Synthesis, Anion Disordering and Electronic Structure of Rb2KWO3F3 Elpasolite
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Synthesis
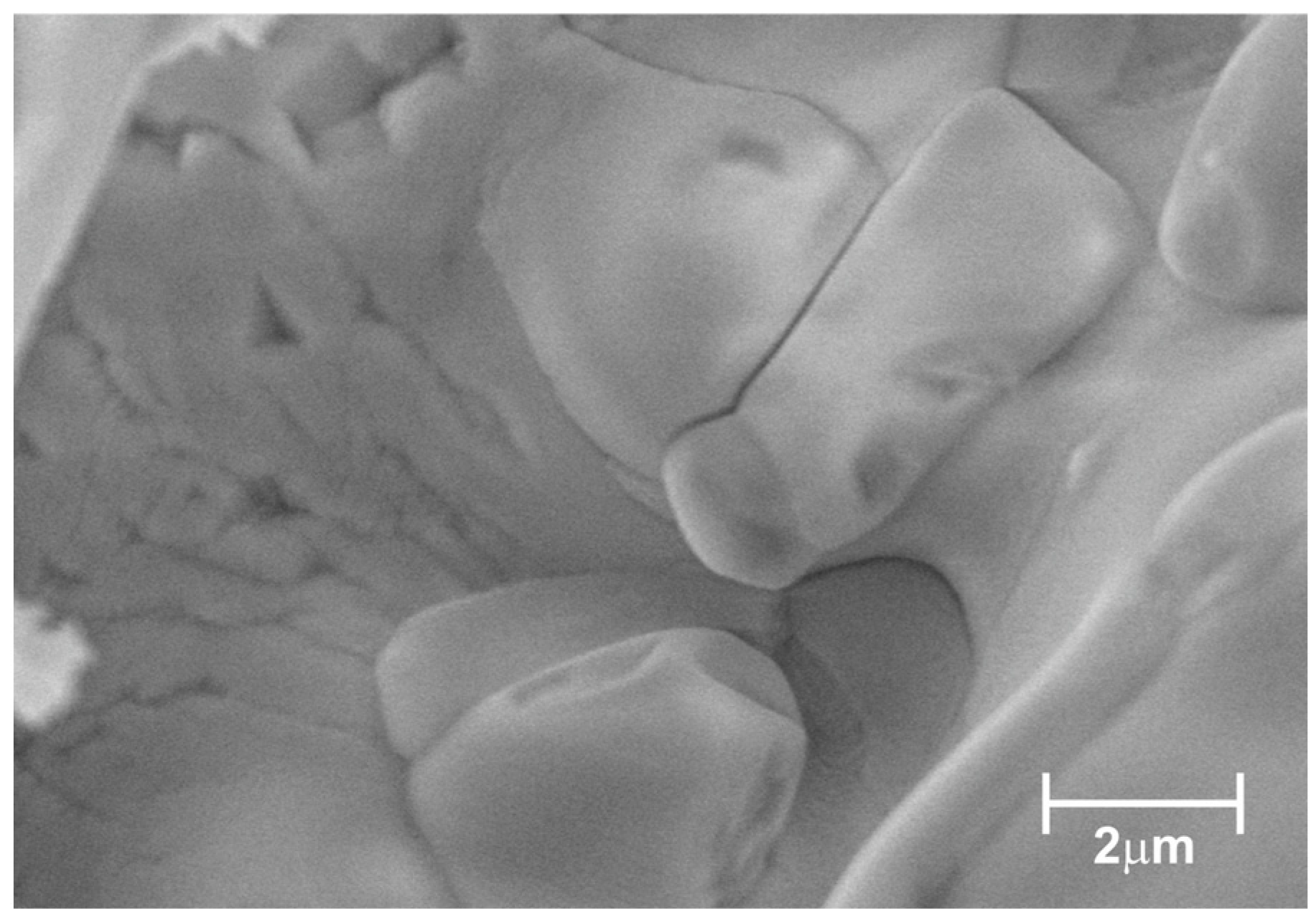
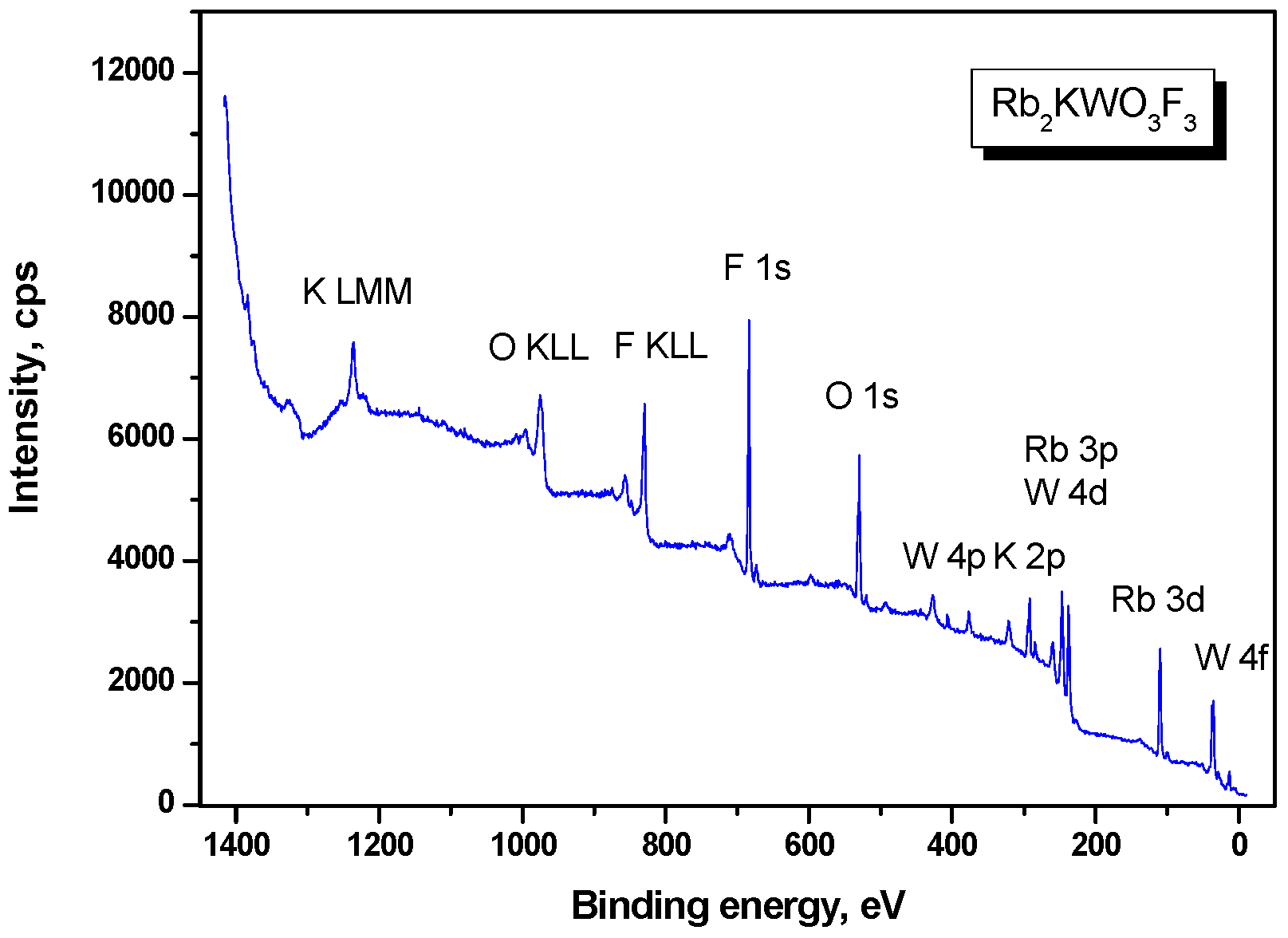
2.2. Characterization Techniques
2.3. Computational Method
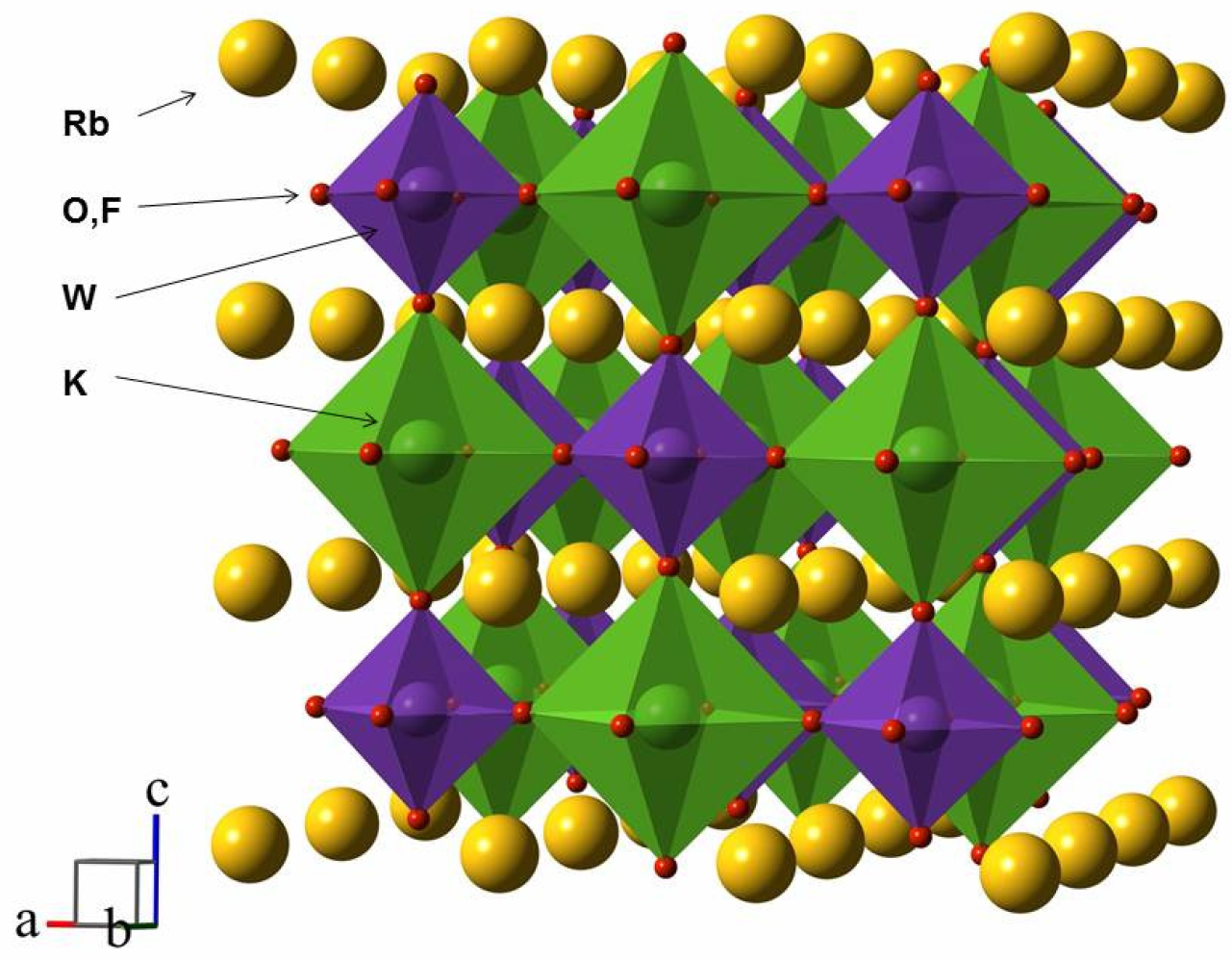
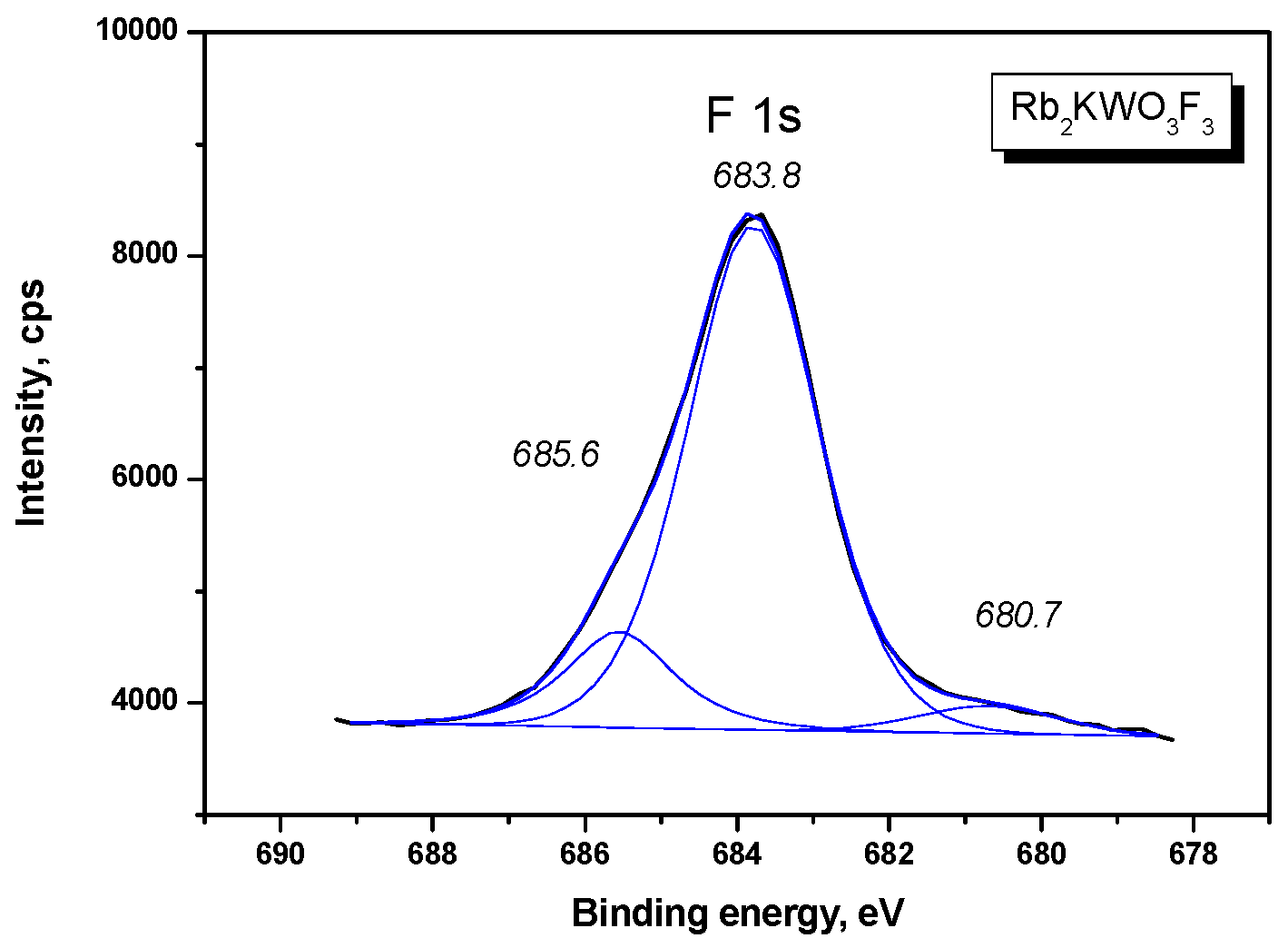
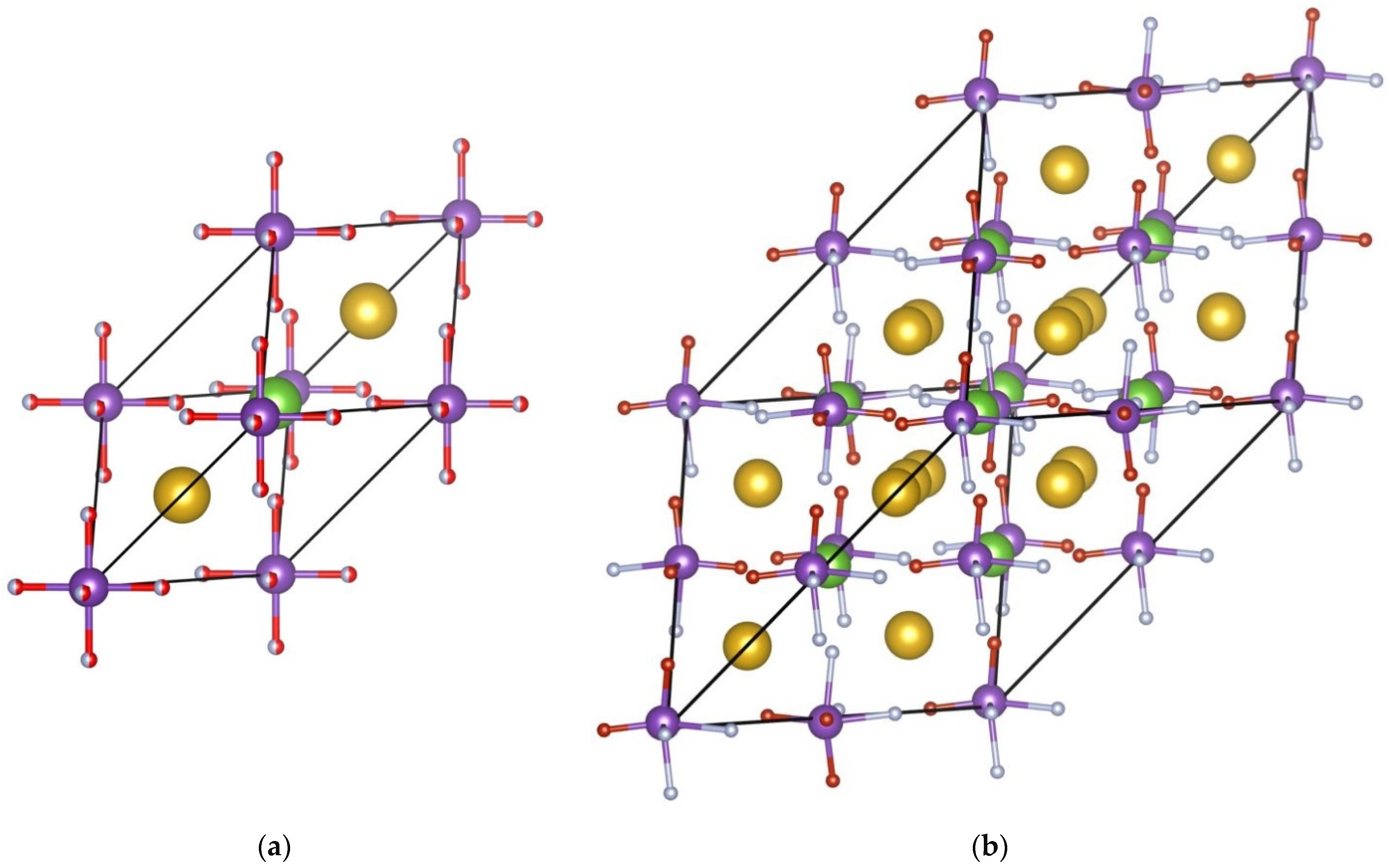
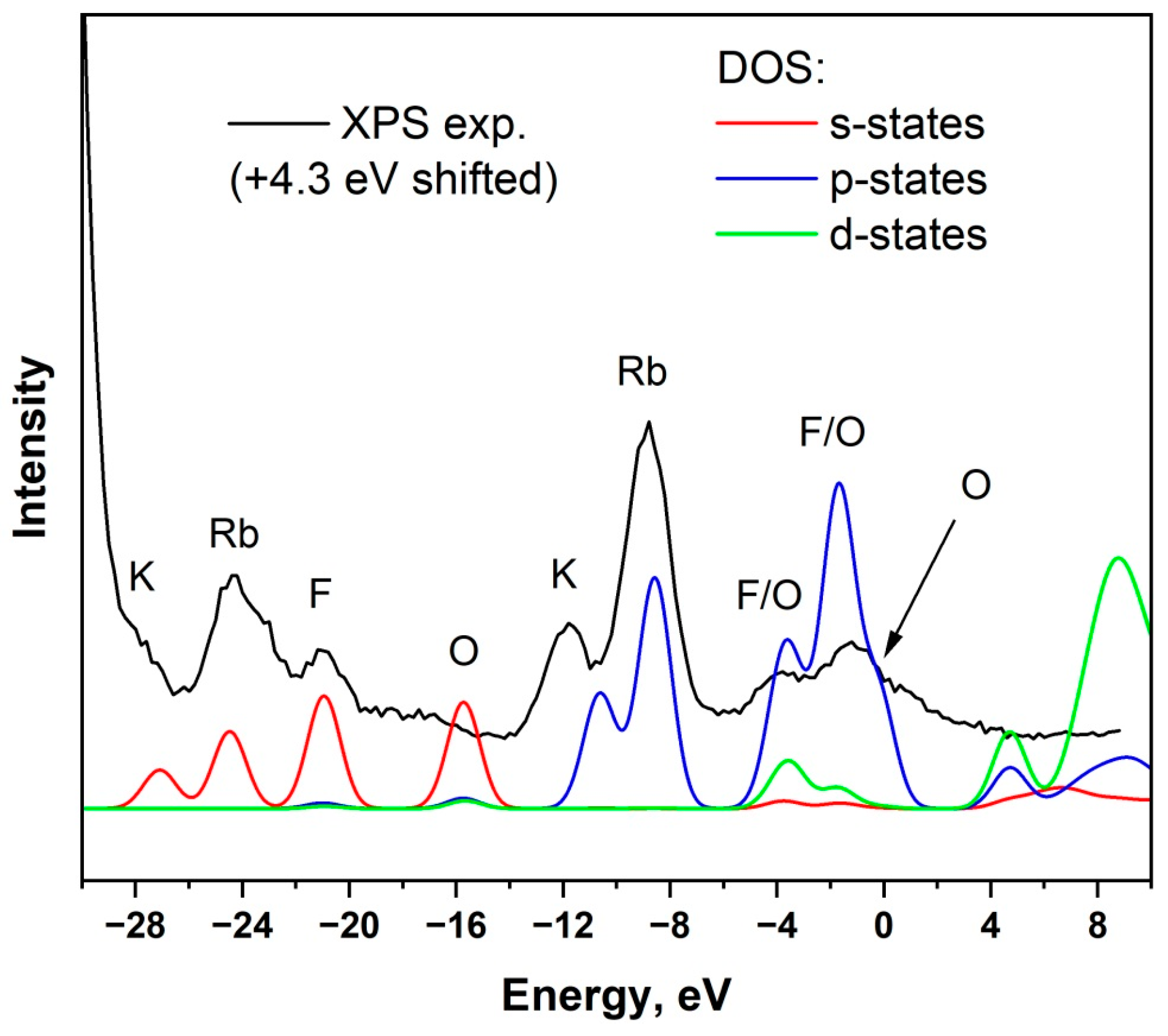
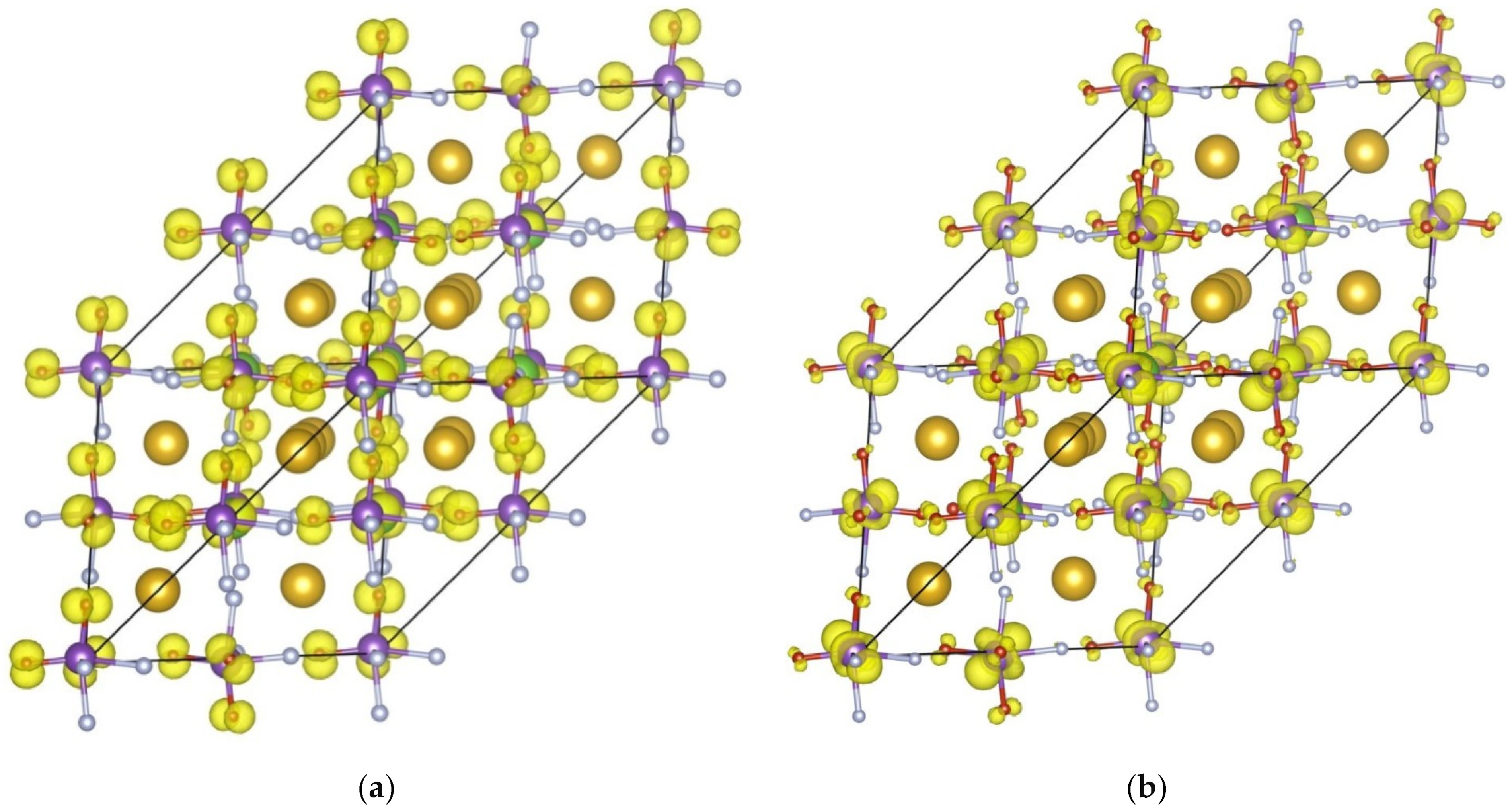
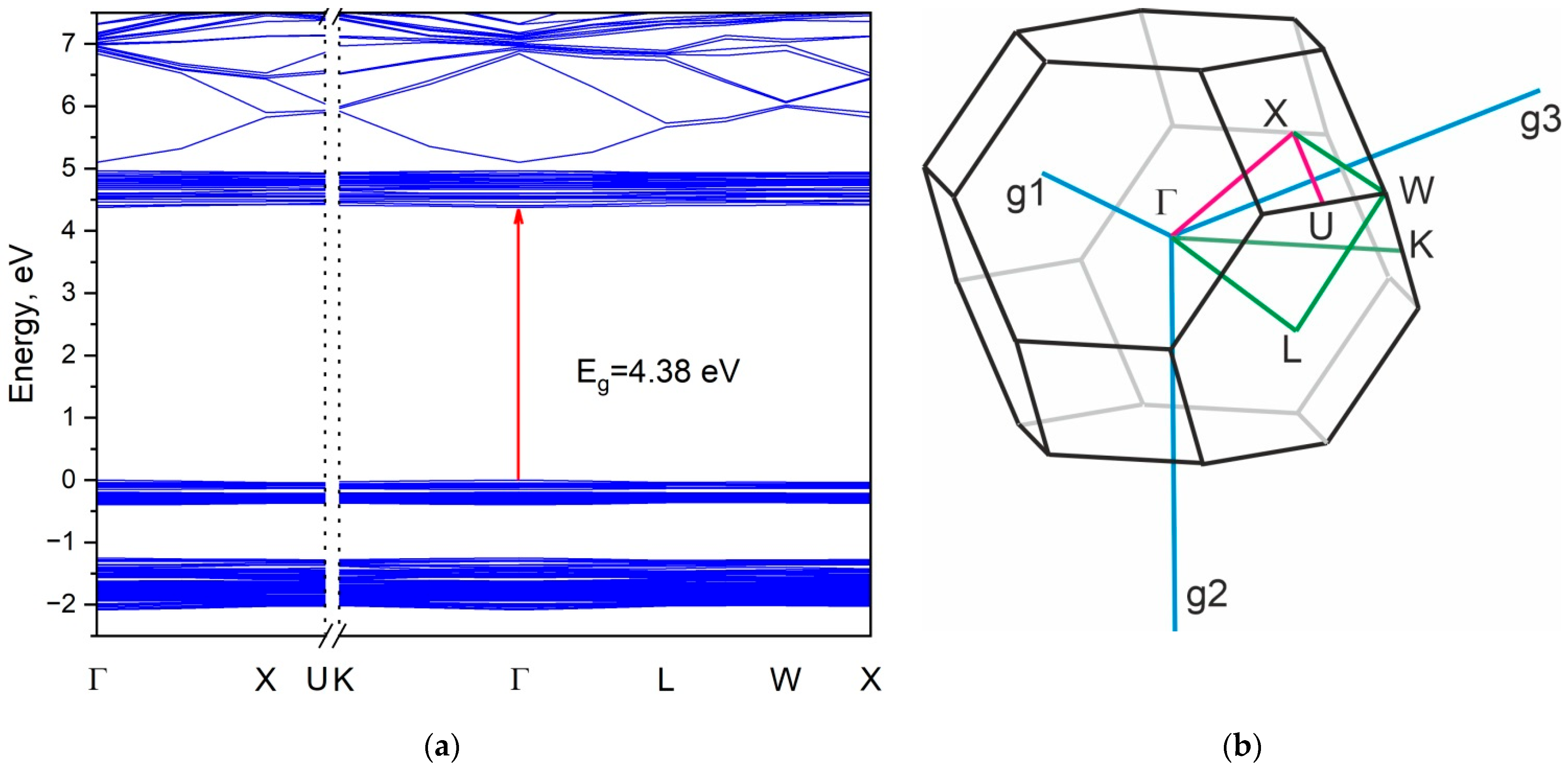
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berg, R.W. Progress in niobium and tantalum coordination chemistry. Coord. Chem. Rev. 1992, 113, 1–130. [Google Scholar] [CrossRef]
- Marvel, M.R.; Lesage, J.; Baek, J.; Halasyamani, P.S.; Stern, C.L.; Poeppelmeier, K.R. Cation-anion interactions and polar structures in the solid state. J. Am. Chem. Soc. 2007, 129, 13963–13969. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Yamaura, K.; Takayama-Muromachi, E. Oxyfluoride chemistry of layered perovskite compounds. Appl. Sci. 2012, 2, 206–219. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Isaenko, L.I.; Kesler, V.G.; Lin, Z.S.; Molokeev, M.S.; Yelisseyev, A.P.; Zhurkov, S.A. Exploration on anion ordering, optical properties and electronic structure in K3WO3F3 elpasolite. J. Solid State Chem. 2012, 187, 159–164. [Google Scholar] [CrossRef]
- Zhuravlev, Y.; Atuchin, V. Chemical bonding effects and physical properties of noncentrosymmetric hexagonal fluorocarbonates ABCO3F (A: K, Rb, Cs; B: Mg, Ca, Sr, Zn, Cd). Molecules 2022, 27, 6840. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.S.; Bai, L.; Liu, L.J.; Lee, M.H.; Xu, J.; Wang, X.Y.; Chen, C.T. Influences of twist boundaries on optical effects: Ab initio studies of the deep ultraviolet nonlinear optical crystal KBe2BO3F2. J. Appl. Phys. 2011, 109, 073721. [Google Scholar] [CrossRef]
- Wu, T.; Jiang, X.; Wu, C.; Hu, Y.; Lin, Z.; Huang, Z.; Humphrey, M.G.; Zhang, C. Ultrawide bandgap and outstanding second-harmonic generation response by a fluorine-enrichment strategy at a transition-metal oxyfluoride nonlinear optical material. Angew. Chem. Int. Ed. 2022, 61, e202203104. [Google Scholar] [CrossRef]
- Wu, H.; Wei, Z.; Hu, Z.; Wang, J.; Wu, Y.; Yu, H. Assembly of π-conjugated [B3O6] units by mer-isomer [YO3F3] octahedra to design a UV nonlinear optical material, Cs2YB3O6F2. Angew. Chem. Int. Ed. 2024, 63, e202406318. [Google Scholar] [CrossRef]
- Pausewang, G.; Rüdorff, W. AI3MeOxF6−x—Verbindungen mit x = 1, 2, 3, Z. Anorg. Allg. Chem. 1969, 364, 69–87. [Google Scholar] [CrossRef]
- Ravez, J. The inorganic fluoride and oxyfluoride ferroelectrics. J. Phys. III 1997, 7, 1129–1144. [Google Scholar]
- Heier, K.R.; Norquist, A.J.; Halasyamani, P.S.; Duarte, A.; Stern, C.L.; Poeppelmeier, K.R. The polar [WO2F4]2− anion in the solid state. Inorg. Chem. 1999, 38, 762–767. [Google Scholar] [CrossRef]
- Molokeev, M.S.; Vasiliev, A.D.; Kocharova, A.G. Crystal structures of room- and low-temperature phases in oxyfluoride (NH4)2KWO3F3. Powder Diffr. 2007, 22, 227–230. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Molokeev, M.S.; Yurkin, G.Y.; Gavrilova, T.A.; Kesler, V.G.; Laptash, N.M.; Flerov, I.N.; Patrin, G.S. Synthesis, structural, magnetic, and electronic properties of cubic CsMnMoO3F3 oxyfluoride. J. Phys. Chem. C 2012, 116, 10162–10170. [Google Scholar] [CrossRef]
