Crystal Structures, Thermal Analysis, and Dissolution Behavior of New Solid Forms of the Antiviral Drug Arbidol with Dicarboxylic Acids
Abstract
:1. Introduction
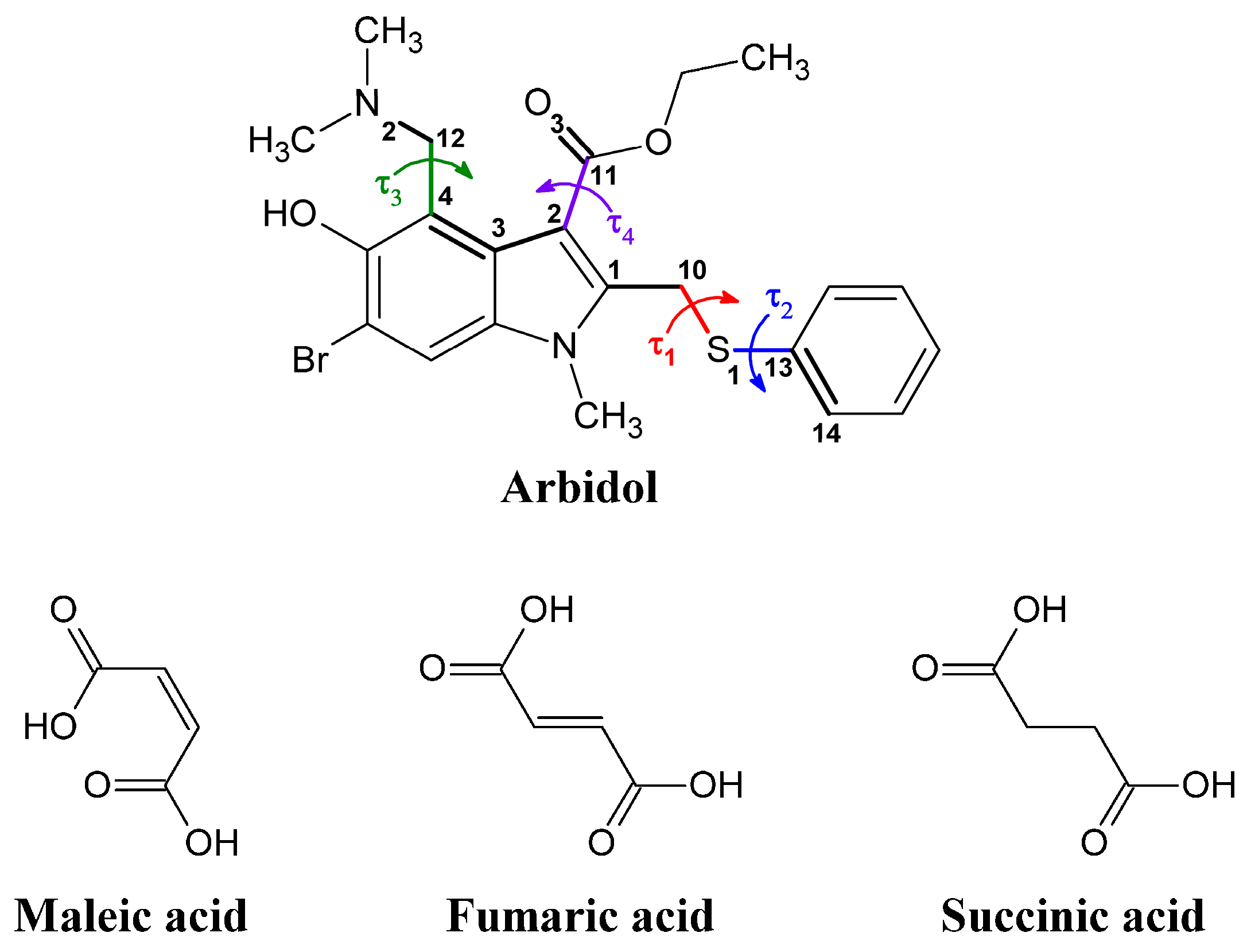
2. Results and Discussion
2.1. Crystal Structures
| Compound Reference | [Arb+Mlc] (1:1) | [Arb+Fum] (2:1) | [Arb+Suc] (1:1) |
|---|---|---|---|
| Chemical formula | C22H26BrN2O3S·C4H3O4 | 2(C22H26BrN2O3S)·C4H2O4 | C22H25BrN2O3S·C4H6O4 |
| Formula Mass | 593.48 | 1070.89 | 595.50 |
| Crystal system | Triclinic | Monoclinic | Monoclinic |
| a/Å | 8.3812(12) | 15.9728(12) | 10.4180(11) |
| b/Å | 13.0305(18) | 14.0080(10) | 14.7052(15) |
| c/Å | 13.2656(19) | 22.8933(17) | 17.8673(18) |
| α/° | 64.054(2) | 90.00 | 90.00 |
| β/° | 83.078(2) | 109.716(1) | 99.129(2) |
| γ/° | 86.643(2) | 90.00 | 90.00 |
| Unit cell volume/Å3 | 1293.2(3) | 4822.0(6) | 2702.6(5) |
