Synthesis, Structure, and Magnetic Properties of New Spin Crossover Fe(II) Complexes Forming Short Hydrogen Bonds with Substituted Dicarboxylic Acids
Abstract
:1. Introduction
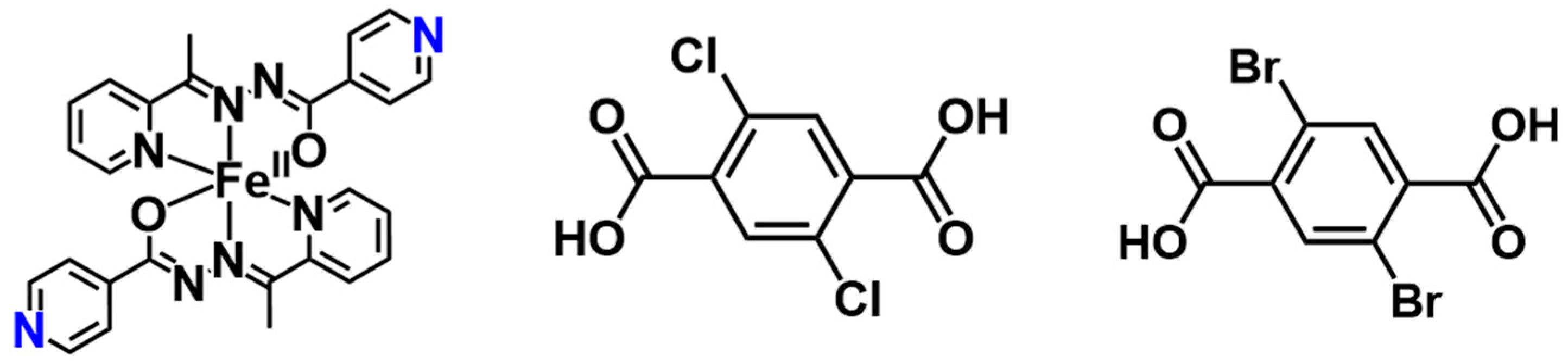
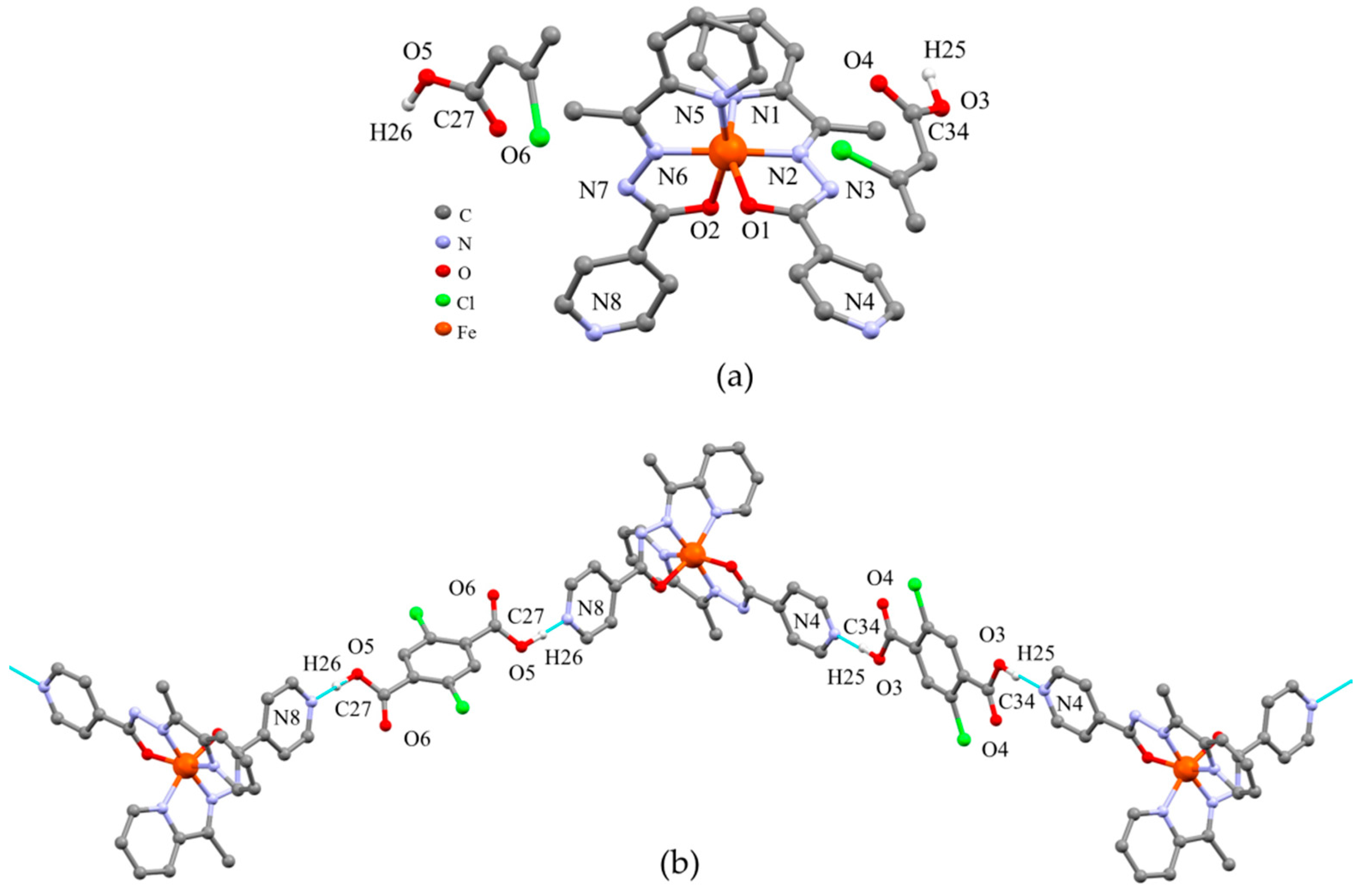
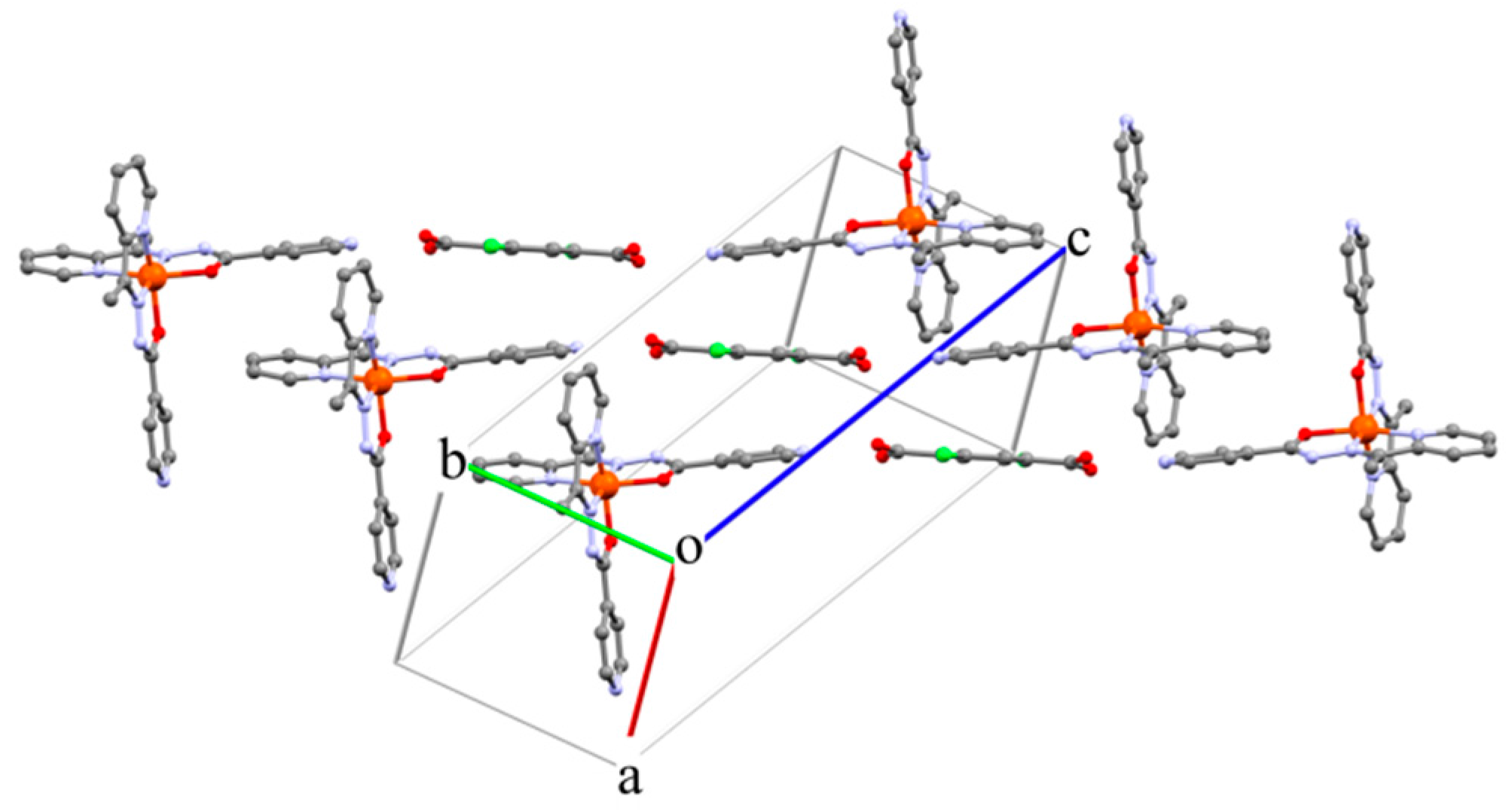
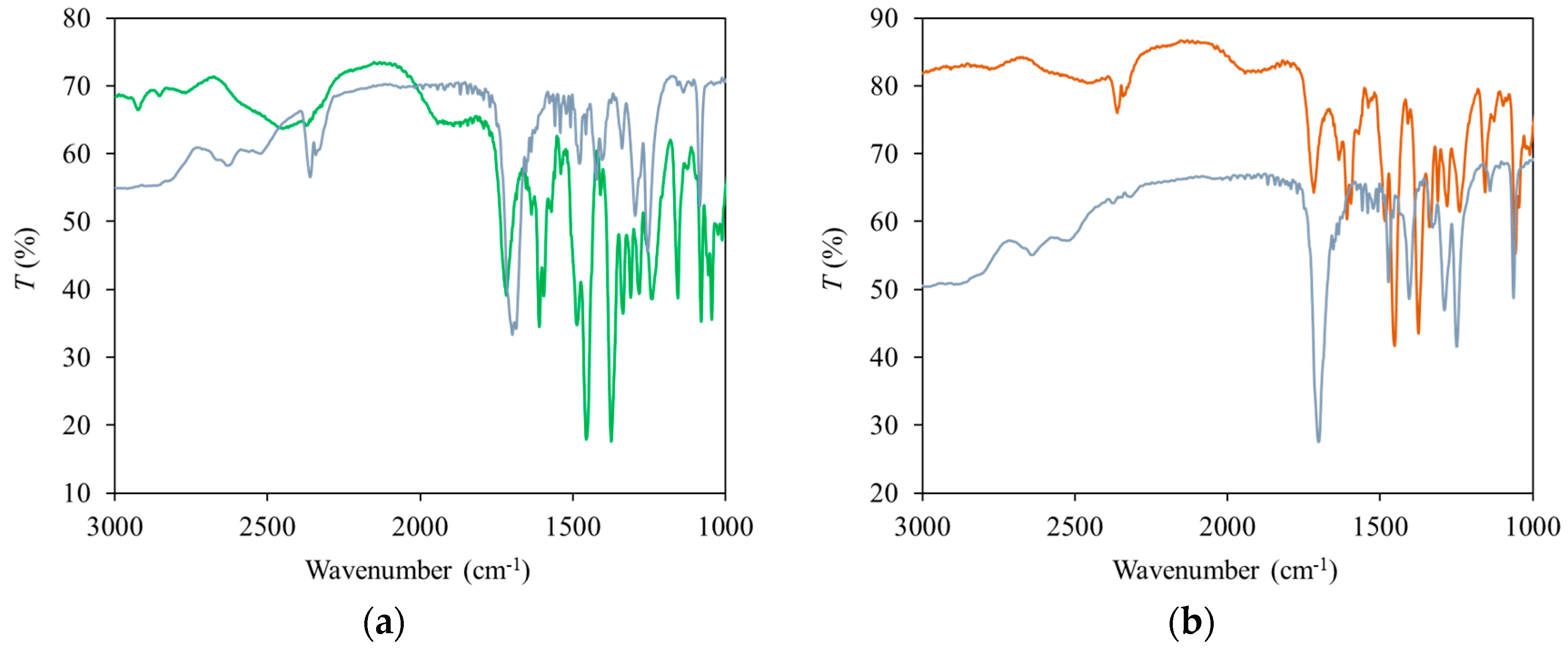
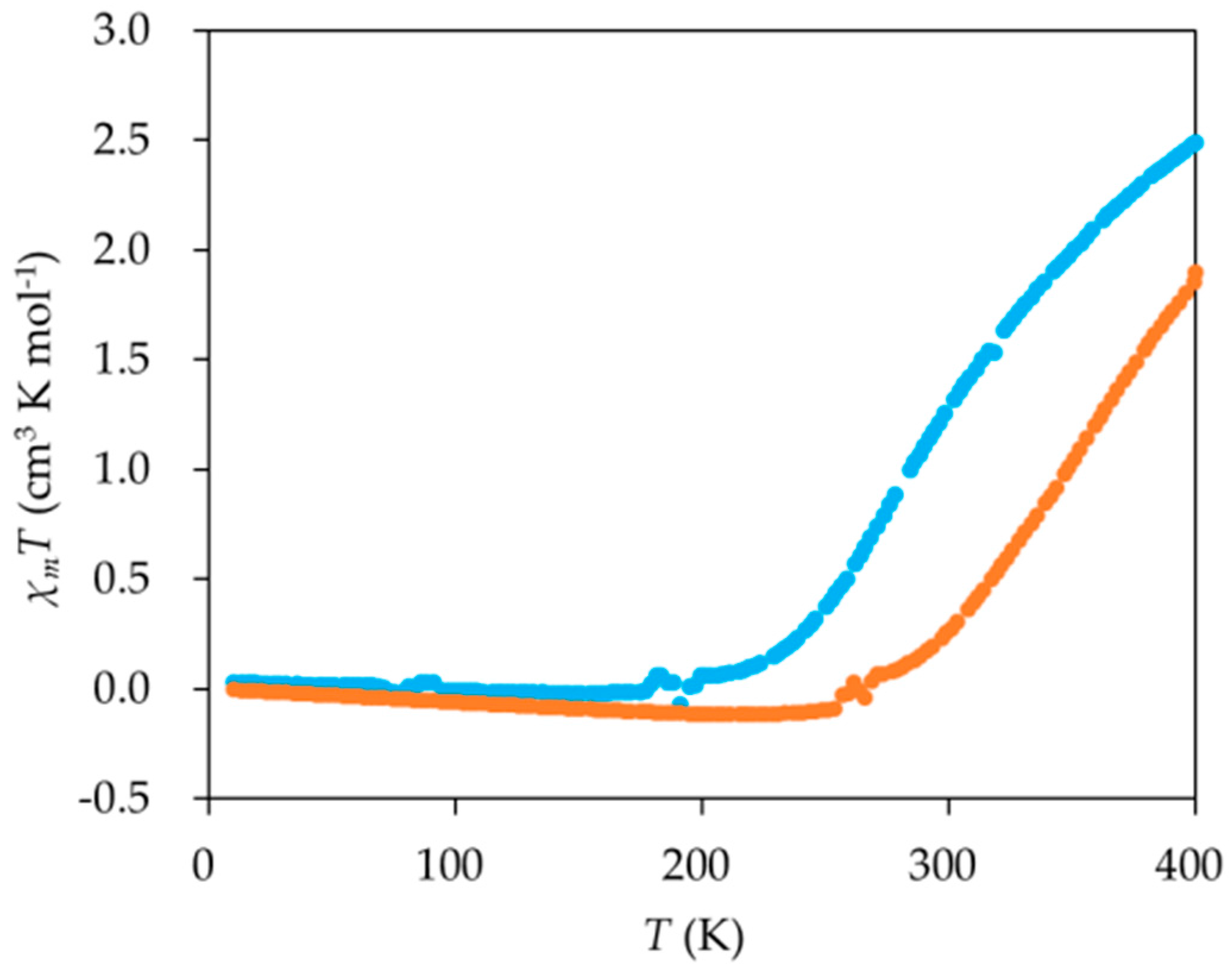
2. Results and Discussion
Experiment
3. Experimental Section
3.1. Synthesis
3.1.1. Synthesis of Ligand HL
3.1.2. Synthesis of [Fe(L)2](H2Cl2TPA) (1-Cl)
3.1.3. Synthesis of [Fe(L)2](H2Br2TPA) (1-Br)
3.2. X-ray Structure Determination
3.3. IR Spectra
3.4. Magnetic Property Measurement
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Yamaguchi, S.; Kamikubo, H.; Kurihara, K.; Kuroki, R.; Niimura, N.; Shimizu, N.; Yamazaki, Y.; Kataoka, M. Low-barrier hydrogen bond in photoactive yellow protein. Proc. Natl. Acad. Sci. USA 2009, 106, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, T.; Shibuguchi, T.; Fukuta, Y.; Shibasaki, M. Catalytic asymmetric phase-transfer reactions using tartrate-derived asymmetric two-center organocatalysts. Tetrahedron 2004, 60, 7743–7754. [Google Scholar] [CrossRef]
- Shan, N.; Bond, A.D.; Jones, W. Crystal engineering using 4,4′-bipyridyl with di- and tricarboxylic acids. Cryst. Eng. 2002, 5, 9–24. [Google Scholar] [CrossRef]
