Flux-Grown Piezoelectric Materials: Application to α-Quartz Analogues
Abstract
:1. Introduction
2. Flux-Growth and Morphology
2.1. Flux-Grown α-GaPO4
2.2. Flux-Grown α-GeO2

3. Impurities Contamination
3.1. Flux-Grown α-GaPO4
3.2. Flux-Grown α-GeO2

4. Thermal Characterizations
4.1. Flux-Grown α-GaPO4
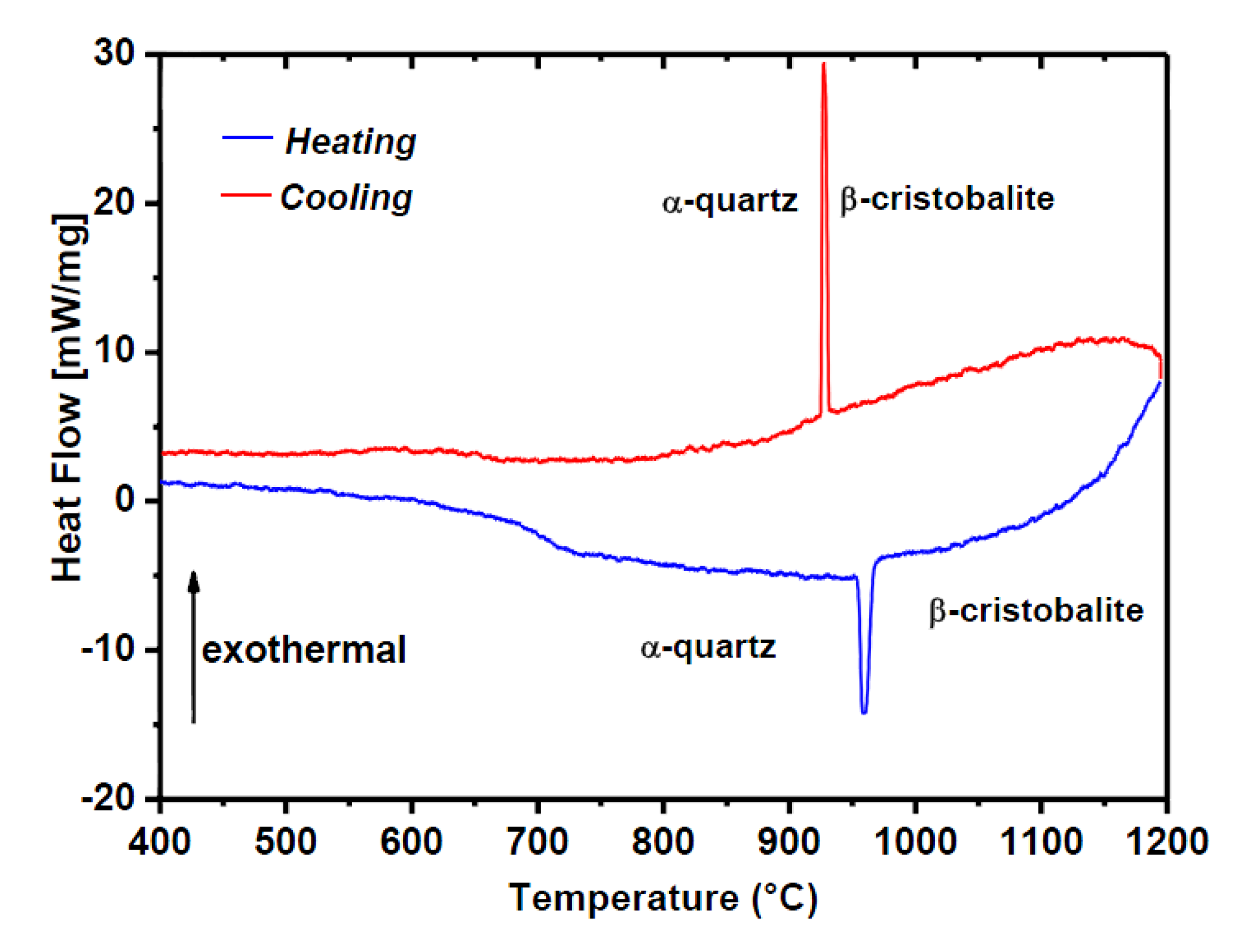
4.2. Flux-Grown α-GeO2
5. Elastic Constants
5.1. Ambient Conditions
5.1.1. Flux-Grown α-GaPO4
| Elastic constant | Hydrothermal-Growth | Computed values (−273 °C) | Flux-Growth |
|---|---|---|---|
| CE11 | 66.58 [112] 66.35 [67] 66.60 [17] 66.58 [113] | 79.80 [111] | 64.01 [46,110] 66.37 [90] 66.52 [103] |
| CE12 [=(CE11 − 2CE66)] | 21.81 [112] 21.65 [67] 21.80 [17] 17.38 [113] | 16.60 [111] | 13.51 [46,110] 21.45 [90] 21.04 [103] |
| |CE14| | 3.91 [112] 4.20 [67] 3.90 [17] 5.14 [113] | 3.20 [111] | 5.52 [46,110] 4.93 [90] 5.53 [103] |
| CE33 | 102.13 [112] 101.31 [67] 102.10 [17] 102.13 [113] | 106.30 [111] | 103.29 [90] 103.88 [103] |
| CE44 | 37.66 [112] 37.80 [67] 37.70 [17] 39.68 [113] | 39.90 [111] | 39.39 [46,110] 37.85 [90] 38.01 [103] |
| CE66 | 22.38 [112] 22.35 [67] 22.40 [17] 24.60 [113] | 31.60 [111] | 21.25 [46,110] 22.46 [90] 22.74 [103] |
5.1.2. Flux-Grown α-GeO2
| Elastic constant | Hydrothermal-Growth | Computed values | Flux-Growth [107] |
|---|---|---|---|
| CE11 CD11 | 64.00 [116] 64.80 [23] 66.40 [24] 64.13 [23] | 62.90 [109] 69.90 [114] 56.15 [115] | 68.1(1) 69.3(1) |
| CE12 | 22.00 [116] 21.30 [24] | 25.50 [109] 8.40 [114] 12.03 [115] | 25.1(1) |
| CE13 | 32.00 [116] | 25.70 [109] 4.10 [114] 19.39 [115] | – |
| | CE14| | 2.00 [116] 11.70 [23] 2.20 [24] | 0.60 [109] 15.6 [114] 0 [115] | ≈0 |
| CE33 | 118.0 [116] 116.0 [24] | 116.80 [109] 91.60 [114] 99.05 [115] | 118.8(2) |
| CE44 | 37.00 [116] 37.84 [23] 26.80 [24] | 35.00 [109] 38.40 [114] 39.99 [115] | 38.6(1) |
| CE66 CD66 | 21.00 [116] 21.10 [23] 22.53 [24] 24.90–25.14 [23] | 18.70 [109] 30.70 [114] 22.06 [115] | 21.5(1) 22.7(1) |
5.2. High Temperature
5.2.1. Flux-Grown α-GaPO4
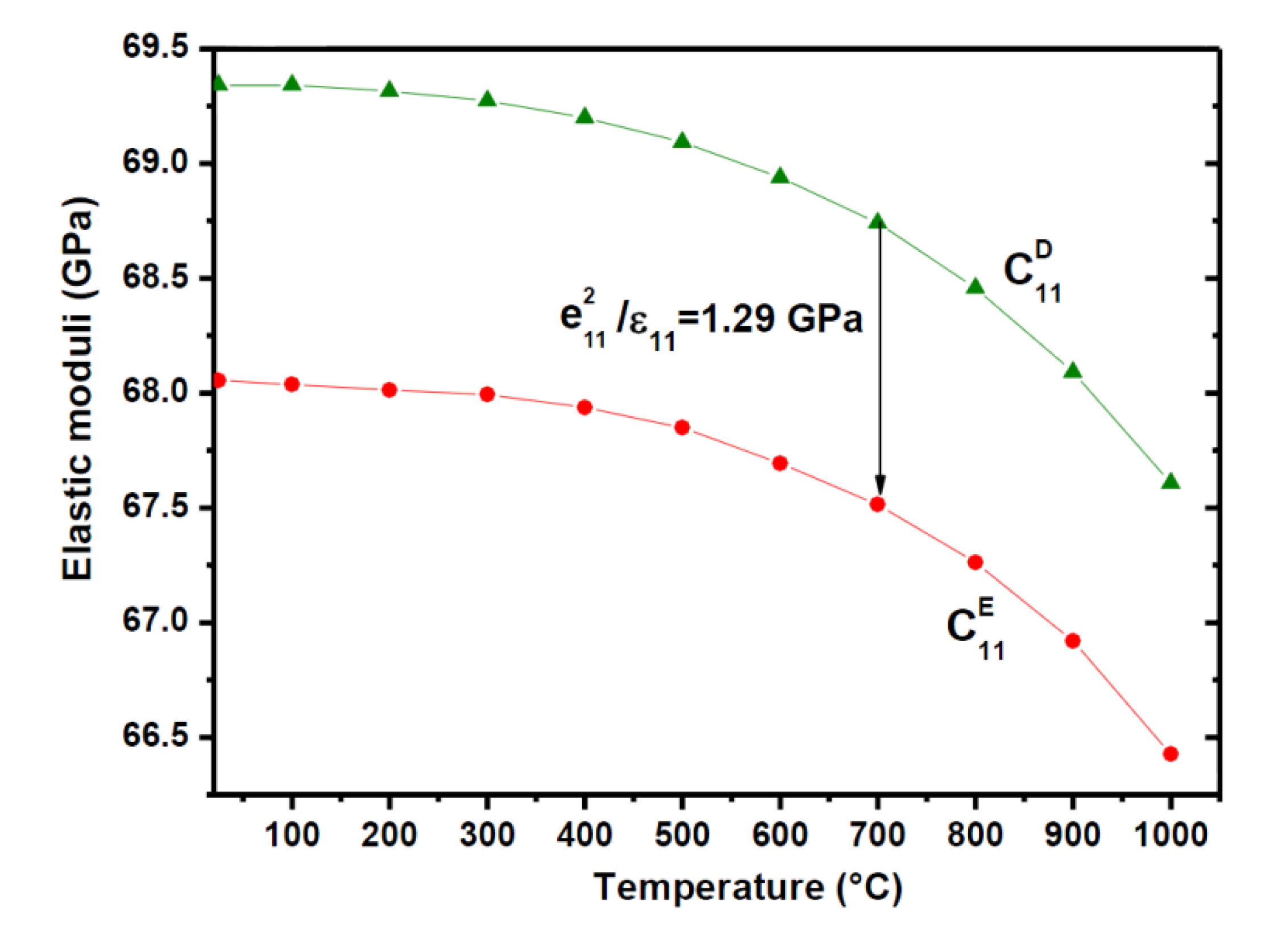
5.2.2. Flux-Grown α-GeO2
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Zhang, S.; Yu, F. Piezoelectric materials for high temperature sensors. J. Am. Ceram. Soc. 2011, 94, 3153–3170. [Google Scholar] [CrossRef]
- Jiang, X.; Kim, K.; Zhang, S.; Johnson, J.; Salazar, F. High temperature piezoelectric sensing. Sensors 2014, 14, 144–169. [Google Scholar] [CrossRef]
- Smith, G.S.; Alexander, L.E. Refinement of the atomic parameters of α-quartz. Acta Cryst. 1963, 16, 462–471. [Google Scholar] [CrossRef]
- Smith, G.S.; Isaacs, P.B. The crystal structure of quartz-like GeO2. Acta Cryst. 1964, 17, 842–846. [Google Scholar] [CrossRef]
- Le Page, Y.; Donnay, G. Refinement of the crystal structure of low-quartz. Acta Cryst. 1976, B32, 2456–2459. [Google Scholar] [CrossRef]
- Le Page, Y.; Calvert, L.D.; Gabe, E.J. Parameter variation in low-quartz between 94 and 298 K. J. Phys. Chem. Solids 1980, 41, 721–725. [Google Scholar] [CrossRef]
- Goiffon, A.; Bayle, G.; Astier, R.; Jumas, J.-C.; Maurin, M.; Philippot, E. Cristallochimie des phases GaPO4, AlAsO4 et GaAsO4. Etude comparée des structures de type quartz-α. Rev. Chim. Minér. 1983, 20, 338–350. (In French) [Google Scholar]
