Microgravity-Grown Crystals as Seeds for Pharmaceutical Compounds
Abstract
1. Introduction
2. Materials and Methods
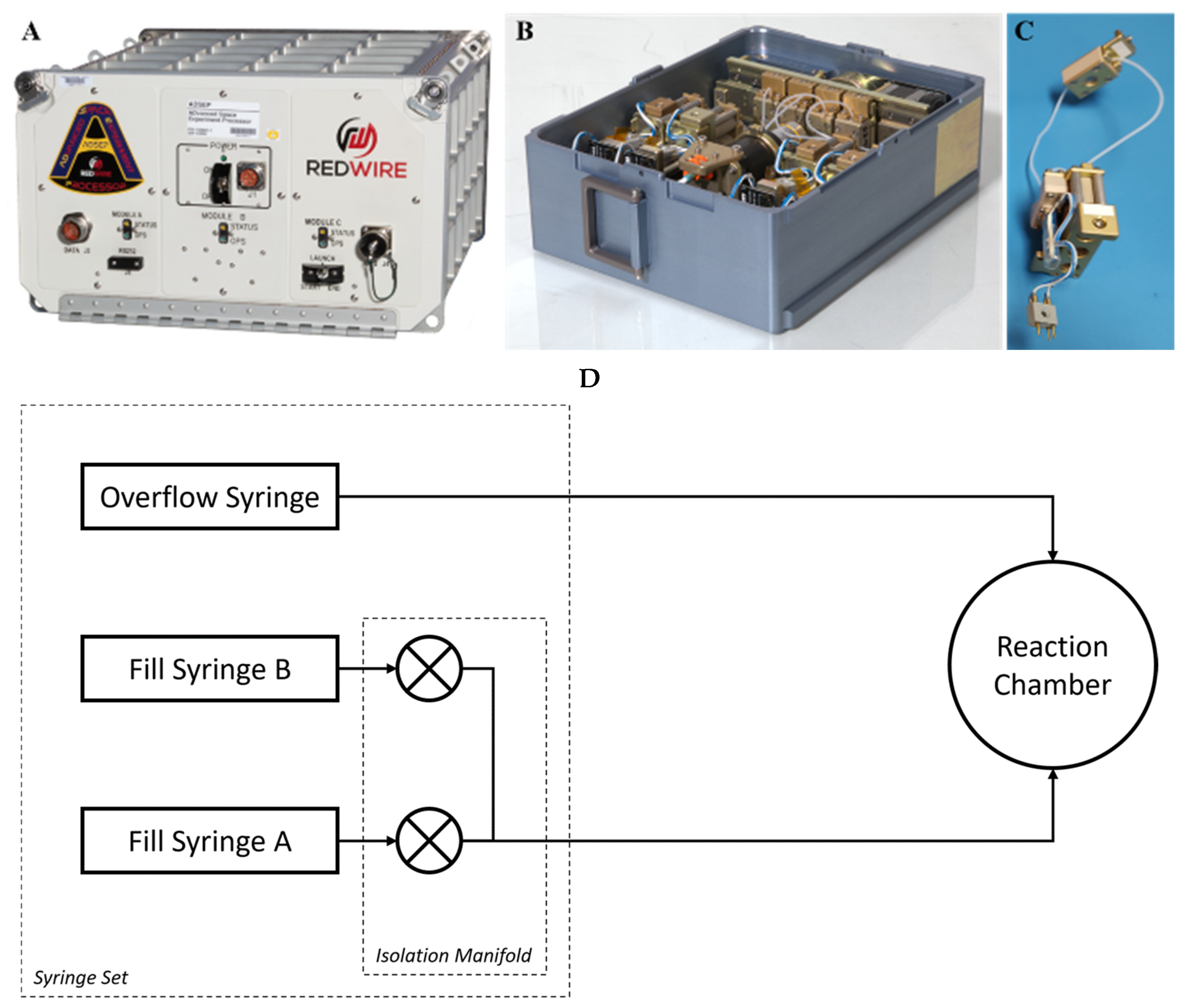
2.1. Microgravity Studies
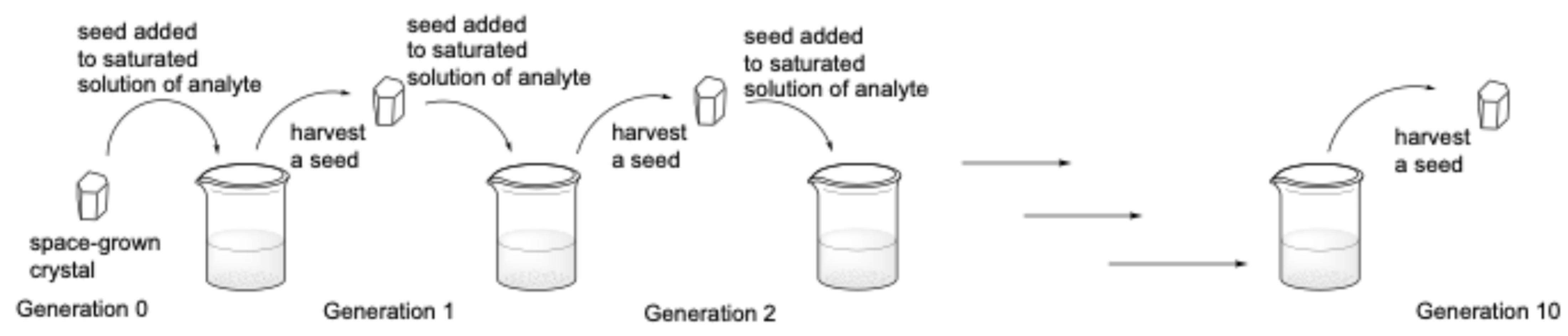
2.2. Seed Studies
3. Results
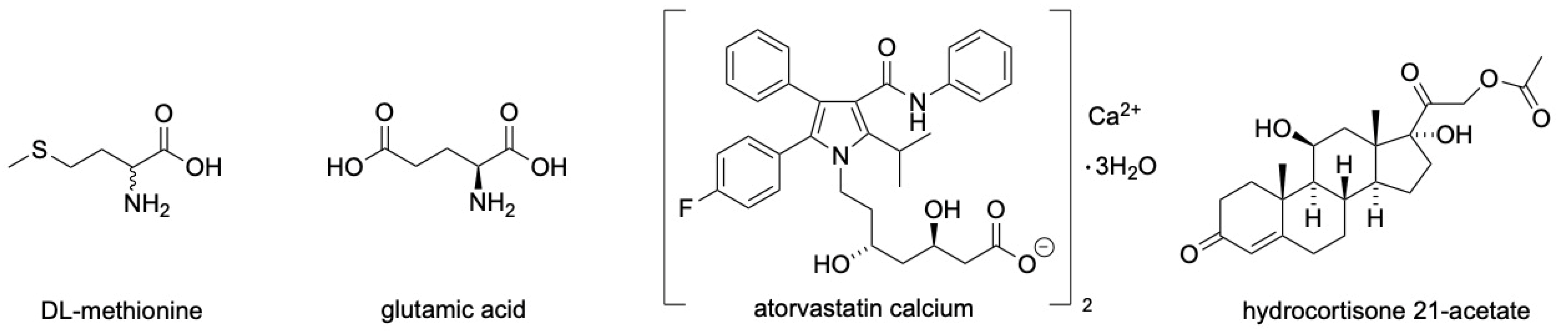
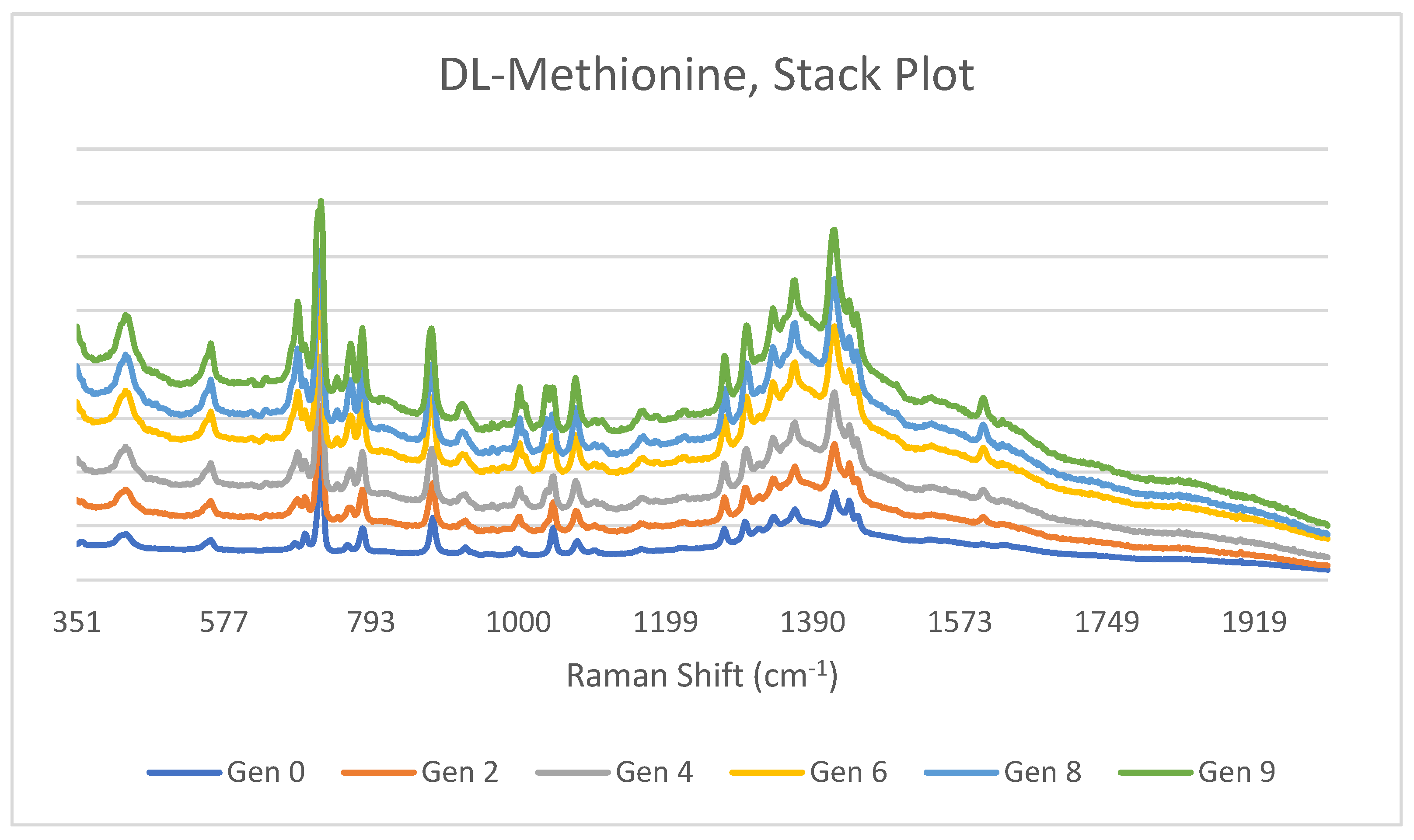
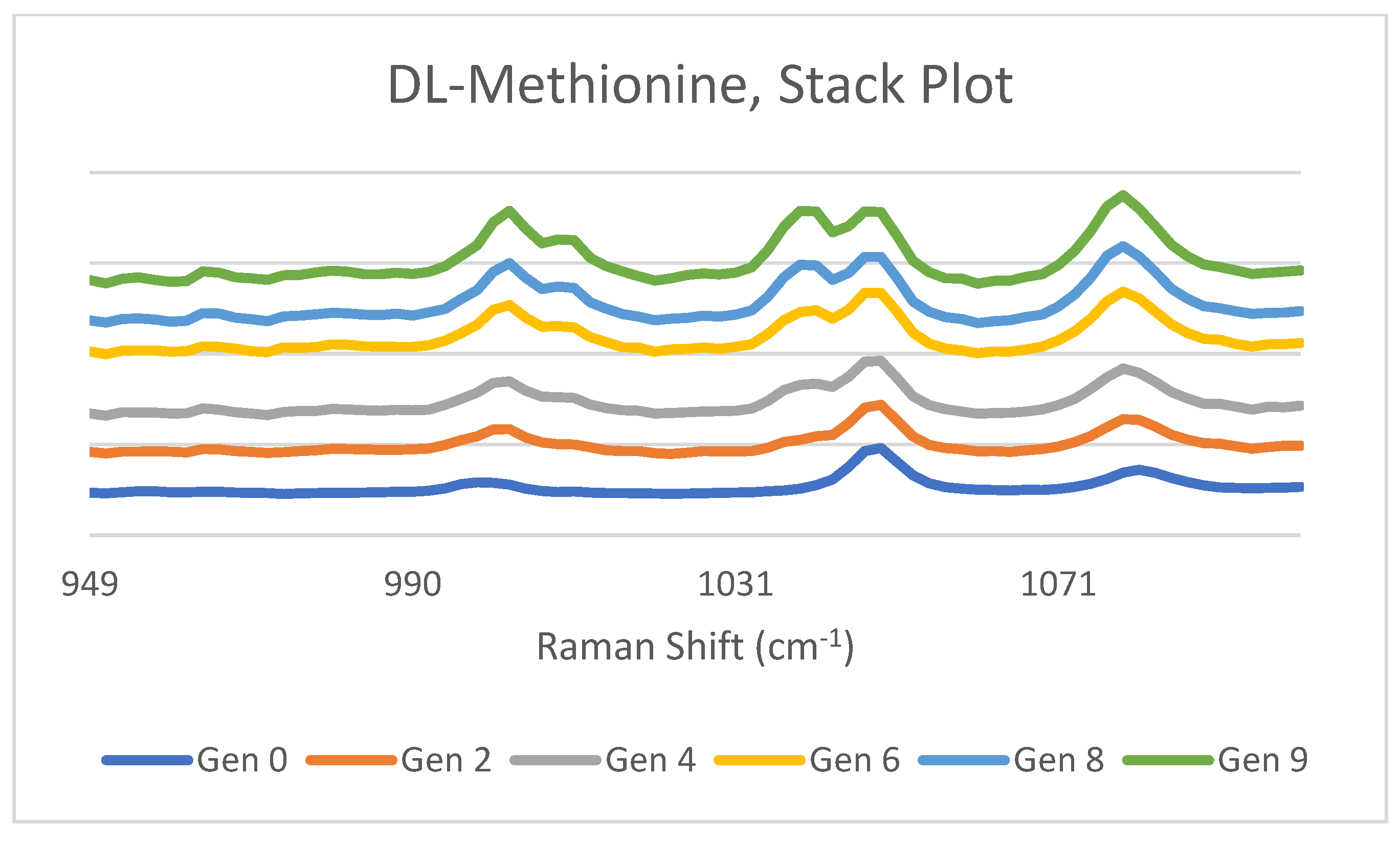
3.1. DL-Methionine
3.2. Glutamic Acid
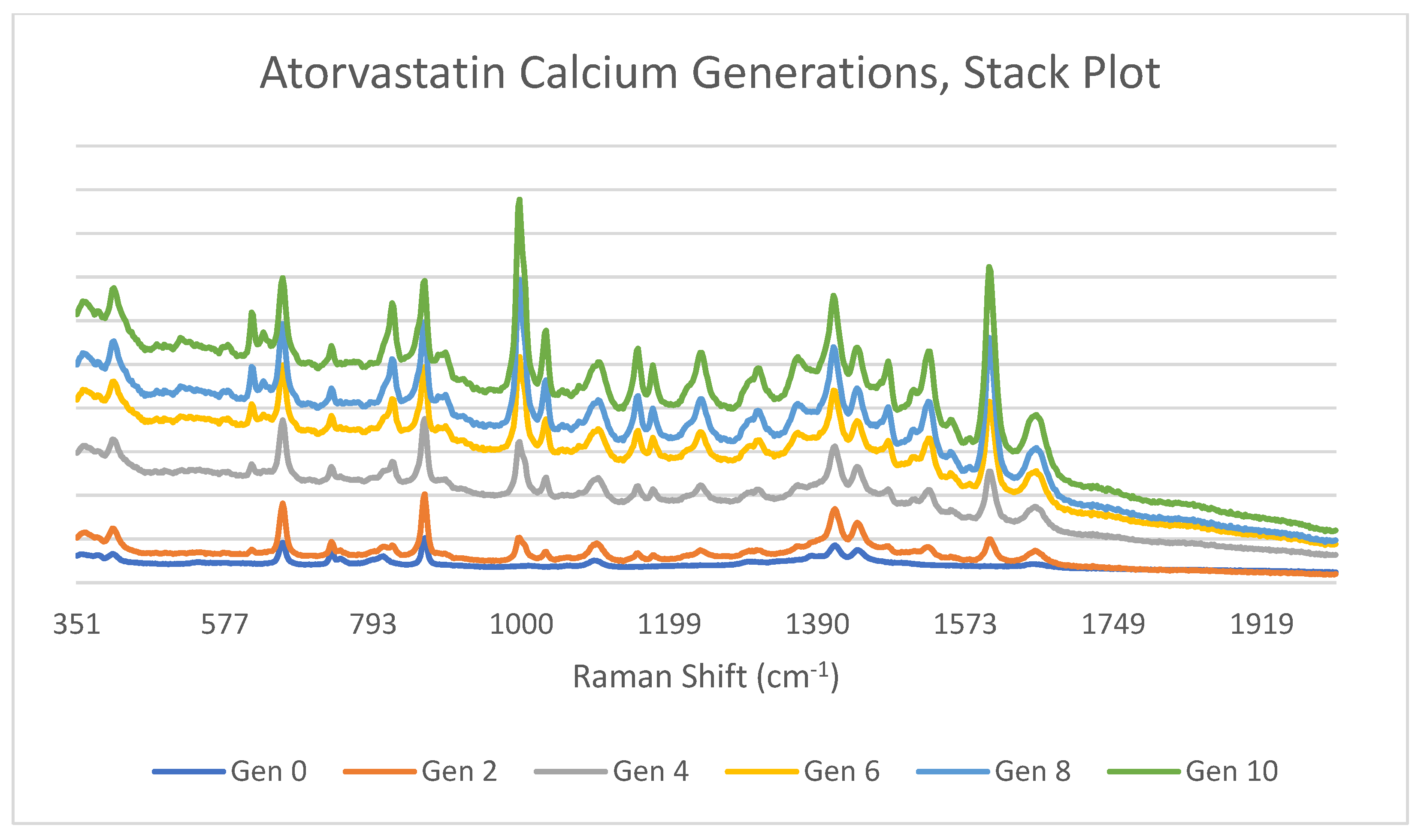
3.3. Atorvastatin Calcium
3.4. Hydrocortisone 21-Acetate
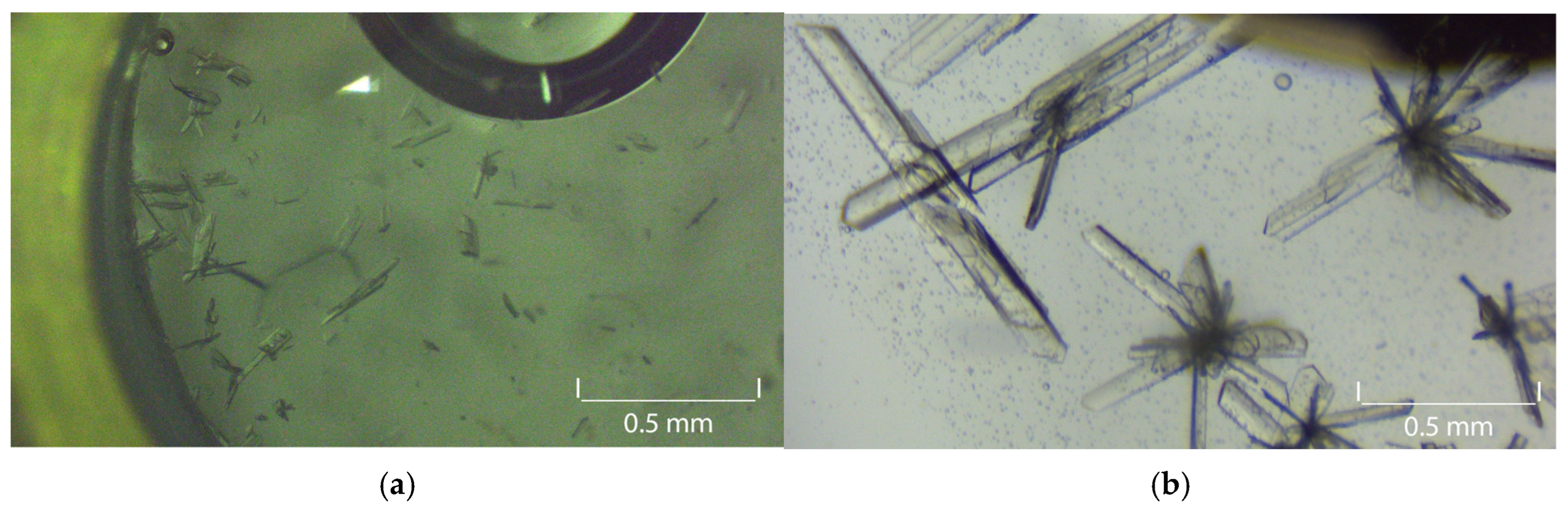
3.5. Carbamazepine Seeds
3.6. Atorvastatin Calcium Seeds
3.7. DL-Methionine Seeds
3.8. Hydrocortisone 21-Acetate Seeds
4. Discussion
4.1. DL-Methionine