| Temperature/K | 180(2) | 183(2) | 180(2) |
| Space group | P | P21/n | P21/n |
| Z | 2 | 4 | 4 |
| No. of reflections measured | 12386 | 49265 | 25637 |
| No. of independent reflections | 5634 | 11638 | 5895 |
| Rint | 0.0276 | 0.0314 | 0.0448 |
| Final R1 values (I > 2σ(I)) | 0.0337 | 0.0317 | 0.0303 |
| Final wR2(F2) values (I > 2σ(I)) | 0.0759 | 0.0715 | 0.0696 |
| Final R1 values (all data) | 0.0477 | 0.0507 | 0.0419 |
| Final wR2(F2) values (all data) | 0.0804 | 0.0786 | 0.0735 |
| Goodness of fit on F2 | 1.071 | 1.011 | 1.055 |
| Largest diff. peak & hole, e·Å−3 | 0.523/−0.305 | 0.519/−0.494 | 0.342/−0.290 |
| CCDC number | 1433018 | 1433017 | 1433016 |
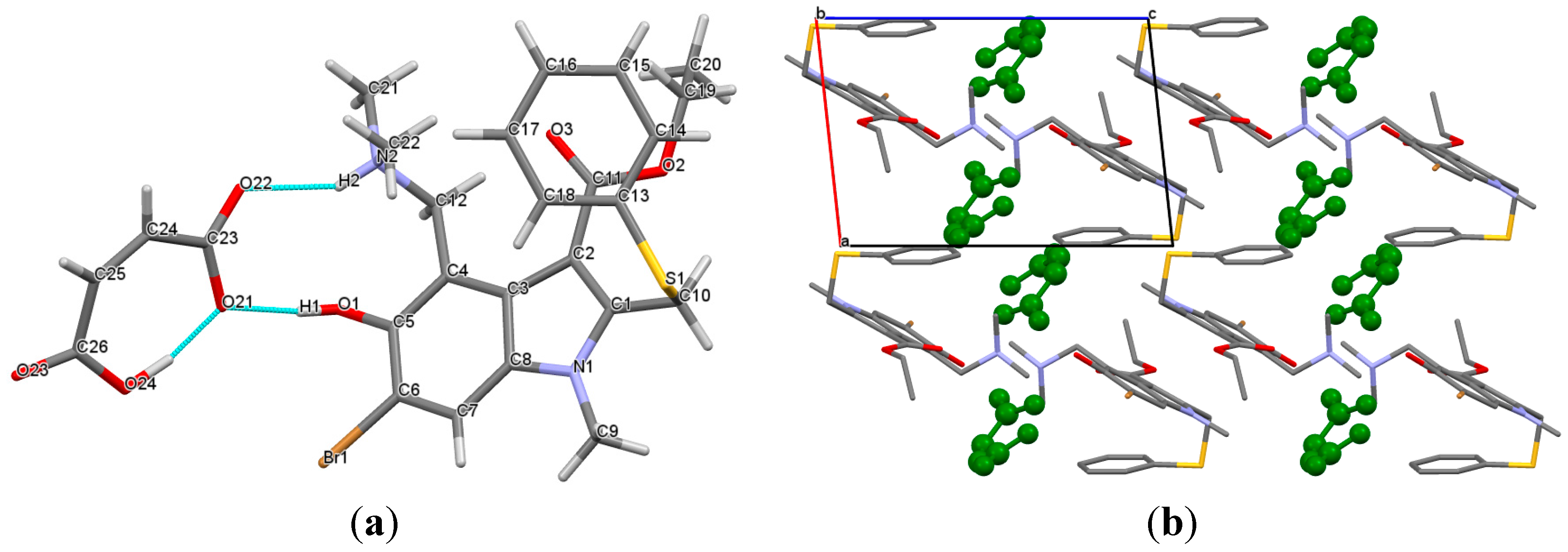
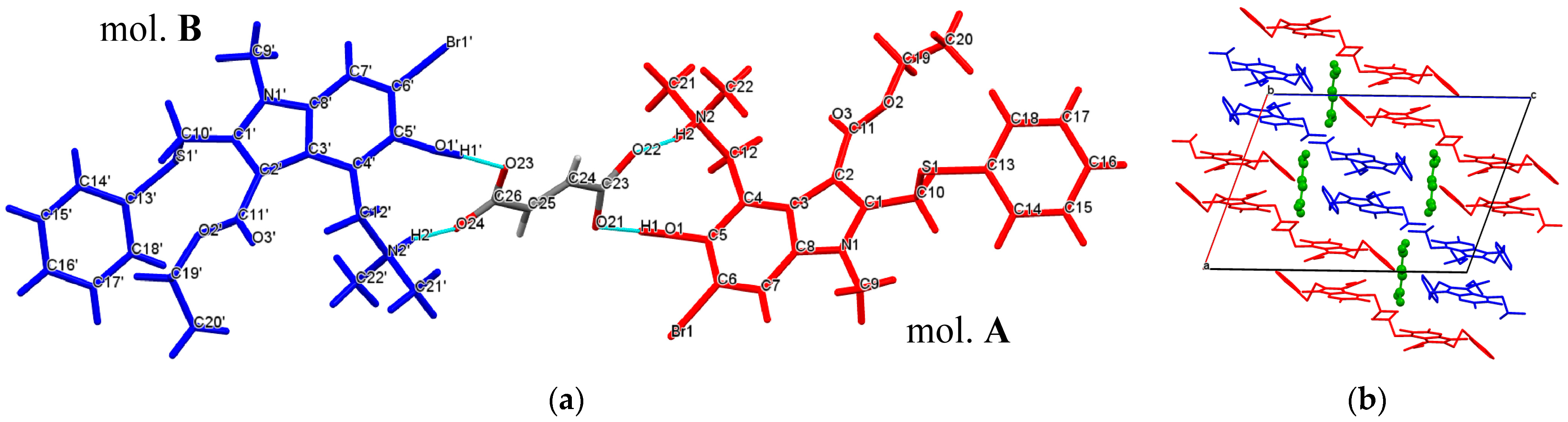
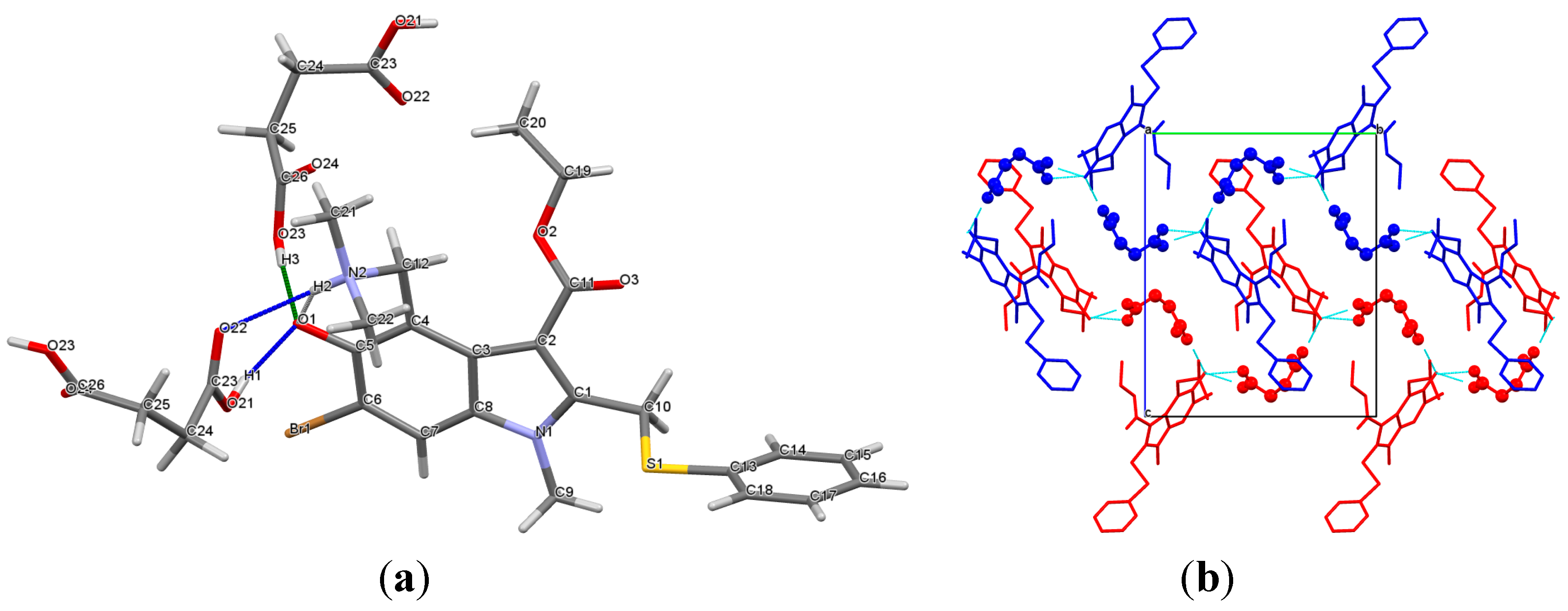
2.2. Conformational Analysis