- Goiffon, A.; Jumas, J.-C.; Philippot, E. Phases de type quartz α: Structure de FePO4 et spectrométrie Mössbauer du fer-57. Rev. Chim. Minér. 1986, 23, 99–109. (In French) [Google Scholar]
- Goiffon, A.; Jumas, J.-C.; Maurin, M.; Philippot, E. Etude comparée à diverses températures (173, 293 et 373 K) des structures de type quartz α des phases MXO4 (MIII = Al, Ga et XV = P, As). J. Solid State Chem. 1986, 61, 384–396. (In French) [Google Scholar] [CrossRef]
- Glinnemann, J.; King, H.E.; Schulz, H.; Hahn, Th.; la Placa, S.J.; Dacol, F. Crystal structures of the low-temperature quartz-type phases of SiO2 and GeO2 at elevated pressure. Z. Kristall. 1992, 198, 177–212. [Google Scholar] [CrossRef]
- Haines, J.; Cambon, O.; Philippot, E.; Chapon, L.; Hull, S. A neutron diffraction study of the thermal stability of the α-quartz-type structure in germanium dioxide. J. Solid State Chem. 2002, 166, 434–441. [Google Scholar] [CrossRef]
- Guillot, R.; Fertey, P.; Hansen, N.K.; Allé, P.; Elkaïm, E.; Lecomte, C. Diffraction study of the piezoelectric properties of low quartz. Eur. Phys. J. B 2004, 42, 373–380. [Google Scholar] [CrossRef]
- Haines, J.; Cambon, O.; Prudhomme, N.; Fraysse, G.; Keen, D.A.; Chapon, L.C.; Tucker, M.G. High-temperature, structural disorder, phase transitions, and piezoelectric properties of GaPO4. Phys. Rev. B 2006, 73, 014103:1–014103:10. [Google Scholar] [CrossRef]
- Labéguerie, P.; Harb, M.; Baraille, I.; Rérat, M. Structural electronic, elastic, and piezoelectric properties of α-quartz and MXO4 (M = Al, Ga, Fe; X = P, As) isomorph compounds: A DFT study. Phys. Rev. B 2010, 81, 045107:1–045107:9. [Google Scholar] [CrossRef]
- Giangrisostomi, E.; Minicucci, M.; Trapananti, A.; di Cicco, A. Multiple-scattering X-ray absorption analysis of quartz-like, rutile-like, and amorphous germanium dioxide. Phys. Rev. B 2011, 84, 214202:1–214202:8. [Google Scholar] [CrossRef]
- Lignie, A.; Granier, D.; Armand, P.; Haines, J.; Papet, P. Modulation of quartz-like GeO2 structure by Si substitution: An X-ray diffraction study of Ge1−xSixO2 (0 ≤ x < 0.2) flux-grown single crystals. J. Appl. Cryst. 2012, 45, 272–278. [Google Scholar]
- Philippot, E.; Palmier, D.; Pintard, M.; Goiffon, A. A general survey of quartz and quartz-like materials: Packing distortions, temperature, and pressure effects. J. Solid State Chem. 1996, 123, 1–13. [Google Scholar] [CrossRef]
- Philippot, E.; Armand, P.; Yot, P.; Cambon, O.; Goiffon, A.; McIntyre, G.J.; Bordet, P. Neutron and X-ray structure refinements between 15 and 1073 K of piezoelectric gallium arsenate, GaAsO4: Temperature and pressure behavior compared with other α-quartz materials. J. Solid State Chem. 1999, 146, 114–123. [Google Scholar] [CrossRef]
- Grimm, H.; Dorner, B. On the mechanism of the α-β phase transformation of quartz. J. Phys. Chem. Solids 1975, 36, 407–413. [Google Scholar] [CrossRef]
- Zwijnenburg, M.A.; Huenerbein, R.; Bell, R.G.; Cora, F. A computational study into the (tetrahedral) distortion of TX2 α-quartz materials: The effect of changing the chemical composition away from SiO2. J. Solid State Chem. 2006, 179, 3429–3436. [Google Scholar]
