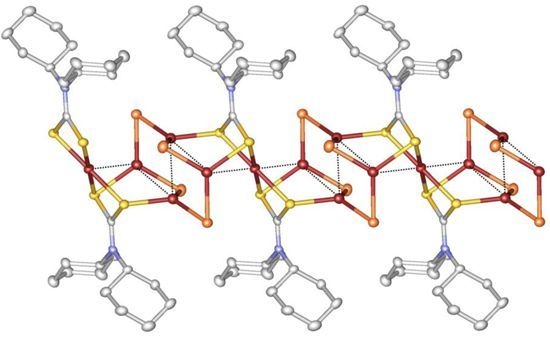
Synthesis, Crystal Structure, and Electroconducting Properties of a 1D Mixed-Valence Cu(I)–Cu(II) Coordination Polymer with a Dicyclohexyl Dithiocarbamate Ligand
Abstract
:1. Introduction
2. Results and Discussion
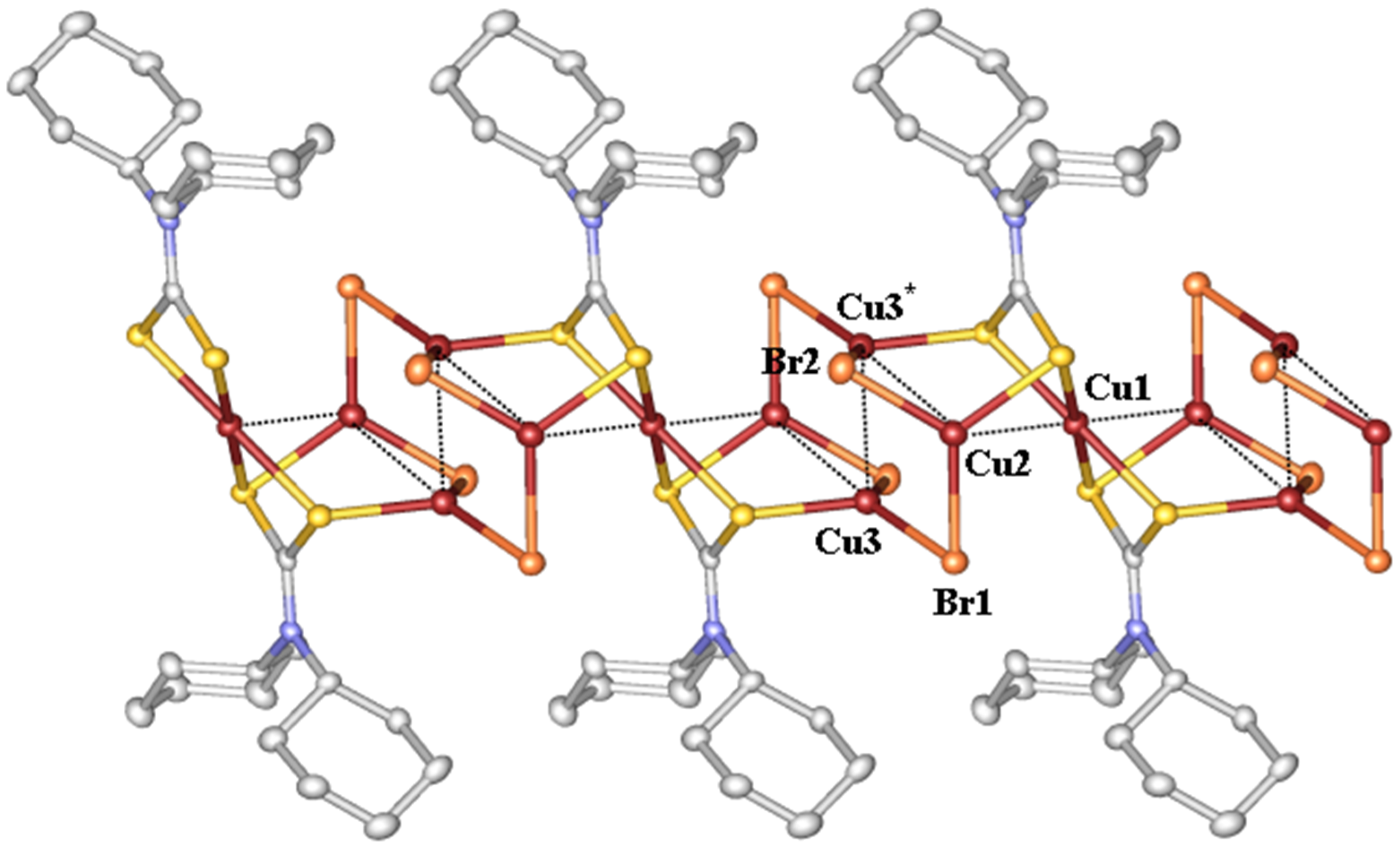
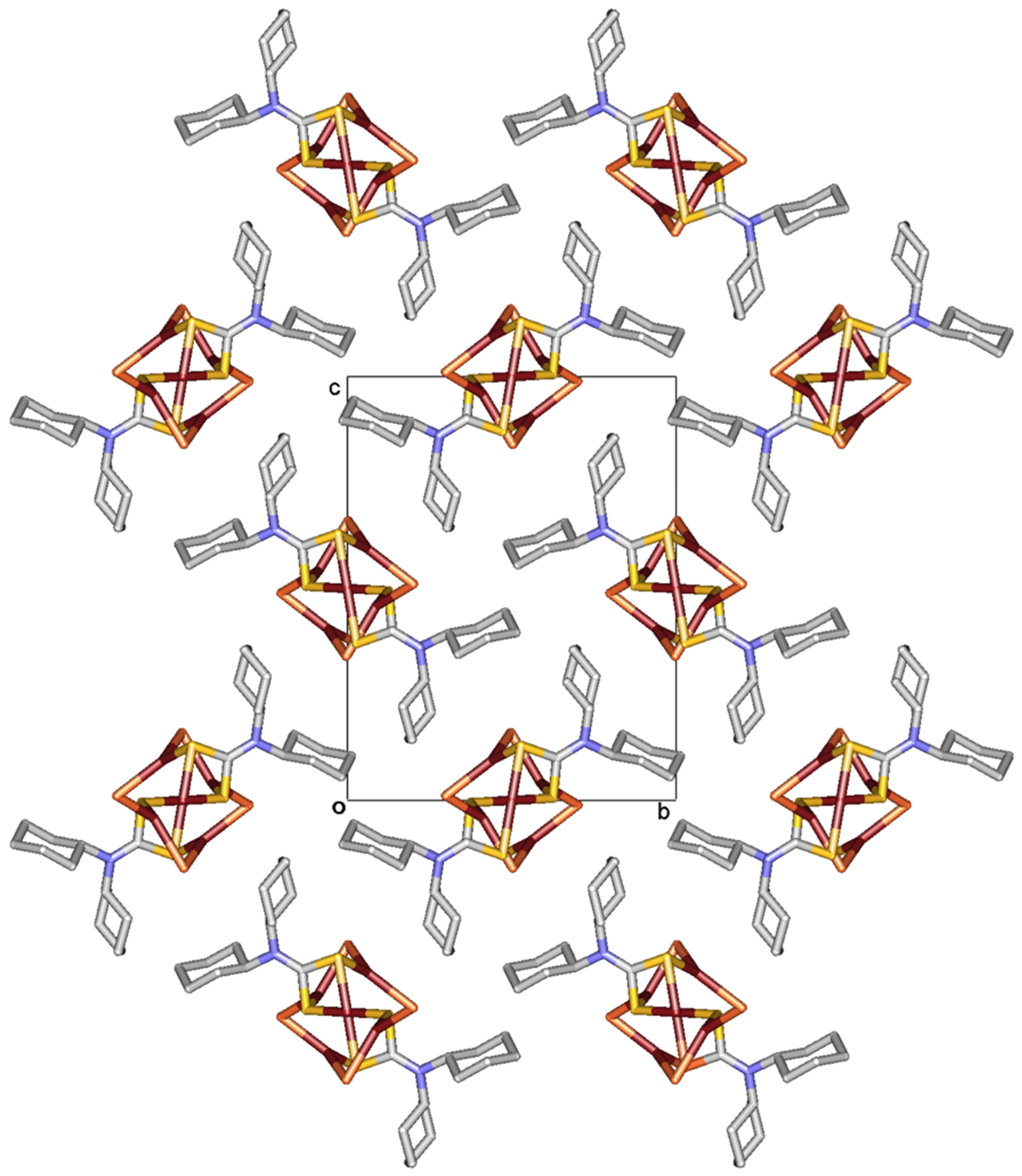
2.1. Description of Crystal Structure
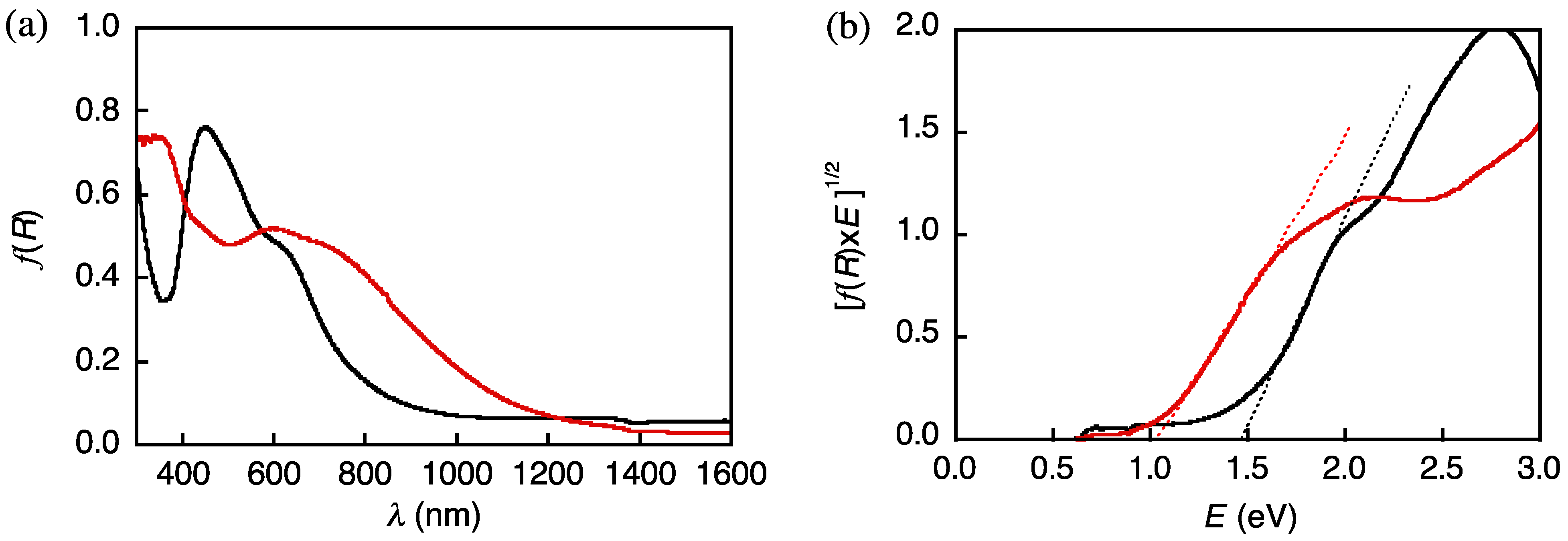
2.2. Spectroscopic Properties of 1
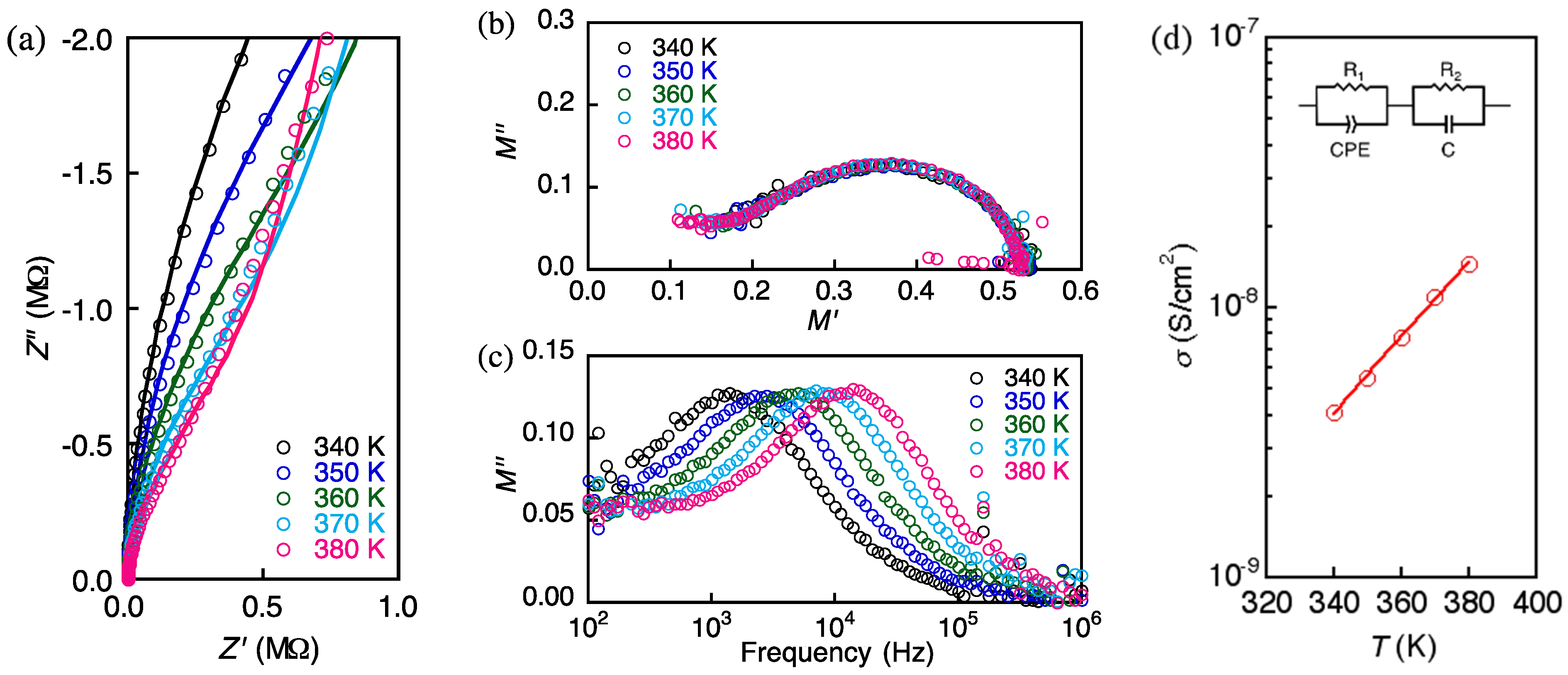
2.3. Electroconducting Properties of 1
3. Experimental Section
3.1. Materials
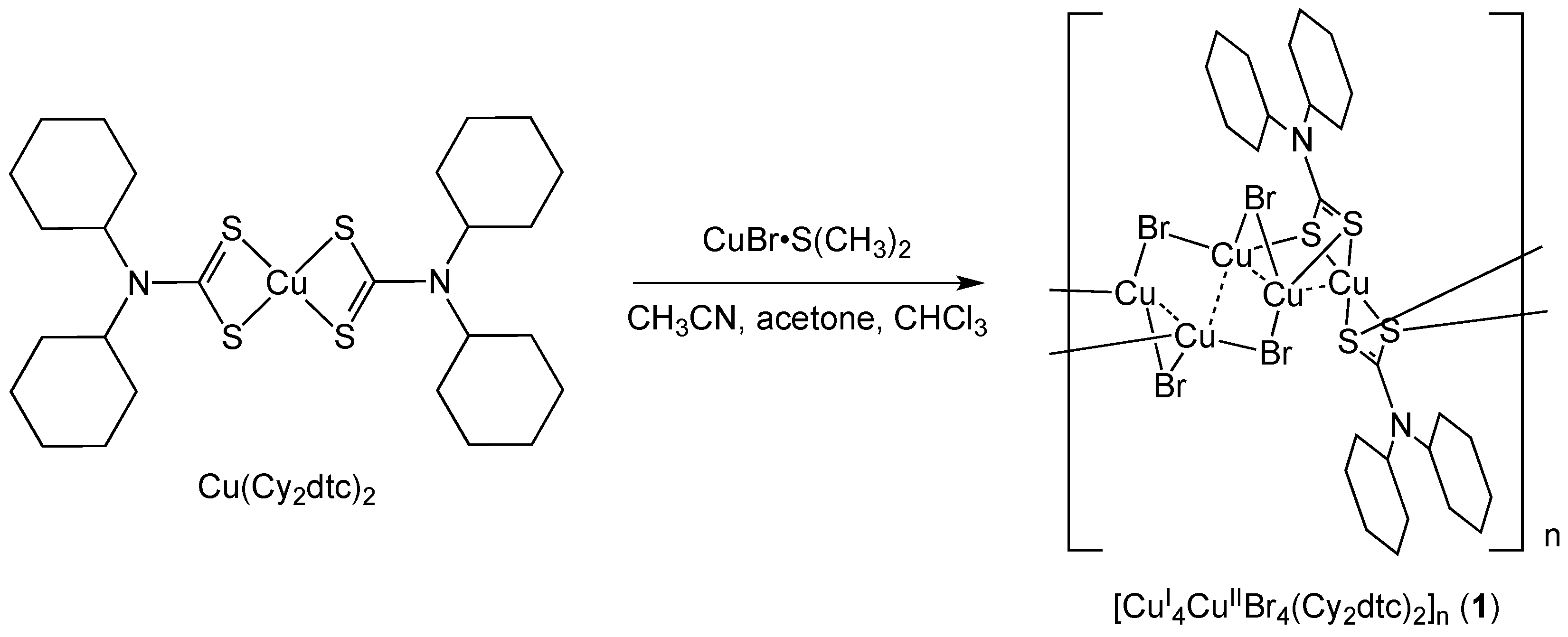
3.2. Synthesis of [CuI4CuIIBr4(Cy2dtc)2]n (1)
3.3. X-Ray Structure Determination
| Atoms | Bond lengths | Atoms | Bond lengths |
|---|---|---|---|
| Cu1–S1 | 2.2991(9) | Cu3*–S2 | 2.321(1) |
| Cu1–S2 | 2.3313(9) | Cu3*–Br2 | 2.3952(4) |
| Cu2–S1 | 2.3533(9) | Cu1···Cu2 | 2.7998(5) |
| Cu2–Br1 | 2.3668(6) | Cu2···Cu3 | 2.8670(6) |
| Cu2–Br2 | 2.3931(7) | Cu2···Cu3* | 2.7567(4) |
| Cu3–Br1 | 2.3859(6) | Cu3···Cu3* | 2.7816(4) |
| Formula | C26H44Br4Cu5N2S4 | Diffractometer | R-AXIS RAPID |
|---|---|---|---|
| Formula weight | 1150.23 | Radiation | Mo-Kα (λ = 0.71075 Å) |
| Crystal system | monoclinic | Temperature | 296 |
| Lattice parameters | a = 7.4743(3) Å | No. Obs. (All reflections) | 4005 |
| b = 13.5090(5) Å | No. Variables | 187 | |
| c = 17.8667(7) Å | Reflection/Parameter Ratio | 21.42 | |
| β = 103.0879(12) ° | Residuals: R1 (I > 2.00 σ(I)) a | 0.0315 | |
| V = 1757.14(12) Å3 | Residuals: wR2 (All reflections) b | 0.0737 | |
| Space group | P21/n (#14) | Goodness of Fit Indicator | 1.080 |
| Z value | 2 | Max Shift/Error in Final Cycle | 0.001 |
