Supramolecular Stabilisation Leads to Challenging Coordination in Fe(III) Hydrazinylpyrazine Schiff Base Complexes
Abstract
1. Introduction
2. Materials and Methods
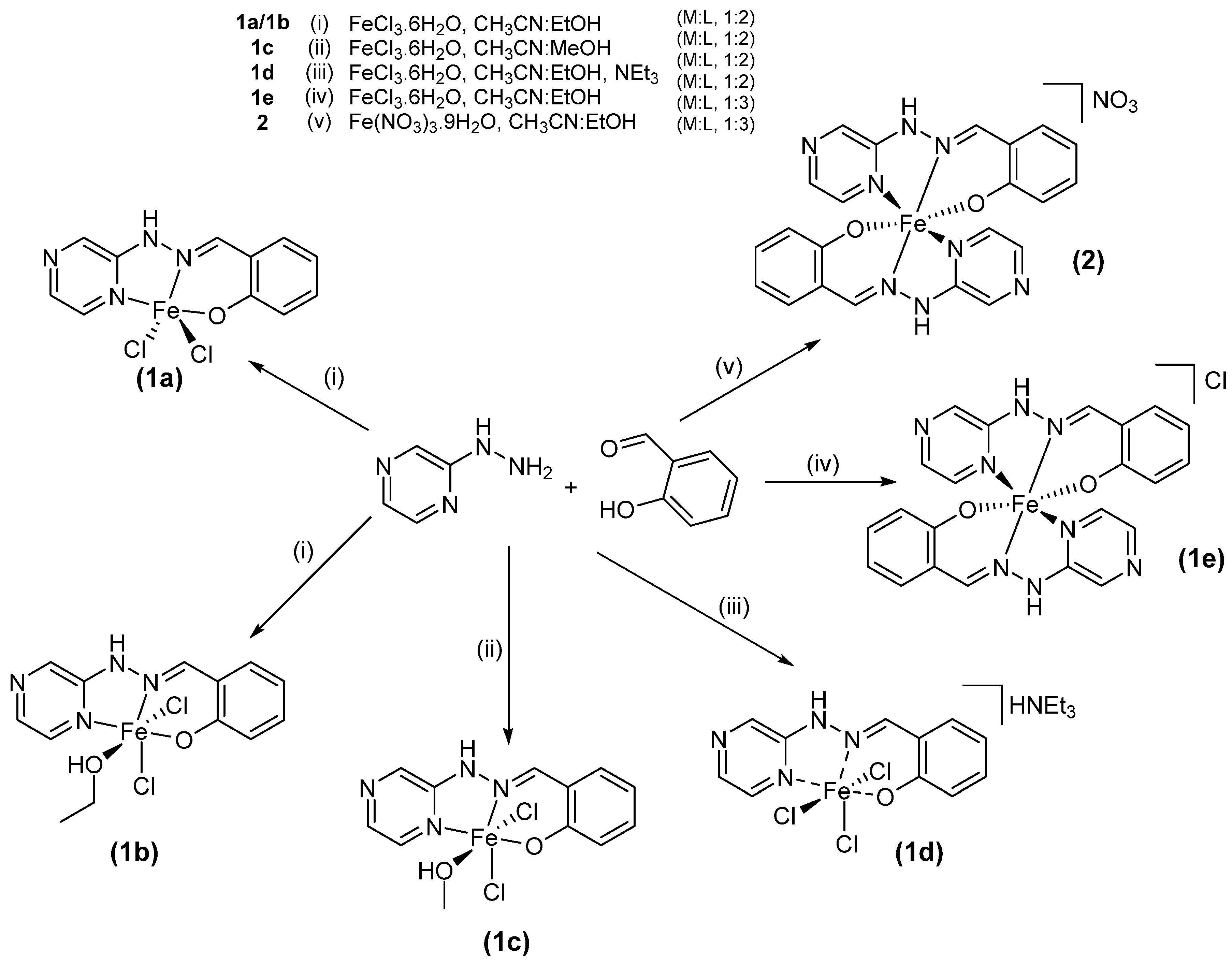
2.1. Synthesis
- Complex 1a and 1b
- Complex 1c
- Complex 1d
- Complex 1e
- Complex 2
2.2. Crystallography
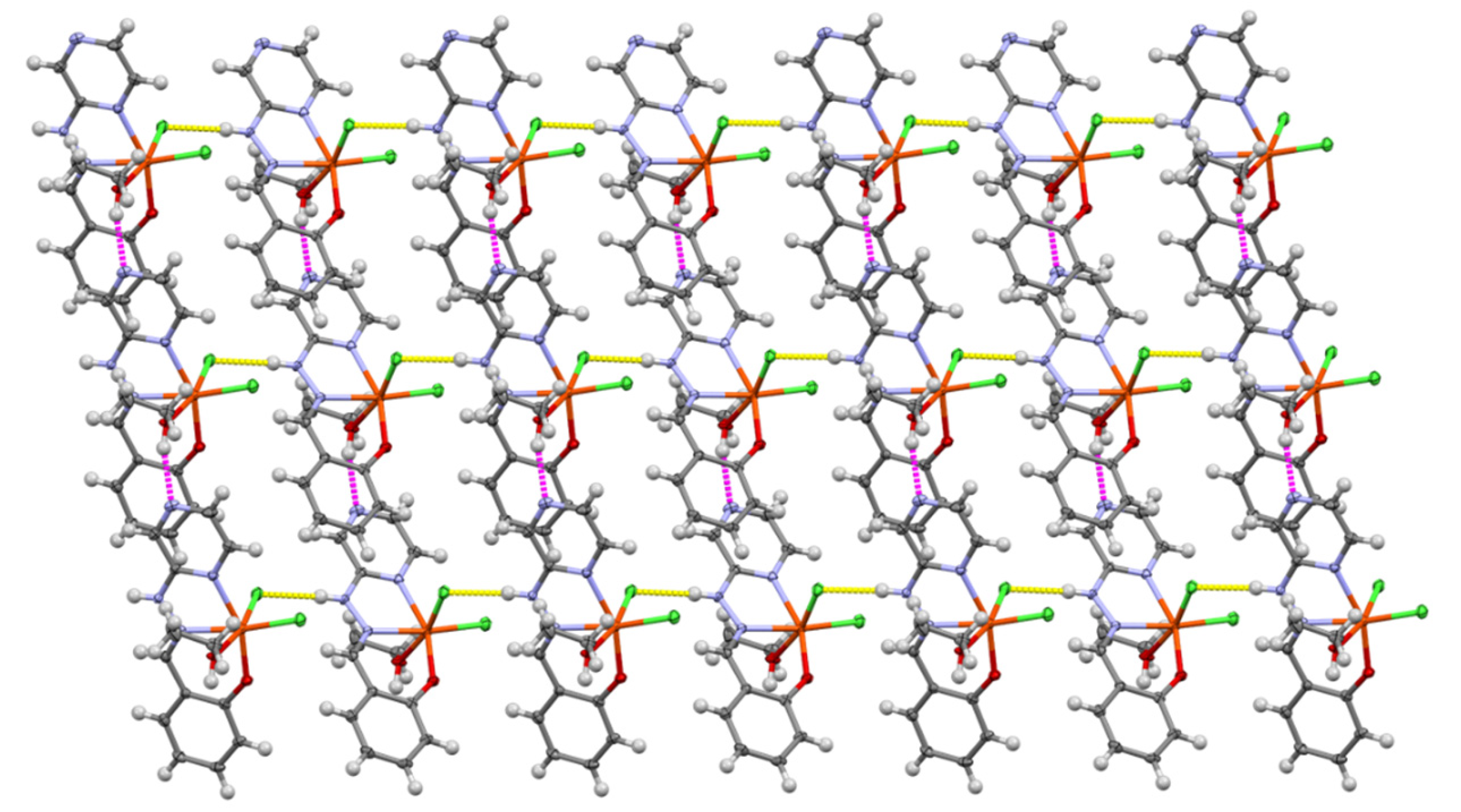
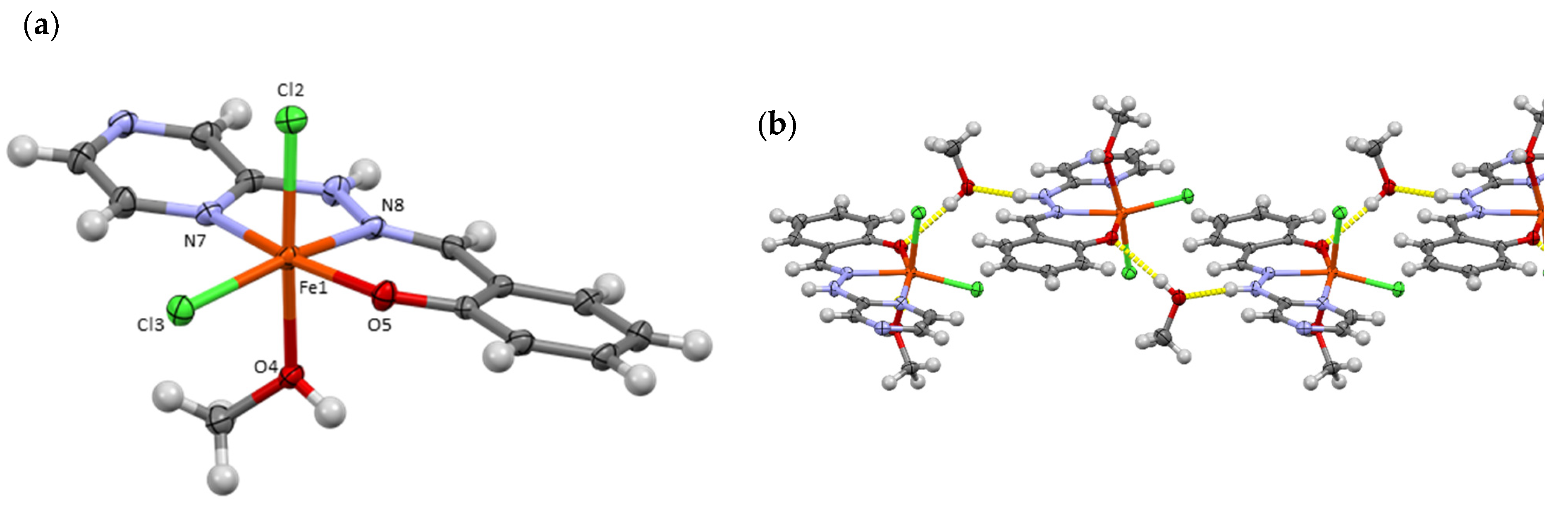
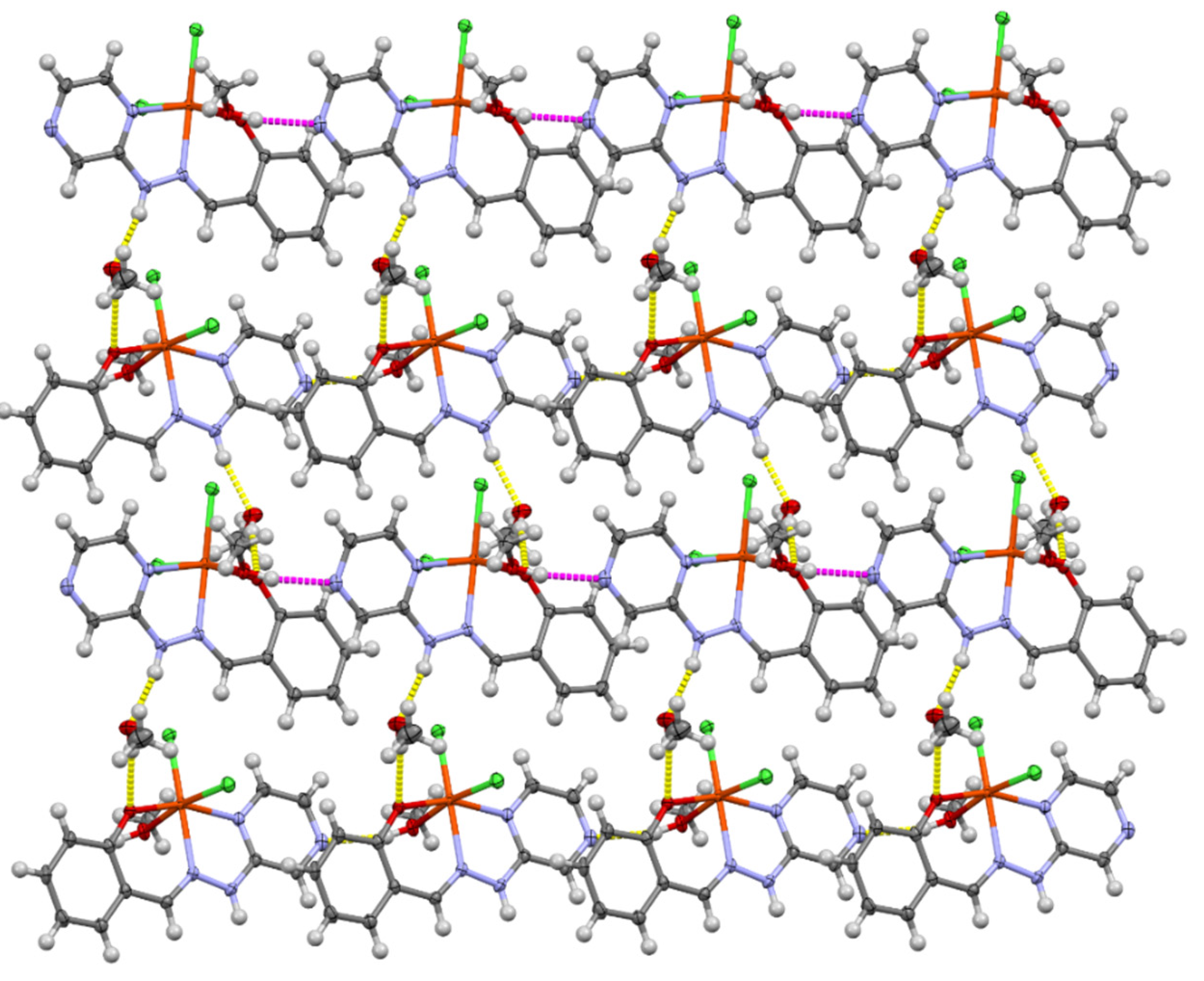
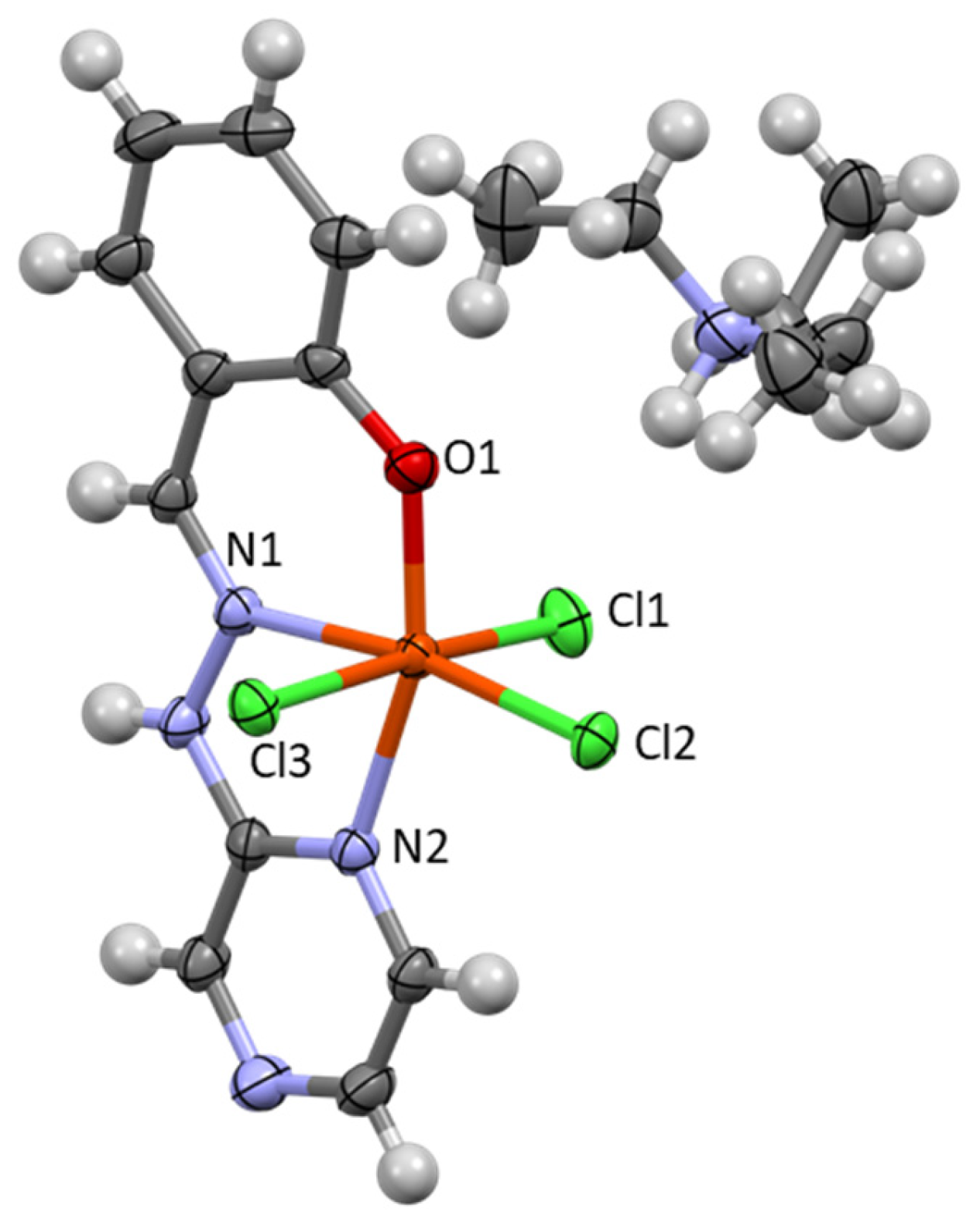
3. Results and Discussion
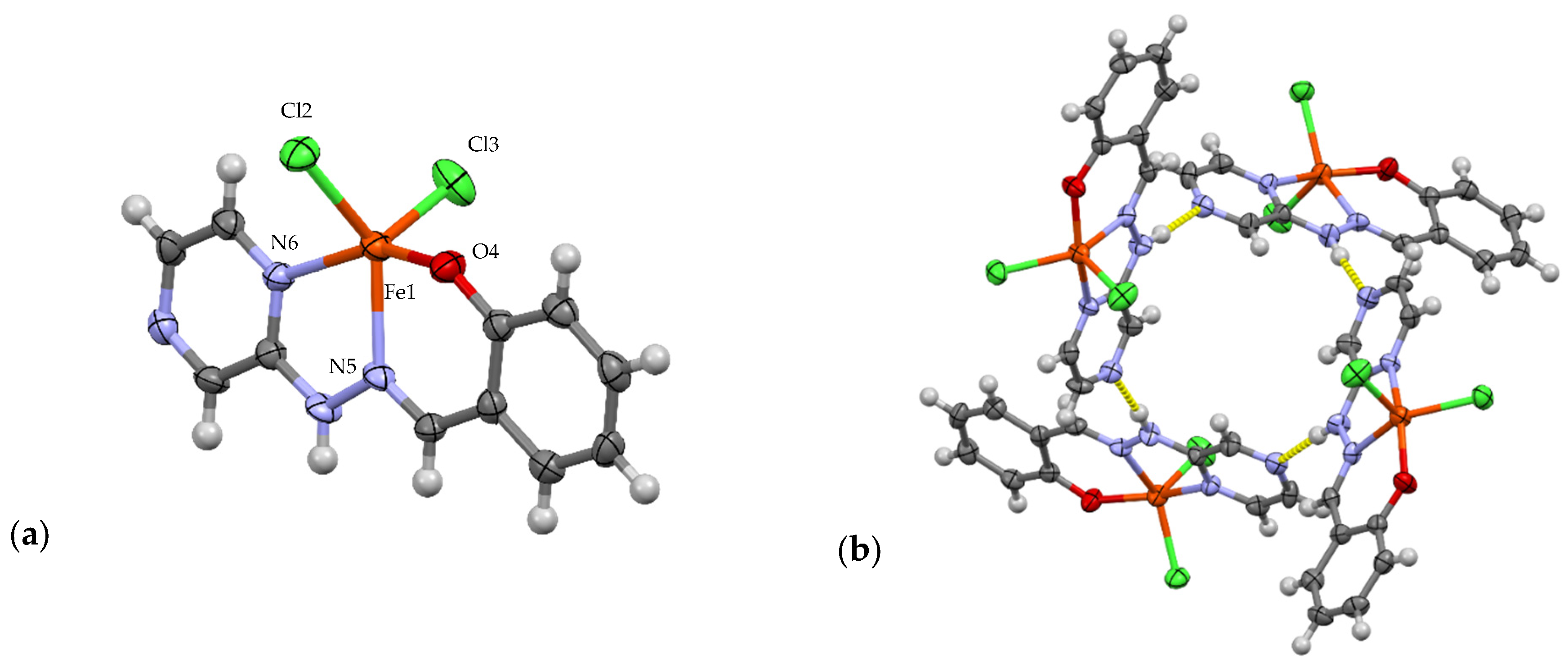
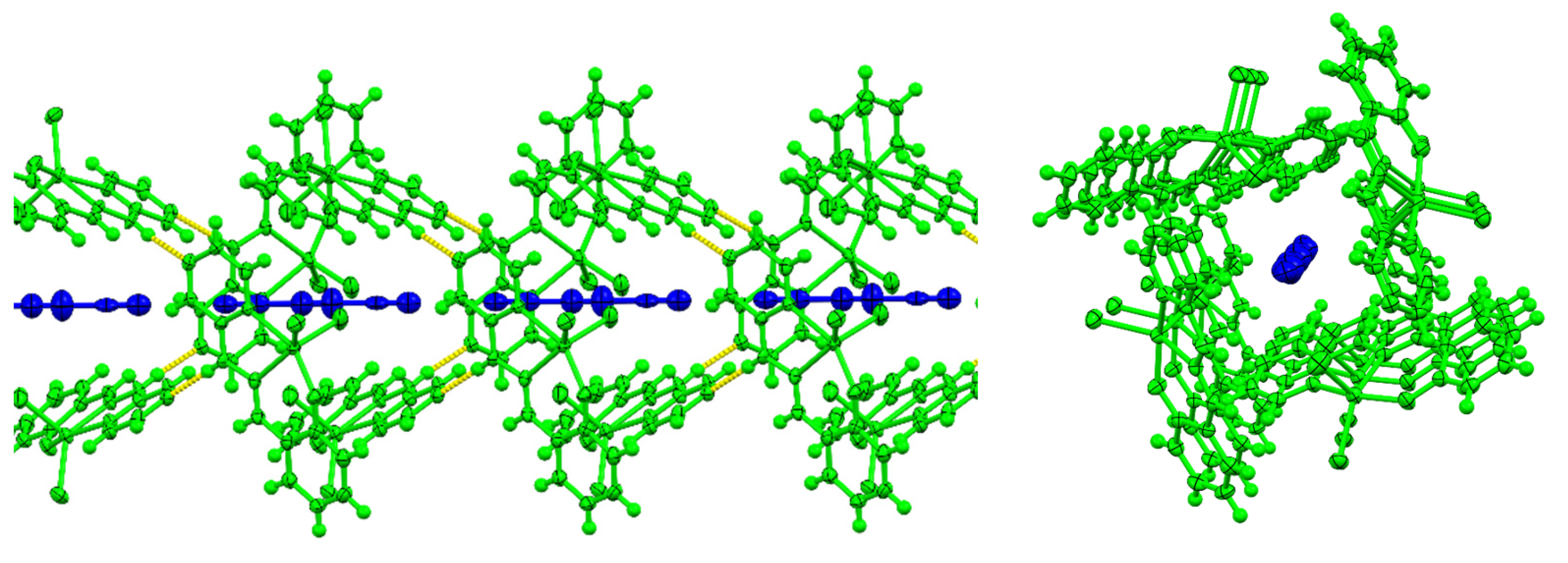
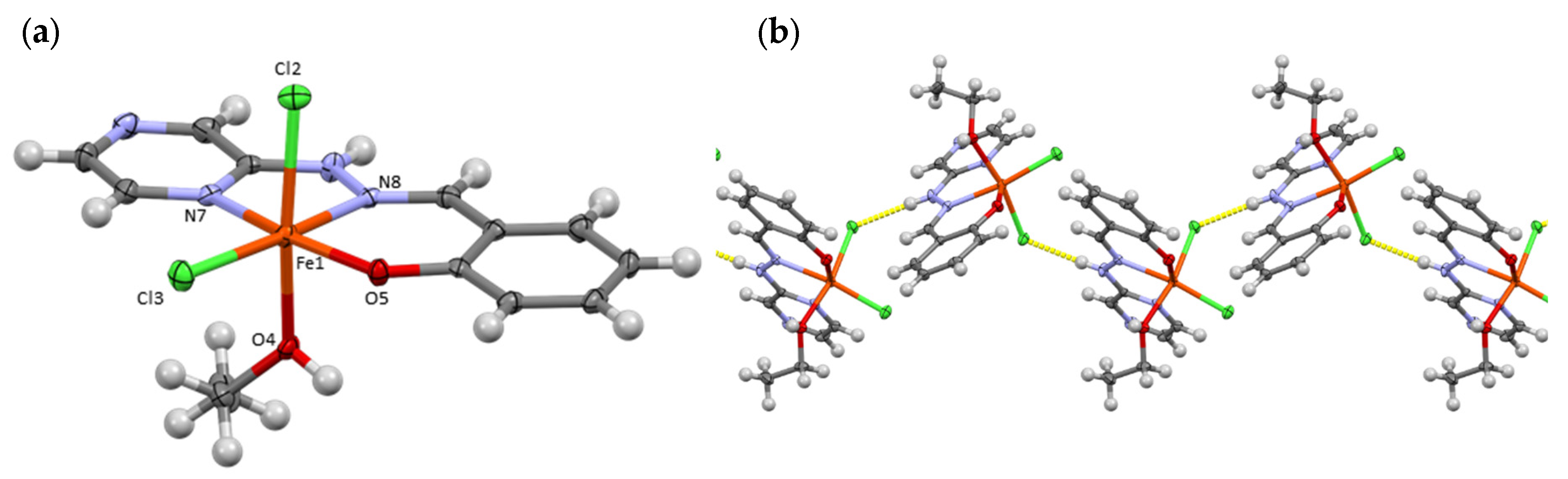
3.1. Crystallographic Analysis
3.2. Continuous Shape Measure Analysis
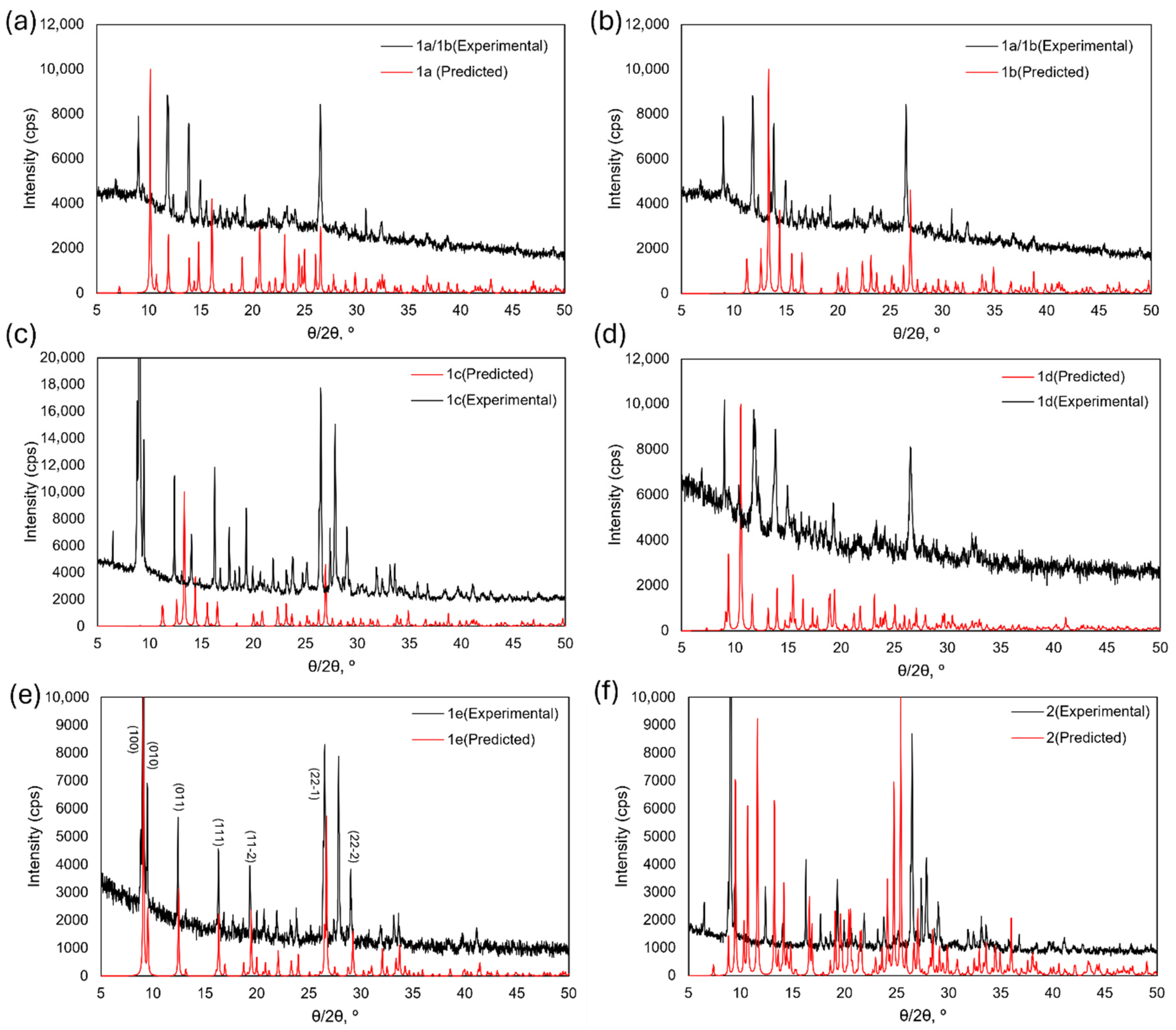
3.3. Powder X-Ray Analysis
3.4. Infrared Analysis
3.5. Synthetic Insights
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinho, P.N.; Martins, F.F.; Bandeira, N.A.G.; Calhorda, M.J. Spin Crossover in 3D Metal Centers Binding Halide-Containing Ligands: Magnetism, Structure and Computational Studies. Sustainability 2020, 12, 2512. [Google Scholar] [CrossRef]
- Boda, S.K.; Pandit, S.; Garai, A.; Pal, D.; Basu, B. Bacterial Siderophore Mimicking Iron Complexes as DNA Targeting Antimicrobials. RSC Adv. 2016, 6, 39245–39260. [Google Scholar] [CrossRef]