- Kihara, K. An X-ray study of the temperature dependence of the quartz structure. Eur. J. Mineral. 1990, 2, 63–77. [Google Scholar]
- Philippot, E.; Goiffon, A.; Ibanez, A.; Pintard, M. Structure deformations and existence of the α-β transition in MXO4 quartz-like materials. J. Solid State Chem. 1994, 110, 356–362. [Google Scholar] [CrossRef]
- Balitsy, D.V.; Silvestrova, O.Y.; Balitsky, V.S.; Pisarevsky, Y.V.; Pushcharovsky, D.Y.; Philippot, E. Elastic, piezoelectric, and dielectric properties of α-GeO2 single crystals. Crystallogr. Rep. 2000, 45, 145–147. [Google Scholar] [CrossRef]
- Pisarevsky, Y.V.; Silvestrova, O.Y.; Philippot, E.; Balitsky, D.V.; Pisharovsky, D.Y.; Balitsky, V.S. Piezoelectric, Dielectric and Elastic Properties of Germanium Dioxide Single Crystals. In Proceedings of the 2000 IEEE/EIA International Frequency Control Symposium and Exhibition, Kansas City, MO, USA, 7–9 June 2000.
- Cambon, O.; Haines, J. Structure-piezoelectric Property Relationships in α-Quartz Isotypes: Design and Characterization of High Performance Piezoelectric Materials. In Proceedings of the 2003 IEEE International Frequency Control Symposium and and PDA Exhibition Jointly with the 17th European Frequency and Time Forum, Tampa, FL, USA, 4–8 May 2003.
- Cambon, O.; Haines, J.; Fraysse, G.; Détaint, J.; Capelle, B.; van der Lee, A. Piezoelectric characterization and thermal stability of a high-performance α-quartz-type material, gallium arsenate. J. Appl. Phys. 2005, 97, 074110:1–074110:7. [Google Scholar] [CrossRef]
- Lignie, A.; Ménaert, B.; Armand, P.; Pena, A.; Debray, J.; Papet, P. Top seeded solution growth and structural characterizations of α-quartz-like structure GeO2 single crystal. Cryst. Growth Des. 2013, 13, 4220–4225. [Google Scholar] [CrossRef]
- Engel, G.F.; Krempl, P.W. Stability of α-phases of quartz-isomorphs. Ferroelectrics 1984, 54, 9–12. [Google Scholar] [CrossRef]
- Kolodiev, B.N.; Makhina, I.B. GeO2—A promising new piezoelectric. Sov. Phys. Crystallogr. 1989, 34, 455–456. [Google Scholar]
- Laubengayer, A.W.; Morton, D.S. The polymorphism of germanium dioxide. J. Am. Chem. Soc. 1932, 54, 2303–2320. [Google Scholar] [CrossRef]
- Baur, W.H.; Khan, A.A. Rutile-type compounds. IV. SiO2, GeO2 and a comparison with other rutile-type structures. Acta Cryst. 1971, B27, 2133–2139. [Google Scholar]
- Rao, K.V.K.; Naidu, S.V.N.; Iyengar, L. Precision lattice parameters and the coefficients of thermal expansion of hexagonal germanium dioxide. J. Appl. Cryst. 1973, 6, 136–138. [Google Scholar] [CrossRef]
- Yamanaka, T.; Kurashima, R.; Mimaki, J. X-ray diffraction study of bond character of rutile-type SiO2, GeO2 and SnO2. Z. Kristallogr. 2000, 215, 424–428. [Google Scholar]
- Micoulaut, M.; Cormier, L.; Henderson, G.S. The structure of amorphous, crystalline and liquid GeO2. J. Phys. Condens. Matter 2006, 18, R753–R784. [Google Scholar] [CrossRef]
- Madon, M.; Gillet, P.; Julien, C.; Price, G.D. A vibrational study of phase transitions among the GeO2 polymorphs. Phys. Chem. Miner. 1991, 18, 7–18. [Google Scholar] [CrossRef]
- Kotera, Y.; Yonemura, M. Kinetics of the transformation of germanium oxide. Trans. Faraday Soc. 1963, 59, 147–155. [Google Scholar] [CrossRef]
