Thienyl-Based Amides of M2 and Neuraminidase Inhibitors: Synthesis, Structural Characterization, and In Vitro Antiviral Activity Against Influenza a Viruses
Abstract
1. Introduction
2. Materials and Methods
2.1. General Remarks
2.2. Boc Protection of Amino Group of Oseltamivir Carboxylate
2.3. Synthesis of Monoamides and Bisamides
General TBTU Coupling Procedure [49,50]
2.4. Characterization Methods
2.4.1. Single-Crystal X-Ray Diffraction (SCXRD)
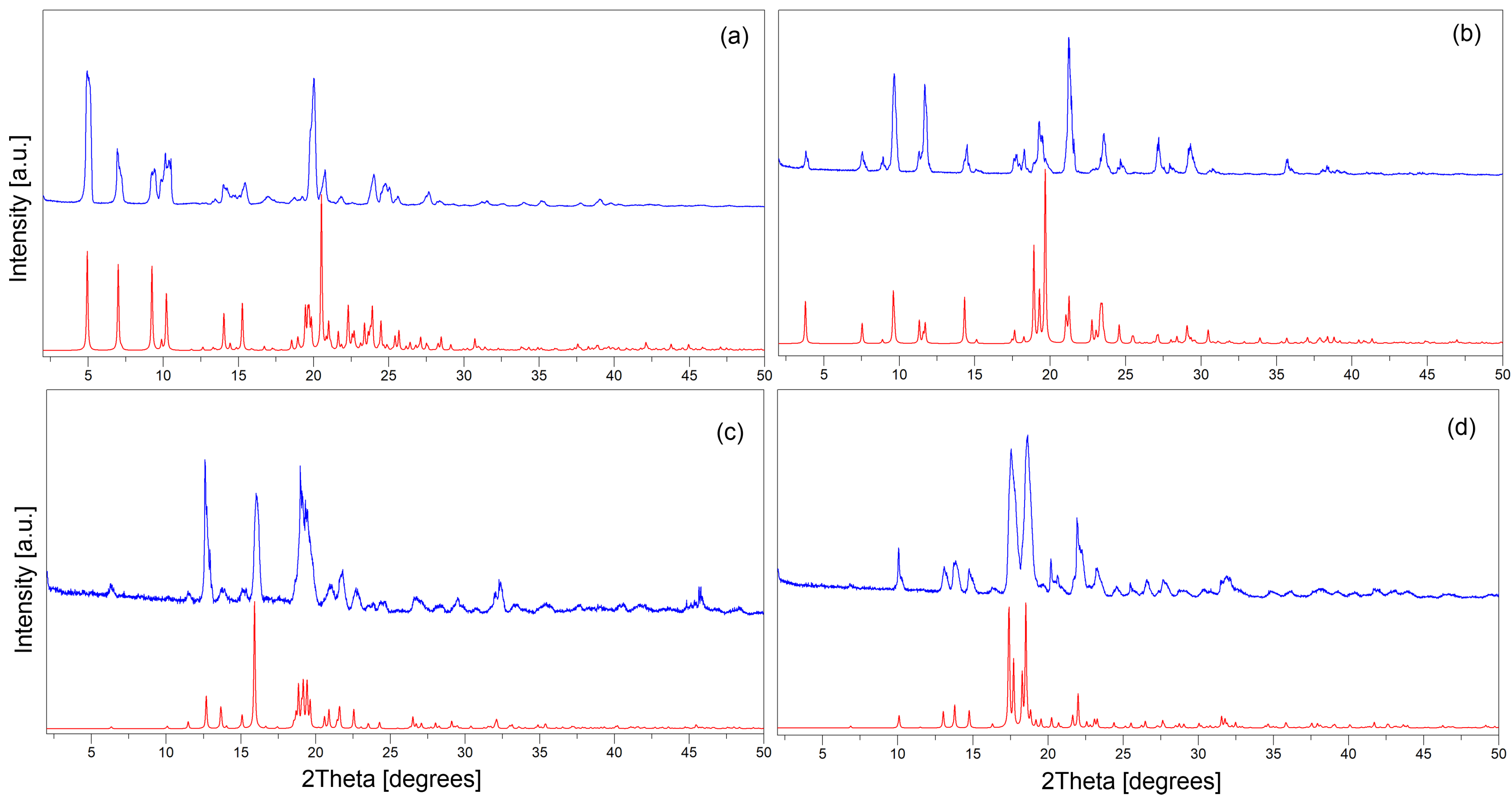
2.4.2. Powder X-Ray Diffraction (PXRD)