- Fokina, V.D.; Flerov, I.N.; Molokeev, M.S.; Pogorel’tsev, E.I.; Bogdanov, E.V.; Krylov, A.S.; Bovina, A.F.; Voronov, V.N.; Laptash, N.M. Heat capacity, p–T phase diagram, and structure of Rb2KTiOF5. Phys. Solid State 2008, 50, 2175–2183. [Google Scholar] [CrossRef]
- Fokina, V.D.; Flerov, I.N.; Gorev, M.V.; Molokeev, M.S.; Vasiliev, A.D.; Laptash, N.M. Effect of cationic substitution on ferroelectric and ferroelastic phase transitions in oxyfluorides A2A′WO3F3 (A, A′: K, NH4, Cs). Ferroelectrics 2007, 347, 60–64. [Google Scholar] [CrossRef]
- Péraudeau, G.; Ravez, J.; Arend, H. Etude des transitions de phases des composes Rb2KMO3F3, Cs2KMO3F3 et Cs2RbMO3F3 (M = Mo, W). Solid State Commun. 1978, 27, 515–518. [Google Scholar] [CrossRef]
- Ravez, J.; Péraudeau, G.; Arend, H.; Abrahams, S.C.; Hagenmüller, P. A new family of ferroelectric materials with composition A2BMO3F3 (A, B = K, Rb, Cs, for rA+ ≥ rB+ and M = Mo, W). Ferroelectrics 1980, 26, 767–769. [Google Scholar] [CrossRef]
- Peraudeau, G.; Ravez, J.; Hagenmuller, P.; Arend, H. Study of phase transitions in A3MO3F3 compounds (A = K, Rb, Cs; M = Mo, W). Solid State Commun. 1978, 27, 591–593. [Google Scholar] [CrossRef]
- Chaminade, J.-P.; Cervera-Marzal, M.; Ravez, J.; Hagenmuller, P. Ferroelastic and ferroelectric behavior of the oxyfluoride Na3MoO3F3. Mater. Res. Bull. 1986, 21, 1209–1214. [Google Scholar] [CrossRef]
- Molokeev, M.S.; Misyul’, S.V.; Fokina, V.D. Structure transitions during phase transitions in the K3WO3F3 oxyfluoride. Phys. Solid State 2011, 53, 834–839. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Bernstein, J.L. Paraelectric-paraelastic Rb2KMoO3F3 structure at 343 and 473 K. Acta Cryst. B 1981, 37, 1332–1336. [Google Scholar] [CrossRef]
- Brink, F.J.; Norén, L.; Withers, R.L. Synthesis, electron diffraction, XRD and DSC study of the new elpasolite-related oxyfluoride, Tl3MoO3F3. J. Solid State Chem. 2003, 174, 44–51. [Google Scholar] [CrossRef]
- Kartashev, A.V.; Molokeev, M.S.; Isaenko, L.I.; Zhurkov, S.A.; Fokina, V.D.; Gorev, M.V.; Flerov, I.N. Heat capacity and structure of Rb2KMeO3F3 (Me: Mo, W) elpasolites. Solid State Sci. 2012, 14, 166–170. [Google Scholar] [CrossRef]
- Bruker AXS TOPAS. V4: General Profile and Structure Analysis Software for Powder Diffraction Data—User’s Manual; Bruker AXS: Karlsruhe, Germany, 2008. [Google Scholar]
- Atuchin, V.V.; Chimitova, O.D.; Gavrilova, T.A.; Molokeev, M.S.; Kim, S.-J.; Surovtsev, N.V.; Bazarov, B.G. Synthesis, structural and vibrational properties of microcrystalline RbNd(MoO4)2. J. Cryst. Growth 2011, 318, 683–686. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Subanakov, A.K.; Aleksandrovsky, A.S.; Bazarov, B.G.; Bazarova, J.G.; Gavrilova, T.A.; Krylov, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; Stefanovich, S.Y. Structural and spectroscopic properties of new noncentrosymmetric self-activated borate Rb3EuB6O12 with B5O10 units. Mater. Des. 2018, 140, 488–494. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. Furst principles methods using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. (Eds.) Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer Corp., Physical Electronics Division: Eden Prairie, MN, USA, 1979. [Google Scholar]