| -- | τ1,°a (∠C1-C10-S1-C13) | τ2,° (∠C10-S1-C13-C14) | τ3,° (∠C3-C4-C12-N2) | τ4,° (∠C3-C2-C11-O3) |
|---|---|---|---|---|
| [Arb + Mlc] (1:1) | −62.3 | −88.9 | −110.5 | 18.4 |
| [Arb + Fum] (2:1) mol. A | 177.0 | −64.4 | −100.6 | −22.4 |
| [Arb + Fum] (2:1) mol. B | −134.5 | −60.8 | −102.7 | −20.5 |
| [Arb + Suc] (1:1) | −170.5 | −21.3 | −126.4 | 164.0 |
| [Arb + Salicylic acid] (1:1)b | −83.0 | −117.5 | −108.3 | 21.3 |
| [Arb + Salicylic+CHCl3] (1:1:1)b | −58.9 | −105.7 | −97.9 | −30.9 |
| [Arb + Gentisic acid] (1:1)b | −45.6 | −62.8 | −101.3 | 30.2 |
| [Arb + Glutaric acid] (1:1)b | −170.2 | −34.6 | −81.4 | −14.7 |
| [Arb+HCl+H2O] (1:1) | −108.9 | 141.4 | −78.7 | 17.7 |
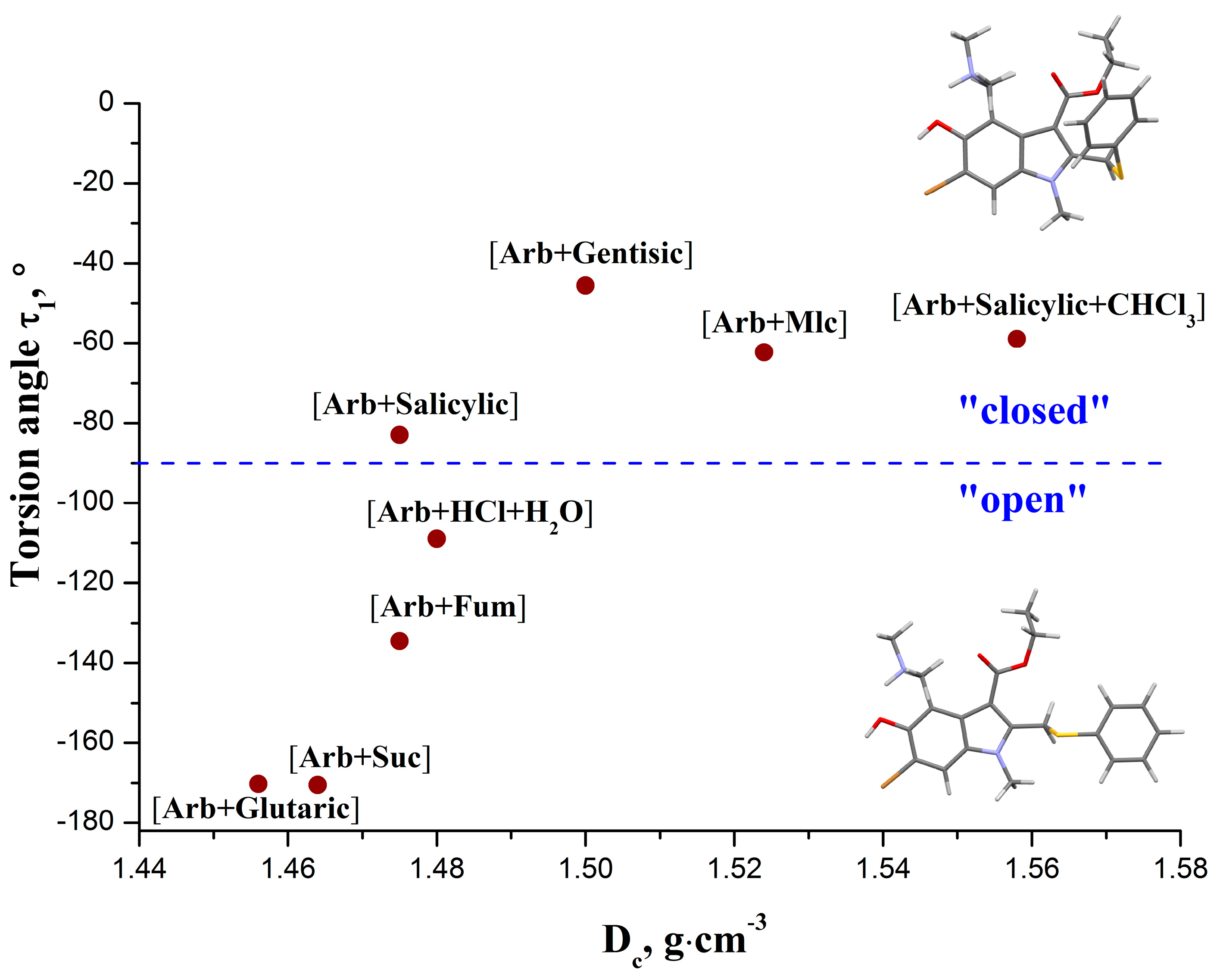
2.3. Thermal Analysis
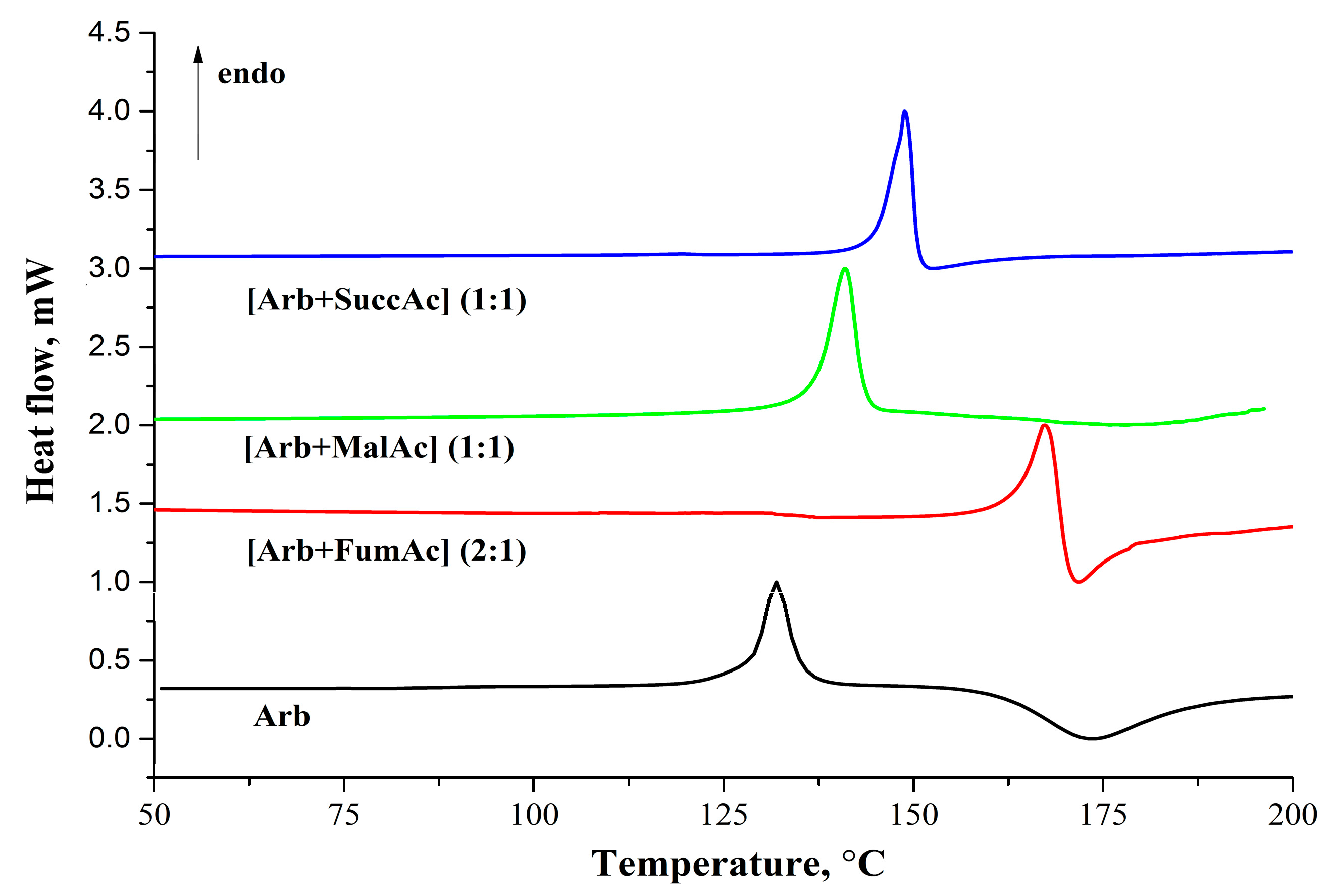
| -- | Arb | [Arb + Fum] (2:1) | [Arb + Mlc] (1:1) | [Arb + Suc] (1:1) |