- Horiuchi, S.; Ishii, F.; Kumai, R.; Okimoto, Y.; Tachibana, H.; Nagaosa, N.; Tokura, Y. Ferroelectricity near room temperature in co-crystals of nonpolar organic molecules. Nat. Mater. 2005, 4, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, S.; Kumai, R.; Tokura, Y. A supramolecular ferroelectric realized by collective proton transfer. Angew. Chem. Int. Ed. Engl. 2007, 46, 3497–3501. [Google Scholar] [CrossRef] [PubMed]
- Sato, O. Dynamic molecular crystals with switchable physical properties. Nat. Chem. 2016, 8, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Ueda, A.; Yamada, S.; Isono, T.; Kamo, H.; Nakao, A.; Kumai, R.; Nakao, H.; Murakami, Y.; Yamamoto, K.; Nishio, Y.; et al. Hydrogen-bond-dynamics-based switching of conductivity and magnetism: A phase transition caused by deuterium and electron transfer in a hydrogen-bonded purely organic conductor crystal. J. Am. Chem. Soc. 2014, 136, 12184–12192. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Gimenez-Lopez, M.C.; Gimenez-Saiz, C.; Romero, F.M. Spin crossover complexes as building units of hydrogen-bonded nanoporous structures. CrystEngComm 2009, 11, 2198–2203. [Google Scholar] [CrossRef]