- Khyzhun, Y. XPS, XES and XAS studies of the electronic structure of tungsten oxides. J. Alloys Compd. 2000, 305, 1–6. [Google Scholar] [CrossRef]
- Khyzhun, Y.; Strunskus, T.; Cramm, S.; Solonin, Y.M. Electronic structure of CuWO4: XPS, XES and NEXAFS studies. J. Alloys Compd. 2005, 389, 14–20. [Google Scholar] [CrossRef]
- Rajagopal, S.; Nataraj, D.; Khyzhun, O.Y.; Djaoued, Y.; Robichaud, J.; Mangalaraj, D. Hydrothermal synthesis and electronic properties of FeWO4 and CoWO4 nanostructures. J. Alloys Compd. 2010, 493, 340–345. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Yao, H.-B.; Zhang, Q.; Gong, J.-Y.; Llu, S.-J.; Yu, S.-H. Hierarchical FeWO4 microcrystals: Solvothermal synthesis and their photocatalytic and magnatic properties. Inorg. Chem. 2009, 48, 1082–1090. [Google Scholar] [CrossRef]
- Ng, K.T.; Hercules, D.M. Studies of nickel-tungsten-alumina catalysts by X-ray photoelectron spectroscopy. J. Phys. Chem. 1976, 80, 2094–2102. [Google Scholar] [CrossRef]
- Wang, W.; Yang, P.; Gai, S.; Niu, N.; He, F.; Lin, J. Fabrication and luminescent properties of CaWO4:Ln3+ (Ln = Eu, Sm, Dy) nanocrystals. J. Nanopart. Res. 2010, 12, 2295–2305. [Google Scholar] [CrossRef]
- Nefedov, V.I. A comparison of results of an ESCA study of nonconducting solids using spectrometers of different constructions. J. Elect. Spectrosc. Relat. Phenom. 1982, 25, 29–47. [Google Scholar] [CrossRef]
- Dong, H.; Li, Z.; Ding, Z.; Pan, H.; Wang, X.; Fu, X. Nanoplates of α-SnWO4 and SnW3O9 prepared via a facile hydrothermal method and their gas-sensing property. Sens. Actuat. B 2009, 140, 623–628. [Google Scholar] [CrossRef]
- Chen, L.; Gao, Y. Fabrication of luminescent SrWO4 thin films by a novel electrochemical method. Mater. Res. Bull. 2007, 42, 1823–1830. [Google Scholar] [CrossRef]
- Charton, P.; Gengembre, L.; Armand, P. TeO2-WO3 glasses: Infrared, XPS and XANES structural characterization. J. Solid State Chem. 2002, 168, 175–183. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Kesler, V.G.; Maklakova, N.Y.; Pokrovsky, L.D. Core level spectroscopy and RHEED analysis of KGd(WO4)2 surface. Solid State Commun. 2005, 133, 347–351. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Kesler, V.G.; Maklakova, N.Y.; Pokrovsky, L.D.; Sheglov, D.V. Core level spectroscopy and RHEED analysis of KGd0.95Nd0.05(WO4)2 surface. Eur. Phys. J. B 2006, 51, 293–300. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Pokrovsky, L.D.; Khyzhun, O.Y.; Sinelnichenko, A.K.; Ramana, C.V. Surface crystallography and electronic structure of potassium yttrium tungstate. J. Appl. Phys. 2008, 104, 033518. [Google Scholar] [CrossRef]
- Chen, S.; Sun, S.; Sun, H.; Fan, W.; Zhao, X.; Sun, X. Experimental and theoretical studies on the enhanced photocatalytic activity of ZnWO4 nanorods by fluorine doping. J. Phys. Chem. C 2010, 114, 7680–7688. [Google Scholar] [CrossRef]