|---|---|---|---|---|
| Tfus, K (onset) | 397.9 ± 1.0 | 432.9 ± 1.0 | 406.5 ± 1.4 | 415.8 ± 1.0 |
| , kJ∙mol−1 | 44.6 ± 2.8 | 47.9 ± 5.0 | 49.4 ± 4.0 | 59.3 ± 6.0 |
| Compound | Aa | Ba | SDa,b | [Arb+acid], K | Δfus(), K |
|---|---|---|---|---|---|
| Fumaric acid | 309.83 ± 23.43 | 0.324 ± 0.053 | 8.78 | 438.7 | 5.8 |
| Maleic acid | 199.26 ± 45.76 | 0.482 ± 0.093 | 13.90 | 391.1 | −15.5 |
| Succinic acid | 263.76 ± 24.56 | 0.392 ± 0.055 | 15.79 | 419.8 | 3.9 |
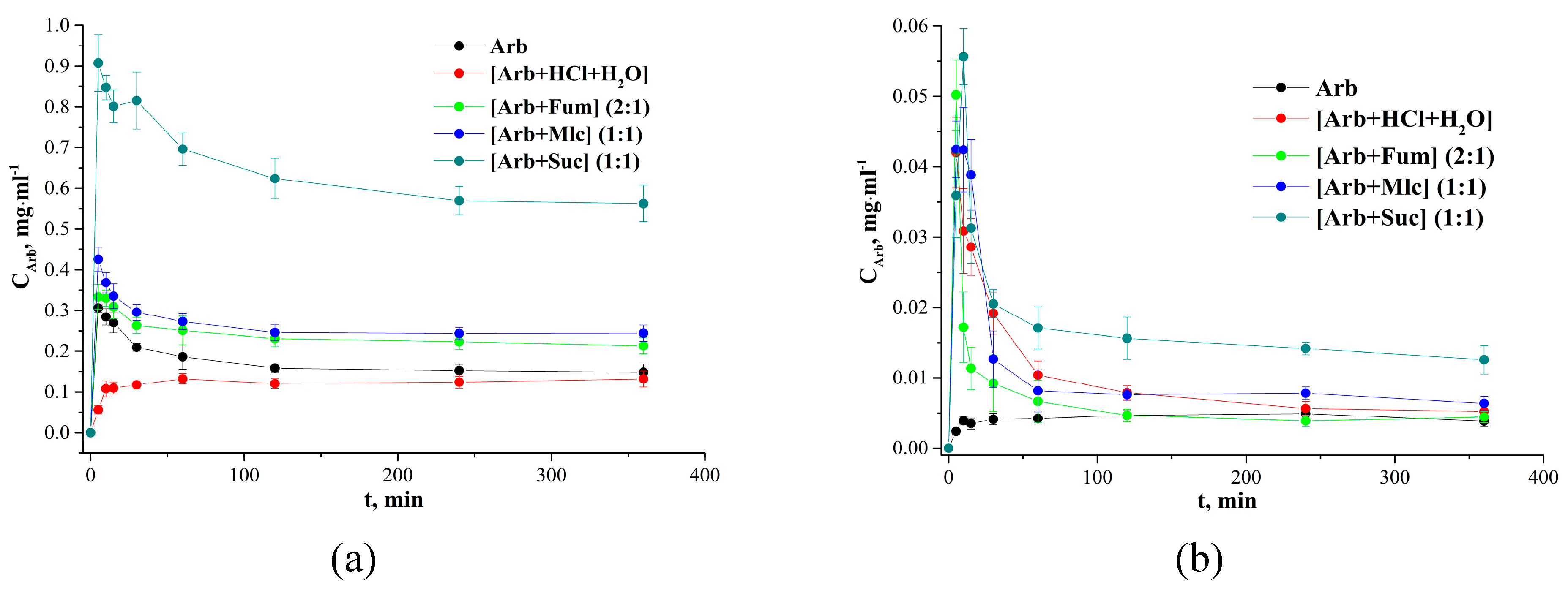
2.4. Aqueous Dissolution Study
| -- | Сmax (mg·mL−1) a | Solubility (mg·mL−1) b | Solid Phase Recovered after Solubility Experiment c |
|---|---|---|---|
| pH 1.2 | |||
| Arb | 0.30 ± 0.01 | 0.15 ± 0.01 | [Arb + HCl + H2O] |
| [Arb + HCl + H2O] | 0.13 ± 0.01 | 0.13 ± 0.01 | [Arb + HCl + H2O] |