| Dcalc | 2.174 g/cm3 | Max. peak in Final Diff. Map | 0.85 |
| F000 | 1126.00 | Min. peak in Final Diff. Map | −0.64 |
| μ(MoKα) | 77.986 cm−1 | CCDC | 1,046,577 |
3.4. Physical Measurements
4. Conclusions
Acknowledgments
Author Contribution
Conflicts of Interest
References
- Kahn, O. Molecular Magnetism; VCH: New York, NY, USA, 1993. [Google Scholar]
- Ferlay, S.; Mallah, T.; Ouahes, R.; Veillet, P.; Verdaguer, M. A room-temperature organometallic magnet based on Prussian blue. Nature 1995, 378, 701–703. [Google Scholar] [CrossRef]
- Sato, O.; Iyoda, T.; Fujishima, A.; Hashimoto, K. Photoinduced magnetization of a cobalt-iron cyanide. Science 1996, 272, 704–705. [Google Scholar] [CrossRef] [PubMed]
- Amo-Ochoa, P.; Castillo, O.; Alexandre, S.S.; Welte, L.; de Pablo, P.J.; Rodriguez-Tapiador, I.; Gomez-Herrero, J.; Zamora, F. Synthesis of Designed Conductive One-Dimensional Coordination Polymers of Ni(II) with 6-Mercaptopurine and 6-Thioguanine. Inorg. Chem. 2009, 48, 7931–7936. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, S.; Kimura, S.; Takahashi, K.; Mori, H.; Yoshida, G.; Manabe, Y.; Matsuda, M.; Tajima, H.; Yamaura, J.-I. Intrinsic carrier doping in antiferromagnetically interacted supramolecular copper complexes with (pyrazino)tetrathiafulvalene (pyra-TTF) as the ligand, [CuIICl2(pyra-TTF)] and (pyra-TTF)2[CuI3Cl4(pyra-TTF)]. Inorg. Chem. 2008, 47, 4140–4145. [Google Scholar] [CrossRef]
- Kishida, H.; Ito, T.; Nakamura, A.; Takaishi, S.; Yamashita, M. Current oscillation originating from negative differential resistance in one-dimensional halogen-bridged nickel compounds. J. Appl. Phys. 2009, 106. [Google Scholar] [CrossRef]
