Nickel-Doped Ceria Nanoparticles: The Effect of Annealing on Room Temperature Ferromagnetism
Abstract
:1. Introduction
2. Results and Discussion
| Sample | Ce[oleate]3 used/g, mmol | Ni[oleate]2 used/g, mmol | Sample Number |
|---|---|---|---|
| CeO2 | 4, 4.06 | 0, 0 | 1 |
| NiO | 0, 0 | 2.52, 4.06 | 2 |
| 50% Ni-CeO2 | 2, 2.03 | 0.662, 2.03 | 3 |
| 10% Ni-CeO2 | 3.59, 3.65 | 0.25, 0.406 | 4 |
| 8% Ni-CeO2 | 3.62, 3.68 | 0.2, 0.325 | 5 |
| 5% Ni-CeO2 | 3.86, 3.8 | 0.125, 0.203 | 6 |
| 4% Ni-CeO2 | 3.84, 3.9 | 0.1, 0.162 | 7 |
| 3% Ni-CeO2 | 3.88, 3.94 | 0.075, 0.122 | 8 |
| 2% Ni-CeO2 | 3.92, 3.98 | 0.05, 0.08 | 9 |
| 1% Ni-CeO2 | 4.02, 3.96 | 0.025, 0.0406 | 10 |
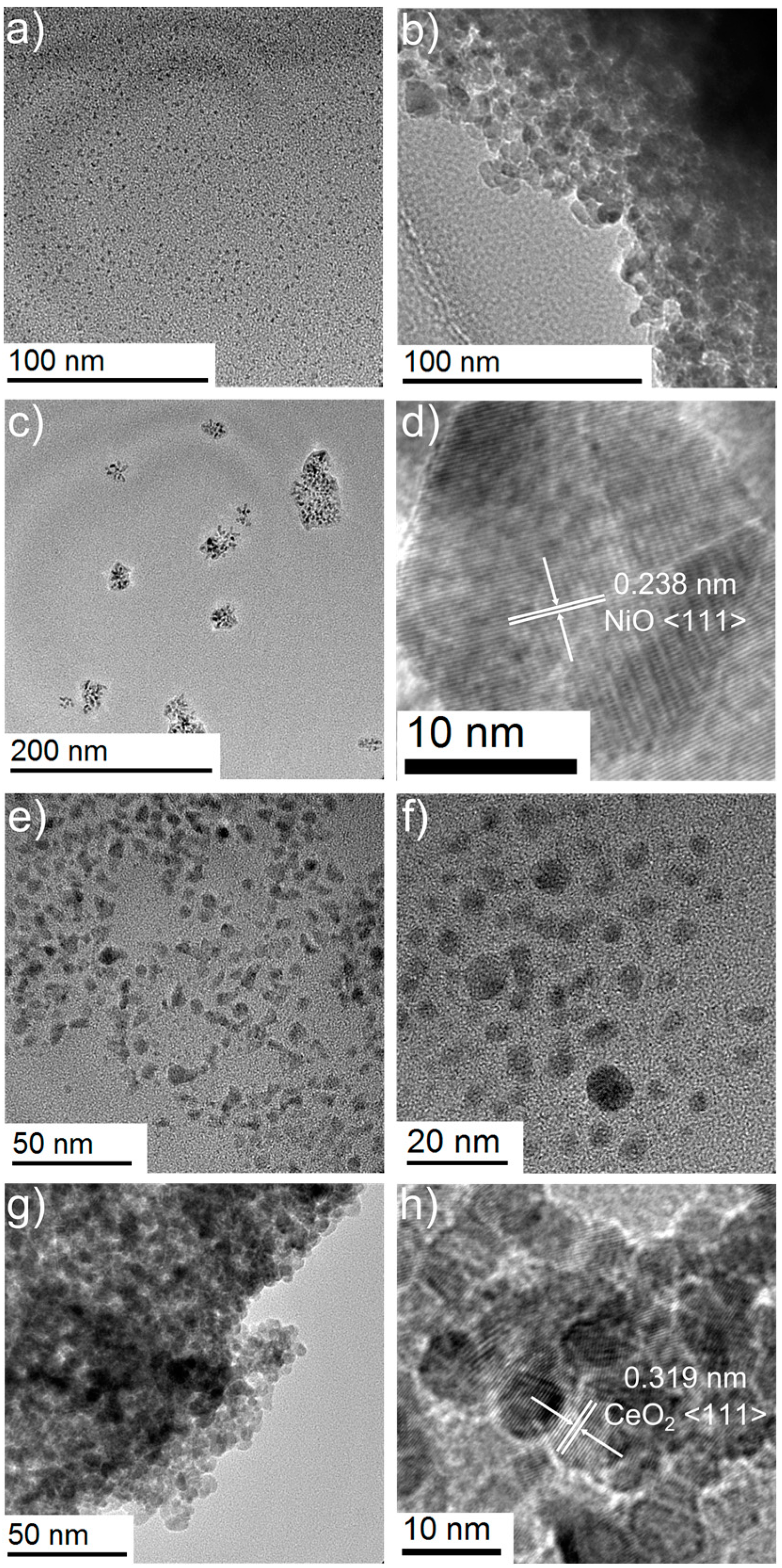
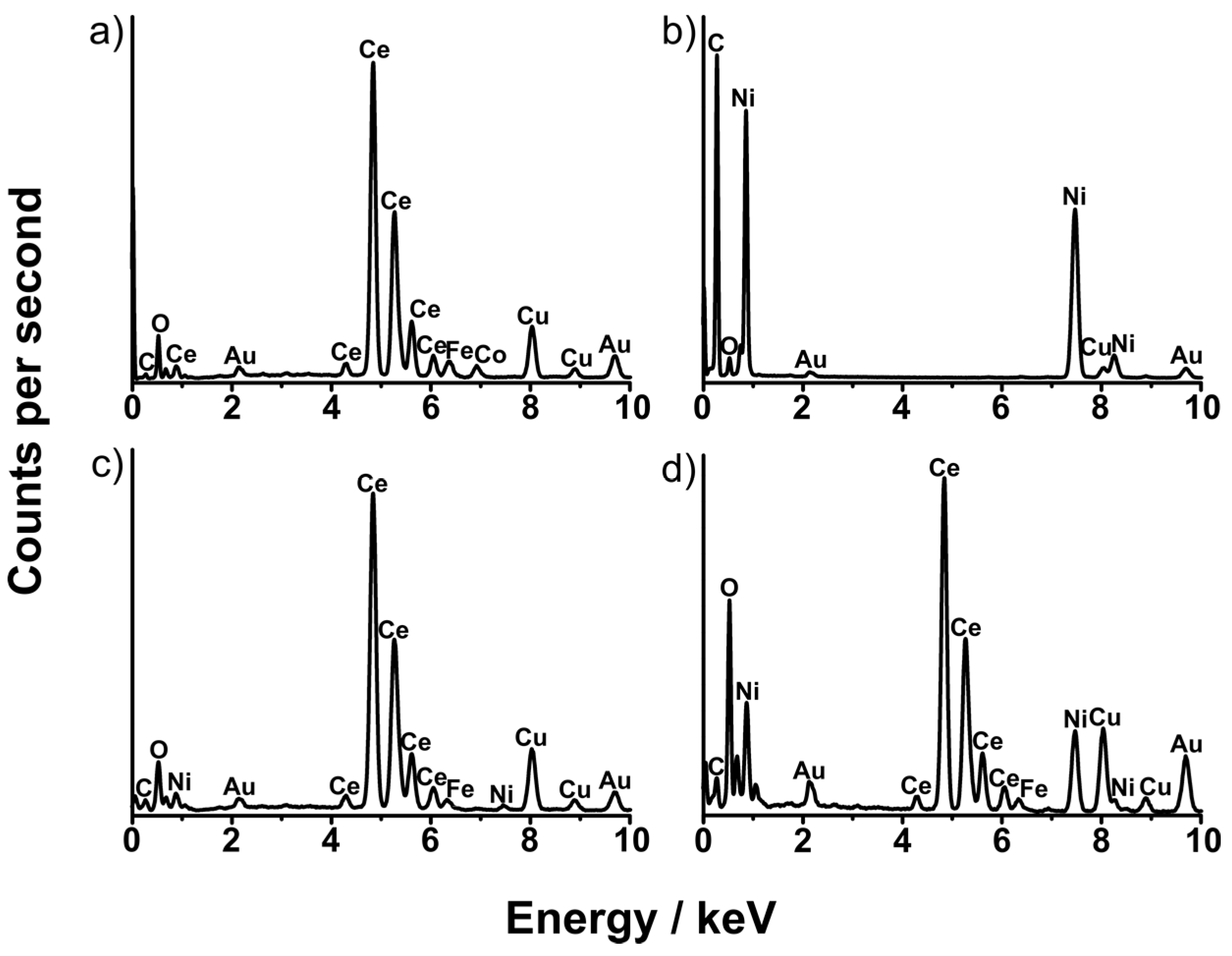
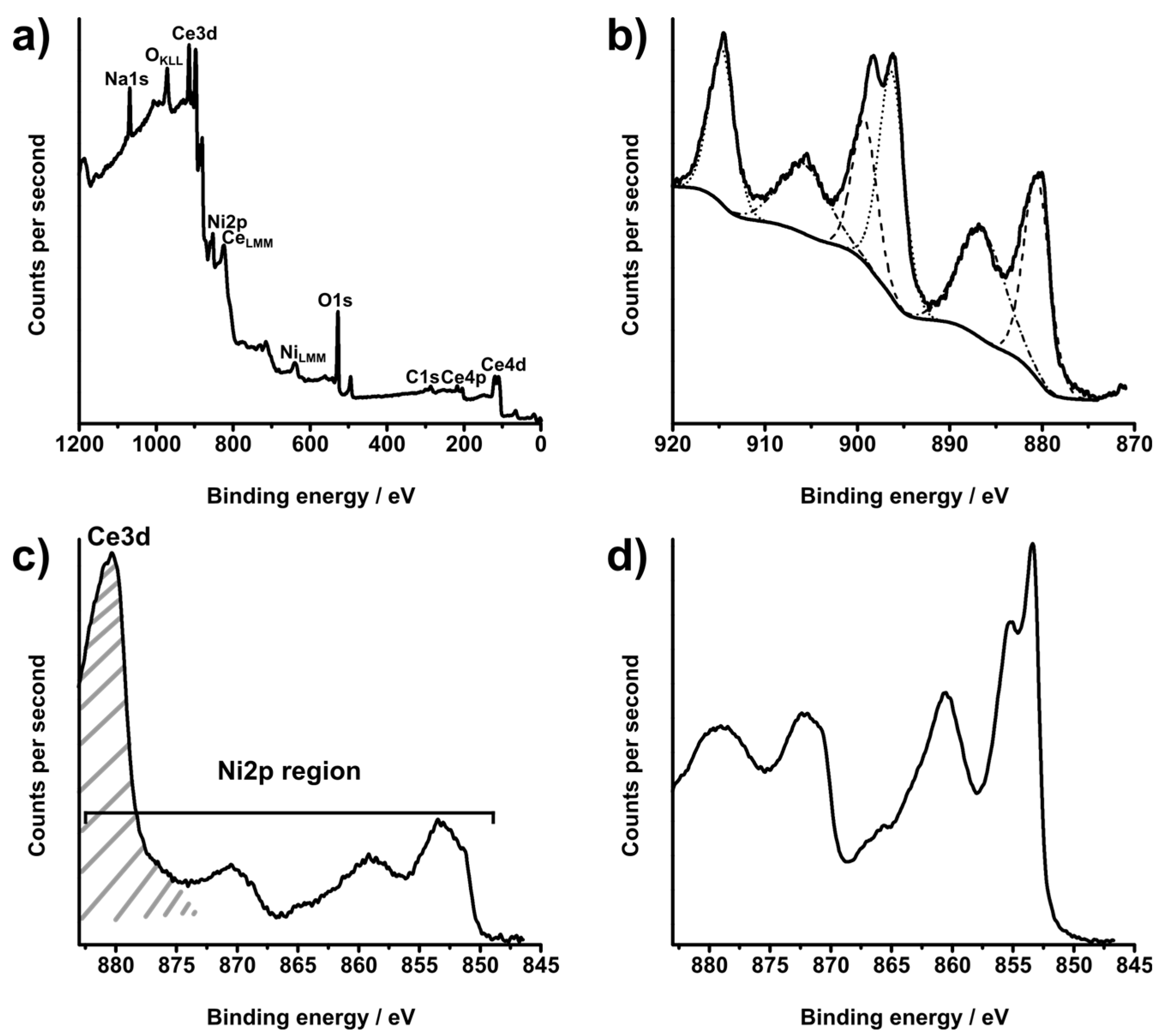
| Sample | Ce Composition by XPS/at.% | O Composition by XPS/at.% | Ni Composition by XPS/at.% |
|---|---|---|---|
| 1 | 37.79 | 62.21 | – |
| 2 | – | 49.52 | 50.48 |
| 3 | 22.02 | 54.57 | 23.40 |
| 4 | 36.51 | 59.64 | 3.86 |
| 5 | 32.72 | 64.80 | 2.48 |
| 6 | 34.26 | 63.88 | 1.86 |
| 7 | 32.92 | 65.81 | 1.27 |
| 8 | 36.98 | 62.13 | 0.90 |
| 9 | 37.95 | 61.47 | 0.58 |
| 10 | 33.43 | 66.32 | 0.25 |
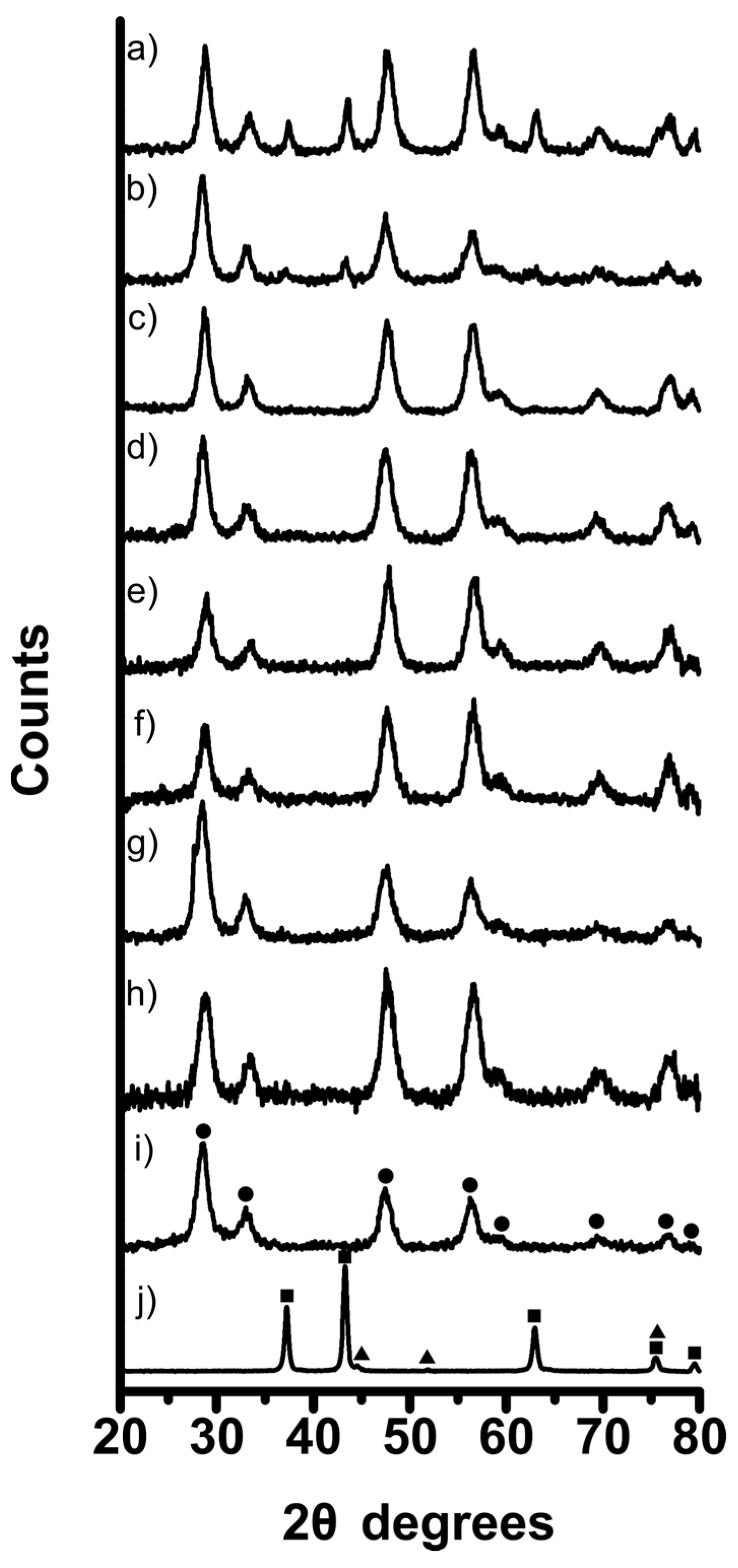
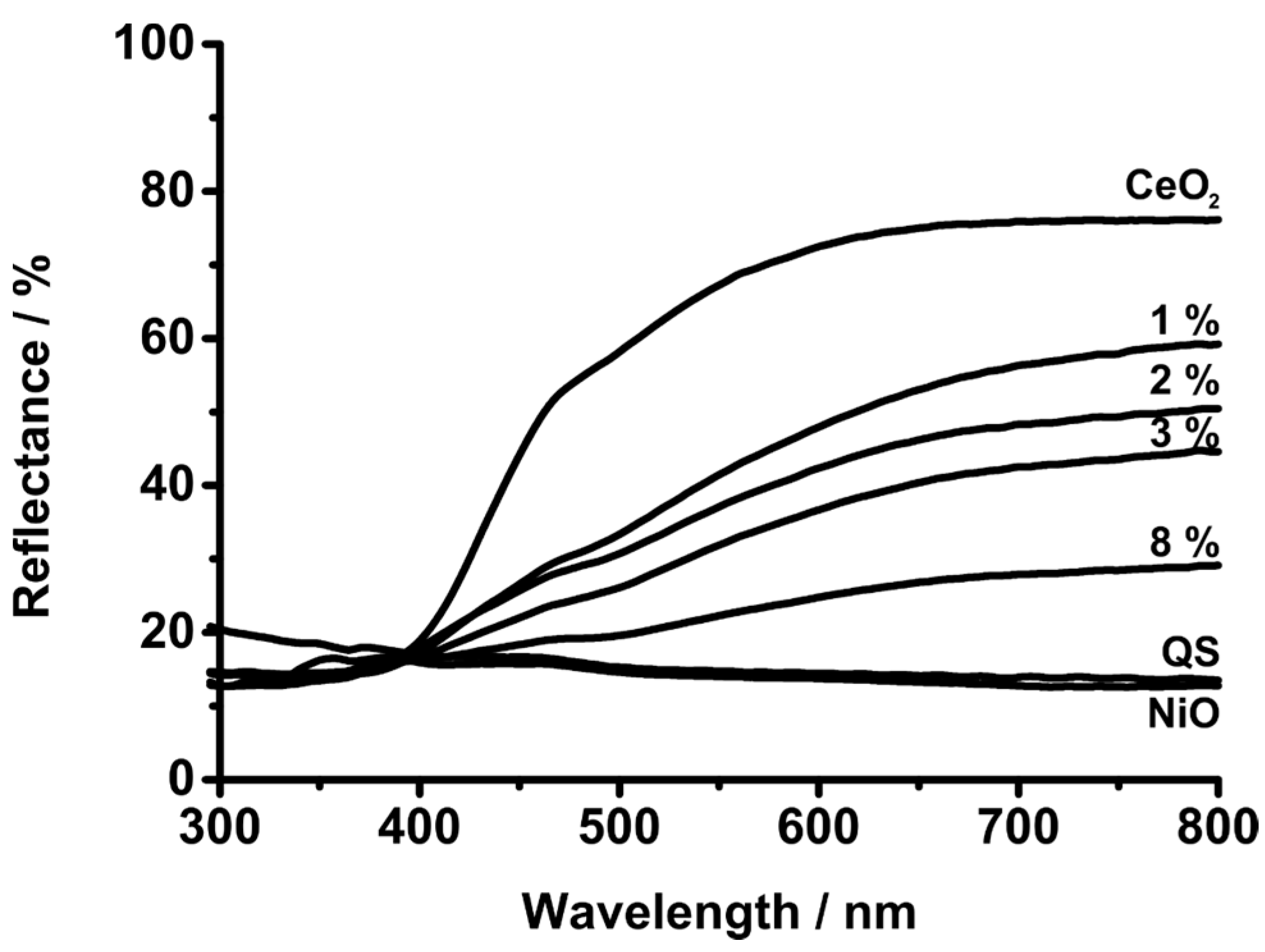
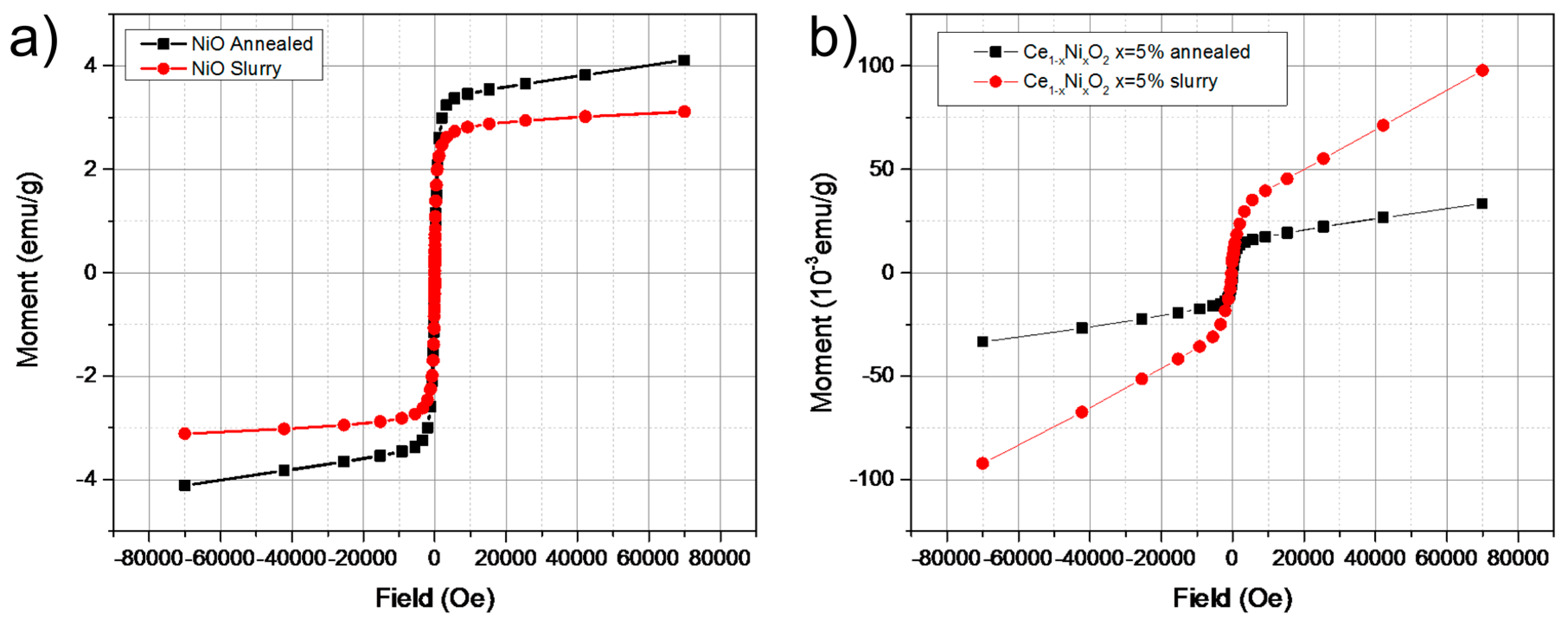
3. Experimental Section
3.1. Materials
3.2. Methods
3.3. Instrumentation
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ozin, G.A.; Arsenault, A.; Cademartiri, L. Nanochemistry: A Chemical Approach to Nanomaterials, 2nd ed.; Royal Society of Chemistry: London, UK, 2008. [Google Scholar]
- Patzke, G.R.; Zhou, Y.; Kontic, R.; Conrad, F. Oxide nanomaterials: Synthetic developments, mechanistic studies, and technological innovations. Angew. Chem. Int. Ed. 2011, 50, 826–859. [Google Scholar] [CrossRef] [PubMed]