- Hill, S.; Datta, S.; Liu, J.; Inglis, R.; Milios, C.J.; Feng, P.L.; Henderson, J.J.; del Barco, E.; Brechin, E.K.; Hendrickson, D.N. Magnetic quantum tunneling: Insights from simple molecule-based magnets. Dalton Trans. 2010, 39, 4693–4707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, G.C.; Xu, H.B.; Zhang, T.; Wang, Z.M.; Yuan, M.; Gao, S. Abrupt spin transition around room temperature and light induced properties in Fe(II) complexes with N4O2 coordination sphere. Chem. Commun. 2010, 46, 2554–2556. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, H.; Duelund, L.; Zimmermann, A.; Averseng, F.; Gerdan, M.; Winkler, H.; Toftlund, H.; Trautwein, A.X. Substituent effects on the spin-transition temperature in complexes with tris(pyrazolyl) ligands. Monatsh. Chem. 2003, 134, 295–306. [Google Scholar] [CrossRef]
- Lemercier, G.; Brefuel, N.; Shova, S.; Wolny, J.A.; Dahan, F.; Verelst, M.; Paulsen, H.; Trautwein, A.X.; Tuchagues, J.P. A range of spin-crossover temperature T1/2 > 300 k results from out-of-sphere anion exchange in a series of ferrous materials based on the 4-(4-imidazolylmethyl)-2-(2-imidazolylmethyl)imidazole (trim) ligand, [Fe(trim)2]X2 (X = F, Cl, Br, I): Comparison of experimental results with those derived from density functional theory calculations. Chem. Eur. J. 2006, 12, 7421–7432. [Google Scholar] [PubMed]