4.2. Glutamic Acid
4.3. Atorvastatin Calcium
4.4. Hydrocortisone 21-Acetate
4.5. Seeds Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| XRD | X-ray diffractometry |
| ISS | International Space Station |
| PIL-BOX | Pharmaceutical In-space Laboratory (PIL)–Biocrystal Optimization eXperiment |
| ADSEP | ADvanced Space Experiment Processor |
| SMALS | Small Molecule Accelerated Laboratory for Structure |
References
- Singh, P.; Sharma, S.; Kumar Sharma, P.; Alam, A. Drug Polymorphism: An Important Pre-formulation Tool in the Formulation Development of a Dosage Form. Curr. Phys. Chem. 2024, 14, 2–19. [Google Scholar] [CrossRef]
- Brittain, H.G. Polymorphism and Solvatomorphism 2008. J. Pharm. Sci. 2010, 99, 3648–3664. [Google Scholar] [CrossRef] [PubMed]
- Saifee, M.; Inamdar, N.; Dhamecha, D.L.; Rathi, A.A. Drug Polymorphism: A Review. Int. J. Health Res. 2009, 2, 291–306. [Google Scholar] [CrossRef]
- Shi, Q.; Chen, H.; Wang, Y.; Xu, J.; Liu, Z.; Zhang, C. Recent advances in drug polymorphs: Aspects of pharmaceutical properties and selective polymorphs. Int. J. Pharm. 2022, 611, 121320. [Google Scholar] [CrossRef]
- Variankaval, N.; Cote, A.S.; Doherty, M.F. From Form to Function: Crystallization of Active Pharmaceutical Ingredients. AIChE J. 2008, 54, 1682–1688. [Google Scholar] [CrossRef]
- Sachin Kumar Sing, R.; Kumar Yadav, A.; Gulati, M.; Mittal, A.; Narang, R.; Garg, V. Polymorph control: Success so far and future expectations. Int. J. PharmTech Res. 2016, 9, 144–165. [Google Scholar]
- Corvis, Y. Solid State Development and Processing of Pharmaceutical Molecules: Salts, Cocrystals, and Polymorphism; Gruss, M., Ed.; Wiley-VCH: Weinheim, Germany, 2022. [Google Scholar]
- Zhou, Y.; Lv, C.; Liu, X.; Gao, J.; Gao, Y.; Wang, T.; Huang, X. An Overview on Polymorph Preparation Methods of Active Pharmaceutical Ingredients. Cryst. Growth Des. 2024, 24, 584–600. [Google Scholar] [CrossRef]
- Lowe, D. Stalking Polymorphs. Science, 14 March 2023. Available online: https://www.science.org/content/blog-post/stalking-polymorphs (accessed on 25 July 2025).