- Bear, J.; Charron, G.; Fernández-Argüelles, M.T.; Massadeh, S.; McNaughter, P.; Nann, T. In Vivo Applications of Inorganic Nanoparticles. In BetaSys: Systems Biology of Regulated Exocytosis in Pancreatic β-Cells; Systems Biology Series; Springer: New York, NY, USA, 2011; Volume 2, pp. 185–220. [Google Scholar]
- Franke, M.E.; Koplin, T.J.; Simon, U. Metal and metal oxide nanoparticles in chemiresistors: Does the nanoscale matter? Small 2006, 2, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Zoolfakar, A.S.; Rani, R.A.; Morfa, A.J.; O’Mullane, A.P.; Kalantar-zadeh, K. Nanostructured copper oxide semiconductors: A perspective on materials, synthesis methods and applications. J. Mater. Chem. C 2014, 2, 5247–5270. [Google Scholar] [CrossRef]
- Hu, Y.H. A Highly Efficient Photocatalyst—Hydrogenated Black TiO2 for the Photocatalytic Splitting of Water. Angew. Chem. Int. Ed. 2012, 51, 12410–12412. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Niklasson, G.A.; Granqvist, C.G. Nanothermochromics with VO2-based core-shell structures: Calculated luminous and solar optical properties. J. Appl. Phys. 2011, 109, 113515. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, Y.; Kang, L.; Cao, C.; Chen, S.; Luo, H. Fine crystalline VO2 nanoparticles: Synthesis, abnormal phase transition temperatures and excellent optical properties of a derived VO2 nanocomposite foil. J. Mater. Chem. A 2014, 2, 2718–2727. [Google Scholar] [CrossRef]
- Son, J.-H.; Wei, J.; Cobden, D.; Cao, G.; Xia, Y. Hydrothermal Synthesis of Monoclinic VO2 Micro- and Nanocrystals in One Step and Their Use in Fabricating Inverse Opals. Chem. Mater. 2010, 22, 3043–3050. [Google Scholar] [CrossRef]
- Zhao, Y.; Dunnill, C.W.; Zhu, Y.; Gregory, D.H.; Kockenberger, W.; Li, Y.; Hu, W.; Ahmad, I.; McCartney, D.G. Low-Temperature Magnetic Properties of Hematite Nanorods. Chem. Mater. 2007, 19, 916–921. [Google Scholar] [CrossRef]