| [Arb + Fum] (2:1) | 0.33 ± 0.03 | 0.21 ± 0.02 | [Arb + HCl + H2O] |
| [Arb + Mlc] (1:1) | 0.42 ± 0.03 | 0.24 ± 0.02 | [Arb + HCl + H2O] |
| [Arb + Suc] (1:1) | 0.91 ± 0.07 | 0.56 ± 0.04 | [Arb + HCl + H2O] |
| pH 6.8 | |||
| Arb | 0.005 ± 0.001 | 0.004 ± 0.001 | Arb |
| [Arb + HCl + H2O] | 0.042 ± 0.005 | 0.005 ± 0.001 | Arb |
| [Arb + Fum] (2:1) | 0.050 ± 0.005 | 0.004 ± 0.001 | Arb |
| [Arb + Mlc] (1:1) | 0.042 ± 0.006 | 0.006 ± 0.001 | Arb |
| [Arb + Suc] (1:1) | 0.055 ± 0.006 | 0.012 ± 0.002 | Arb |
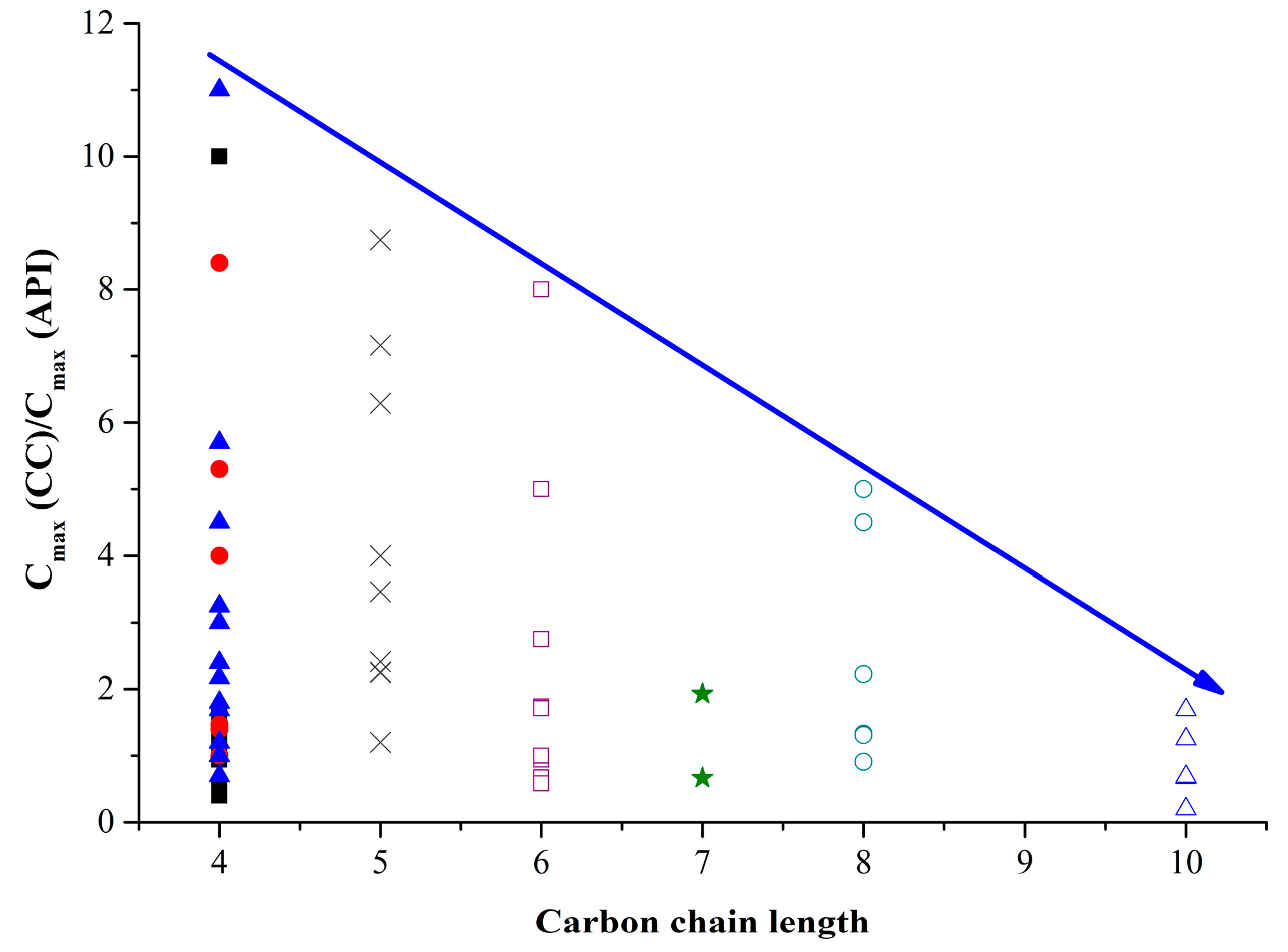
 ); with glutaric (×); with adipinic (□); with pimelic (★); with suberic (○); with sebacic (△) acids. The green dash line corresponds to the cases when the cocrystal/salt solubility is equal to API solubility. The blue dash line corresponds to the case when the cocrystal/salt melting temperatures are equal to the API one.