- Mitsumi, M.; Murase, T.; Kishida, H.; Yoshinari, T.; Ozawa, Y.; Toriumi, K.; Sonoyama, T.; Kitagawa, H.; Mitani, T. Metallic behavior and periodical valence ordering in a MMX chain compound, Pt2(EtCS2)4I. J. Am. Chem. Soc. 2001, 123, 11179–11192. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, H.; Motokawa, N.; Matsunaga, S.; Yamashita, M.; Sugimoto, K.; Mori, T.; Toyota, N.; Dunbar, K.R. Control of charge transfer in a series of Ru2(II,II)/TCNQ two-dimensional networks by tuning the electron affinity of TCNQ units: A route to synergistic magnetic/conducting materials. J. Am. Chem. Soc. 2010, 132, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Otsubo, K.; Kobayashi, A.; Kitagawa, H.; Hedo, M.; Uwatoko, Y.; Sagayama, H.; Wakabayashi, Y.; Sawa, H. Most Stable Metallic Phase of the Mixed-Valence MMX-Chain, Pt2(dtp)4I (dtp = C2H5CS2−), in Purely d-Electronic Conductors Based on the Transition-Metal Complex. J. Am. Chem. Soc. 2006, 128, 8140–8141. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, M.; Yasuzuka, S.; Nakamura, M.; Shinoda, T.; Tatenuma, T.; Mitsumi, M.; Ozawa, Y.; Toriumi, S.; Tatenuma, T.; Mitsumi, M.; et al. A high-conductivity crystal containing a copper(I) coordination polymer bridged by the organic acceptor TANC. Angew. Chem. Int. Ed. 2006, 45, 5144–5147. [Google Scholar]
- Zhong, J.C.; Misaki, Y.; Munakata, M.; Kuroda-Sowa, T.; Maekawa, M.; Suenaga, Y.; Konaka, H. Silver(I) Coordination Polymer of 2,5-Bis-(4′,5′-bis(methylthio)-1′,3′-dithiol-2′-ylidene)-1,3,4,6-tetrathiapentalene (TTM-TTP) and Its Highly Conductive Iodine Derivative. Inorg. Chem. 2001, 40, 7096–7098. [Google Scholar] [CrossRef] [PubMed]