- Peveler, W.J.; Bear, J.C.; Southern, P.; Parkin, I.P. Organic–inorganic hybrid materials: Nanoparticle containing organogels with myriad applications. Chem. Commun. 2014, 50, 14418–14420. [Google Scholar] [CrossRef] [PubMed]
- Crick, C.R.; Bear, J.C.; Southern, P.; Parkin, I.P. A general method for the incorporation of nanoparticles into superhydrophobic films by aerosol assisted chemical vapour deposition. J. Mater. Chem. A 2013, 1, 4336–4344. [Google Scholar] [CrossRef]
- Bear, J.C.; Yu, B.; Blanco-Andujar, C.; McNaughter, P.D.; Southern, P.; Mafina, M.-K.; Pankhurst, Q.A.; Parkin, I.P. A low cost synthesis method for functionalised iron oxide nanoparticles for magnetic hyperthermia from readily available materials. Faraday Discuss. 2014, 175, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Scholz, R.; Wust, P.; Fähling, H.; Felix, R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 1999, 201, 413–419. [Google Scholar] [CrossRef]
- Dobson, J. Magnetic nanoparticles for drug delivery. Drug Dev. Res. 2006, 67, 55–60. [Google Scholar] [CrossRef]
- Dunnill, C.W.; Ansari, Z.; Kafizas, A.; Perni, S.; Morgan, D.J.; Wilson, M.; Parkin, I.P. Visible light photocatalysts—N-doped TiO2 by sol-gel, enhanced with surface bound silver nanoparticle islands. J. Mater. Chem. 2011, 21, 11854–11861. [Google Scholar] [CrossRef]
- Dunnill, C.W.; Parkin, I.P. Nitrogen-doped TiO2 thin films: Photocatalytic applications for healthcare environments. Dalton Trans. 2011, 40, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Na, H.; Song, I.; Hyeon, T. Inorganic Nanoparticles for MRI Contrast Agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Bridot, J.; Dayde, D.; Riviere, C.; Mandon, C.; Billotey, C.; Lerondel, S. Hybrid gadolinium oxide nanoparticles combining imaging and therapy. J. Mater. Chem. 2009, 19, 2328–2335. [Google Scholar] [CrossRef]