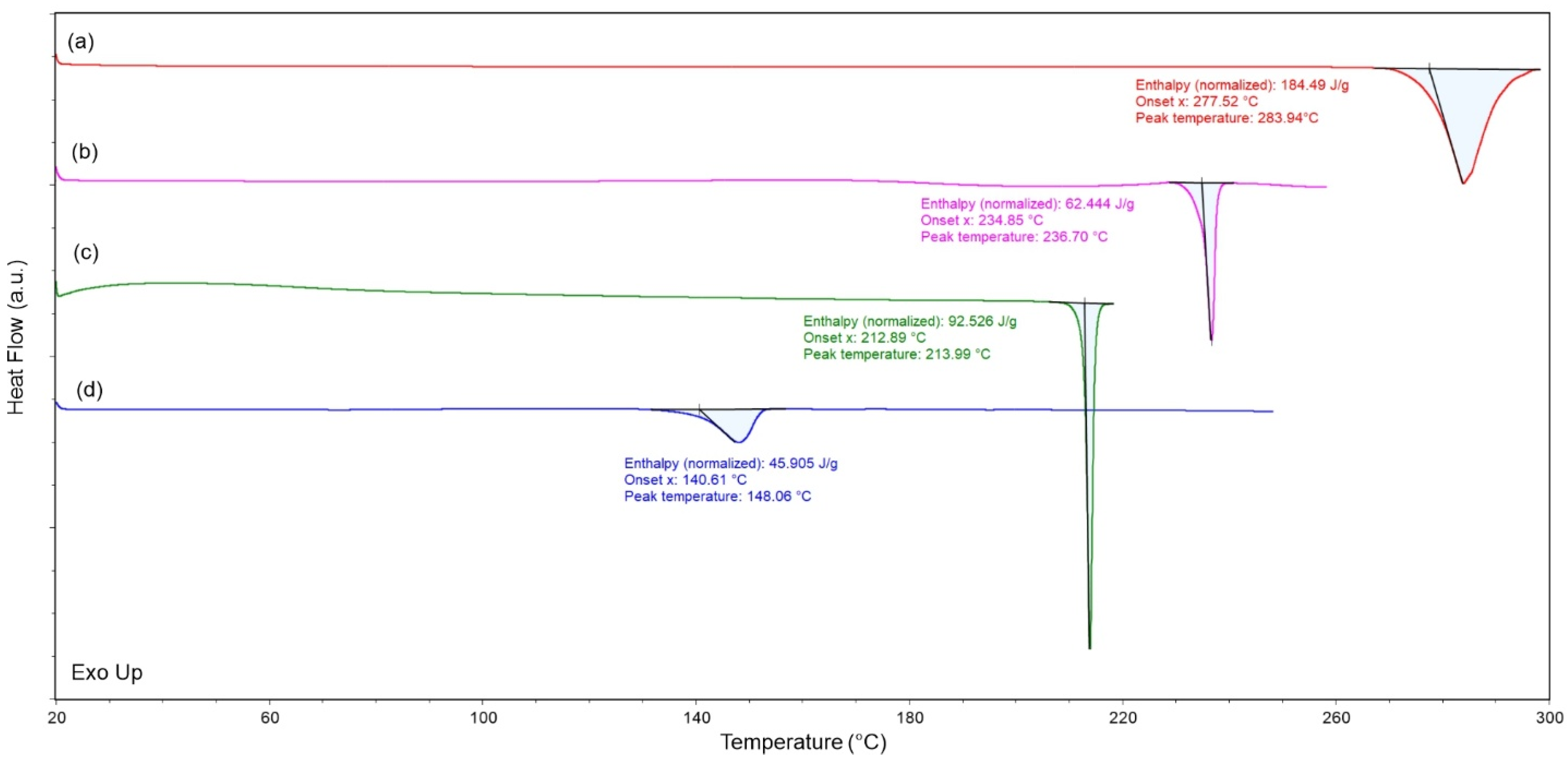
2.4.3. Thermal Analysis (DSC)
2.5. Antiviral Studies In Vitro
2.5.1. Cells and Viruses
2.5.2. Cytotoxicity Assay
2.5.3. Cytopathic Effect (CPE) Reduction Assay