- Newns, G.R.; Hanks, R. Thermal behavior of germanium dioxide. J. Chem. Soc. A 1966, 954–957. [Google Scholar] [CrossRef]
- Sarver, J.F. Polymorphism and subsolidus equilibria in the system GeO2-TiO2. Am. J. Sci. 1961, 259, 709–718. [Google Scholar] [CrossRef]
- Bielz, T.; Soisuwan, S.; Kaindl, R.; Tessadri, R.; Többens, D.M.; Klötzer, B.; Penner, S. A high-resolution diffraction and spectroscopic study of the low-temperature phase transformation of hexagonal to tetragonal GeO2 with and without alkali hydroxide promotion. J. Phys. Chem. C 2011, 115, 9706–9712. [Google Scholar] [CrossRef]
- Perloff, A. Temperature inversions of anhydrous gallium orthophosphate. J. Am. Ceram. Soc. 1956, 39, 83–88. [Google Scholar] [CrossRef]
- Barz, R.-U.; Schneider, J.; Gille, P. High-temperature phase transitions of gallium orthophosphate (GaPO4). Z. Kristallogr. 1999, 214, 845–849. [Google Scholar] [CrossRef]
- Jacobs, K.; Hofmann, P.; Klimm, D.; Reichow, J.; Schneider, M. Structural phase transformations in crystalline gallium orthophosphate. Solid State Chem. 2000, 149, 180–188. [Google Scholar] [CrossRef]
- Pey, F. Synthèse et Caractérisations de Matériaux Homéotypes du Quartz. Ph.D. Thesis, Université Montpellier II, Montpellier, France, October 2004. [Google Scholar]
- Beaurain, M.; Armand, P.; Papet, P. Synthesis and characterization of α-GaPO4 single crystals grown by the flux method. J. Cryst. Growth 2006, 294, 396–400. [Google Scholar] [CrossRef]
- Angot, E.; le Parc, R.; Levelut, C.; Beaurain, M.; Armand, P.; Cambon, O.; Haines, J. A high temperature Raman scattering study of the phase transitions in GaPO4 and the AlPO4-GaPO4 system. J. Phys. Condens. Matter 2006, 18, 4315–4327. [Google Scholar] [CrossRef] [PubMed]
- Beaurain, M.; Armand, P.; Balitsky, D.; Papet, P. Physical Characterizations of α-GaPO4 Single Crystals Grown by the Flux Method. In Proceedings of the 2007 Joint Meeting of the European Time and Frequency Forum (EFTF) and the IEEE International Frequency Control Symposium (IEEE-FCS), Geneva, Switzerland,, 29 May–1 June 2007.
- Hirano, S.; Miwa, K.; Naka, S. Hydrothermal synthesis of gallium orthophosphate crystals. J. Cryst. Growth 1986, 79, 215–218. [Google Scholar] [CrossRef]
- Hirano, S.; Kim, P. Hydrothermal synthesis of gallium orthophosphate crystals. Bull. Chem. Soc. Jpn. 1989, 62, 275–278. [Google Scholar] [CrossRef]
- Engel, G.; Klapper, H.; Krempl, P.; Mang, H. Growth twinning in quartz-homeotypic gallium orthophosphate crystals. J. Cryst. Growth 1989, 94, 597–606. [Google Scholar] [CrossRef]
- Cochez, M. Cristallogénèse et Caractérisations du Phosphate de Gallium, GaPO4, Matériau Piézoélectrique à Fort Coefficient de Couplage. Ph.D. Thesis, Université Montpellier II, Montpellier, France, September 1994. [Google Scholar]
- Hirano, S.; Kim, P.C. Growth of gallium orthophosphate single crystals in acidic hydrothermal solutions. J. Mater. Sci. 1991, 26, 2805–2808. [Google Scholar] [CrossRef]
- Philippot, E.; Ibanez, A.; Goiffon, A.; Cochez, M.; Zarka, A.; Capelle, B.; Schwartzel, J.; Détaint, J. A quartz-like material: Gallium phosphate (GaPO4); crystal growth and characterization. J. Cryst. Growth 1993, 130, 195–208. [Google Scholar] [CrossRef]