- Atanasov, M.; Aravena, D.; Suturina, E.; Bill, E.; Maganas, D.; Neese, F. First Principles Approach to the Electronic Structure, Magnetic Anisotropy and Spin Relaxation in Mononuclear 3d-Transition Metal Single Molecule Magnets. Coord. Chem. Rev. 2015, 289–290, 177–214. [Google Scholar] [CrossRef]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Coordination Polymers, Metal–Organic Frameworks and the Need for Terminology Guidelines. CrystEngComm 2012, 14, 3001. [Google Scholar] [CrossRef]
- Esra, D.; Pilia, L.; Marchiò, L.; Mercuri, M.L.; Serpe, A.; Barsella, A.; Fort, A.; Dalgleish, S.J.; Robertson, N.; Deplano, P. Redox-Switchable Chromophores Based on Metal (Ni, Pd, Pt) Mixed-Ligand Dithiolene Complexes Showing Molecular Second-Order Nonlinear-Optical Activity. Inorg. Chem. 2011, 50, 2058–2060. [Google Scholar] [CrossRef]
- Brooker, S. Spin Crossover with Thermal Hysteresis: Practicalities and Lessons Learnt. Chem. Soc. Rev. 2015, 44, 2880–2892. [Google Scholar] [CrossRef] [PubMed]
- Büldt, L.A.; Wenger, O.S. Chromium Complexes for Luminescence, Solar Cells, Photoredox Catalysis, Upconversion, and Phototriggered NO Release. Chem. Sci. 2017, 8, 7359–7367. [Google Scholar] [CrossRef] [PubMed]
- Belair, S.D.; Maupin, C.L.; Logue, M.W.; Riehl, J.P. Analysis of the Temperature Dependence of the Racemization of Eu(III) Complexes through Measurement of Steady-State Circularly Polarized Luminescence. J. Lumin. 2000, 86, 61–66. [Google Scholar] [CrossRef]