- Okubo, T.; Tanaka, N.; Kim, K.H.; Yone, H.; Maekawa, M.; Kuroda-Sowa, T. Magnetic and Conducting Properties of New Halide-Bridged Mixed-Valence CuI-CuII 1D Coordination Polymers Including a Hexamethylene Dithiocarbamate Ligand. Inorg. Chem. 2010, 49, 3700–3702. [Google Scholar] [CrossRef] [PubMed]
- Okubo, T.; Tanaka, N.; Kim, K.H.; Anma, H.; Seki, S.; Saeki, A.; Maekawa, M.; Kuroda-Sowa, T. Crystal structure and carrier transport properties of a new 3D mixed-valence Cu(I)–Cu(II) coordination polymer including pyrrolidine dithiocarbamate ligand. Dalton Trans. 2011, 40, 2218–2224. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Ueta, T.; Okubo, T.; Hayami, S.; Anma, H.; Kato, K.; Shimizu, T.; Fujimori, J.; Maekawa, M.; Kuroda-Sowa, T.; et al. Synthesis and conducting properties of a new mixed-valence Cu(I)–Cu(II) 1-D coordination polymer bridged by morpholine dithiocarbamate. Chem. Lett. 2011, 40, 1184–1186. [Google Scholar]
- Okubo, T.; Anma, H.; Tanaka, N.; Himoto, K.; Seki, S.; Saeki, A.; Maekawa, M.; Kuroda-Sowa, T. Crystal structure and carrier transport properties of a new semiconducting 2D coordination polymer with a 3,5-dimethylpiperidine dithiocarbamate ligand. Chem. Commum. 2013, 49, 4316–4318. [Google Scholar] [CrossRef]
- Tanaka, N.; Okubo, T.; Anma, H.; Kim, K.H.; Inuzuka, Y.; Maekawa, M.; Kuroda-Sowa, T. New Halide-Bridged 1D Mixed-Valence CuI–CuII Coordination Polymers Bearing a Piperidine-1-carbodithioato Ligand: Crystal Structure, Magnetic and Conductive Properties, and Application in Dye-Sensitized Solar Cells. Eur. J. Inorg. Chem. 2013, 19, 3384–3391. [Google Scholar] [CrossRef]
- Okubo, T.; Anma, H.; Nakahashi, Y.; Maekawa, M.; Kuroda-Sowa, T. New one-dimensional mixed-valence coordination polymers including an iodine-bridged pentanuclear copper(I) cluster unit. Polyhedron 2014, 69, 103–109. [Google Scholar] [CrossRef]