- Palmier, D.; Goiffon, A.; Capelle, B.; Détaint, J.; Philippot, E. Crystal growth and characterization of quartz-like material: Gallium phosphate (GaPO4). J. Cryst. Growth 1996, 166, 347–353. [Google Scholar] [CrossRef]
- Motchany, A.I.; Chvanski, P.P.; Leonyuk, N.I. Synthesis and solubility of GaPO4 crystals in acid solutions under hydrothermal conditions. J. Cryst. Growth 2000, 211, 506–508. [Google Scholar] [CrossRef]
- Yot, P.; Cambon, O.; Balitsky, D.; Goiffon, A.; Philippot, E.; Capelle, B.; Détaint, J. Advances in crystal growth and characterizations of gallium phosphate, GaPO4. J. Cryst. Growth 2001, 224, 294–302. [Google Scholar] [CrossRef]
- Demianets, L.N. Gallium orthophosphate hydrothermal growth at high temperatures (320 °C). Ann. Chim. Sci. Mater. 2001, 26, 67–74. [Google Scholar]
- Jacobs, K.; Hofmann, P.; Reichow, J. Physico-chemical aspects of the hydrothermal growth of GaPO4. Ann. Chim. Sci. Mater. 2001, 26, 85–90. [Google Scholar] [CrossRef]
- Barz, R.-U.; Grassl, M.; Gille, P. Study of anisotropic effects in hydrothermal growth of gallium orthophosphate single crystals. Ann. Chim. Sci. Mater. 2001, 26, 95–98. [Google Scholar] [CrossRef]
- Grassel, M.; Barz, R.-U.; Gille, P. Reducing inversion twinning in single crystal growth of GaPO4. Cryst. Res. Technol. 2002, 37, 531–539. [Google Scholar] [CrossRef]
- Barz, R.-U.; Grassel, M.; Gille, P. Studies on the solubility of GaPO4 in phosphoric acid. J. Cryst. Growth 2002, 245, 273–277. [Google Scholar] [CrossRef]
- Balitsky, D.V.; Philippot, E.; Papet, P.; Balitsky, V.S.; Pey, F. Comparative crystal growth of GaPO4 crystals in the retrograde and direct solubility range by hydrothermal methods of temperature gradient. J. Cryst. Growth 2005, 275, e887–e894. [Google Scholar] [CrossRef]
- Byrappa, K.; Yoshimura, M. Hydrothermal Growth of Some Selected Crystals. In Handbook of Hydrothermal Technology, 2nd ed.; Noyes Publications: Park Ridge, NJ, USA; William Andrew Publishing, LLC: Norwich, NY, USA, 2001. [Google Scholar]
- Philippot, E.; Goiffon, A.; Maurin, M.; Détaint, J.; Schwartzel, J.; Toudic, B.; Capelle, B.; Zarka, A. Evaluation of high quality berlinite crystals grown in sulphuric acid medium. J. Cryst. Growth 1990, 104, 713–726. [Google Scholar] [CrossRef]
- Zvereva, O.V.; Demianets, L.N. Substrate orientation and hydrothermal growth of GaPO4 single crystals. Crystallogr. Rep. 1995, 40, 990–993. [Google Scholar]
- Krispel, F.; Krempl, P.W.; Knoll, P.; Wallnöfer, W. OH Impurities in Gallium Phosphate. In Proceedings of the 11th European Frequency and Time Forum, Neuchâtel, Switzerland, 4–6 March 1997.
- Détaint, J.; Zarka, A.; Capelle, B.; Palmier, D.; Philippot, E. Optimisation of the Design of the Resonators Using the New Materials: Application to Gallium Phosphate and Langasite. In Proceedings of the 1997 IEEE International, Orlando, FL, USA, 28–30 May 1997.
- Palmier, D. Optimisation de la Cristallogénèse et de la Caractérisation des Propriétés Piézoélectriques du Phosphate de gallium. Ph.D. Thesis, Université Montpellier II, Montpellier, France, November 1996. [Google Scholar]
- Marhino, E.; Palmier, D.; Goiffon, A.; Philippot, E. Spatial-OH impurity distribution in gallium phosphate crystals. J. Mater. Sci. 1998, 33, 2825–2830. [Google Scholar] [CrossRef]