- Na, H.B.; Lee, J.H.; An, K.; Park, Y.I.; Park, M.; Lee, I.S.; Nam, D.-H.; Kim, S.T.; Kim, S.-H.; Kim, S.-W.; et al. Development of a T1 Contrast Agent for Magnetic Resonance Imaging Using MnO Nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 5397–5401. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Terris, B.D.; Thomson, T. Nanofabricated and self-assembled magnetic structures as data storage media. J. Phys. Appl. Phys. 2005, 38, R199–R222. [Google Scholar] [CrossRef]
- Reiss, G.; Hütten, A. Magnetic nanoparticles: Applications beyond data storage. Nat. Mater. 2005, 4, 725–726. [Google Scholar] [CrossRef] [PubMed]
- Chng, L.L.; Erathodiyil, N.; Ying, J.Y. Nanostructured Catalysts for Organic Transformations. Acc. Chem. Res. 2013, 46, 1825–1837. [Google Scholar] [CrossRef] [PubMed]
- Crick, C.R.; Bear, J.C.; Kafizas, A.; Parkin, I.P. Superhydrophobic Photocatalytic Surfaces through Direct Incorporation of Titania Nanoparticles into a Polymer Matrix by Aerosol Assisted Chemical Vapor Deposition. Adv. Mater. 2012, 24, 3505–3508. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Karunakaran, R.G.; Guo, J.; Yang, S. Transparent, Superhydrophobic Surfaces from One-Step Spin Coating of Hydrophobic Nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, U.M.; Ross, I.M.; Saghi, Z.; Stringfellow, A.; Sayle, D.; Sayle, T.X.T.; Karakoti, A.; Reid, D.; Seal, S.; Möbus, G. Atomic motion on various surfaces of ceria nanoparticles in comparison. J. Phys. Conf. Ser. 2012, 371. [Google Scholar] [CrossRef]
- Chattopadhyay, J.; Son, J.E.; Pak, D. Preparation and characterizations of Ni-alumina, Ni-ceria and Ni-alumina/ceria catalysts and their performance in biomass pyrolysis. Korean J. Chem. Eng. 2011, 28, 1677–1683. [Google Scholar] [CrossRef]
- Zhang, S.; Muratsugu, S.; Ishiguro, N.; Tada, M. Ceria-Doped Ni/SBA-16 Catalysts for Dry Reforming of Methane. ACS Catal. 2013, 3, 1855–1864. [Google Scholar] [CrossRef]