- Heng, T.; Yang, D.; Wang, R.; Zhang, L.; Lu, Y.; Du, G. Progress in Research on Artificial Intelligence Applied to Polymorphism and Cocrystal Prediction. ACS Omega 2021, 6, 15543–15550. [Google Scholar] [CrossRef]
- Burcham, C.L.; Doherty, M.F.; Peters, B.G.; Price, S.L.; Salvalagilio, M.; Reutzel-Edens, S.M.; Price, L.S.; Reddy Addula, R.K.; Francia, N.; Khanna, V.; et al. Pharmaceutical Digital Design: From Chemical Structure through Crystal Polymorph to Conceptual Crystallization Process. Cryst. Growth Des. 2024, 24, 5417–5438. [Google Scholar] [CrossRef]
- Parent, S.D.; Smith, P.A.; Purcell, D.K.; Smith, D.T.; Bogdanowich-Knipp, S.J.; Bhavsar, A.S.; Chan, L.R.; Croom, J.M.; Bauser, H.C.; McCalip, A.; et al. Ritanovir Form III: A Coincidental Concurrent Discovery. Cryst. Growth Des. 2023, 23, 320–325. [Google Scholar] [CrossRef]
- Surov, A.O.; Drozd, K.V.; Ramazanova, A.G.; Churakov, A.V.; Vologzhania, A.V.; Kulikova, E.S.; Perlovich, G.L. Polymorphism of Carbamazepine Pharmaceutical Cocrystal: Structural Analysis and Solubility Performance. Pharmaceutics 2023, 15, 1747. [Google Scholar] [CrossRef]
- Salazar-Rojas, D.; Kaufman, T.S.; Maggio, R.M. A comprehensive approach toward concomitant triclabendazole polymorphism in pharmaceutical products. J. Drug Deliv. Sci. Tech. 2021, 62, 102386. [Google Scholar] [CrossRef]
- Wirgowska, G.; Stasilowicz, A.; Miklaszewski, A.; Lewandowska, K.; Cielecka-Piontek, J. Structural Polymorphism of Sorafenib Tosylate as a Key Factor in Its Solubility Differentiation. Pharmaceutics 2021, 13, 384. [Google Scholar] [CrossRef] [PubMed]
- Toukabri, I.; Bahri, S.; Sfar, S.; Ali Lassoued, M. Impact of crystal polymorphism of rifaximin on dissolution behavior. Heliyon 2024, 10, e27131. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; McKenna, G.B. Isothermal Crystallization and Time-Temperature Transformation of Amorphous Nifedipine: A Case of Polymorphism Formation and Conversion. Molec. Pharm. 2021, 18, 2786–2802. [Google Scholar] [CrossRef]
- Li, M.; Xu, W.; Su, Y. Solid-state NMR spectroscopy in pharmaceutical sciences. Trends Anal. Chem. 2021, 135, 116152. [Google Scholar] [CrossRef]
- Souza Almeida, L.; Carneiro, J.; Alberto Colnago, L. Time domain NMR for polymorphism characterization: Current status and future prospects. Int. J. Pharm. 2025, 669, 125027. [Google Scholar] [CrossRef]
- Helena Mazurek, A.; Szeleszczuk, Ł.; Maciej Pisklak, D. Periodic DFT Calculations—Review of Applications in the Pharmaceutical Sciences. Pharmaceutics 2020, 12, 415. [Google Scholar] [CrossRef]
- Yan, T.; Deng, Y.; Yu, Z.; John, E.; Han, R.; Yao, Y.; Liu, Y. Exploring the Polymorphism of Cinchomeronic Acid at High Pressure. J. Phys. Chem. C 2021, 125, 8582–8588. [Google Scholar] [CrossRef]
- Guerain, M.; Willart, J.-F. Polymorphic Transformations of Pharmaceutical Materials Induced by Mechanical Milling: A Review. Pharmaceutics 2025, 17, 946. [Google Scholar] [CrossRef]
- Chakraborty, J.; Subash, M.; Thorat, B.N. Drying induced polymorphic transformation of pharmaceutical ingredients: A critical review of recent progresses and challenges. Dry. Technol. 2022, 40, 2817–2835. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.-S.; Hong, S.; Ha, E.-S.; Nie, H.; Zhou, Q.T.; Kim, M.-S. Tableting process-induced solid-state polymorphic transition. J. Pharm. Investig. 2022, 52, 175–194. [Google Scholar] [CrossRef]
- Maruyama, M.; Yoshikawa, H.Y.; Takano, K.; Yoshimura, M.; Mori, Y. Solution-mediated phase transition of pharmaceutical compounds: Case studies of acetaminophen and aspirin. J. Cryst. Growth 2023, 602, 126990. [Google Scholar] [CrossRef]
- Roy, S.; Chamberlin, B.; Matzger, A.J. Polymorph Discrimination Using Low Wavenumber Raman Spectroscopy. Org. Proc. Res. Dev. 2013, 17, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Fateixa, S.; Nogueira, H.I.S.; Trindade, T. Carbamazepine polymorphism: A re-visitation using Raman imaging. Int. J. Pharm. 2022, 617, 121632. [Google Scholar] [CrossRef]
- Aragon, F.F.H.; Haeck, C.M.; Morals, P.C.; Variano, B. Polymorphism characterization of segesterone acetate: A comprehensive study using CRPD, FT-IR and Raman spectroscopy. Int. J. Pharm. 2021, 596, 120234. [Google Scholar] [CrossRef]
- Du, Y.; Frank, D.; Chen, Z.; Struppe, J.; Su, Y. Ultrafast magic angle spinning NMR characterization of pharmaceutical solid polymorphism: A posaconazole example. J. Magn. Reson. 2023, 346, 107352. [Google Scholar] [CrossRef]
- Wright, H.; Williams, A.; Wilkinson, A.; Harper, L.; Savin, K.; Wilson, A.M. An Analysis of Publicly Available Microgravity Crystallization Data; Emergent Themes Across Crystal Types. Cryst. Growth Des. 2022, 22, 6849–6851. [Google Scholar] [CrossRef]
- Jackson, K.; Brewer, F.; Wilkinson, A.; Williams, A.; Whiteside, B.; Wright, H.; Harper, L.; Wilson, A.M. Microgravity Crystal Formation. Crystals 2024, 14, 12. [Google Scholar] [CrossRef]
- NASA; Sands, K.; Bowman, A. “What Is Microgravity?”. 2023. Available online: https://www.nasa.gov/centers-and-facilities/glenn/what-is-microgravity/#:~:text=But%20they’re%20not%20falling,%C3%9710%2D6%20g (accessed on 4 September 2025).
