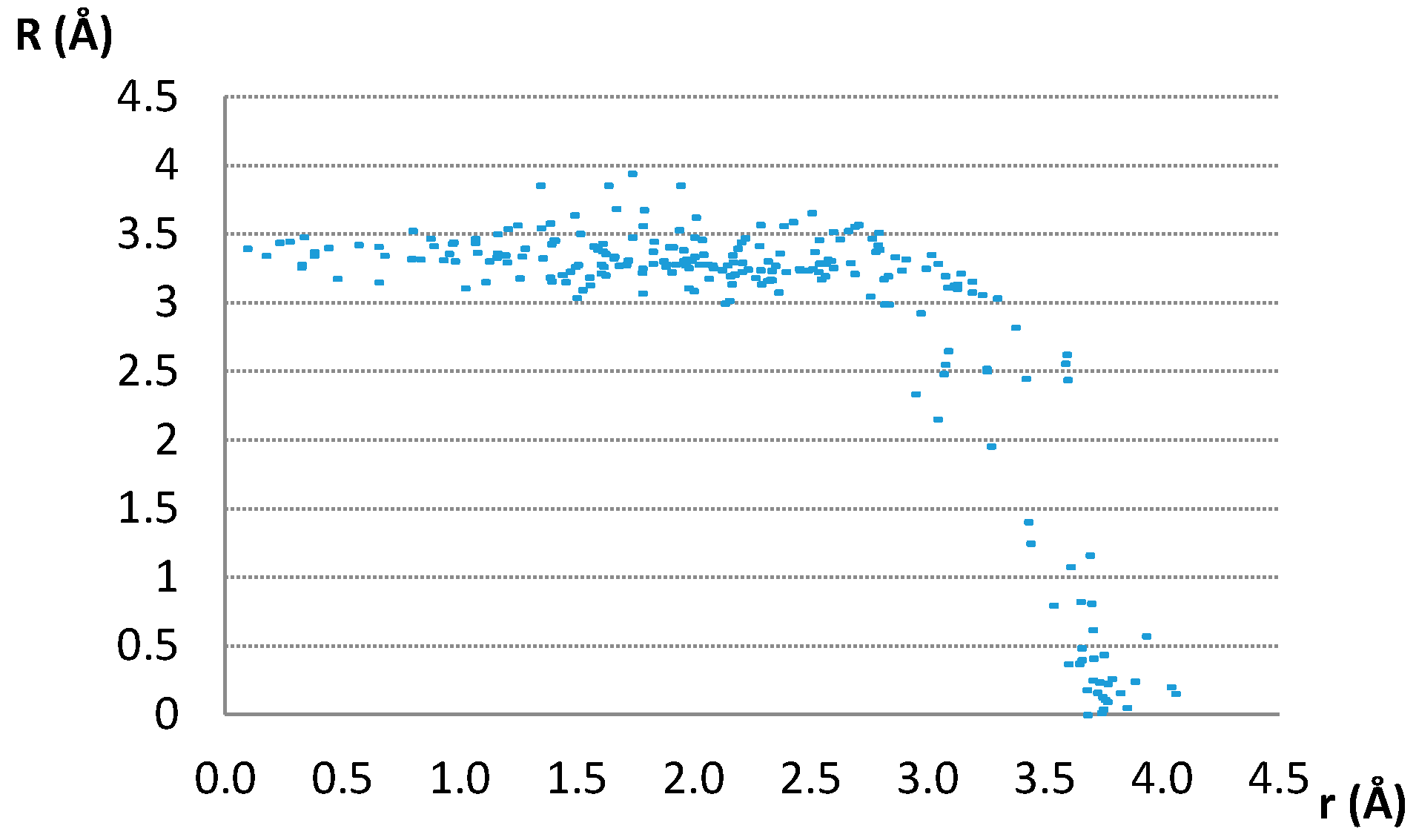

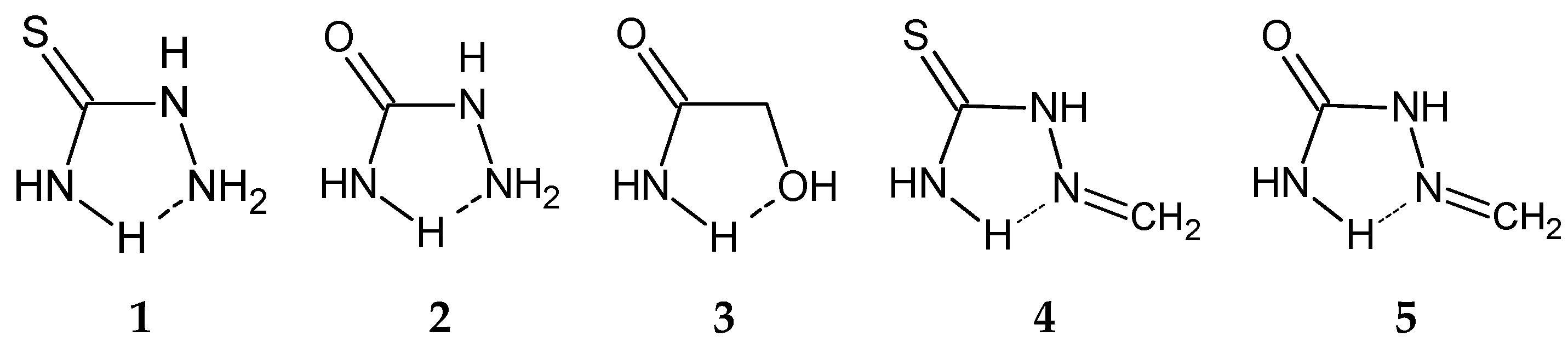
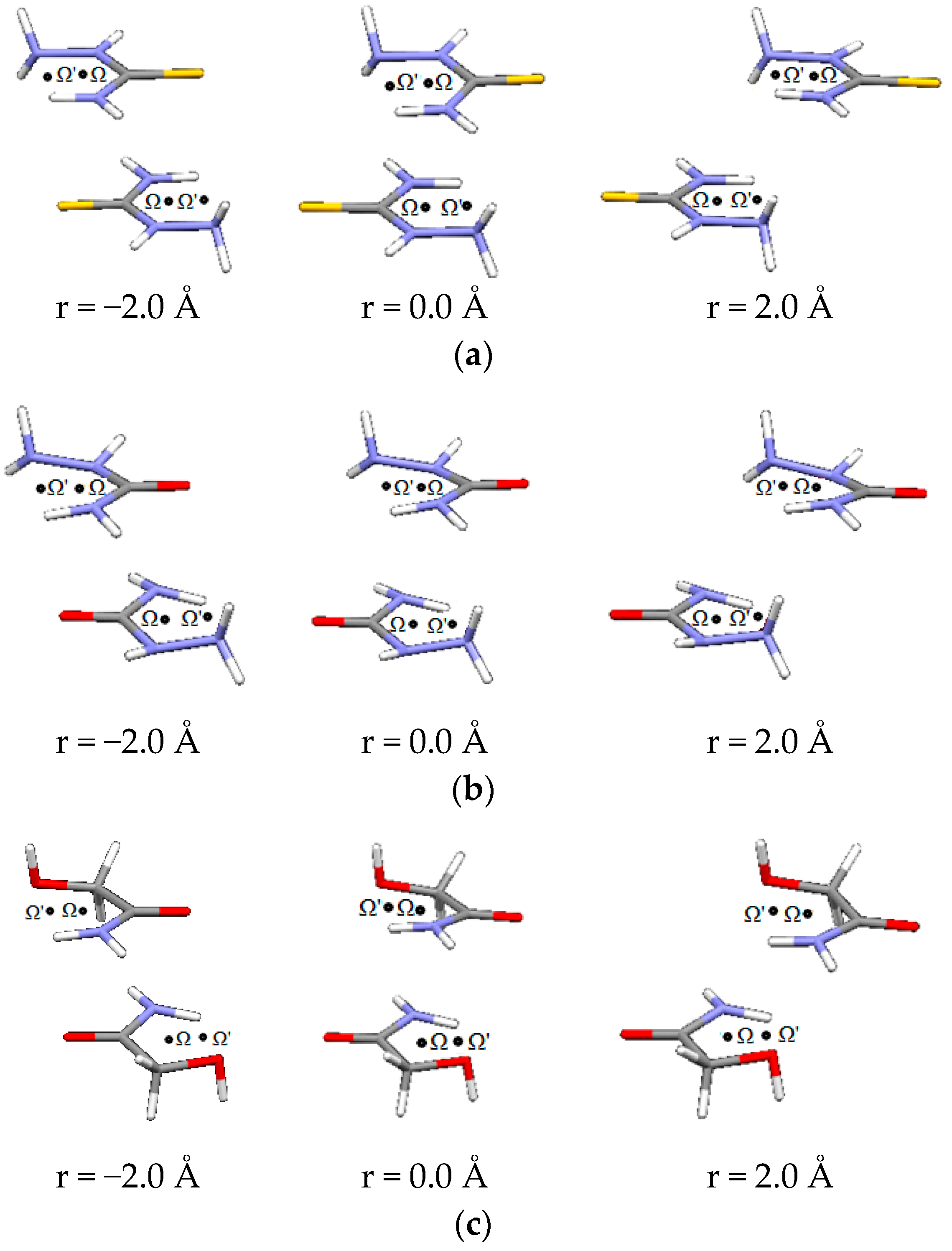
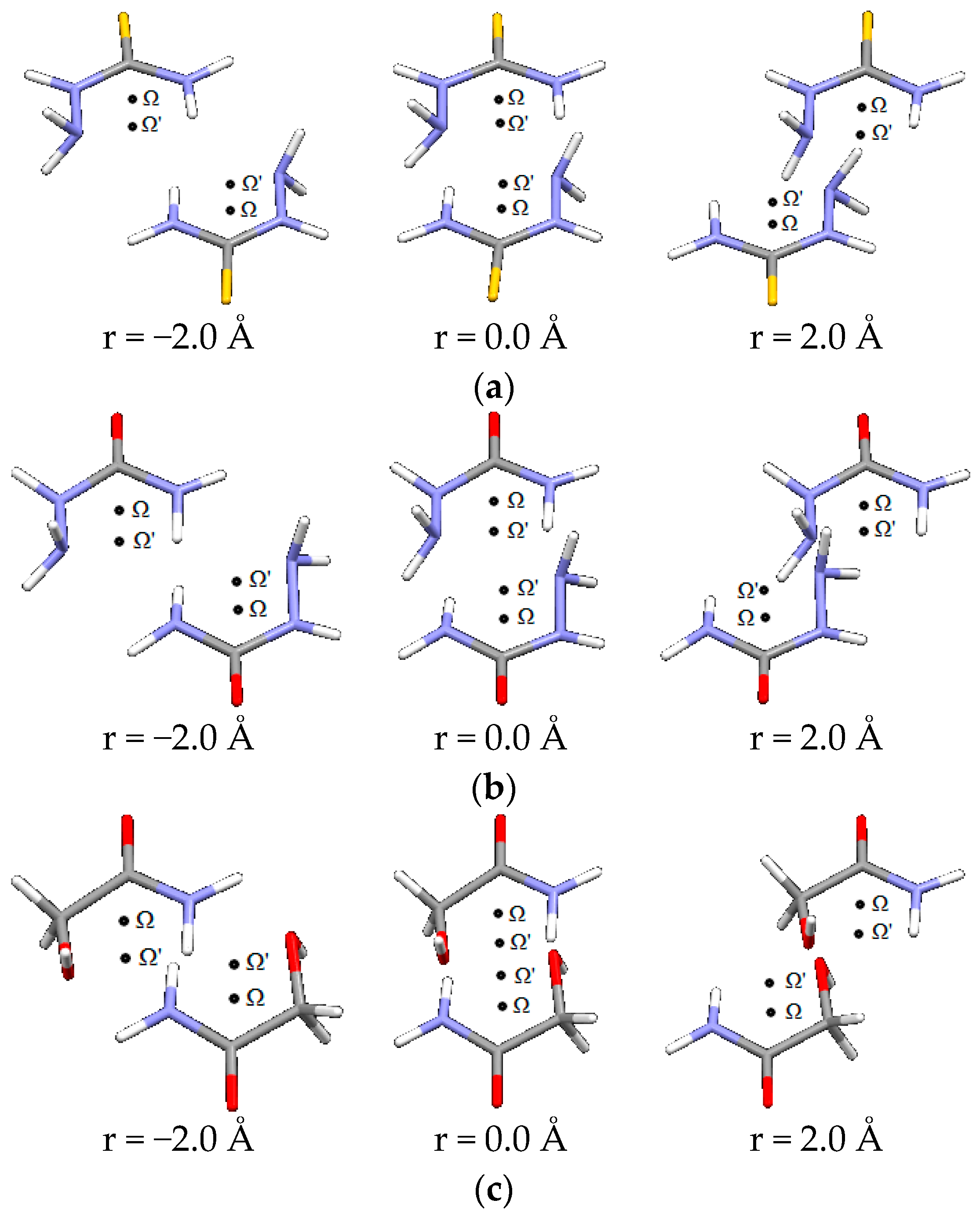
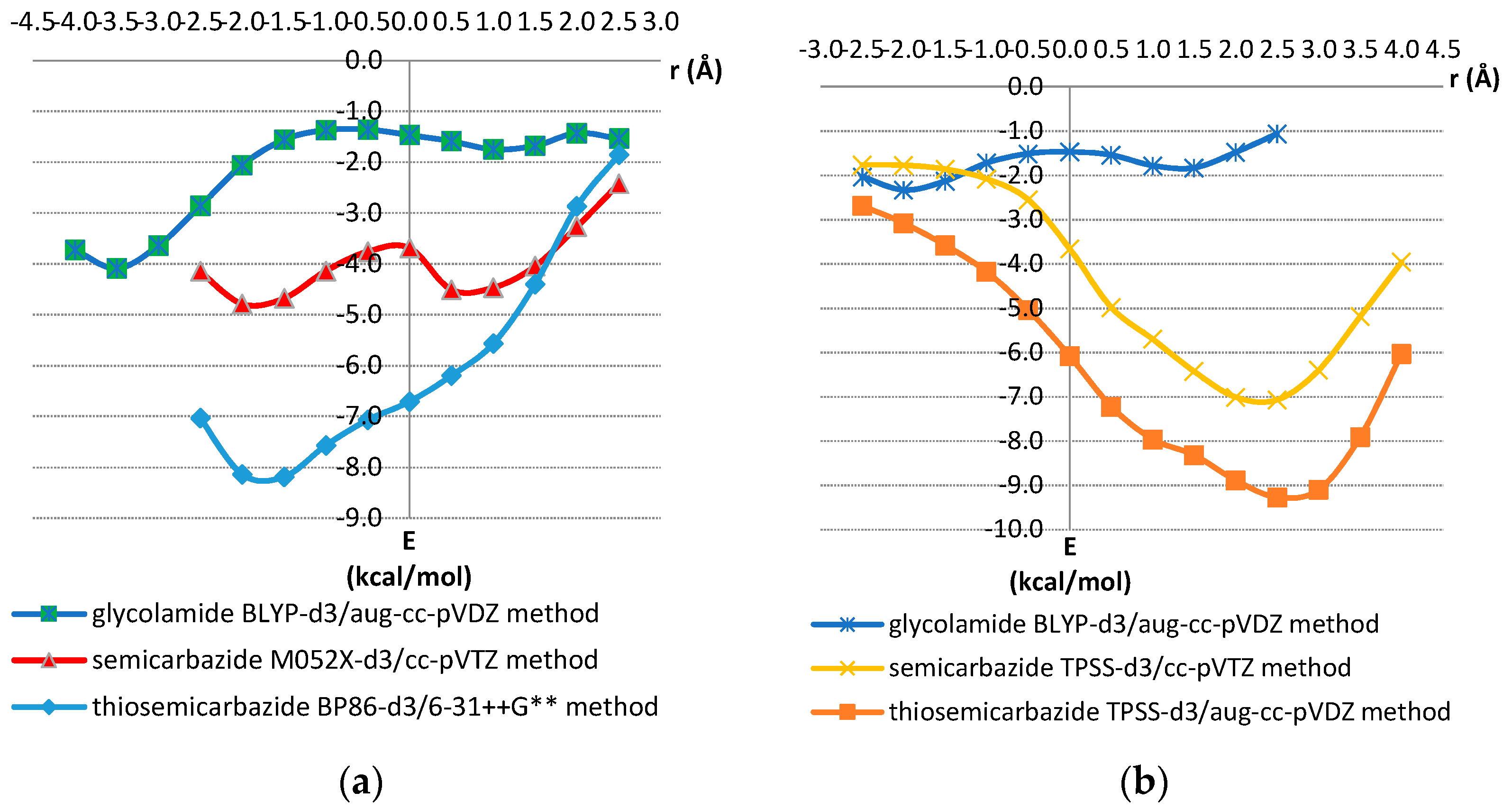
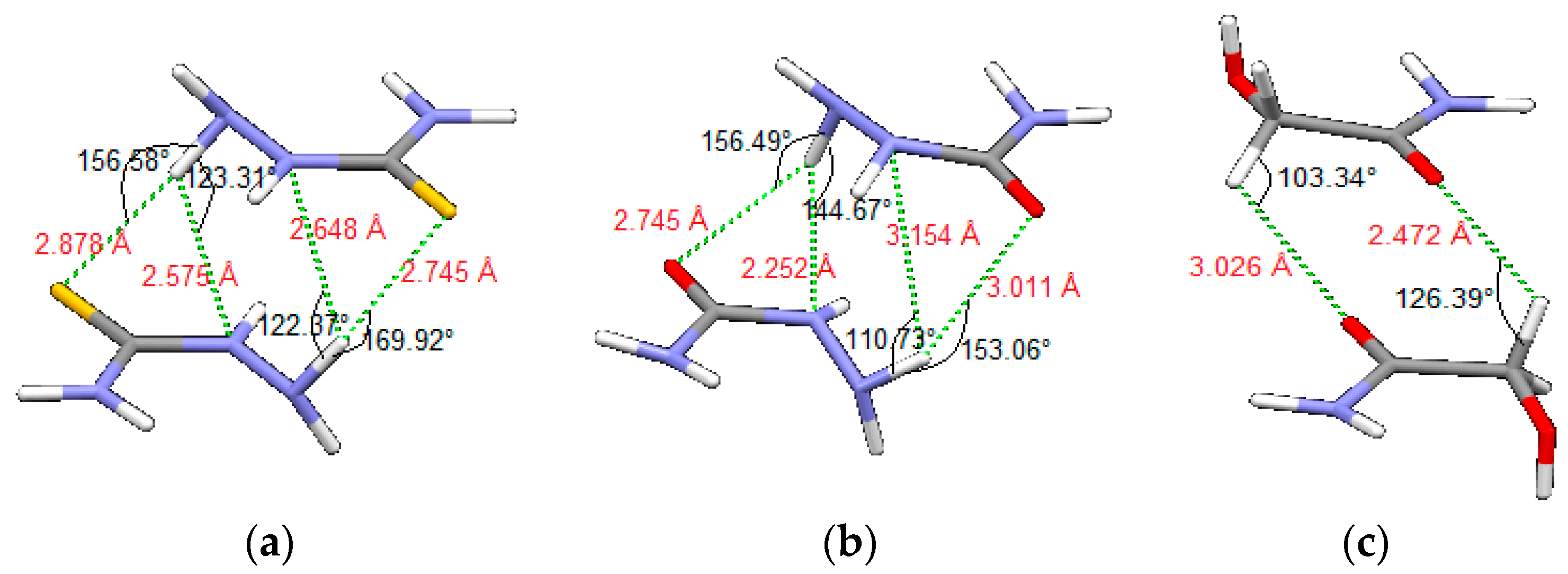


| D,Y,X,A | Percentage of Total Number of Contacts |
|---|---|
| N,C,N,N | 49.1 |
| N,C,C,O | 18.8 |
| N,C,C, Z | 8.0 |
| N,C,N,O | 4.3 |