- Bi, J.; Wu, L.; Li, Z.; Ding, Z.; Wang, X.; Fu, X. A facile microwave solvothermal process to synthesize ZnWO4 nanoparticles. J. Alloys Compd. 2009, 480, 684–688. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Galashov, E.N.; Khyzhun, O.Y.; Kozhukhov, A.S.; Pokrovsky, L.D.; Shlegel, V.N. Structural and electronic properties of ZnWO4(010) cleaved surface. Cryst. Growth Des. 2011, 11, 2479–2484. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Galashov, E.N.; Khyzhun, O.Y.; Bekenev, V.L.; Pokrovsky, L.D.; Borovle, Y.A.; Zhdankov, V.N. Low thermal gradient Czochralski growth of large CdWO4 crystals and electronic properties of (010) cleaved surface. J. Solid State Chem. 2016, 236, 24–31. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Gavrilova, T.A.; Kesler, V.G.; Molokeev, M.S.; Aleksandrov, K.S. Structural and electronic parameters of ferroelectric K3WO3F3. Solid State Commun. 2010, 150, 2085–2088. [Google Scholar] [CrossRef]
- Morgan, W.E.; Van Wazer, J.R.; Stec, W.J. Inner-orbital photoelectron spectroscopy of the alkali metal halides, perchlorates, phosphates, and pyrophosphates. J. Am. Chem. Soc. 1973, 95, 751–755. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Isaenko, L.I.; Kesler, V.G.; Pokrovsky, L.D.; Tarasova, A.Y. Electronic parameters and top surface chemical stability of RbPb2Br5. Mater. Chem. Phys. 2012, 132, 82–86. [Google Scholar] [CrossRef]
- Tarasova, Y.; Isaenko, L.I.; Kesler, V.G.; Pashkov, V.M.; Yelisseyev, A.P.; Denysyuk, N.M.; Khyzhun, O.Y. Electronic structure and fundamental absorption edges of KPb2Br5, K0.5Rb0.5Pb2Br5, and RbPb2Br5 single crystals. J. Phys. Chem. Solids 2012, 73, 674–682. [Google Scholar] [CrossRef]
- Shchukarev, A.V.; Korolkov, D.V. XPS study of Group IA carbonates. Cent. Eur. J. Chem. 2004, 2, 347–362. [Google Scholar] [CrossRef]
- Wahlqvist, M.; Shchukarev, A. XPS spectra and electronic structure of Group IA sulfates. J. Elect. Spectrosc. Relat. Phenom. 2007, 156–158, 310–314. [Google Scholar] [CrossRef]
- Slobodin, B.V.; Sarat, L.L.; Zubkov, V.G.; Tyutyunnik, A.P.; Berger, I.F.; Kuznetsov, M.V.; Perelyaeva, L.A.; Shein, I.R.; Ivanovskii, A.L.; Shulgin, B.V.; et al. Structural, luminescence, and electronic properties of the alkaline metal-strontium cyclotetravanadates M2Sr(VO3)4. Phys. Rev. B 2005, 72, 155205. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Aleksandrovsky, A.S.; Chimitova, O.D.; Diao, C.-P.; Gavrilova, T.A.; Kesler, V.G.; Molokeev, M.S.; Krylov, A.S.; Bazarov, B.G.; Bazarova, J.G.; et al. Electronic structure of β-RbSm(MoO4)2 and chemical bonding in molybdates. Dalton Trans. 2015, 44, 1805–1815. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Khyzhun, O.Y.; Chimitova, O.D.; Molokeev, M.S.; Gavrilova, T.A.; Bazarov, B.G.; Bazarova, J.G. Electronic structure of β-RbNd(MoO4)2 by XPS and XES. J. Phys. Chem. Solids 2015, 77, 101–108. 55. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Kesler, V.G.; Meng, G.S.; Lin, Z.S. The electronic structure of RbTiOPO4 and the effects of the A-site cation substitution in KTiOPO4-family crystals. J. Phys. Condens. Matter 2012, 24, 405503. [Google Scholar] [CrossRef] [PubMed]