- Wallnöfer, W.; Krempl, P.W.; Krispel, F.; Willfurth, V. Segregation forming and growth defect characterization by heat treatment of hydrothermally grown GaPO4. J. Cryst. Growth 1999, 198–199, 487–491. [Google Scholar]
- Grassl, M.; Barz, R.-U.; Gille, P. Etch studies on GaPO4 single crystals. J. Cryst. Growth 2000, 220, 522–530. [Google Scholar] [CrossRef]
- Jacobs, K.; Hofmann, P.; Klimm, D. OH impurities in GaPO4 crystals: Correlation between infrared absorption and mass loss during thermal treatment. J. Cryst. Growth 2002, 237–239, 837–842. [Google Scholar]
- Prud’homme, N. Cristallisation-dissolution de GaPO4: Phénomènes à l’interface Cristal-Solvant. Etude de la Dissolution Contrôlée de GaPO4 pour la Réalisation de Résonateurs Piézoélectriques à Haute Fréquence. Ph.D. Thesis, Université Montpellier II, Montpellier, France, December 2005. [Google Scholar]
- Hofmann, P.; Juda, U.; Jacobs, K. Characterization of etch figures on as-grown surfaces of GaPO4 crystals. J. Cryst. Growth 2005, 275, e1883–e1888. [Google Scholar] [CrossRef]
- Defregger, S.; Engel, G.F.; Krempl, P.W. Linear and nonlinear optical properties of quartz-type GaPO4. Phys. Rev. B 1991, 43, 6733–6738. [Google Scholar] [CrossRef]
- Barz, R.U.; Ghemen, S.V. Water-free gallium phosphate single-crystal growth from the flux. J. Cryst. Growth 2005, 275, e921–e926. [Google Scholar] [CrossRef]
- Hill, V.G.; Chang, L.L.Y. Hydrothermal investigation of GeO2. Am. Miner. 1968, 53, 1744–1748. [Google Scholar]
- Roy, R.; Theokritoff, S. Crystal growth of metastable phases. J. Cryst. Growth 1972, 12, 69–72. [Google Scholar] [CrossRef]
- Demianets, L.N. Hydrothermal synthesis of new compounds. Prog. Cryst. Growth Charact. 1990, 22, 299–355. [Google Scholar]
- Glushkova, T.M.; Kiselev, D.F.; Makhina, I.B.; Firsova, M.M.; Shtyrkova, A.P. Trigonal germanium dioxide: Its preparation and optical parameters. Moscow Univ. Phys. Bull. 1992, 47, 55–58. [Google Scholar]
- Balitsky, D.V.; Balitsky, V.S.; Pisarevley, Y.V.; Philippot, E.; Silvestrova, O.Y.; Pushcharovsky, D.Y. Growth of germanium dioxide single crystals with α-quartz structure and investigation of their crystal structure, optical, elastic, piezoelectric, dielectric and mechanical properties. Ann. Chim. Sci. Mater. 2001, 26, 183–192. [Google Scholar] [CrossRef]
- Balitsky, D.V.; Balitsky, V.S.; Pushcharovsky, D.Y.; Bondarenko, G.V.; Kosenko, A.V. Growth and characterization of GeO2 single crystals with the quartz structure. J. Cryst. Growth 1997, 180, 212–219. [Google Scholar] [CrossRef]
- Laurent, Y. Obtention de monocristaux au sein d’un flux fondu. Rev. Chim. Miner. 1969, 6, 1145–1186. (In French) [Google Scholar]
- Elwell, D.; Neate, B.W. Review: Mechanisms of crystal growth from fluxed melts. J. Mater. Sci. 1971, 6, 1499–1519. [Google Scholar] [CrossRef]
- Tolksdorf, W. Crystal growth and epitaxy from high-temperature solutions. In Synthesis, Crystal Growth and Characterization; Lal, K., Ed.; North-Holland: Oxford, UK, 1982; pp. 197–211. [Google Scholar]