- Yoo, H.-D.; Wilcox, W.R.; Lal, R.; Trolinger, J.D. Modeling the growth of triglycine sulphate crystals in spacelab 3. J. Cryst. Growth 1988, 92, 101–117. [Google Scholar] [CrossRef]
- Lal, R.B.; Batra, A.K.; Trolinger, J.D.; Wilcox, W.R.; Steiner, B. Growth and characteristics of tgs crystals grown aboard first international microgravity laboratory (IML-1). Ferroelectrics 1994, 158, 81–85. [Google Scholar] [CrossRef]
- Aggarwal, M.D.; Batra, A.K.; Lal, R.B.; Penn, B.G.; Frazier, D.O. Growth and Characteristics of Bulk Single Crystals Grown from Solution on Earth and in Microgravity. NASA Technical Reports Server. Available online: https://ntrs.nasa.gov/citations/20110006347 (accessed on 10 September 2024).
- Kroes, R.L.; Reiss, D.A.; Lehoczky, S.L. Nucleation of Crystals from Solution in Microgravity-USML-1 Glovebox (GBX) Investigation. In Proceedings of the Joint Launch + One Year Science Review of USML-1 and USMP-1 with the Microgravity Measurement Group, Huntsville, AL, USA, 22–24 September 1993; Volume 2. [Google Scholar]
- Nielsen, K.F.; Lind, M.D. Results of the TTF-TCNQ- and the Calcium Carbonate-Crystallization on the Long Duration Exposure Facility. In Proceedings of the First LDEF Post-Retrieval Symposium Abstracts, Hyatt Orlando, Kissimmee, FL, USA, 2–8 June 1991; NASA Langley Research Center: Hampton, VA, USA, 1991; pp. 725–731. [Google Scholar]
- Bauser, H.C.; Smith, P.A.; Parent, S.D.; Chan, L.R.; Bhavsar, A.S.; Condon, K.H.; McCalip, A.; Croom, J.M.; Purcell, D.K.; Bogdanowich-Knipp, S.J.; et al. Return of Ritanovir: A Study on the Stability of Pharmaceuticals Processed in Orbit and Returned to Earth. ChemRxiv 2024, 3. Available online: https://chemrxiv.org/engage/chemrxiv/article-details/65faecbee9ebbb4db91b4ac1 (accessed on 10 September 2024).
- Miller, L.; Mulligan, M.K.; Savin, K.A.; Tuma, S.; Wilson, A.M. Crystallization of Small Molecules in Microgravity Using Pharmaceutical In-Space Laboratory-Biocrystal Optimization eXperiment (PIL-BOX). Crystals 2025, 15, 527. [Google Scholar] [CrossRef]
- Jackson, K.; Hoff, R.; Wright, H.; Wilkinson, A.; Brewer, F.; Williams, A.; Whiteside, B.; Macbeth, M.R.; Wilson, A.M. An Analysis of Protein Crystals Grown under Microgravity Conditions. Crystals 2024, 14, 652. [Google Scholar] [CrossRef]
- Wilkinson, A.; Brewer, F.; Wright, H.; Whiteside, B.; Williams, A.; Harper, L.; Wilson, A.M. A meta-analysis of semiconductor materials fabricated in microgravity. npj Microgravity 2024, 10, 73. [Google Scholar] [CrossRef]
- DeLucas, L.J.; Smith, C.D.; Smith, H.W.; Vijay-Kumar, S.; Senadhi, S.E.; Ealick, S.E.; Carter, D.C.; Salemme, F.R.; Ohlendorf, D.H.; Einspahr, H.M.; et al. Protein Crystal Growth in Microgravity. Science 1989, 246, 651–654. [Google Scholar] [CrossRef]
- McPherson, A.; DeLucas, L.J. Microgravity protein crystallization. npj Microgravity 2015, 1, 15010. [Google Scholar] [CrossRef]
- Maes, D.; Decanniere, K.; Zegers, I.; Vanhee, C.; Sleutel, M.; Willaert, R.; Weerdt, C.; Martial, J.; Declercq, J.; Evrard, C.; et al. Protein crystallization under microgravity conditions: What did we learn on TIM crystallization from the Soyuz missions? Microgravity Sci. Technol. 2007, 19, 90–94. [Google Scholar]