- Pino, L.; Vita, A.; Cipitì, F.; Laganà, M.; Recupero, V. Hydrogen production by methane tri-reforming process over Ni-ceria catalysts: Effect of La-doping. Appl. Catal. B Environ. 2011, 104, 64–73. [Google Scholar] [CrossRef]
- Xu, J.; Xue, B.; Liu, Y.-M.; Li, Y.-X.; Cao, Y.; Fan, K.-N. Mesostructured Ni-doped ceria as an efficient catalyst for styrene synthesis by oxidative dehydrogenation of ethylbenzene. Appl. Catal. Gen. 2011, 405, 142–148. [Google Scholar] [CrossRef]
- Qureshi, U.; Dunnill, C.W.; Parkin, I.P. Nanoparticulate cerium dioxide and cerium dioxide–titanium dioxide composite thin films on glass by aerosol assisted chemical vapour deposition. Appl. Surf. Sci. 2009, 256, 852–856. [Google Scholar] [CrossRef]
- Gomez, V.; Clemente, A.; Irusta, S.; Balas, F.; Santamaria, J. Identification of TiO2 nanoparticles using La and Ce as labels: Application to the evaluation of surface contamination during the handling of nanosized matter. Environ. Sci. Nano 2014, 1, 496–503. [Google Scholar] [CrossRef]
- Naganuma, T.; Traversa, E. Stability of the Ce3+ valence state in cerium oxide nanoparticle layers. Nanoscale 2012, 4, 4950–4953. [Google Scholar] [CrossRef] [PubMed]
- Trovarelli, A. Catalytic Properties of Ceria and CeO2-Containing Materials. Catal. Rev. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Tao, Z.; Hou, G.; Xu, N.; Zhang, Q. A highly coking-resistant solid oxide fuel cell with a nickel doped ceria: Ce1−xNixO2−y reformation layer. Int. J. Hydrog. Energy 2014, 39, 5113–5120. [Google Scholar] [CrossRef]
- Sun, C.; Li, H.; Chen, L. Nanostructured ceria-based materials: Synthesis, properties, and applications. Energy Environ. Sci. 2012, 5, 8475–8505. [Google Scholar] [CrossRef]
- Dohčević-Mitrović, Z.D.; Paunović, N.; Radović, M.; Popović, Z.V.; Matović, B.; Cekić, B.; Ivanovski, V. Valence state dependent room-temperature ferromagnetism in Fe-doped ceria nanocrystals. Appl. Phys. Lett. 2010, 96, 203104. [Google Scholar] [CrossRef]