- Chimitova, O.D.; Bazarov, B.G.; Bazarova, J.G.; Atuchin, V.V.; Azmi, R.; Sarapulova, A.E.; Mikhailova, D.; Balachandran, G.; Fiedler, A.; Geckle, U.; et al. The crystal growth and properties of novel magnetic double molybdate RbFe5(MoO4)7 with mixed Fe3+/Fe2+ states and 1D negative thermal expansion. CrystEngComm 2021, 23, 3297–3307. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Isaenko, L.I.; Kesler, V.G.; Kang, L.; Lin, Z.; Molokeev, M.S.; Yelisseyev, A.P.; Zhurkov, S.A. Structural, spectroscopic, and electronic properties of cubic G0-Rb2KTiOF5 oxyfluoride. J. Phys. Chem. C 2013, 117, 7269–7278. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Gavrilova, T.A.; Isaenko, L.I.; Kesler, V.G.; Molokeev, M.S.; Zhurkov, S.A. Synthesis and structural properties of cubic G0-Rb2KMoO3F3 oxyfluoride. Ceram. Int. 2012, 38, 2455–2459. [Google Scholar] [CrossRef]
- Zhavoronkov, N.M.; Nefedov, V.I.; Buslaev, Y.A.; Kokunov, Y.V.; Porai-Koshits, M.A.; Ilin, E.G.; Mihailov, Y.N. XPS study of complex fluorides and oxyfluorides of elements from IV-VI groups. Izv. Academii Nauk USSR Phys. 1972, 36, 376–380. [Google Scholar]
- Nemoshkalenko, V.V.; Senkevich, A.I.; Aleshin, V.G. Photoelectron spectra and band structure of alkali hallide crystals. Dokl. Akad. Nauk. USSR 1972, 206, 593–596. [Google Scholar]
- Zhang, M.; Wang, Z.H.; Mo, M.S.; Chen, X.Y.; Zhang, R.; Yu, W.C.; Qian, Y.T. A simple approach to synthesize KNiF3 hollow spheres by solvothermal method. Mater. Chem. Phys. 2005, 89, 373–378. [Google Scholar] [CrossRef]
- Huang, B.; Hong, J.M.; Chen, X.T.; Yu, Z.; You, X.Z. Mild solvothermal synthesis of KZnF3 and KCdF3 nanocrystals. Mater. Lett. 2005, 59, 430–433. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Isaenko, L.I.; Kesler, V.G.; Tarasova, A.Y. Single crystal growth and surface chemical stability of KPb2Br5. J. Cryst. Growth 2011, 318, 1000–1004. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Kesler, V.G.; Maklakova, N.Y.; Pokrovsky, L.D.; Semenenko, V.N. Study of KTiOPO4 surface by X-ray photoelectron spectroscopy and reflection high-energy electron diffraction. Surf. Interface Anal. 2002, 34, 320–323. [Google Scholar] [CrossRef]
- Ramana, C.V.; Atuchin, V.V.; Becker, U.; Ewing, R.C.; Isaenko, L.I.; Khyzhun, O.Y.; Merkulov, A.A.; Pokrovsky, L.D.; Sinelnichenko, A.K.; Zhurkov, S.A. Low-energy Ar+ ion-beam-induced amorphization and chemical modification of potassium titanyl arsenate (001) crystal surfaces. J. Phys. Chem. C 2007, 111, 2702–2708. [Google Scholar] [CrossRef]
- Engelhard, M.; Evans, C.; Land, T.A.; Nelson, A.J. A study of potassium dihydrogen phosphate (KDP) crystal surfaces by XPS. Surf. Sci. Spectra 2001, 8, 56–80. [Google Scholar] [CrossRef]
- Ratcliff, L.E.; Oshima, T.; Nippert, F.; Janzen, B.M.; Kluth, E.; Goldhahn, R.; Feneberg, M.; Mazzolini, P.; Bierwagen, O.; Wouters, C.; et al. Tackling disorder in γ-Ga2O3. Adv. Mater. 2022, 34, 2204217. [Google Scholar] [CrossRef]
- Kahk, J.M.; Lischner, J. Core electron binding energies of adsorbates on Cu(111) from first-principles calculations. Phys. Chem. Chem. Phys. 2018, 20, 30403–30411. [Google Scholar] [CrossRef]
- Ozaki, T.; Lee, C.-C. Absolute binding energies of core levels in solids from first principles. Phys. Rev. Lett. 2017, 118, 026401. [Google Scholar] [CrossRef]
- Pauling, L. The crystal structures of ammonium fluoferrate, fluo-aluminate and oxyfluomolybdate. J. Am. Chem. Soc. 1924, 46, 2738–2751. [Google Scholar] [CrossRef]