- Xie, Y.-M.; Liu, J.-H.; Wu, X.-Y.; Zhao, Z.-G.; Zhang, Q.-S.; Wang, F.; Chen, S.-C.; Lu, C.-Z. New Ferroelectric and Nonlinear Optical Porous Coordination Polymer Constructed from a Rare (CuBr)∞ Castellated Chain. Cryst. Growth Des. 2008, 8, 3914–3916. [Google Scholar] [CrossRef]
- Ye, Q.; Song, Y.-M.; Wang, G.-X.; Chen, K.; Fu, D.-W.; Chan, P.W.H.; Zhu, J.-S.; Huang, S.D.; Xiong, R.-G. Ferroelectric Metal-Organic Framework with a High Dielectric Constant. J. Am. Chem. Soc. 2006, 128, 6554–6555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiong, R.-G.; Huang, S.D. 3D Framework Containing Cu4Br4 Cubane as Connecting Node with Strong Ferroelectricity. J. Am. Chem. Soc. 2008, 130, 10468–10469. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Kitaura, R.; Noro, S.-I. Functional porous coordination polymers. Angew. Chem. Int. Ed. Engl. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Noro, S.-I. Rational synthesis and characterization of porous Cu(II) coordination polymers. Phys. Chem. Chem. Phys. 2010, 12, 2519–2531. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-H.; Ma, B.; Nguyen, S.T.; Hupp, J.T.; Albrecht-Schmitt, T.E. A metal-organic framework material that functions as an enantioselective catalyst for olefin epoxidation. Chem. Commun. 2006, 24, 2563–2565. [Google Scholar] [CrossRef]
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal-Organic Frameworks: Opportunities for Catalysis. Angew. Chem. Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef]