); with glutaric (×); with adipinic (□); with pimelic (★); with suberic (○); with sebacic (△) acids. The green dash line corresponds to the cases when the cocrystal/salt solubility is equal to API solubility. The blue dash line corresponds to the case when the cocrystal/salt melting temperatures are equal to the API one.
 ); with glutaric (×); with adipinic (□); with pimelic (★); with suberic (○); with sebacic (△) acids. The green dash line corresponds to the cases when the cocrystal/salt solubility is equal to API solubility. The blue dash line corresponds to the case when the cocrystal/salt melting temperatures are equal to the API one.
); with glutaric (×); with adipinic (□); with pimelic (★); with suberic (○); with sebacic (△) acids. The green dash line corresponds to the cases when the cocrystal/salt solubility is equal to API solubility. The blue dash line corresponds to the case when the cocrystal/salt melting temperatures are equal to the API one.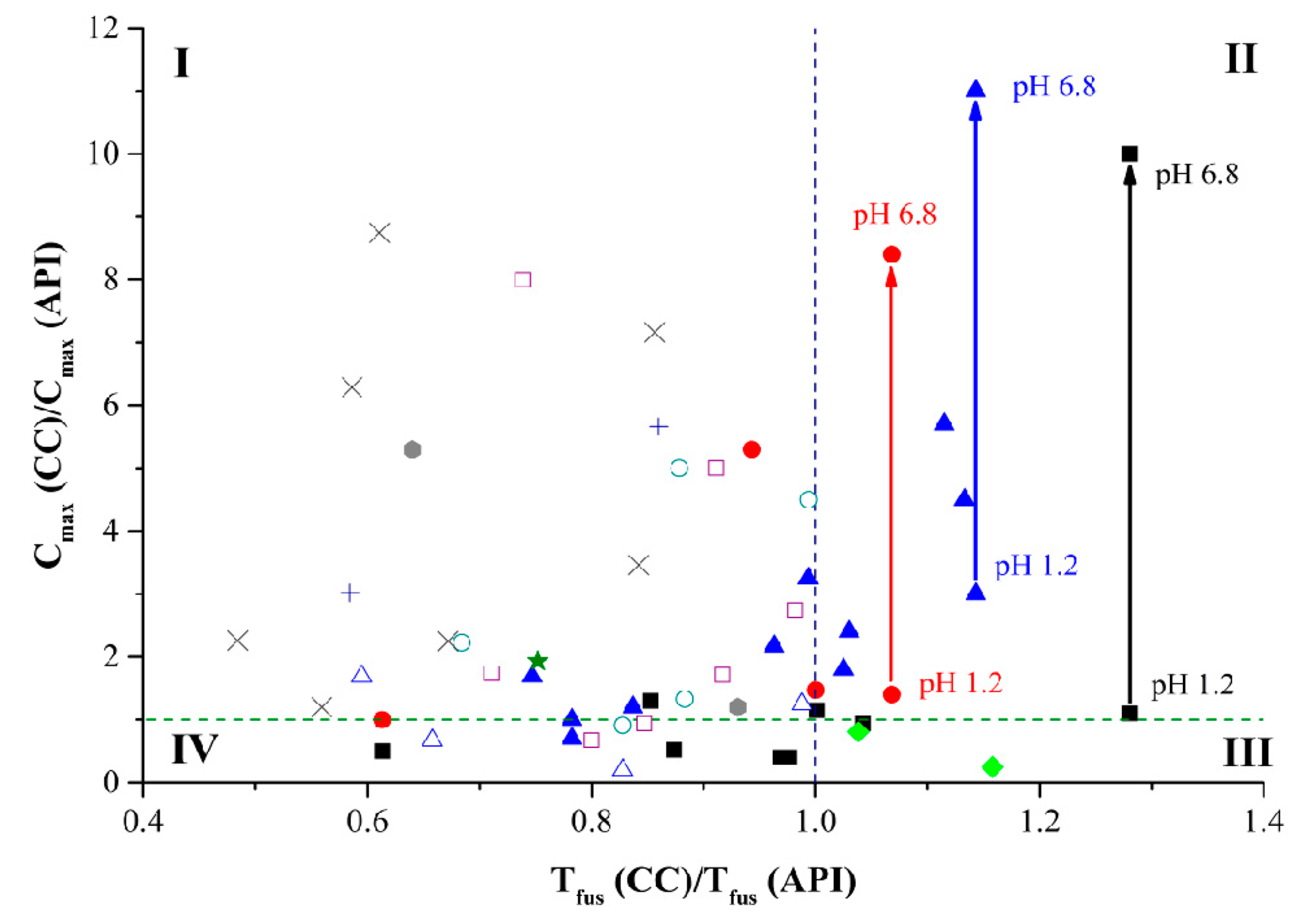
3. Experimental Section
3.1. Compounds and Solvents
3.2. Crystallization Procedure
3.3. X-Ray Diffraction Experiments
3.4. Differential Scanning Calorimetry (DSC)
3.5. TG/Mass-Spectrometry Experiment
3.6. Aqueous Dissolution Experiments
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Desiraju, G.R. Crystal engineering: a holistic view. Angew. Chem. Int. Ed. 2007, 46, 8342–8356. [Google Scholar] [CrossRef] [PubMed]
- Blagden, N.; Matas, M.D.; Gavan, P.T.; York, P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv. Drug Delivery Rev. 2007, 59, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, N.; Newman, A. Pharmaceutical cocrystals and their physicochemical properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef] [PubMed]
- Stahl, P.H.; Wermuth, C.G. Handbook of Pharmaceutical Salts: Properties, Selection and Use; Wiley-VCH: Zurich, Switzerland, 2002; p. 388. [Google Scholar]
- Sacchetti, M. General equations for in situ salt screening of multibasic drugs in multiprotic acids. Pharm. Dev. Technol. 2000, 3, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Childs, S.L.; Chyall, L.J.; Dunlap, J.T.; Smolenskaya, V.N.; Stahly, B.C.; Stahly, G.P. Crystal engineering approach to forming cocrystals of amine hydrochlorides with organic acids. molecular complexes of fluoxetine hydrochloride with benzoic, succinic, and fumaric acids. J. Am. Chem. Soc. 2004, 126, 13335–13342. [Google Scholar] [CrossRef] [PubMed]
- Trask, A.V. An overview of pharmaceutical cocrystals as intellectual property. Mol. Pharm. 2007, 4, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Trask, A.V.; Motherwell, W.D.; Jones, W. Pharmaceutical cocrystallization: Engineering a remedy for caffeine hydration. Cryst. Growth Des. 2005, 5, 1013–1021. [Google Scholar] [CrossRef]
- Vishweshwar, P.; McMahon, J.A.; Bis, J.A.; Zaworotko, M.J. Pharmaceutical co-crystals. J. Pharm. Sci. 2006, 95, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Archakov, A.I.; Guseva, M.K.; Uchaykin, V.F.; Ipatova, O.M.; Dochshitsin, Y.F.; Tikhonova, Y.G.; Medvedeva, N.V.; Prozorovsky, V.N.; Strekalova, O.S.; Shironin, A.V. Pharmaceutical composition containing arbidol in the form of phospholipid nanoparticles. U.S. Patent 20120052099 A1, 1 March 2012. [Google Scholar]
- Brooks, M.J.; Burtseva, E.J.; Ellery, P.J.; Marsh, G.A.; Lew, A.M.; Slepushkin, A.N.; Crowe, S.M.; Tannock, G.A. Antiviral activity of arbidol, a broad-spectrum drug for use against respiratory viruses, varies according to test conditions. J. Med. Virol. 2012, 84, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Semenenko, T.A.; Sel’kova, E.P.; Gotvianskaia, T.P.; Gaidarenko, A.D.; Polezhaeva, N.A.; Evseeva, L.F.; Nikolaeva, O.G. Characteristics of the immune status in specific and nonspecific prophylaxis of influenza in elderly persons. Zhurnal Mikrobiologii Epidemiologii I Immunobiologii 2005, 6, 24–28. (in Russian). [Google Scholar]