- Udovenko, A.A.; Laptash, N.M. Orientational disorder in crystals of (NH4)3MoO3F3 and (NH4)3WO3F3. Acta Cryst. B 2008, 64, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Krylov, A.S.; Sofronova, S.N.; Kolesnikova, E.M.; Vtyurin, A.N.; Isaenko, L.I. Lattice dynamics of oxyfluoride Rb2KMoO3F3. Ferroelectrics 2012, 441, 52–60. [Google Scholar] [CrossRef]
- Crowley, J.M.; Tahir-Kheli, J.; Goddard, W.A.I. Resolution of the band gap prediction problem for materials design. J. Phys. Chem. Lett. 2016, 7, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Borlido, P.; Aull, T.; Huran, A.W.; Tran, F.; Marques, M.A.L.; Botti, S. Large-scale benchmark of exchange–correlation functionals for the determination of electronic band gaps of solids. J. Chem. Theory Comput. 2019, 15, 5069–5079. [Google Scholar] [CrossRef] [PubMed]
- Garza, A.J.; Scuseria, G.E. Predicting band gaps with hybrid density functionals. J. Phys. Chem. Lett. 2016, 7, 4165–4170. [Google Scholar] [CrossRef] [PubMed]
- Adolph, B.; Furthmüller, J.; Bechstedt, F. Optical properties of semiconductors using projector-augmented waves. Phys. Rev. B 2001, 63, 125108. [Google Scholar] [CrossRef]
- Chen, J.; Wu, Q.; Tian, H.; Jiang, X.; Xu, F.; Zhao, X.; Lin, Z.; Luo, M.; Ye, N. Uncovering a vital band gap mechanism of pnictides. Adv. Sci. 2022, 9, 2105787. [Google Scholar] [CrossRef]
- Wu, T.; Liu, J.; Liu, M.; Li, X.; Zhang, M.; Cao, W.; Gu, J.; Wang, D. Recent achievements of d0 transition-metal-based oxyfluorides: Crystal chemistry and application in second-order NLO materials. Coord. Chem. Rev. 2026, 549, 217347. [Google Scholar] [CrossRef]
- Pei, C.; Wang, L.; Gao, X.; Yao, G.; Quan, L.; Chang, J.; Liu, J.; Yang, D.; Liu, P.; Jia, Y.; et al. Structural phase transformation and microwave dielectric properties of polymorphic Li3Mg2SbO6−xF2x oxyfluoride nanoceramics. Ceram. Int. 2025, 51, 64045–64051. [Google Scholar] [CrossRef]
- Peng, C.; Tian, H.; Li, K.; Zhao, Z.; Liu, C. Luminescence properties of Tm3+/Yb3+ ions doped oxyfluoride glass-ceramic containing Y5O4F7 and NaYF4 nanocrystals. J. Alloys Compd. 2025, 1037, 182533. [Google Scholar] [CrossRef]
- He, P.; Li, Y.; Zuo, J.; Zhang, B.; Yang, F.; Peng, J.; Liu, S.; Wang, W.; Huang, D.; Xiao, Y.; et al. Less is more: An oxyfluoride garnet broadband cyan-green phosphor towards highly efficient full-spectrum WLEDs. J. Alloys Compd. 2024, 985, 173997. [Google Scholar] [CrossRef]
- Sen, B.; Paul, S.; Das, S.; Chattopadhyay, A.P.; Ali, S.I. Synthesis, crystal structure and photocatalytic studies of new oxyfluoride Cu5AsO5F5. J. Mol. Struct. 2023, 1286, 135610. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Y.; Zhang, L.; Liu, X.; Feng, Q.; Deng, L.; Li, J.; Han, S. A3ZnNO3X4 (A = Rb, NH4, X = Cl, I): Regulating cations and halides yields birefringent crystals with significantly enhanced optical anisotropy. Inorg. Chem. 2025, 64, 17098–17103. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Cheng, X.; Minaud, C.; Mentré, O.; Vieira, B.J.C.; Waerenborgh, J.C.; Li, F.; Li, Y.; Lü, M. Fluorinated phosphates, BaMPO4F (M = Mn, Fe), with one-dimensional channels: Structure and magnetism. Inorg. Chem 2025, 64, 11731–11743. [Google Scholar] [CrossRef]