- Bünzli, J.C.G. On the Design of Highly Luminescent Lanthanide Complexes. Coord. Chem. Rev. 2015, 293–294, 19–47. [Google Scholar] [CrossRef]
- Rajput, A.; Mukherjee, R. Coordination Chemistry with Pyridine/Pyrazine Amide Ligands. Some Noteworthy Results. Coord. Chem. Rev. 2013, 257, 350–368. [Google Scholar] [CrossRef]
- Abedin, N.; Alshehri, A.H.A.; Almughrbi, A.M.A.; Moore, O.; Alyza, S.; Rusbridge, E.K.; Masood, N.; Egbowon, B.F.; Hargreaves, A.J.; Dafhnis-Calas, F.; et al. Expanding the Family of Tetrahalide Iron Complexes: Synthesis, Structure and Biological Applications. Polyhedron 2020, 190, 114755. [Google Scholar] [CrossRef]
- Li, J.-Y.; He, C.-T.; Chen, Y.-C.; Zhang, Z.-M.; Liu, W.; Ni, Z.-P.; Tong, M.-L. Tunable Cooperativity in a Spin-Crossover Hoffman-like Metal–Organic Framework Material by Aromatic Guests. J. Mater. Chem. C 2015, 3, 7830–7835. [Google Scholar] [CrossRef]
- Shepherd, H.J.; Bartual-Murgui, C.; Molnar, G.; Real, J.A.; Munoz, M.C.; Salmon, L.; Bousseksou, A. Thermal and Pressure-Induced Spin Crossover in a Novel Three-Dimensional Hoffman-like Clathrate Complex. New J. Chem. 2011, 35, 1205–1210. [Google Scholar] [CrossRef]