- Uemura, T.; Kitaura, R.; Ohta, Y.; Nagaoka, M.; Kitagawa, S. Nanochannel-promoted polymerization of substituted acetylenes in porous coordination polymers. Angew. Chem. Int. Ed. 2006, 45, 4112–4116. [Google Scholar] [CrossRef]
- Zou, R.-Q.; Sakurai, H.; Xu, Q. Preparation, adsorption properties, and catalytic activity of three-dimensional porous metal-organic frameworks composed of cubic building blocks and alkali-metal ions. Angew. Chem. Int. Ed. 2006, 45, 2542–2546. [Google Scholar] [CrossRef]
- Patel, R.N.; Kumar, S.; Pandeya, K.B. Synthesis and spectral studies of mixed-valence binary copper(II)–copper(I) complexes. Indian J. Chem. 2001, 40, 1104–1109. [Google Scholar]
- Batsanov, S.S. Van der Waals Radii of Elements. Inorg. Mater. 2001, 37, 1031–1046. [Google Scholar]
- Jian, F.; Wang, Z.; Bai, Z.; You, X.; Fun, H.-K.; Chinnakali, K.; Razak, I.R. The crystal structure, equilibrium and spectroscopic studies of bis(dialkyldithiocarbamate) copper(II) complexes [Cu2(R2dtc)4] (dtc = dithiocarbamate). Polyhedron 1999, 18, 3401–3406. [Google Scholar] [CrossRef]
- Ngo, S.C.; Banger, K.K.; DelaRosa, M.J.; Toscano, P.J.; Welch, J.T. Thermal and structural characterization of a series of homoleptic Cu(II) dialkyldithiocarbamate complexes: Bigger is only marginally better for potential MOCVD performance. Polyhedron 2003, 22, 1575–1583. [Google Scholar] [CrossRef]
- Hogarth, G.; Pateman, A.; Redmond, S.P. Functionalised dithiocarbamate complexes: Synthesis and molecular structures of bis(2-methoxyethyl)dithiocarbamate complexes [M{S2CN(CH2CH2OMe)2}2] (M = Ni, Cu, Zn) and [Cu{S2CN(CH2CH2OMe)2}2][ClO4]. Inorg. Chim. Acta 2000, 306, 232–236. [Google Scholar] [CrossRef]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Cryst. 1991, B47, 192–197. [Google Scholar] [CrossRef]