- Cowan, J.A.; Howard, J.A.; McIntyre, G.J.; Lo, S.M.; Williams, I.D. Variable-temperature neutron diffraction studies of the short, strong hydrogen bonds in the crystal structure of pyridine-3,5-dicarboxylic acid. Acta Crystallogr. B 2005, 61, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T.; Majerz, I.; Wilson, C.C. First O–H–N hydrogen fond with a centered proton obtained by thermally induced proton migration. Angew. Chem. Int. Ed. 2001, 40, 2651–2654. [Google Scholar] [CrossRef]
- Cowan, J.A.; Howard, J.A.K.; McIntyre, G.J.; Lo, S.M.F.; Williams, I.D. Variable-temperature neutron diffraction studies of the short, strong n center dot center dot center dot o hydrogen bonds in the 1:2 co-crystal of benzene-1,2,4,5-tetracarboxylic acid and 4,4′-bipyridyl. Acta Crystallogr. Sect. B-Struct. Sci. 2003, 59, 794–801. [Google Scholar] [CrossRef]
- Grobelny, P.; Mukherjee, A.; Desiraju, G.R. Drug-drug co-crystals: Temperature-dependent proton mobility in the molecular complex of isoniazid with 4-aminosalicylic acid. CrystEngComm 2011, 13, 4358–4364. [Google Scholar] [CrossRef]
- Cherukuvada, S.; Nangia, A. Fast dissolving eutectic compositions of two anti-tubercular drugs. CrystEngComm 2012, 14, 2579–2588. [Google Scholar] [CrossRef]
- Aitipamula, S.; Wong, A.B.H.; Chow, P.S.; Tan, R.B.H. Novel solid forms of the anti-tuberculosis drug, isoniazid: Ternary and polymorphic cocrystals. CrystEngComm 2013, 15, 5877–5887. [Google Scholar] [CrossRef]