- Goze, C.; Dupont, N.; Beitler, E.; Leiggener, C.; Jia, H.; Monbaron, P.; Liu, S.; Neels, A.; Hauser, A.; Decurtins, S. Ruthenium (II) Coordination Chemistry of a Fused Donor—Acceptor Ligand: Synthesis, Characterization, and Photoinduced Electron-Transfer Reactions of [{Ru(bpy)2}n(TTF-ppb)](PF6)2n (n = 1, 2). Inorg. Chem. 2008, 47, 11010–11017. [Google Scholar] [CrossRef]
- Beddoe, S.V.F.; Fitzpatrick, A.J.; Price, J.R.; Mallo, N.; Beves, J.E.; Morgan, G.G.; Kitchen, J.A.; Keene, T.D. A Bridge Too Far: Testing the Limits of Polypyridyl Ligands in Bridging Soluble Subunits of a Coordination Polymer. Cryst. Growth Des. 2017, 17, 6603–6612. [Google Scholar] [CrossRef]
- Fitzpatrick, A.J.; Trzop, E.; Müller-Bunz, H.; Dîrtu, M.M.; Garcia, Y.; Collet, E.; Morgan, G.G.; Muller-Bunz, H.; Dirtu, M.M.; Garcia, Y.; et al. Electronic vs. Structural Ordering in a Manganese(III) Spin Crossover Complex. Chem. Commun. 2015, 51, 17540–17543. [Google Scholar] [CrossRef]
- Kelly, C.T.; Dunne, S.; Kühne, I.A.; Barker, A.; Esien, K.; Felton, S.; Müller-Bunz, H.; Ortin, Y.; Morgan, G.G. Proton-Induced Spin State Switching in an FeIII Complex. Angew. Chem. 2023, 135, e202217388. [Google Scholar] [CrossRef]
- Gualandi, A.; Calogero, F.; Potenti, S.; Cozzi, P.G. Al(Salen) Metal Complexes in Stereoselective Catalysis. Molecules 2019, 24, 1716. [Google Scholar] [CrossRef] [PubMed]
- Shyu, H.-L.; Wei, H.-H.; Wang, Y. Structure and Magnetic Properties of Dinuclear [Mn(III)(salen)(H2O)]2(ClO4)2 and polynuclear [Mn(III)(salen)(NO3)]n. Inorganica Chim. Acta 1999, 290, 8–13. [Google Scholar] [CrossRef]