- Kubelka, P. New contributions to the optics of intensely light-scattering materials. J. Opt. Soc. Am. 1948, 38, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Cookson, J.; Evans, E.A.L.; Maher, J.P.; Serpell, C.J.; Paul, R.L.; Cowley, A.R.; Drew, M.G.B.; Beer, P.D. Metal-directed assembly of large dinuclear copper(II) dithiocarbamate macrocyclic complexes. Inorg. Chim. Acta 2010, 363, 1195–1203. [Google Scholar] [CrossRef]
- Sarova, G.H.; Jeliazkova, B.G. Effect of solvent and remote ligand substituents on the photochemical behavior of copper(II) dithiocarbamates and dithiophosphates. Transit. Met. Chem. 2001, 26, 388–394. [Google Scholar] [CrossRef]
- Sakthivel, S.; Kisch, H. Daylight photocatalysis by carbon-modified titanium dioxide. Angew. Chem. Int. Ed. Engl. 2003, 42, 4908–4911. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Nayak, P.; Choudhary, R.N.P. Study of complex impedance spectroscopic properties of LiBa2Nb5O15 ceramics. Mater. Chem. Phys. 2007, 106, 193–197. [Google Scholar] [CrossRef]
- Szu, S.-P.; Lin, C.-Y. AC impedance studies of copper doped silica glass. Mater. Chem. Phys. 2003, 82, 295–300. [Google Scholar] [CrossRef]
- Chandra, K.P.; Prasad, K.; Gupta, R.N. Impedance spectroscopy study of an organic semiconductor: Alizarin. Physica B Condens. Matter 2007, 388, 118–123. [Google Scholar] [CrossRef]
- Sural, M.; Ghosh, A. Electric conductivity and relaxation in ZnF2-AlF3-PbF2-LiF glasses. Solid State Ion. 2000, 130, 259–266. [Google Scholar] [CrossRef]
- Nadeem, M.; Akhtar, M.J.; Khan, A.Y. Effects of low frequency near metal-insulator transition temperatures on polycrystalline La0.65Ca0.35Mn1−yFeyO3 (where y = 0.05–0.10) ceramic oxides. Solid State Commun. 2005, 134, 431–436. [Google Scholar]