- Nicoud, N.; Licordari, F.; Myerson, A.S. Estimation of the Solubility of Metastable Polymorphs: A Critical Review. Cryst. Growth Des. 2018, 18, 7228–7237. [Google Scholar] [CrossRef]
- Singhal, D.; Curatolo, W. Drug polymorphism and dosage form design: A practical perspective. Adv. Drug Deliv. Rev. 2004, 56, 335–347. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Y.; Lin, J.; Gao, Z.; Wu, S.; Li, W.; Han, D.; Gong, J.; Wang, J. The polymorph and crystal habit control of dl-methionine assisted by ultrasound. J. Cryst. Growth 2022, 596, 126818. [Google Scholar] [CrossRef]
- Srinivasan, K.; Dhanasekaran, P. Nucleation control and crystallization of l-glutamic acid polymorphs by swift cooling process and their characterization. J. Cryst. Growth 2011, 318, 1080–1084. [Google Scholar] [CrossRef]
- Skorda, D.; Kontoyannis, C.G. Identification and quantitative determination of atorvastatin calcium polymorph in tablets using FT-Raman spectroscopy. Talanta 2008, 74, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Callow, R.K.; Kennard, O. Polymorphism of Cortisone Acetate. J. Pharm. Pharmacol. 1961, 13, 723–733. [Google Scholar] [CrossRef]
- Wantha, L.; Flood, A.E. Crystal growth rates and secondary nucleation threshold for γ-DL-methionine in aqueous solution. J. Cryst. Growth 2011, 318, 117–121. [Google Scholar] [CrossRef]
- Jin, Y.S.; Ulrich, J. New Crystalline Solvates of Atorvastatin Calcium. Chem. Eng. Technol. 2010, 33, 839–844. [Google Scholar] [CrossRef]
- Deeley, C.M.; Spragg, R.A.; Threlfall, T.L. A comparison of Fourier transform infrared and near-infrared Fourier transform Raman spectroscopy for quantitative measurements: An application in polymorphism. Spectrochim. Acta 1991, 47A, 1217–1223. [Google Scholar] [CrossRef]
- Matsuoka, M.; Yamanobe, M.; Tezuka, N.; Takiyama, H.; Ishii, H. Polymorphism, morphologies and bulk densities of DL-methionine agglomerate crystals. J. Cryst. Growth 1999, 198/199, 1299–1306. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, T.; Yang, Y.; Ma, C.Y.; Wang, X.E.; Ni, X. In Situ Measurement of 3D Crystal Size Distribution by Double-View Image Analysis with Case Study on L-Glutamic Acid Crystallization. Ind. Eng. Chem. Res. 2020, 59, 4646–4658. [Google Scholar]
- Shete, G.; Puri, V.; Kumar, L.; Bansal, A.K. Solid State Characterization of Commercial Crystalline and Amorphous Atorvastatin Calcium Samples. AAPS PharmSciTech 2010, 11, 598–609. [Google Scholar] [CrossRef]
- Kurakula, M.; El-Helw, A.M.; Sobahi, T.R.; Abdelaal, M.Y. Chitosan based atorvastatin nanocrystals: Effect of cationic charge on particle size, formulation stability, and in-vivo efficacy. Int. J. Nanomed. 2015, 15, 321–334. [Google Scholar] [CrossRef]
- Hench, P.S.; Kendall, E.C.; Slocumb, C.H.; Polley, H.F. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone; compound E and of pituitary adrenocorticotropic hormone on rheumatoid arthritis. Proc. Staff Meet. Mayo Clin 1949, 24, 181–197. [Google Scholar] [CrossRef]