- Bis, J.A.; Zaworotko, M.J. The 2-aminopyridinium-carboxylate supramolecular heterosynthon: A robust motif for generation of multiple-component crystals. Cryst. Growth Des. 2005, 5, 1169–1179. [Google Scholar] [CrossRef]
- Bhunia, M.K.; Das, S.K.; Bhaumik, A. Temperature induced proton transfer in a hydrogen bonded supramolecule. Chem. Phys. Lett. 2010, 498, 145–150. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Hussain, I.; Forbes, S.; Desper, J. Exploring the hydrogen-bond preference of N–H moieties in co-crystals assembled via O–H(acid)∙∙∙N(py) intermolecular interactions. CrystEngComm 2007, 9, 46–54. [Google Scholar] [CrossRef]
- Ababei, L.V.; Kriza, A.; Andronescu, C.; Musuc, A.M. Synthesis and characterization of new complexes of some divalent transition metals with 2-acetyl-pyridyl-isonicotinoylhydrazone. J. Therm. Anal. Calorim. 2011, 107, 573–584. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
| Crystallographic Data | ||||
|---|---|---|---|---|
| 1-Cl | 1-Bt | |||
| Formula | C34H26Cl2FeN8O6 | C34H26Br2FeN8O6 | ||
| Formula weight | 769.38 | 858.28 | ||
| Crystal system | Tricrinic | Tricrinic | ||
| T (K) | 123 | 320 | 123 | 320 |
| Space group | P-1 | P-1 | P-1 | P-1 |
| a (Å) | 8.336(3) | 8.1849(16) | 8.3141(12) | 8.2936(18) |
| b (Å) | 8.606(3) | 8.6570(17) | 8.7502(14) | 8.7689(15) |
| c (Å) | 23.279(8) | 23.026(5) | 23.465(4) | 24.150(4) |
| α (°) | 99.212(9) | 97.818(5) | 77.589(8) | 77.177(11) |