- Blaising, J.; Polyak, S.J.; Pécheur, E.-I. Arbidol as a broad-spectrum antiviral: An update. Antiviral Res. 2014, 107, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Gatich, R.Z.; Kolobukhina, L.V.; Vasil’ev, A.N.; Isaeva, E.I.; Burtseva, E.I.; Orlova, T.G.; Voronina, F.V.; Kol’tsov, V.D.; Malinovskaia, V.V. Viferon suppositories in the treatment of influenza in adult. Antibiot. Khimioter. 2008, 53, 13–17. (in Russian). [Google Scholar] [PubMed]
- Kolobukhina, L.V.; Malinovskaia, V.V.; Gatich, R.Z.; Merkulova, L.N.; Burtseva, E.I.; Isaeva, E.I.; Parshina, O.V.; Guseva, T.S.; Orlova, T.G.; Voronina, F.V. Evaluation of the efficacy of viferon and arbidol in adult influenza. Vopr. Virusol. 2008, 53, 31–33. (in Russian). [Google Scholar] [PubMed]
- Leneva, I.A.; Shuster, A.M. Antiviral etiotropic chemicals: Efficacy against influenza A viruses A subtype H5N1. Vopr. Virusol. 2006, 51, 4–7. (in Russian). [Google Scholar] [PubMed]
- Arastoo, M.; Khorram Khorshid, H.R.; Radmanesh, R.; Gharibdoust, F. Combination of IMOD™ and Arbidol to increase their immunomodulatory effects as a novel medicine to prevent and cure influenza and some other infectious diseases. J. Med. Hypotheses Ideas 2014, 8, 53–56. [Google Scholar] [CrossRef]
- Glushkov, R.G. Arbidol antiviral, immunostimulant, interferon inducer. Drug Future 1992, 17, 1079–1081. [Google Scholar] [CrossRef]
- Vorobei, A.M.; Pokrovskiy, O.I.; Ustinovich, K.B.; Krotova, L.I.; Parenago, O.O.; Lunin, V.V. Effect of solvent type and concentration on size and morphology of arbidol microparticles obtained by supercritical antisolvent precipitation. Supercrit. Fluids Theory Pract. 2015, 10, 51–59. (in Russian). [Google Scholar]
- Eropkin, M.Y.; Solovskii, M.V.; Smirnova, M.Y.; Bryazzhikova, T.S.; Gudkova, T.M.; Konovalova, N.I. Synthesis and biological activity of water-soluble polymer complexes of arbidol. Pharm. Chem. J. 2009, 43, 563–567. [Google Scholar] [CrossRef]
- Babkin, V.A.; Kiselev, O.I. Method for Preparing Antiviral Water-Soluble Polymer Complexes of Arbidol. RU Patent 2475255C1, 20 February 2013. [Google Scholar]
- Orola, L.; Sarcevica, I.; Kons, A.; Actins, A.; Veidis, M.V. Conformation of the umifenovir cation in the molecular and crystal structures of four carboxylic acid salts. J. Mol. Struct. 2014, 1056–1057, 63–69. [Google Scholar] [CrossRef]
- Surov, A.O.; Manin, A.N.; Churakov, A.V.; Perlovich, G.L. New solid forms of the antiviral drug arbidol: Crystal structures, thermodynamic stability, and solubility. Mol. Pharm. 2015, 12, 4154–4165. [Google Scholar] [CrossRef] [PubMed]
- Childs, S.L.; Hardcastle, K.I. Cocrystals of piroxicam with carboxylic acids. Cryst. Growth Des. 2007, 7, 1291–1304. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Iida, M.; Tada, N.; Kojima, T.; Ikeda, Y.; Moriwaki, T.; Higashi, K.; Moribe, K.; Yamamoto, K. Characterization and evaluation of miconazole salts and cocrystals for improved physicochemical properties. Int. J. Pharm. 2011, 421, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.-Q. Salt screening and selection: New challenges and considerations in the modern pharmaceutical research and development paradigm. In Developing Solid Oral Dosage Forms. Pharmaceutical Theory and Practice; Qiu, Y., Chen, Y., Zhang, G.G.Z., Eds.; Academic Press: New York, NY, USA, 2009; pp. 75–86. [Google Scholar]
- Berendsen, B.J.A.; Wegh, R.S.; Essers, M.L.; Stolker, A.A.M.; Weigel, S. Quantitative trace analysis of a broad range of antiviral drugs in poultry muscle using column-switch liquid chromatography coupled to tandem mass spectrometry. Anal. Bioanal. Chem. 2012, 402, 1611–1623. [Google Scholar] [CrossRef] [PubMed]
- Bhogala, B.R.; Basavoju, S.; Nangia, A. Tape and layer structures in cocrystals of some di- and tricarboxylic acids with 4,4′-bipyridines and isonicotinamide. From binary to ternary cocrystals. CrystEngComm 2005, 7, 551–562. [Google Scholar] [CrossRef]