| other | 19.8 |
| Model System | Ω-Ω’ Direction | Orthogonal to Ω-Ω’ Direction | ||||
|---|---|---|---|---|---|---|
| r | Em | CCSD(T)/CBS | r | Em | CCSD(T)/CBS | |
| 1 | −1.5 | −8.19 | −7.66 | 2.5 | −9.27 | −9.68 |
| 2 | −2.0 | −4.79 | −4.90 | 2.5 | −7.07 | −7.12 |
| 3 | −3.5 | −4.09 | −4.25 | −2.0 | −2.33 | −2.21 |
| 4 | 0.0 | −4.49 | −4.84 | −1.0 | −4.91 | −4.89 |
| 5 | 0.0 | −2.88 | −2.95 | −1.0 | −3.19 | −2.95 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blagojević, J.P.; Janjić, G.V.; Zarić, S.D. Very Strong Parallel Interactions Between Two Saturated Acyclic Groups Closed with Intramolecular Hydrogen Bonds Forming Hydrogen-Bridged Rings. Crystals 2016, 6, 34. https://doi.org/10.3390/cryst6040034
Blagojević JP, Janjić GV, Zarić SD. Very Strong Parallel Interactions Between Two Saturated Acyclic Groups Closed with Intramolecular Hydrogen Bonds Forming Hydrogen-Bridged Rings. Crystals. 2016; 6(4):34. https://doi.org/10.3390/cryst6040034
Chicago/Turabian StyleBlagojević, Jelena P., Goran V. Janjić, and Snežana D. Zarić. 2016. "Very Strong Parallel Interactions Between Two Saturated Acyclic Groups Closed with Intramolecular Hydrogen Bonds Forming Hydrogen-Bridged Rings" Crystals 6, no. 4: 34. https://doi.org/10.3390/cryst6040034
APA StyleBlagojević, J. P., Janjić, G. V., & Zarić, S. D. (2016). Very Strong Parallel Interactions Between Two Saturated Acyclic Groups Closed with Intramolecular Hydrogen Bonds Forming Hydrogen-Bridged Rings. Crystals, 6(4), 34. https://doi.org/10.3390/cryst6040034