- Thurber, A.; Reddy, K.M.; Shutthanandan, V.; Engelhard, M.H.; Wang, C.; Hays, J.; Punnoose, A. Ferromagnetism in chemically synthesized CeO2 nanoparticles by Ni doping. Phys. Rev. B 2007, 76, 165206. [Google Scholar] [CrossRef]
- Xia, C.; Hu, C.; Chen, P.; Wan, B.; He, X.; Tian, Y. Magnetic properties and photoabsorption of the Mn-doped CeO2 nanorods. Mater. Res. Bull. 2010, 45, 794–798. [Google Scholar] [CrossRef]
- Brito, P.C.A.; Santos, D.A.A.; Duque, J.G.S.; Macêdo, M.A. Structural and magnetic study of Fe-doped CeO2. Phys. B Condens. Matter 2010, 405, 1821–1825. [Google Scholar] [CrossRef]
- Ferrari, V.; Llois, A.M.; Vildosola, V. Co-doped ceria: Tendency towards ferromagnetism driven by oxygen vacancies. J. Phys. Condens. Matter 2010, 22. [Google Scholar] [CrossRef] [PubMed]
- Thurber, A.; Reddy, K.M. High-temperature magnetic-field-induced activation of room-temperature ferromagnetism in Ce1−xNixO2. J. Appl. Phys. 2007, 101, 09N506. [Google Scholar] [CrossRef]
- Fernandes, V.; Mossanek, R.J.O.; Schio, P.; Klein, J.J.; de Oliveira, A.J.A.; Ortiz, W.A.; Mattoso, N.; Varalda, J.; Schreiner, W.H.; Abbate, M.; et al. Dilute-defect magnetism: Origin of magnetism in nanocrystalline CeO2. Phys. Rev. B 2009, 80. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Fong, K.-W.; Peng, T.-T.; Dong, C.-L.; Gloter, A.; Yan, D.-C.; Chen, C.-L.; Lin, H.-J.; Chen, C.-T. Enhancement of Ferromagnetism in CeO2 Nanoparticles by Nonmagnetic Cr3+ Doping. J. Phys. Chem. C 2012, 116, 26570–26576. [Google Scholar] [CrossRef]