| β (°) | 97.935(8) | 98.149(10) | 82.085(10) | 81.980(10) |
| γ (°) | 91.648(7) | 91.536(6) | 87.203(10) | 87.835(13) |
| V (Å3) | 1630.60(10) | 1667.7(6) | 1651.0(4) | 1695.7(5) |
| Z | 2 | 2 | 2 | 2 |
| Dcal (g/cm3) | 1.563 | 1.528 | 1.726 | 1.681 |
| F (000) | 855 | 784 | 860 | 860 |
| Data collected | 27257 | 28574 | 29119 | 29629 |
| Unique data | 7389 | 7590 | 7545 | 7715 |
| R(int) | 0.0499 | 0.0500 | 0.0672 | 0.0687 |
| GOF on F2 | 1.131 | 1.160 | 1.084 | 1.146 |
| R1 [I > 2σ(I)] | 0.0600 | 0.0733 | 0.0463 | 0.0738 |
| Bond Lengths (Å) and Angles (°) | ||||
| 1-Cl (123 K) | 1-Cl (320 K) | 1-Br (123 K) | 1-Br (320 K) | |
| Fe1–N1 | 1.966(3) | 2.077(4) | 1.959(3) | 2.011(4) |
| Fe1–N2 | 1.878(2) | 1.989(3) | 1.873(2) | 1.923(4) |
| Fe1–O1 | 1.999(2) | 2.047(3) | 1.991(2) | 2.010(3) |
| Fe1–N5 | 1.947(3) | 2.089(3) | 1.943(3) | 1.974(4) |
| Fe1–N6 | 1.879(2) | 1.988(4) | 1.869(3) | 1.916(4) |
| Fe1–O2 | 1.989(2) | 2.043(3) | 1.991(2) | 2.016(4) |
| C34–O3 | 1.318(4) | 1.315(4) | 1.316(4) | 1.306(7) |
| C34–O4 | 1.210(4) | 1.187(6) | 1.204(4) | 1.173(6) |
| C27–O5 | 1.305(4) | 1.299(5) | 1.302(4) | 1.312(6) |
| C27–O6 | 1.217(4) | 1.208(5) | 1.212(4) | 1.199(6) |
| C13–N4–C11 | 117.6(3) | 116.6(4) | 117.3(3) | 115.8(5) |
| C24–N8–C26 | 118.7(3) | 118.3(3) | 117.9(3) | 117.7(5) |
| Hydrogen Bond Geometries (Å, °) | ||||
| N4–O3 | 2.663(4) | 2.692(5) | 2.681(4) | 2.694(4) |