- Fitzpatrick, A.J.; Stepanovic, S.; Müller-Bunz, H.; Gruden-Pavlović, M.A.; García-Fernández, P.; Morgan, G.G.; Falvello, L.R.; Kliment, I.K.; Khomskiĭ, D.I.; Rao, C.N.R.; et al. Challenges in Assignment of Orbital Populations in a High Spin Manganese(III) Complex. Dalton Trans. 2016, 45, 6702–6708. [Google Scholar] [CrossRef]
- Ammeter, J.; Buergi, H.B.; Gamp, E.; Meyer-Sandrin, V.; Jensen, W.P. Static and Dynamic Jahn-Teller Distortions in CuN6 Complexes. Crystal Structures and EPR Spectra of Complexes between Copper(II) and Rigid, Tridentate Cis,Cis-1,3,5-Triaminocyclohexane (Tach: Cu(Tach)2(ClO4)2, Cu(Tach)2(NO3)2. Crystal Structure of Ni(Tach)2(NO3)2. Inorg. Chem. 1979, 18, 733–750. [Google Scholar] [CrossRef]
- Persson, I.; Persson, P.; Sandstroem, M.; Ullstroem, A.-S. Structure of Jahn-Teller Distorted Solvated Copper(II) Ions in Solution, and in Solids with Apparently Regular Octahedral Coordination Geometry. J. Chem. Soc. Dalton Trans. 2002, 1256–1265. [Google Scholar] [CrossRef]
- Realista, S.; Fitzpatrick, A.J.; Santos, G.; Ferreira, L.P.; Barroso, S.; Pereira, L.C.J.; Bandeira, N.A.G.; Neugebauer, P.; Hrubý, J.; Morgan, G.G.; et al. A Mn(III) Single Ion Magnet with Tridentate Schiff-Base Ligands. Dalton Trans. 2016, 45, 12301–12307. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, E.S.; Antinarelli, L.M.R.; de A. Crispi, M.; Nogueira, T.C.M.; Pinheiro, A.C.; de Souza, M.V.N. Synthesis, Biological Activity, and Mechanism of Action of 2-Pyrazyl and Pyridylhydrazone Derivatives, New Classes of Antileishmanial Agents. ChemMedChem 2018, 13, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Golding, D.J.L.; Carter, N.; Robinson, D.; Fitzpatrick, A.J. Crystallisation-Induced Emission Enhancement in Zn(II) Schiff Base Complexes with a Tuneable Emission Colour. Sustainability 2020, 12, 9599. [Google Scholar] [CrossRef]
- Tang, J.; Sánchez Costa, J.; Smulders, S.; Molnár, G.; Bousseksou, A.; Teat, S.J.; Li, Y.; van Albada, G.A.; Gamez, P.; Reedijk, J. Two-Step Spin-Transition Iron(III) Compound with a Wide [High Spin-Low Spin] Plateau. Inorg. Chem. 2009, 48, 2128–2135. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. IUCr SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Kelly, C.T.; Griffin, M.; Esien, K.; Felton, S.; Müller-Bunz, H.; Morgan, G.G. Crystallographic Detection of the Spin State in FeIII Complexes. Cryst. Growth Des. 2022, 22, 6429–6439. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Bofill, J.M.; Alvarez, S.; Pinsky, M.; AvnirJerusalem, D.U.d.B. The University of Barcelona. 2003. SHAPE, Version 2.0 2003. Available online: https://www.ee.ub.edu/ (accessed on 1 August 2025).