- Suitchmezian, V.; Jeß, I.; Näther, C. Structural, Thermodynamic, and Kinetic Aspects of the Trimorphism of Hydrocortisone. J. Pharm. Sci. 2008, 97, 4516–4527. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Ulrich, J.; Yin, Q.; Xue, L. Effect of Solvent on the Crystal Structure and Habit of Hydrocortisone. Cryst. Growth Des. 2008, 8, 1490–1494. [Google Scholar] [CrossRef]
- He, Y.; Gao, Z.; Zhang, T.; Sun, J.; Ma, Y.; Tian, N.; Gong, J. Seeding Techniques and Optimization of Solution Crystallization Processes. Org. Proc. Res. Dev. 2020, 24, 1839–1849. [Google Scholar] [CrossRef]
- Long, Y.; Ment, A.; Xu, Q.; Shan, B.; Wang, Y.; Zhang, F.; Yu, Z.-Q. Uncertainty analysis of seed recipe for optimal control of crystal size distribution in batch cooling crystallization. Chem. Eng. Res. Des. 2024, 204, 601–611. [Google Scholar] [CrossRef]
- Czernicki, W.; Baranska, M. Carbamazepine polymorphs: Theoretical and experimental vibrational spectroscopy studies. Vib. Spect. 2013, 65, 12–23. [Google Scholar] [CrossRef]
- Van de Streek, J.; Firaha, D.; Kaduk, J.A.; Blanton, T.N. From ‘crystallographic accuracy’ to ‘thermodynamic accuracy’: A redetermination of the crystal structure of calcium atorvastatin trihydrate (Lipitor®). Acta Cryst. 2024, B80, 682–687. [Google Scholar] [CrossRef]
- Corvis, Y. Impact of Solid Forms on API Scale-Up. In Solid State Development and Processing of Pharmaceutical Molecules: Salts, Cocrystals, and Polymorphism; Gruss, M., Ed.; Wiley-VCH: Weinheim, Germany, 2022; p. 307. [Google Scholar]
- Yamanobe, M.; Takiyama, H.; Matsuoka, M. Polymorphic transformation of DL-methionine crystals in aqueous solutions. J. Cryst. Growth 2002, 237–239 Pt 3, 2221–2226. [Google Scholar] [CrossRef]

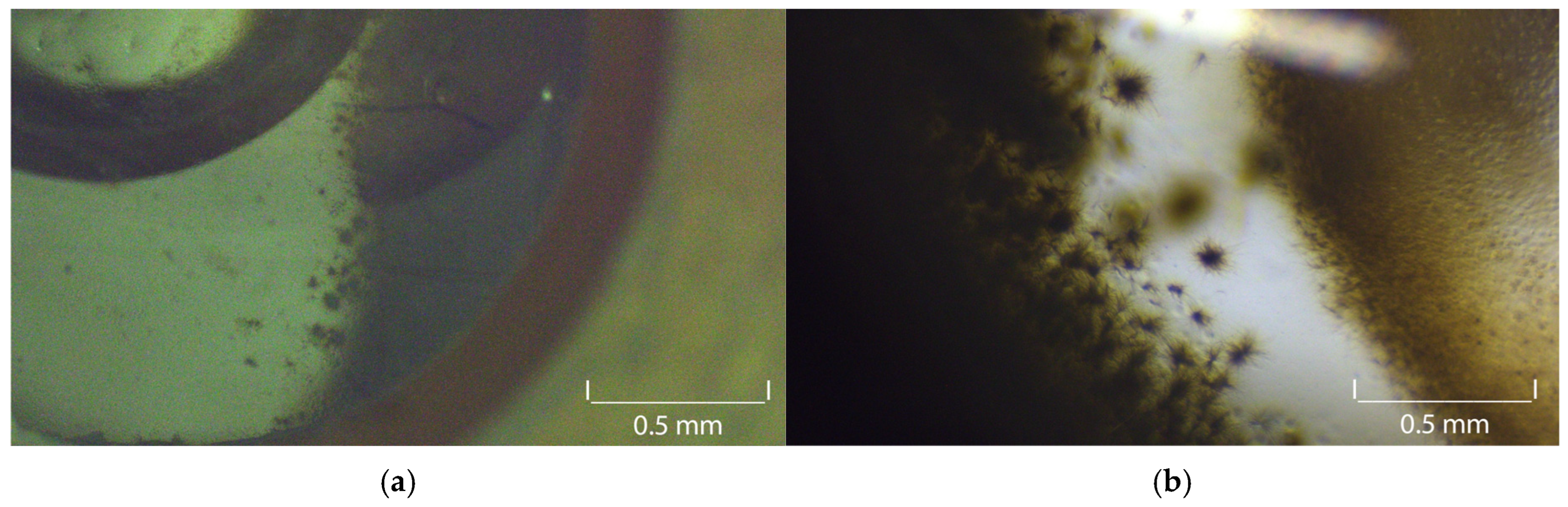

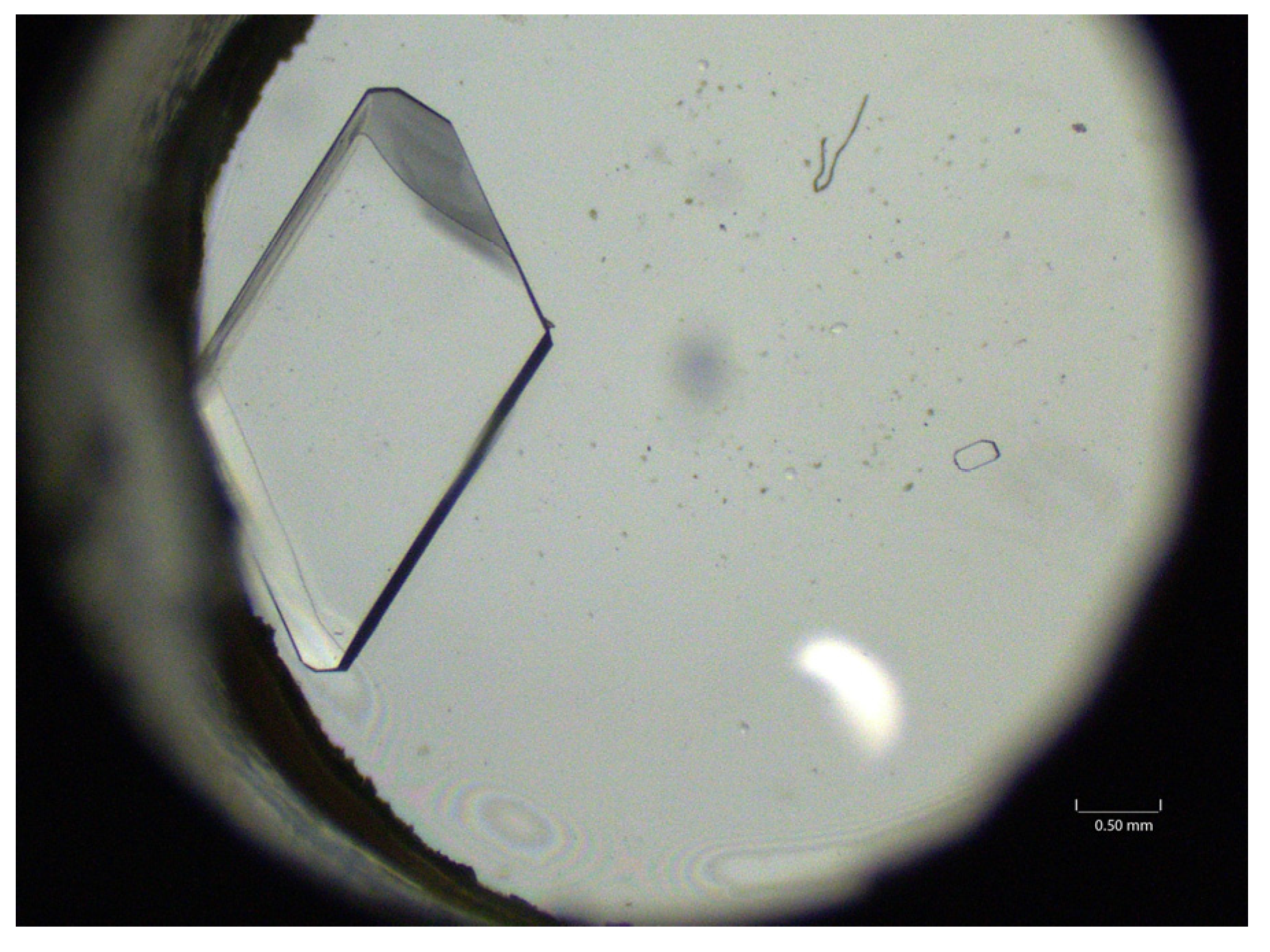

| Substrate (Amount) | Solvent (Amount) | Antisolvent |
|---|---|---|
| DL-Methionine (24.6 mg) | Water (1 mL) | Ethanol |
| Glutamic Acid (7.9 mg) | Water (1 mL) | Ethanol |
| Atorvastatin Calcium (28.9 mg) | Dimethylformamide (1 mL) | Water |
| Hydrocortisone 21-Acetate (58.8 mg) | Dichloromethane (1 mL) | Ethyl Acetate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulson, J.; Miller, L.; Tuma, S.; Mulligan, M.K.; Savin, K.A.; Wilson, A.M. Microgravity-Grown Crystals as Seeds for Pharmaceutical Compounds. Crystals 2025, 15, 825. https://doi.org/10.3390/cryst15090825
Paulson J, Miller L, Tuma S, Mulligan MK, Savin KA, Wilson AM. Microgravity-Grown Crystals as Seeds for Pharmaceutical Compounds. Crystals. 2025; 15(9):825. https://doi.org/10.3390/cryst15090825
Chicago/Turabian StylePaulson, Jessica, Lillian Miller, Stephen Tuma, Molly K. Mulligan, Kenneth A. Savin, and Anne M. Wilson. 2025. "Microgravity-Grown Crystals as Seeds for Pharmaceutical Compounds" Crystals 15, no. 9: 825. https://doi.org/10.3390/cryst15090825
APA StylePaulson, J., Miller, L., Tuma, S., Mulligan, M. K., Savin, K. A., & Wilson, A. M. (2025). Microgravity-Grown Crystals as Seeds for Pharmaceutical Compounds. Crystals, 15(9), 825. https://doi.org/10.3390/cryst15090825