- Nielse, J.W. Recent development in crystal growth from high-temperature solutions. In Growth of Crystals; Chernov, A.A., Ed.; Consultant Bureau: New York, NY, USA, 1984; Volume 12, pp. 143–154. [Google Scholar]
- Roy, R.; White, W.B. High temperature solution (flux) and high pressure solution (hydrothermal) crystal growth. J. Cryst. Growth 1968, 3–4, 33–42. [Google Scholar]
- Beaurain, M.; Armand, P.; Papet, P. Growth of piezoelectric single crystals by the flux method. J. Cryst. Growth 2005, 2975, e279–e282. [Google Scholar] [CrossRef]
- Shvanskii, E.; Armand, P.; Balitsky, D.; Philippot, E.; Papet, E. Flux growth gallium orthophosphate crystals. Ann. Chim. Sci. Mater. 2006, 31, 97–102. [Google Scholar] [CrossRef]
- Beaurain, M. Monocristaux de α-GaPO4: Croissance par la Technique du Flux et Caractérisations Physiques. Ph.D. Thesis, Université Montpellier II, Montpellier, France, December 2006. [Google Scholar]
- Armand, P.; Beaurain, M.; Rufflé, B.; Ménaert, B.; Balitsky, D.; Clément, S.; Papet, P. Characterizations of piezoelectric GaPO4 single crystals grown by the flux method. J. Cryst. Growth 2008, 310, 1455–1459. [Google Scholar] [CrossRef]
- Finch, C.B.; Clark, G.W. Flux growth and characterization of hexagonal germanium dioxide single crystals. Am. Miner. 1968, 53, 1394–1398. [Google Scholar]
- Goodrum, J.W. Solution top-seeding: Growth of GeO2 polymorphs. J. Cryst. Growth 1972, 13–14, 604–607. [Google Scholar]
- Lignie, A. Matériaux Piézoélectriques Pour Applications Hautes Températures: Etude de la Croissance de Monocristaux de Ge1−xSixO2 (0 ≤ x ≤ 0,2) et de Leurs Propriétés. Ph.D. Thesis, Université Montpellier II, Montpellier, France, September 2012. [Google Scholar]
- Lignie, A.; Armand, P.; Papet, P. Growth of piezoelectric water-free GeO2 and SiO2-substituted GeO2 single-crystals. Inorg. Chem. 2011, 50, 9311–9317. [Google Scholar] [CrossRef] [PubMed]
- Tarr, W.A.; Lonsdale, J.T. Pseudo-cubic quartz crystals from Artesia, New Mexico. Am. Miner. 1929, 14, 50–53. [Google Scholar]
- Hartman, P. Sur la morphologie des cristaux. Bull. Miner. 1978, 101, 195–201. (In French) [Google Scholar]
- Iwasaki, H.; Iwasaki, F. Morphological variations of quartz crystals as deduced from computer experiments. J. Cryst. Growth 1995, 151, 348–358. [Google Scholar] [CrossRef]
- Philippot, E.; Goiffon, A.; Ibanez, A. Comparative crystal habit study of quartz and MPO4 isomorphous compounds (M = Al, Ga). J. Cryst. Growth 1996, 160, 268–278. [Google Scholar] [CrossRef]
- Philippot, E.; Ibanez, A.; Goiffon, A.; Capelle, B.; Zarka, A.; Schwartzel, J.; Détaint, J. Crystal Growth and Physical Characterizations of GaPO4. In Proceedings of the 6th European Frequency and Time Forum, Noordwijk, NL, USA,, 17–19 March 1992.
- Cochez, M.; Ibanez, A.; Goiffon, A.; Philippot, E. Crystal growth and infrared characterization of GaPO4 in phosphoric-sulphuric media. Eur. J. Solid State Inorg. Chem. 1993, 30, 509–519. [Google Scholar]
- Beaurain, M.; Armand, P.; Papet, P. Growth of α-GaPO4 and α-GeO2 single crystals by the flux method. J. Phys. IV 2005, 126, 23–26. [Google Scholar]
- Krempl, P.W.; Krispel, F.; Wallnöfer, W.; Leuprecht, G. GaPO4: A Critical Review of Material Data. In Proceedings of the Ninth European Frequency and Time Forum, Besançon, France, 8–10 March 1995.