- Alvarez, S.; Avnir, D.; Llunell, M.; Pinsky, M. Continuous Symmetry Maps and Shape Classification. The Case of Six-Coordinated Metal Compounds. New J. Chem. 2002, 26, 996–1009. [Google Scholar] [CrossRef]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape Maps and Polyhedral Interconversion Paths in Transition Metal Chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
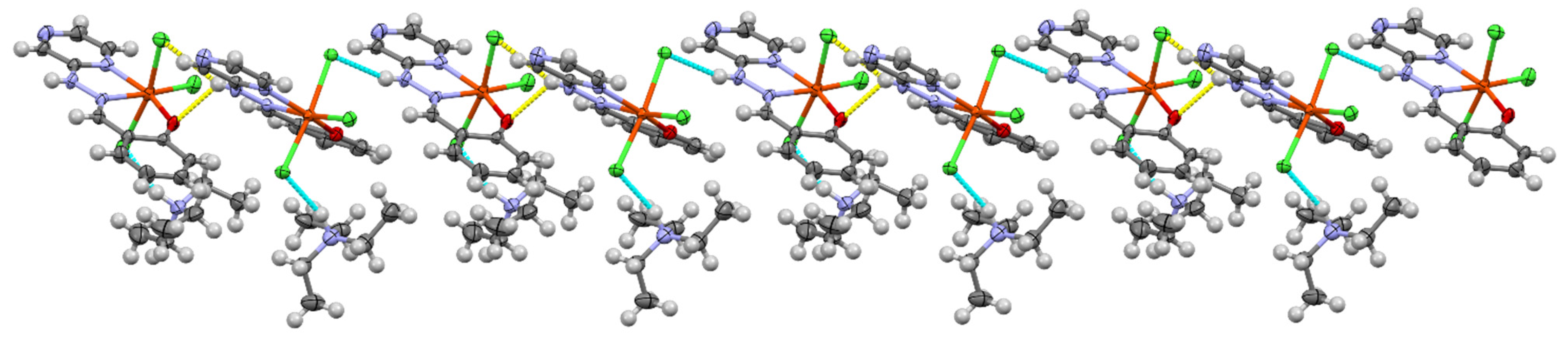
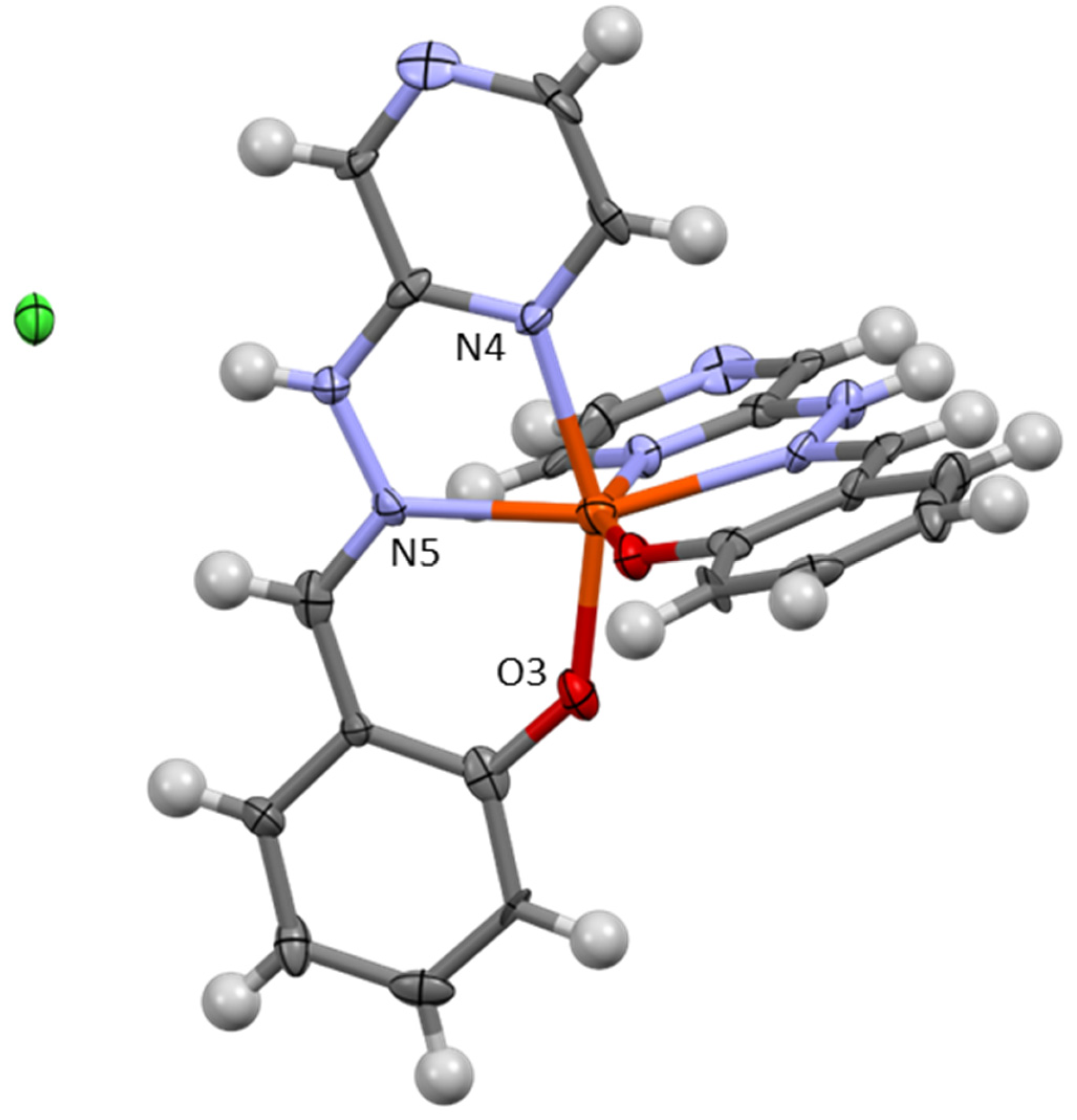
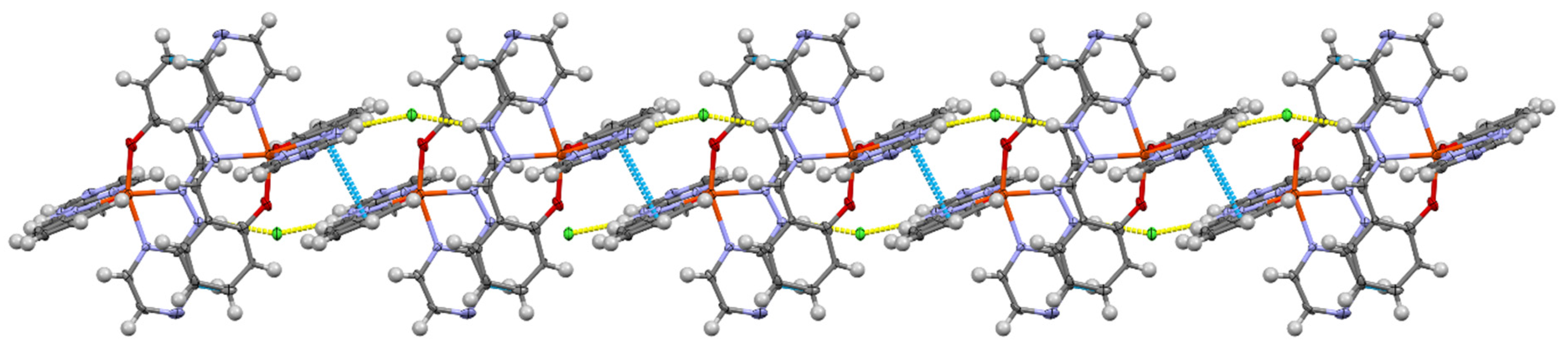
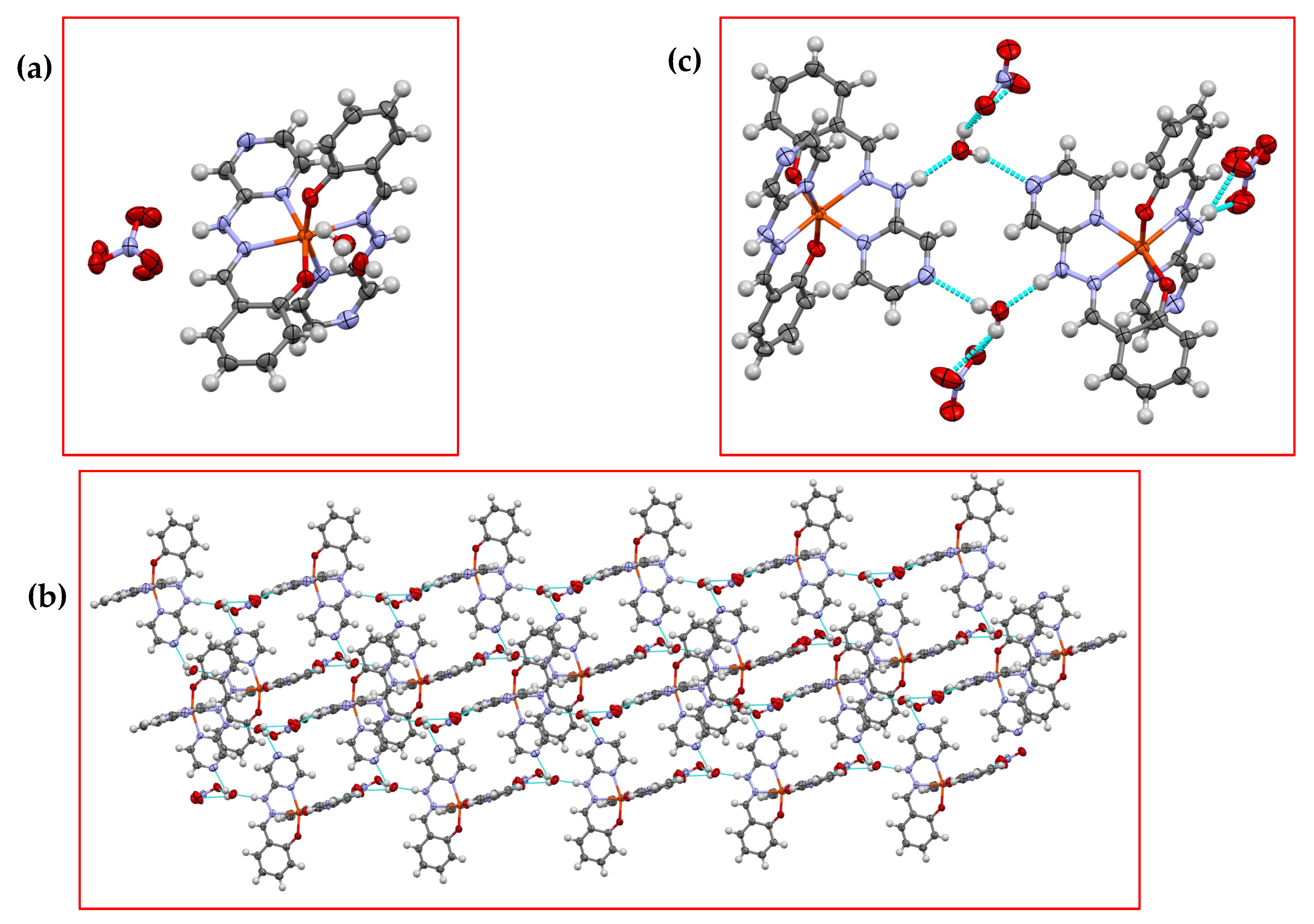
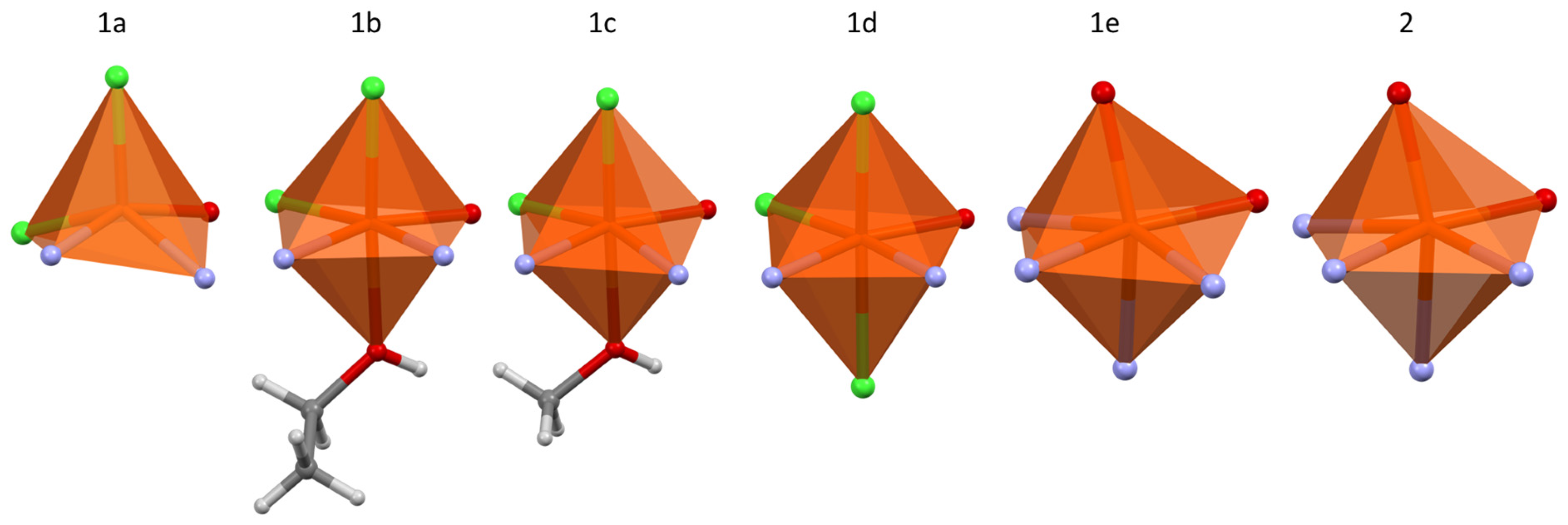
| Complex | CShM (OCT) | % Deviation from Ideal |
|---|---|---|
| 1b | 1.312 | 7.8 |
| 1c | 1.345 | 8.0 |
| 1d | Fe1 1.362 Fe 2 1.281 | 8.0 8.1 |
| 1e | 3.522 | 21.0 |
| 2 | 2.851 | 17.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coughlin, O.; Benjamin, S.L.; Fitzpatrick, A.J. Supramolecular Stabilisation Leads to Challenging Coordination in Fe(III) Hydrazinylpyrazine Schiff Base Complexes. Crystals 2025, 15, 805. https://doi.org/10.3390/cryst15090805
Coughlin O, Benjamin SL, Fitzpatrick AJ. Supramolecular Stabilisation Leads to Challenging Coordination in Fe(III) Hydrazinylpyrazine Schiff Base Complexes. Crystals. 2025; 15(9):805. https://doi.org/10.3390/cryst15090805
Chicago/Turabian StyleCoughlin, Omar, Sophie L. Benjamin, and Anthony J. Fitzpatrick. 2025. "Supramolecular Stabilisation Leads to Challenging Coordination in Fe(III) Hydrazinylpyrazine Schiff Base Complexes" Crystals 15, no. 9: 805. https://doi.org/10.3390/cryst15090805
APA StyleCoughlin, O., Benjamin, S. L., & Fitzpatrick, A. J. (2025). Supramolecular Stabilisation Leads to Challenging Coordination in Fe(III) Hydrazinylpyrazine Schiff Base Complexes. Crystals, 15(9), 805. https://doi.org/10.3390/cryst15090805