- Bader, J.; Fischer, L.; Hoffmann, K.F.; Limberg, N.; Millanvois, A.; Oesten, F.; Pérez-Bitrián, A.; Schlögl, J.; Toraman, A.N.; Wegener, D.; et al. On pentafluoroorthotellurates and related compounds. Chem. Rev. 2025, 125, 9140–9186. [Google Scholar] [CrossRef]
- Carone, D.; Klepov, V.V.; Misture, S.T.; Schaeperkoetter, J.C.; Jacobsohn, L.G.; Aziziha, M.; Schorne-Pinto, J.; Thomson, S.A.J.; Hines, A.T.; Besmann, T.M.; et al. Luminescence and scintillation in the niobium doped oxyfluoride Rb4Ge5O9F6:Nb. Inorganics 2022, 10, 83. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, X.; Gao, H.; Duanmu, K.; Wu, C.; Lin, Z.; Huang, Z.; Humphrey, M.G.; Zhang, C. Breaking the deep-UV transparency/optical nonlinearity trade-off: Three-parameter optimization in oxyfluorides by tailoring d0-metal incorporation. Angew. Chem. Int. Ed. 2025, 46, e202513438. [Google Scholar]
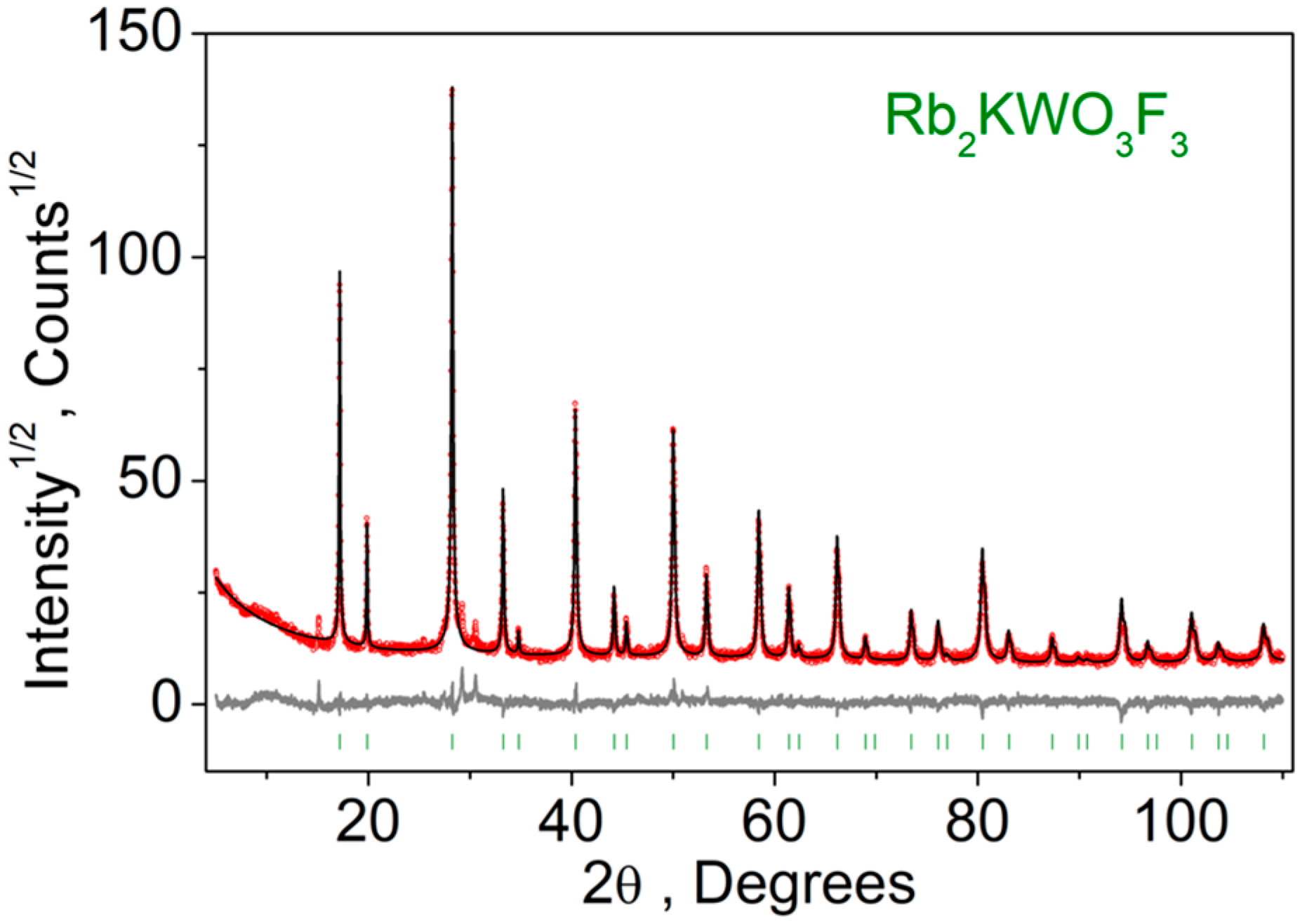
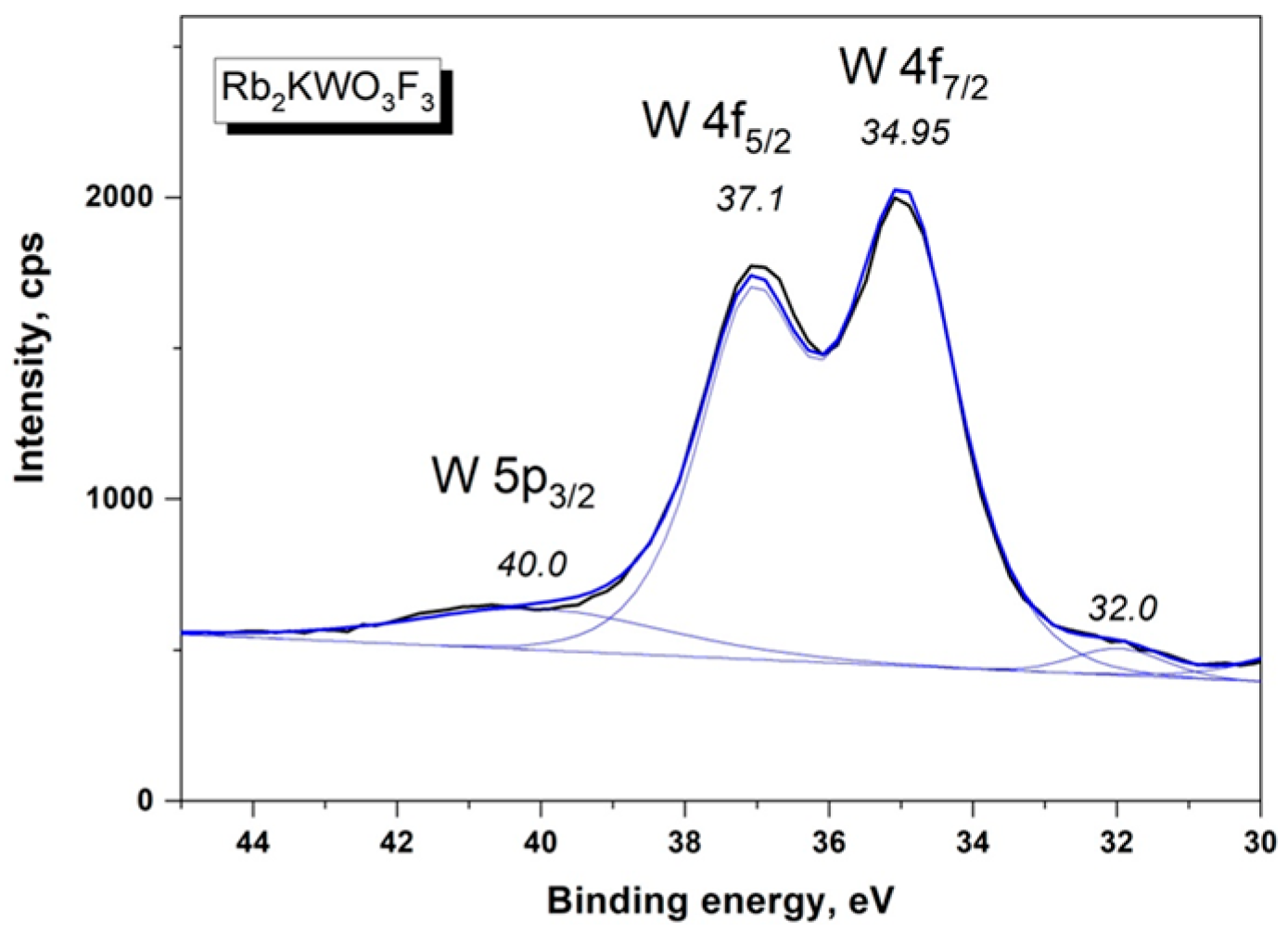
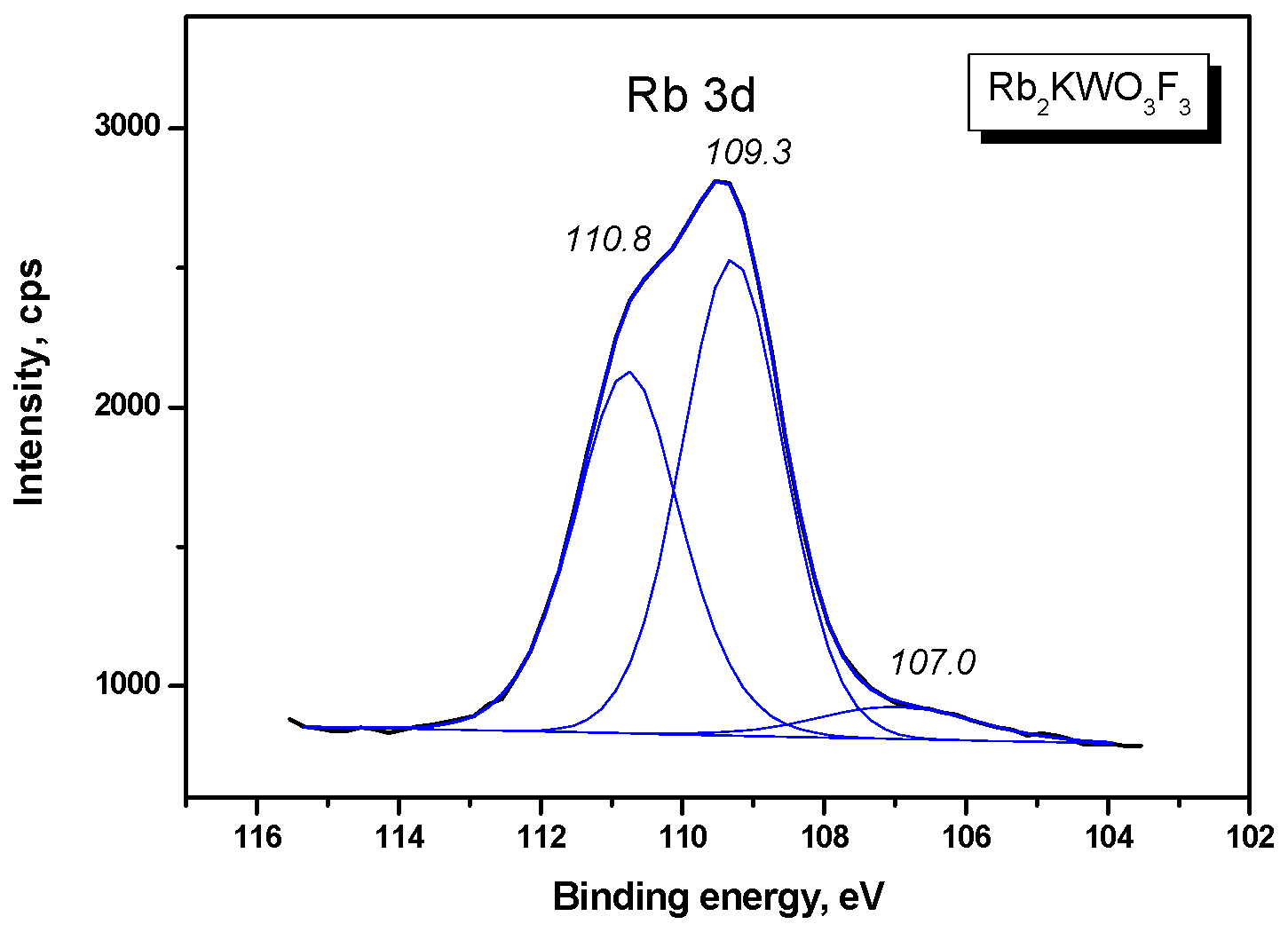
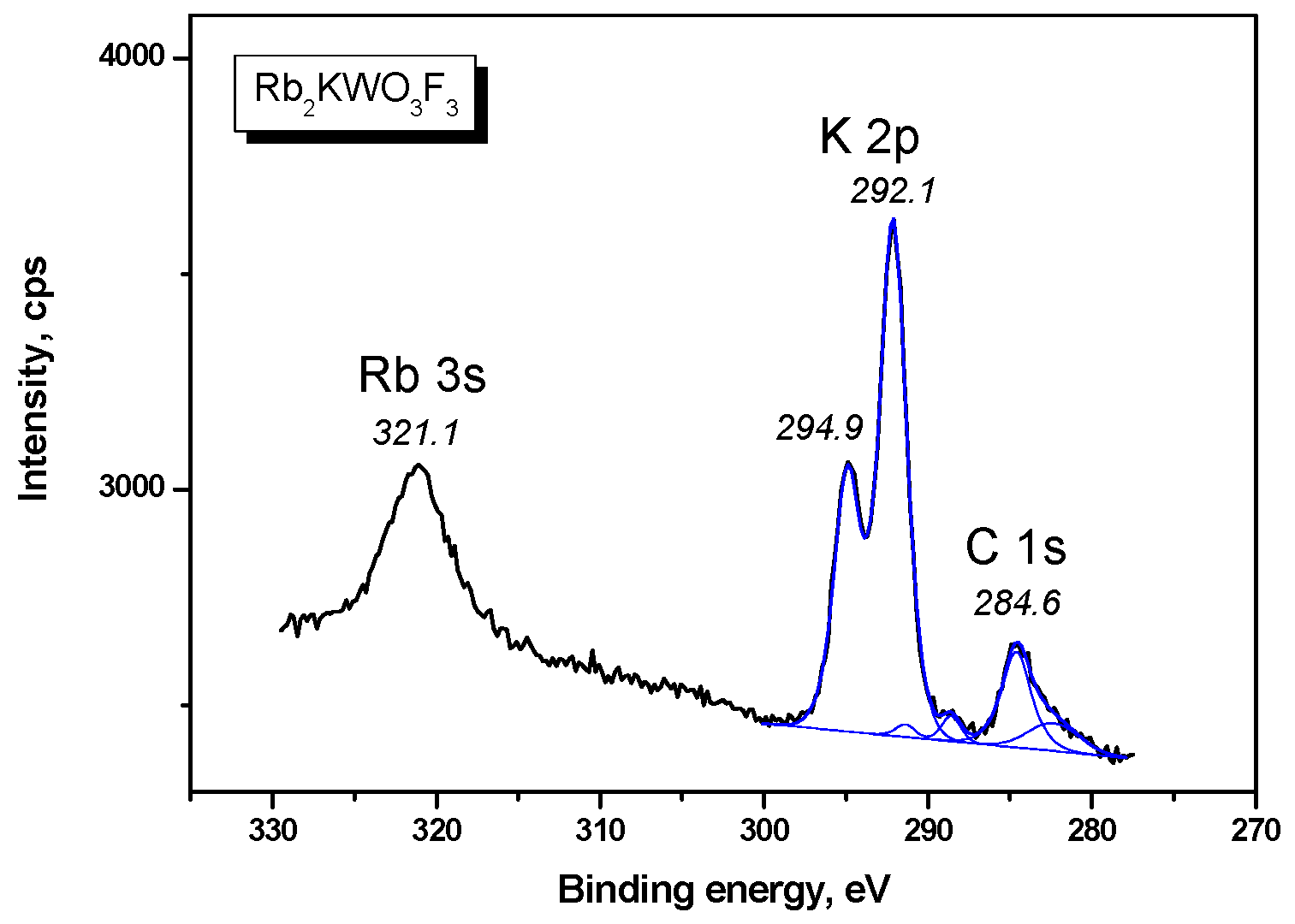
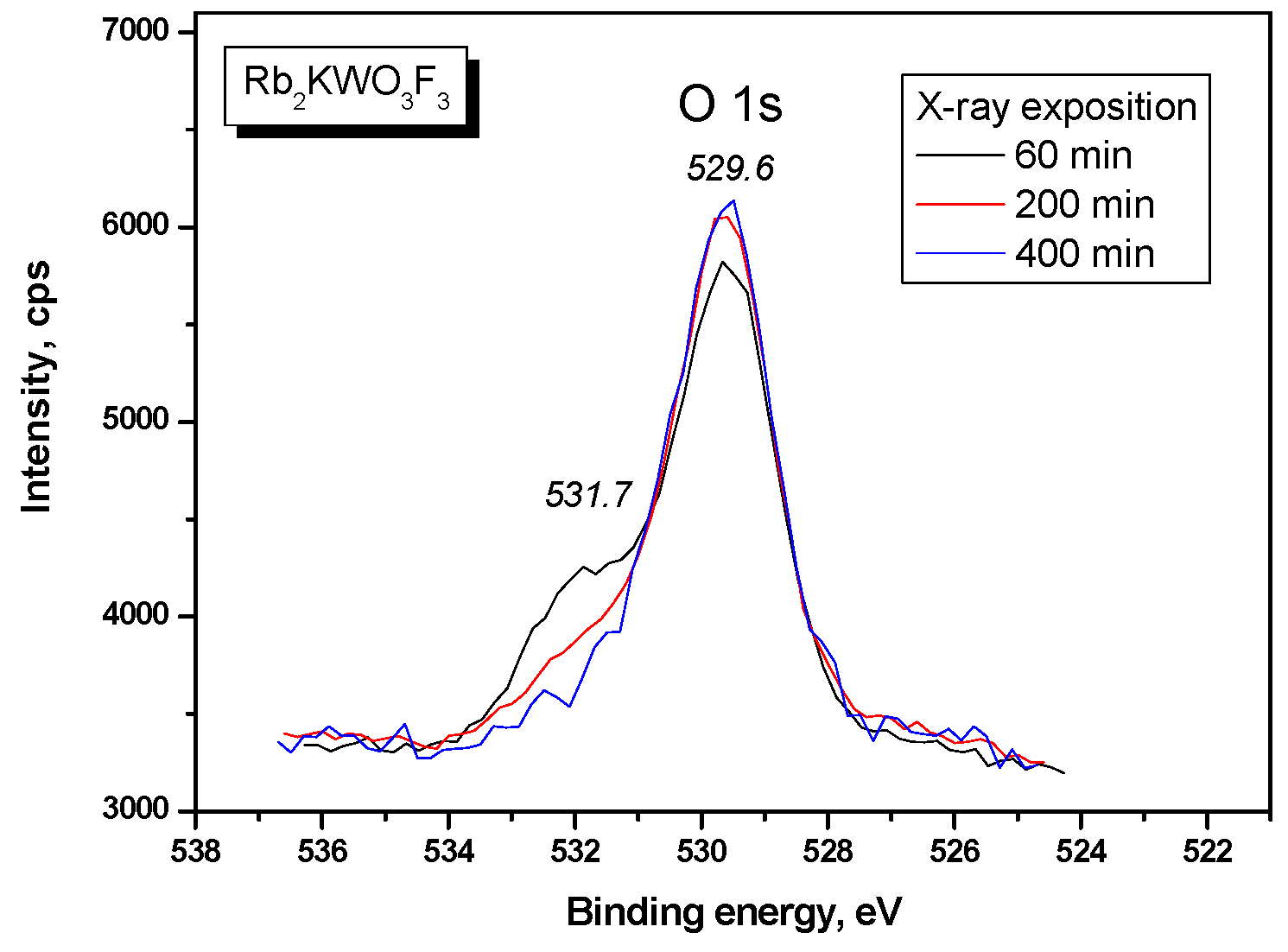
| Compound | Rb2KWO3F3 |
|---|---|
| Space group | Fm-3m |
| a (Å) | 8.92413(17) |
| V (Å3) | 710.72(4) |
| Z | 2 |
| 2θ-interval, º | 5–110 |
| Rwp,% | 10.78 |
| Rp,% | 8.05 |
| χ2 | 1.72 |
| RB,% | 4.0 |
| Core Level | Binding Energy, eV Rb2KWO3F3 | Binding Energy, eV K3WO3F3 |
|---|---|---|
| VB | 4.0, 5.6, 8.2 | 5.9, 8.4 |
| Rb 4p | 13.1 | - |
| K 3p | 16.1 | ~16.6 |
| O 2s | 21.9 | ~22.9 (3.6) |
| F 2s | 28.5 | ~28.2 (2.2) |
| W 4f7/2 | 34.95 (1.77) | 35.4 (1.8) |
| W 4f5/2 | 37.1 | 37.6 (1.8) |
| W 5p3/2 | 40.0 | 41.2 (2.6) |
| W 5p1/2 | 50.7 | ~50.9 (4.6) |
| Rb 3d | 109.29/110.77 (1.69) | - |
| Rb 3p3/2 | 237.6 | - |
| W 4d5/2 | ** | 247.1 (4.7) |
| W 4d3/2 | 259.6 | 259.6 (4.9) |
| C 1s | Fixed at 284.6 | Fixed at 284.6 (2.0) |
| K 2p3/2 | 292.14 (1.92) | 292.6 (2.1) |
| K 2p1/2 | 294.9 | 295.4 (1.8) |
| Rb 3s | 321.1 | - |
| K 2s | 376.7 | 377.1 (3.8) |
| W 4p3/2 | 427.2 | 427.3 (~5.0) |
| W 4p1/2 | 493.8 | 492.5 (~5.0) |
| O 1s | 529.58 (2.01) | 530.0 (2.0) |
| F 1s | 683.79 (2.04) | 684.2 (2.0) |
| F KL23L23 | 829.7 | 830.0 |
| F KL1L23 | - | 856.1 |
| O KL23L23 | 975.0 | 975.8 |
| K L23M23M23 | 1235.7 | 1235.7, 1237.9 |
| Rb Auger | 1375.6, 1383.6 | - |
| Compound | BE(O 1s), eV | BE(W 4f7/2), eV | Reference |
|---|---|---|---|
| WO3 | 530.5 | 35.7 | [30] |
| CuWO4 | 530.6 | 35.5 | [31] |
| FeWO4 | 530.6 | 35.6 | [32] |
| 530.3 | 35.1 | [33] | |
| CoWO4 | 530.7 | 35.7 | [32] |
| NiWO4 | 531.5 | 35.2 | [34] |
| CaWO4 | 530.3 | 34.8 | [35] |
| 530.1 | 35.1 | [36] | |
| α-SnWO4 | 530.5 | 35.5 | [37] |
| SrWO4 | 529.9 | 35.5 | [38] |
| β-PbWO4 | 530.7 | 35.4 | [39] |
| KGd(WO4)2 | 530.1 | 35.2 | [40] |
| KGd0.95Nd0.05(WO4)2 | 530.3 | 35.2 | [41] |
| KY(WO4)2 | 530.8 | 35.3 | [42] |
| ZnWO4 | 530.6 | 35.6 | [43] |
| 530.1 | 35.1 | [44] | |
| 530.95 | 35.95 | [45] | |
| CdWO4 | 529.68 | 34.83 | [46] |
| K3WO3F3 | 530.0 | 35.4 | [47] |
| Rb2KWO3F3 | 529.58 | 34.95 | This study |
| Compound | Rb 3d, Rb 3d5/2 (eV) | Rb 3p3/2 (eV) | O 1s (eV) | Reference |
|---|---|---|---|---|
| RbF | 110.0 * | - | - | [48] |
| RbCl | 110.1 * | - | - | [48] |
| RbBr | 110.2 * | - | - | [48] |
| RbPb2Br5 | 109.5 109.20 | 237.9 - | - - | [49] [50] |
| K0.5Rb0.5Pb2Br5 | 109.22 | - | - | [50] |
| RbI | 110.6 * | - | - | [48] |