- Johnson, D. ZView: A Software Program for IES Analysis, Version 3.1; Scribner Associates Inc.: Southern Pines, NC, USA, 2009. [Google Scholar]
- Crystal Structure Analysis Package; Rigaku Corporation: Tokyo, Japan, 2010.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakatani, K.; Himoto, K.; Kono, Y.; Nakahashi, Y.; Anma, H.; Okubo, T.; Maekawa, M.; Kuroda-Sowa, T. Synthesis, Crystal Structure, and Electroconducting Properties of a 1D Mixed-Valence Cu(I)–Cu(II) Coordination Polymer with a Dicyclohexyl Dithiocarbamate Ligand. Crystals 2015, 5, 215-225. https://doi.org/10.3390/cryst5020215
Nakatani K, Himoto K, Kono Y, Nakahashi Y, Anma H, Okubo T, Maekawa M, Kuroda-Sowa T. Synthesis, Crystal Structure, and Electroconducting Properties of a 1D Mixed-Valence Cu(I)–Cu(II) Coordination Polymer with a Dicyclohexyl Dithiocarbamate Ligand. Crystals. 2015; 5(2):215-225. https://doi.org/10.3390/cryst5020215
Chicago/Turabian StyleNakatani, Kenji, Kento Himoto, Yuki Kono, Yuuki Nakahashi, Haruho Anma, Takashi Okubo, Masahiko Maekawa, and Takayoshi Kuroda-Sowa. 2015. "Synthesis, Crystal Structure, and Electroconducting Properties of a 1D Mixed-Valence Cu(I)–Cu(II) Coordination Polymer with a Dicyclohexyl Dithiocarbamate Ligand" Crystals 5, no. 2: 215-225. https://doi.org/10.3390/cryst5020215
APA StyleNakatani, K., Himoto, K., Kono, Y., Nakahashi, Y., Anma, H., Okubo, T., Maekawa, M., & Kuroda-Sowa, T. (2015). Synthesis, Crystal Structure, and Electroconducting Properties of a 1D Mixed-Valence Cu(I)–Cu(II) Coordination Polymer with a Dicyclohexyl Dithiocarbamate Ligand. Crystals, 5(2), 215-225. https://doi.org/10.3390/cryst5020215