- Sundaresan, A.; Bhargavi, R.; Rangarajan, N.; Siddesh, U.; Rao, C.N.R. Ferromagnetism as a universal feature of nanoparticles of the otherwise nonmagnetic oxides. Phys. Rev. B 2006, 74. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Saravanan, P.; Chen, S.-H.; Dong, C.-L.; Chen, C.L.; Chen, S.-Y.; Asokan, K.; Rajendra Kumar, R.T. Enhanced Room-Temperature Ferromagnetism on Co-Doped CeO2 Nanoparticles: Mechanism and Electronic and Optical Properties. J. Phys. Chem. C 2014, 118, 27039–27047. [Google Scholar] [CrossRef]
- Wang, Z.; Quan, Z.; Lin, J. Remarkable Changes in the Optical Properties of CeO2 Nanocrystals Induced by Lanthanide Ions Doping. Inorg. Chem. 2007, 46, 5237–5242. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Babu, S.; Karakoti, A.S.; Schulte, A.; Seal, S. Luminescence Properties of Europium-Doped Cerium Oxide Nanoparticles: Role of Vacancy and Oxidation States. Langmuir 2009, 25, 10998–11007. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, G.; Su, Y.; Qiu, X.; Li, L.; Zou, Z. Synthesis and room-temperature ferromagnetism of CeO2 nanocrystals with nonmagnetic Ca2+ doping. Nanotechnology 2009, 20. [Google Scholar] [CrossRef] [PubMed]
- NIST X-ray Photoelectron Spectroscopy (XPS) Database, Version 3.5. Available online: http://srdata.nist.gov/xps/ (accessed on 23 February 2015).
- Burroughs, P.; Hamnett, A.; Orchard, A.F.; Thornton, G. Satellite structure in the X-ray photoelectron spectra of some binary and mixed oxides of lanthanum and cerium. J. Chem. Soc. Dalton Trans. 1976, 1686–1698. [Google Scholar] [CrossRef]
- Mullins, D.R.; Overbury, S.H.; Huntley, D.R. Electron spectroscopy of single crystal and polycrystalline cerium oxide surfaces. Surf. Sci. 1998, 409, 307–319. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of Xps Data; Physical Electronics: Eden Prairie, MN, USA, 1995; pp. 142–143. [Google Scholar]
- Taguchi, M.; Takami, S.; Adschiri, T.; Nakane, T.; Sato, K.; Naka, T. Supercritical hydrothermal synthesis of hydrophilic polymer-modified water-dispersible CeO2 nanoparticles. CrystEngComm 2011, 13, 2841–2848. [Google Scholar] [CrossRef]