| RbClO4 | 110.6 * | - | 533.0 | [48] |
| Rb3PO4 | 110.2 * | - | 530.5 | [49] |
| Rb4P2O7 | 110.2 * | - | 530.9 | [51] |
| Rb2SO4 | 109.8 | - | 531.3 | [52] |
| Rb2CO3 | 109.4 | - | 530.1 | [51] |
| RbHCO3 | 109.2 | - | 530.7 | [51] |
| Rb2Sr(VO3)4 | - | 237.8 | 530.2 | [53] |
| RbSm(MoO4)2 | 108.9 | 237.2 | 529.8 | [54] |
| RbNd(MoO4)2 | 109.45 | 237.5 | 530.11 | [55] |
| RbTiOPO4 | 108.9 | 237.1 | 530.4 | [56] |
| RbFe3(MoO4)7 | 109.9 | - | 530.9 | [57] |
| Rb2KTiOF5 | 109.4 | 237.7 | 529.6 | [58] |
| Rb2KMoO3F3 | - | 237.6 | - | [59] |
| Rb2KWO3F3 | 109.3 | 237.6 | 529.6 | This study |
| Compound | K 2p3/2 | O 1s | F 1s | Ref. |
|---|---|---|---|---|
| KF | 293.3 294.0 | - - | 684.0 684.5 | [46] [61] |
| K2SiF6 | 293.4 | - | 685.8 | [60] |
| K2GeF6 | 293.1 | - | 685.4 | [60] |
| K2TiF6 | 293.2 | - | 685.1 | [60] |
| K2ZrF6 | 292.8 | - | 684.8 | [60] |
| K3ZrF7 | 293.0 | - | 684.5 | [60] |
| K2NbF7 | 293.5 | - | 685.4 | [60] |
| K2TaF7 | 293.2 | - | 685.3 | [60] |
| KNiF3 | 293.15 | - | 685.1 | [62] |
| KZnF3 | 292.2 | - | 684.3 | [63] |
| KPb2Br5 | 292.8 292.62 | - - | - - | [64] [50] |
| K0.5Rb0.5Pb2Br5 | 292.56 | - | - | [51] |
| KClO4 | 293.6 | 532.4 | - | [48] |
| KClO3 | 293.4 | 532.5 | - | [48] |
| K3PO4 | 292.7 | 530.6 | - | [48] |
| K4P2O7 | 292.4 | 530.3 | - | [48] |
| K2SO4 | 293 | 531.6 | - | [52] |
| K2CO3 | 292.2 | 530.2 | - | [51] |
| KHCO3 | 292.9 | 531.5 | - | [51] |
| KTiOPO4 | 292.3 | 530.9 | - | [65] |
| KTiOAsO4 | 292.8 | 531.2 | - | [66] |
| K2Sr(VO3)4 | 292.6 | 530.2 | - | [53] |
| KGd(WO4)2 | 292.3 | 530.1 | - | [40] |
| KY(WO4)2 | 292.7 | 530.8 | - | [42] |
| KH2PO4 | 291.66 | 530.24 | - | [67] |
| KWO3F3 | 292.6 | 530.0 | 684.2 | [47] |
| K3NbOF6 | 293.1 | 531.6 | 685.1 | [60] |
| K2NbOF5 | 293.4 | 531.8 | 685.4 | [60] |
| K4Ta4O5F14 | 293.4 | 531.3 | 685.1 | [60] |
| Rb2KTiOF5 | 292.1 | 529.6 | 683.9 | [56] |
| Rb2KWO3F3 | 292.1 | 529.6 | 683.8 | This study |
| Configuration | mer- | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|---|
| fac- | 8 | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 | |
| Relative total energy, eV/f.u. | 0 | +0.10 | +0.21 | +0.31 | +0.42 | +0.54 | +0.64 | +0.75 | +0.85 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Atuchin, V.; Gavrilova, T.; Isaenko, L.; Kesler, V.; Molokeev, M.; Oreshonkov, A.; Zhurkov, S. Synthesis, Anion Disordering and Electronic Structure of Rb2KWO3F3 Elpasolite. Crystals 2026, 16, 18. https://doi.org/10.3390/cryst16010018
Atuchin V, Gavrilova T, Isaenko L, Kesler V, Molokeev M, Oreshonkov A, Zhurkov S. Synthesis, Anion Disordering and Electronic Structure of Rb2KWO3F3 Elpasolite. Crystals. 2026; 16(1):18. https://doi.org/10.3390/cryst16010018
Chicago/Turabian StyleAtuchin, Victor, Tatyana Gavrilova, Ludmila Isaenko, Valery Kesler, Maxim Molokeev, Aleksandr Oreshonkov, and Sergey Zhurkov. 2026. "Synthesis, Anion Disordering and Electronic Structure of Rb2KWO3F3 Elpasolite" Crystals 16, no. 1: 18. https://doi.org/10.3390/cryst16010018
APA StyleAtuchin, V., Gavrilova, T., Isaenko, L., Kesler, V., Molokeev, M., Oreshonkov, A., & Zhurkov, S. (2026). Synthesis, Anion Disordering and Electronic Structure of Rb2KWO3F3 Elpasolite. Crystals, 16(1), 18. https://doi.org/10.3390/cryst16010018