| N8–O5 | 2.552(4) | 2.574(4) | 2.560(4) | 2.578(4) |
| O3–H25 | 1.03(7) | – | 0.82(4) | – |
| N4–H25 | 1.64(7) | – | 1.86(4) | – |
| O5–H26 | 1.09(6) | – | 1.04(7) | – |
| N8–H26 | 1.47(6) | – | 1.52(7) | – |
| N4–H25–O3 | 174(6) | – | 177(4) | – |
| N8–H26–O5 | 175(6) | – | 173(6) | – |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakanishi, T.; Sato, O. Synthesis, Structure, and Magnetic Properties of New Spin Crossover Fe(II) Complexes Forming Short Hydrogen Bonds with Substituted Dicarboxylic Acids. Crystals 2016, 6, 131. https://doi.org/10.3390/cryst6100131
Nakanishi T, Sato O. Synthesis, Structure, and Magnetic Properties of New Spin Crossover Fe(II) Complexes Forming Short Hydrogen Bonds with Substituted Dicarboxylic Acids. Crystals. 2016; 6(10):131. https://doi.org/10.3390/cryst6100131
Chicago/Turabian StyleNakanishi, Takumi, and Osamu Sato. 2016. "Synthesis, Structure, and Magnetic Properties of New Spin Crossover Fe(II) Complexes Forming Short Hydrogen Bonds with Substituted Dicarboxylic Acids" Crystals 6, no. 10: 131. https://doi.org/10.3390/cryst6100131
APA StyleNakanishi, T., & Sato, O. (2016). Synthesis, Structure, and Magnetic Properties of New Spin Crossover Fe(II) Complexes Forming Short Hydrogen Bonds with Substituted Dicarboxylic Acids. Crystals, 6(10), 131. https://doi.org/10.3390/cryst6100131