- Childs, S.L.; Stahly, G.P.; Park, A. The salt-cocrystal continuum: the influence of crystal structure on ionization state. Mol. Pharm. 2007, 4, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Cabeza, A.J. Acid-base crystalline complexes and the pKa Rule. CrystEngComm 2012, 14, 6362–6365. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and Decoding Hydrogen-Bond Patterns of Organic Compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Tilborg, A.; Norberg, B.; Wouters, J. Pharmaceutical salts and cocrystals involving amino acids: A brief structural overview of the state-of-art. Eur. J. Med. Chem. 2014, 74, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Abramov, Y.; Bordner, J.; Leonard, J.; Medek, A.; Trask, A.V. Solid-State Acid-Base Interactions in Complexes of Heterocyclic Bases with Dicarboxylic Acids: Crystallography, Hydrogen Bond Analysis, and 15N NMR Spectroscopy. J. Am. Chem. Soc. 2006, 128, 8199–8210. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.S.; Bethune, S.J.; Kampf, J.W.; Rodríguez-Hornedo, N. Cocrystals and Salts of Gabapentin: pH Dependent Cocrystal Stability and Solubility. Cryst. Growth Des. 2009, 9, 378–385. [Google Scholar] [CrossRef]
- Patel, J.R.; Carlton, R.A.; Needham, T.E.; Chichester, C.O.; Vogt, F.G. Preparation, structural analysis, and properties of tenoxicam cocrystals. Int. J. Pharm. 2012, 436, 685–706. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.H.; Klapwijk, A.R.; Wales, C.; Wilson, C.C. Intermolecular hydrogen transfer and solubility tuning in multi-component molecular crystals of the API piroxicam. CrystEngComm 2014, 16, 5924–5932. [Google Scholar] [CrossRef]
- Chernyshev, V.V.; Davlyatshin, D.I.; Shpanchenko, R.V.; Nosyrev, P.V. Structural Characterization of Arbidol®. Z. Kristallogr. 2011, 226, 832–836. [Google Scholar] [CrossRef]
- Perlovich, G.L. Thermodynamic characteristics of cocrystal formation and melting points for rational design of pharmaceutical two-component systems. CrystEngComm 2015, 17, 7019–7028. [Google Scholar] [CrossRef]
- Babu, N.J.; Nangia, A. Solubility advantage of amorphous drugs and pharmaceutical cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. [Google Scholar] [CrossRef]
- Kumar, S.S.; Nangia, A. A solubility comparison of neutral and zwitterionic polymorphs. Cryst. Growth Des. 2014, 14, 1865–1881. [Google Scholar] [CrossRef]
- Yalkowsky, S.H.; Valvani, S.C. Solubility and partitioning I: Solubility of nonelectrolytes in water. J. Pharm. Sci. 1980, 69, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Perlovich, G.L.; Ryzhakov, A.M.; Tkachev, V.V.; Hansen, L.Kr.; Raevsky, O.A. Sulfonamide molecular crystals: structure, sublimation thermodynamic characteristics, molecular packing, hydrogen bonds network. Cryst. Growth Des. 2013, 13, 4002–4016. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Fasulo, M.E.; Desper, J. Cocrystal or salt: does it really matter? Mol. Pharm. 2007, 4, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SADABS, Program for Scaling and Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manin, A.N.; Surov, A.O.; Churakov, A.V.; Perlovich, G.L. Crystal Structures, Thermal Analysis, and Dissolution Behavior of New Solid Forms of the Antiviral Drug Arbidol with Dicarboxylic Acids. Crystals 2015, 5, 650-669. https://doi.org/10.3390/cryst5040650
Manin AN, Surov AO, Churakov AV, Perlovich GL. Crystal Structures, Thermal Analysis, and Dissolution Behavior of New Solid Forms of the Antiviral Drug Arbidol with Dicarboxylic Acids. Crystals. 2015; 5(4):650-669. https://doi.org/10.3390/cryst5040650
Chicago/Turabian StyleManin, Alex N., Artem O. Surov, Andrei V. Churakov, and German L. Perlovich. 2015. "Crystal Structures, Thermal Analysis, and Dissolution Behavior of New Solid Forms of the Antiviral Drug Arbidol with Dicarboxylic Acids" Crystals 5, no. 4: 650-669. https://doi.org/10.3390/cryst5040650
APA StyleManin, A. N., Surov, A. O., Churakov, A. V., & Perlovich, G. L. (2015). Crystal Structures, Thermal Analysis, and Dissolution Behavior of New Solid Forms of the Antiviral Drug Arbidol with Dicarboxylic Acids. Crystals, 5(4), 650-669. https://doi.org/10.3390/cryst5040650