- Armand, P.; Beaurain, M.; Rufflé, B.; Ménaert, B.; Papet, P. Temperature dependence of single-crystal elastic constants of flux-grown α-GaPO4. Inorg. Chem. 2009, 48, 4988–4996. [Google Scholar] [CrossRef] [PubMed]
- Sendova-Vassileva, M.; Tzenov, N.; Dimova-Malinovskia, D.; Rosenbauer, M.; Stutzmann, M.; Josepovits, K.V. Structural and luminescence studies of stain-etched and electrochemically etched germanium. Thin Solid Films 1995, 255, 282–285. [Google Scholar] [CrossRef]
- Kaindl, R.; Többens, D.M.; Penner, S.; Bielz, T.; Soisuwan, S.; Klötzer, B. Quantum mechanical calculations of the vibrational spectra of quartz- and rutile-type GeO2. Phys. Chem. Miner. 2012, 39, 47–55. [Google Scholar] [CrossRef]
- Fraysse, G.; Lignie, A.; Armand, P.; Bourgogne, D.; Haines, J.; Ménaert, B.; Papet, P. Vibrational origin of the thermal stability in the highly distorted α-quartz type material GeO2: An experimental and theoretical study. Inorg. Chem. 2013, 52, 7271–7279. [Google Scholar] [CrossRef] [PubMed]
- Hermet, P.; Fraysse, G.; Lignie, A.; Armand, P.; Papet, Ph. Density functional theory predictions of the nonlinear optical properties in α-quartz-type germanium dioxide. J. Phys. Chem. C 2012, 116, 8692–8698. [Google Scholar] [CrossRef]
- Hermet, P.; Lignie, A.; Fraysse, G.; Armand, P.; Papet, P. Thermodynamic properties of the α-quartz-type and rutile-type GeO2 from first-principles calculations. Phys. Chem. Chem. Phys. 2013, 15, 15943–15948. [Google Scholar] [CrossRef] [PubMed]
- Lignie, A.; Zhou, W.; Armand, P.; Rufflé, B.; Mayet, R.; Debray, J.; Hermet, P.; Ménaert, B.; Thomas, P.; Papet, P. High-temperature elastic moduli of flux-grown α-GeO2 single crystal. ChemPhysChem 2014, 15, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Beaurain, M.; Armand, P.; Detaint, J.; Ménaert, B.; Balitsky, D.; Papet, P. Elastic characterizations of the α-GaPO4 single crystals grown by the flux method. J. Phys. Condens. Matter 2008, 20, 025226:1–025226:7. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, B. Theoretical investigation of mechanical and thermal properties of MPO4 (M = Al, Ga). J. Eur. Ceram. Soc. 2013, 33, 2817–2821. [Google Scholar] [CrossRef]
- Krempl, P.W.; Schleinzer, G.; Wallnöfer, W. Gallium phosphate: A new piezoelectric crystal material for high-temperature sensorics. Sens. Actuator A 1997, 61, 361–363. [Google Scholar] [CrossRef]
- Krempl, P.W.; Krispel, F.; Wallnöfer, W. Industrial development and prospects of GaPO4. Ann. Chim. Sci. Mater. 1997, 22, 623–626. [Google Scholar]
- Ghobadi, E.; Capobianco, J.A. Crystal properties of α-quartz type GeO2. Phys. Chem. Chem. Phys. 2000, 2, 5761–5763. [Google Scholar] [CrossRef]
- Liu, Q.-J.; Liu, Z.-T.; Feng, L.-P.; Tian, H. First-principles study of structural, elastic, electronic and optical properties of rutile GeO2 and α-quartz GeO2. Solid State Sci. 2010, 12, 1748–1755. [Google Scholar] [CrossRef]
- Grimsditch, M.; Polian, A.; Brazhkin, V.; Balitskii, D. Elastic constants of α-GeO2. J. Appl. Phys. 1998, 83, 3018–3020. [Google Scholar] [CrossRef]
- Krempl, P.W. Piezoelectricity in quartz analogues. J. Phys. IV 2005, 126, 95–100. [Google Scholar]
- Wallnöfer, W.; Stadler, J.; Krempl, P. Temperature Dependence of Elastic Constants of GaPO4 and Its Influence on BAW and SAW Devices. In Proceedings of the 7th European Frequency and Time Forum, Neuchâtel, Switzerland, 16–18 March 1993.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Armand, P.; Lignie, A.; Beaurain, M.; Papet, P. Flux-Grown Piezoelectric Materials: Application to α-Quartz Analogues. Crystals 2014, 4, 168-189. https://doi.org/10.3390/cryst4020168
Armand P, Lignie A, Beaurain M, Papet P. Flux-Grown Piezoelectric Materials: Application to α-Quartz Analogues. Crystals. 2014; 4(2):168-189. https://doi.org/10.3390/cryst4020168
Chicago/Turabian StyleArmand, Pascale, Adrien Lignie, Marion Beaurain, and Philippe Papet. 2014. "Flux-Grown Piezoelectric Materials: Application to α-Quartz Analogues" Crystals 4, no. 2: 168-189. https://doi.org/10.3390/cryst4020168
APA StyleArmand, P., Lignie, A., Beaurain, M., & Papet, P. (2014). Flux-Grown Piezoelectric Materials: Application to α-Quartz Analogues. Crystals, 4(2), 168-189. https://doi.org/10.3390/cryst4020168