- Zhang, F.; Chan, S.-W.; Spanier, J.E.; Apak, E.; Jin, Q.; Robinson, R.D.; Herman, I.P. Cerium oxide nanoparticles: Size-selective formation and structure analysis. Appl. Phys. Lett. 2002, 80, 127–129. [Google Scholar] [CrossRef]
- Zhou, X.D.; Huebner, W. Size-induced lattice relaxation in CeO2 nanoparticles. Appl. Phys. Lett. 2001, 79, 3512–3514. [Google Scholar] [CrossRef]
- Mogensen, M.; Sammes, N.M.; Tompsett, G.A. Physical, chemical and electrochemical properties of pure and doped ceria. Solid State Ion. 2000, 129, 63–94. [Google Scholar] [CrossRef]
- Griffiths, T.R.; Davies, M.J.; Hubbard, H.V.S.A. Spectroscopic studies on single crystals having the fluorite lattice. Part 1—The fundamental absorption edge; Urbach’s rule and the Debye temperature in CeO2. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1976, 72, 765–772. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bear, J.C.; McNaughter, P.D.; Southern, P.; O’Brien, P.; Dunnill, C.W. Nickel-Doped Ceria Nanoparticles: The Effect of Annealing on Room Temperature Ferromagnetism. Crystals 2015, 5, 312-326. https://doi.org/10.3390/cryst5030312
Bear JC, McNaughter PD, Southern P, O’Brien P, Dunnill CW. Nickel-Doped Ceria Nanoparticles: The Effect of Annealing on Room Temperature Ferromagnetism. Crystals. 2015; 5(3):312-326. https://doi.org/10.3390/cryst5030312
Chicago/Turabian StyleBear, Joseph C., Paul D. McNaughter, Paul Southern, Paul O’Brien, and Charles W. Dunnill. 2015. "Nickel-Doped Ceria Nanoparticles: The Effect of Annealing on Room Temperature Ferromagnetism" Crystals 5, no. 3: 312-326. https://doi.org/10.3390/cryst5030312
APA StyleBear, J. C., McNaughter, P. D., Southern, P., O’Brien, P., & Dunnill, C. W. (2015). Nickel-Doped Ceria Nanoparticles: The Effect of Annealing on Room Temperature Ferromagnetism. Crystals, 5(3), 312-326. https://doi.org/10.3390/cryst5030312