2.6. QSAR/ADMET In Silico Analysis
3. Results
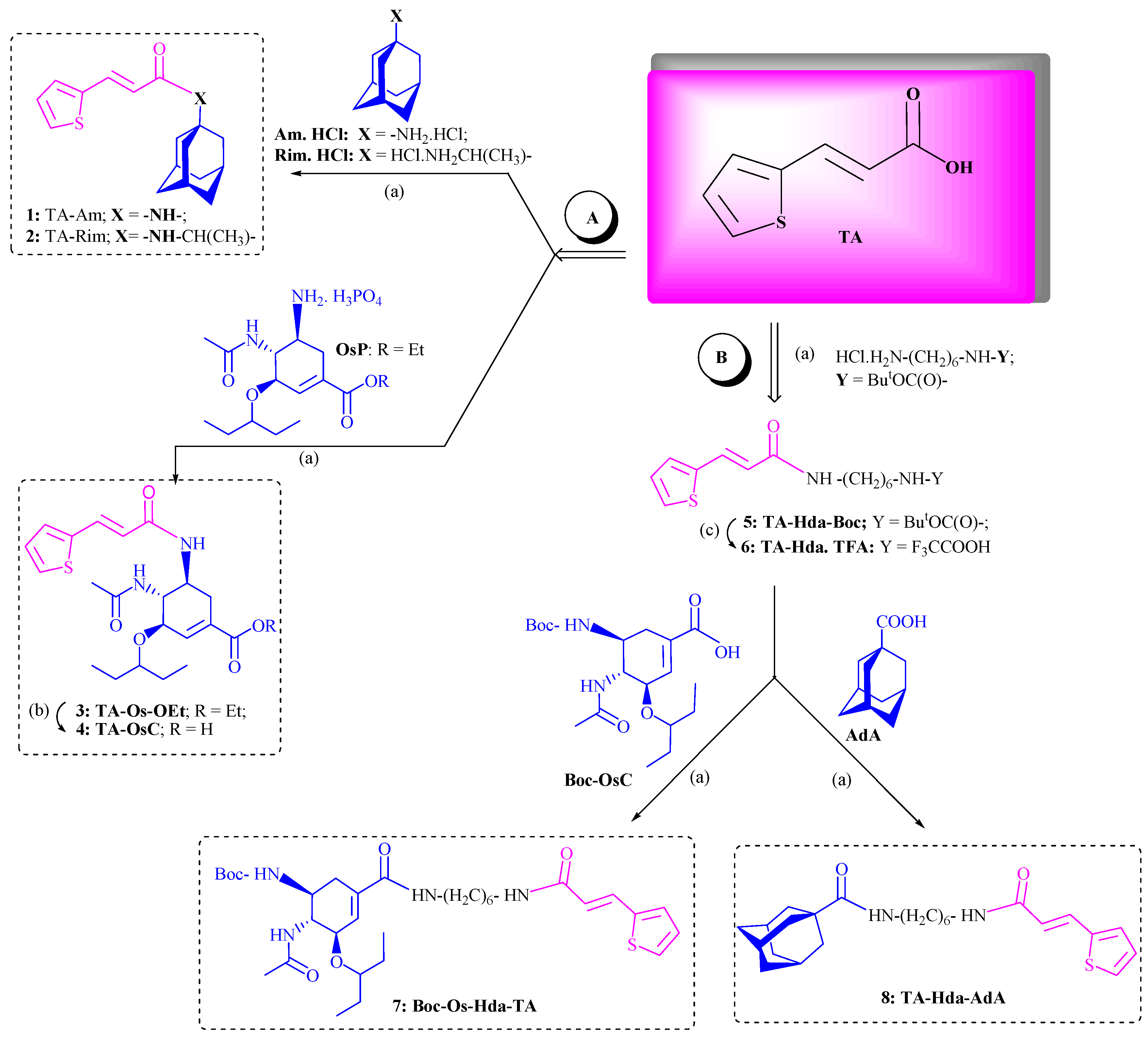
3.1. Chemistry
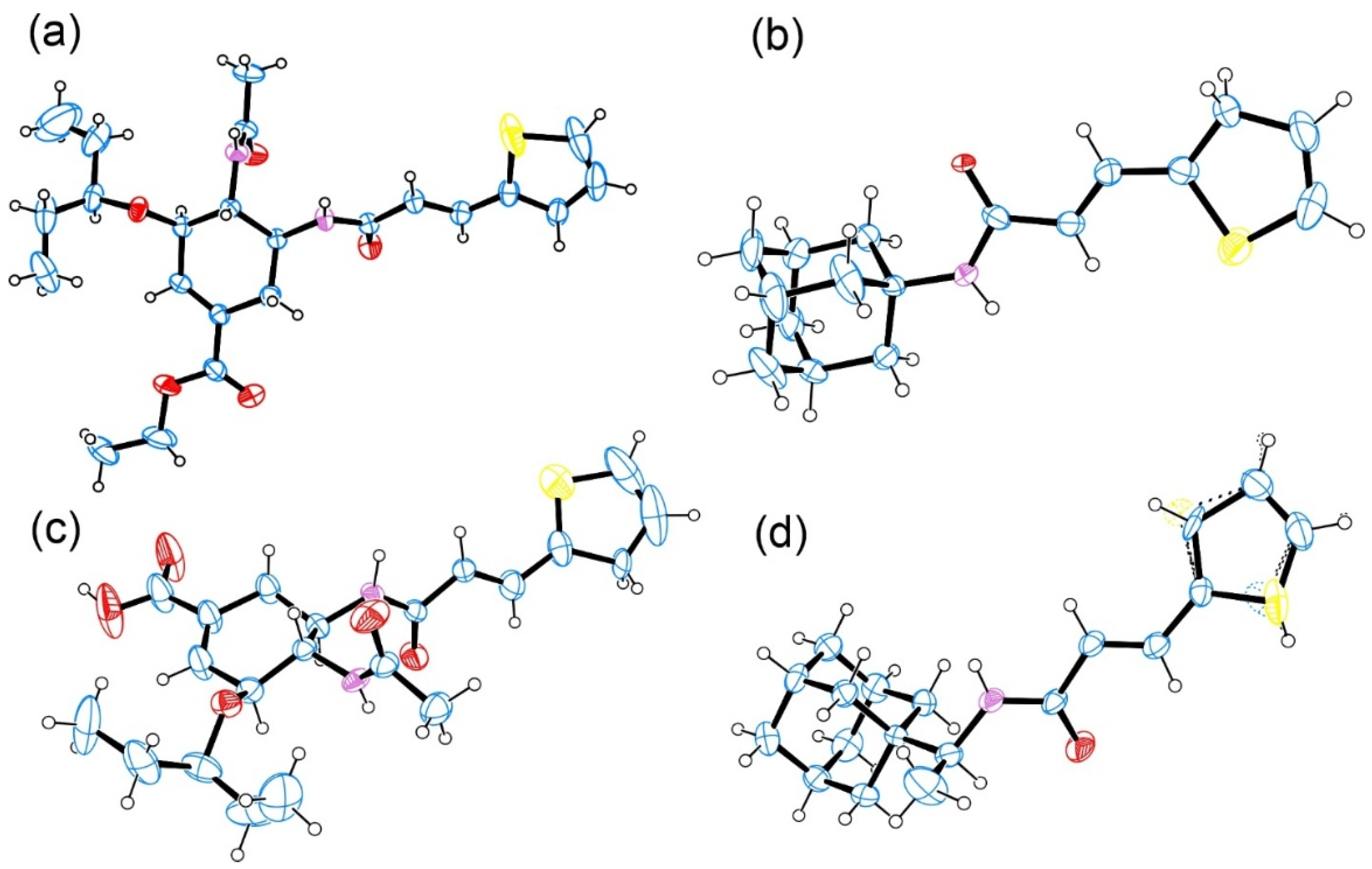
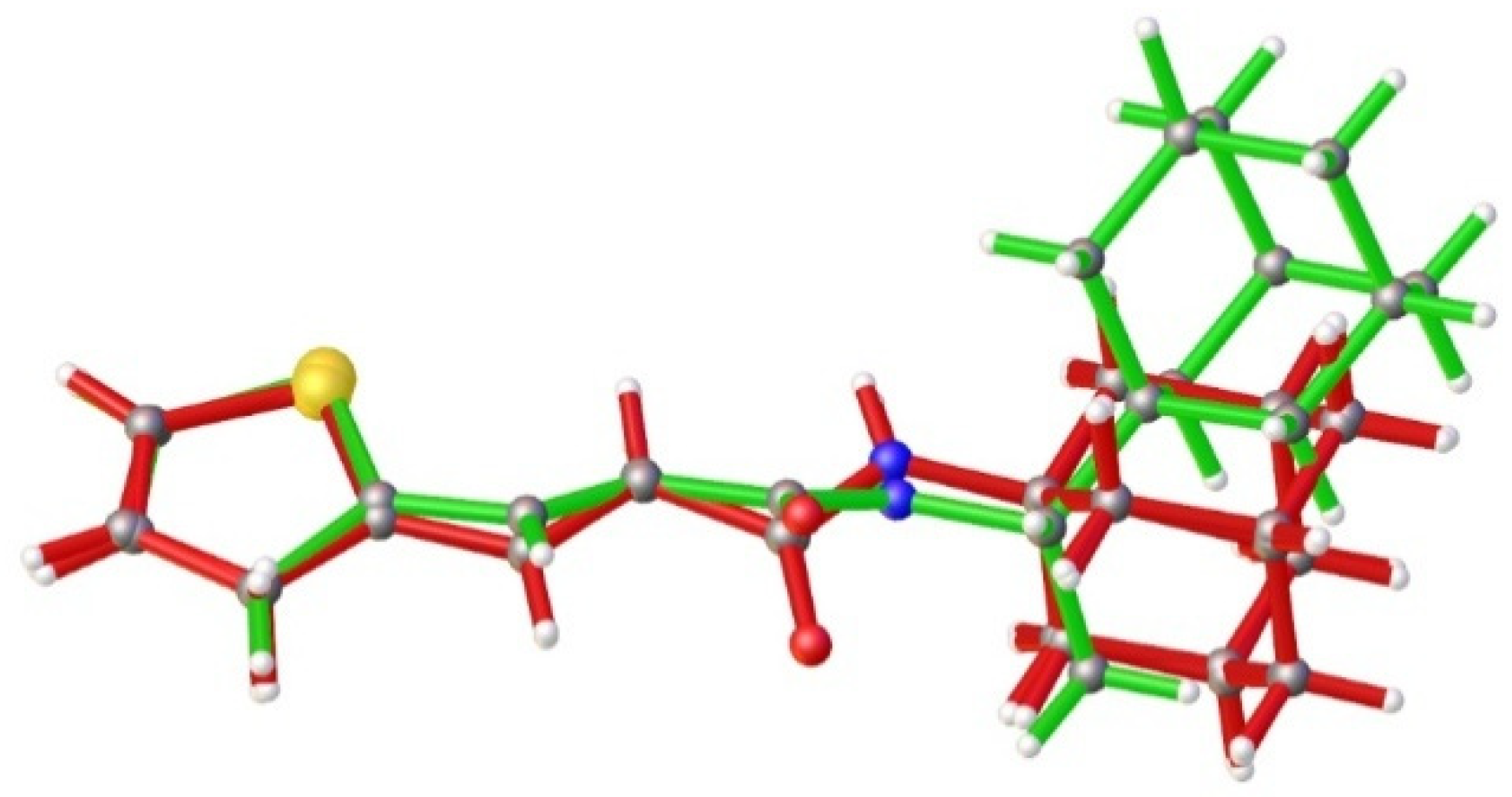
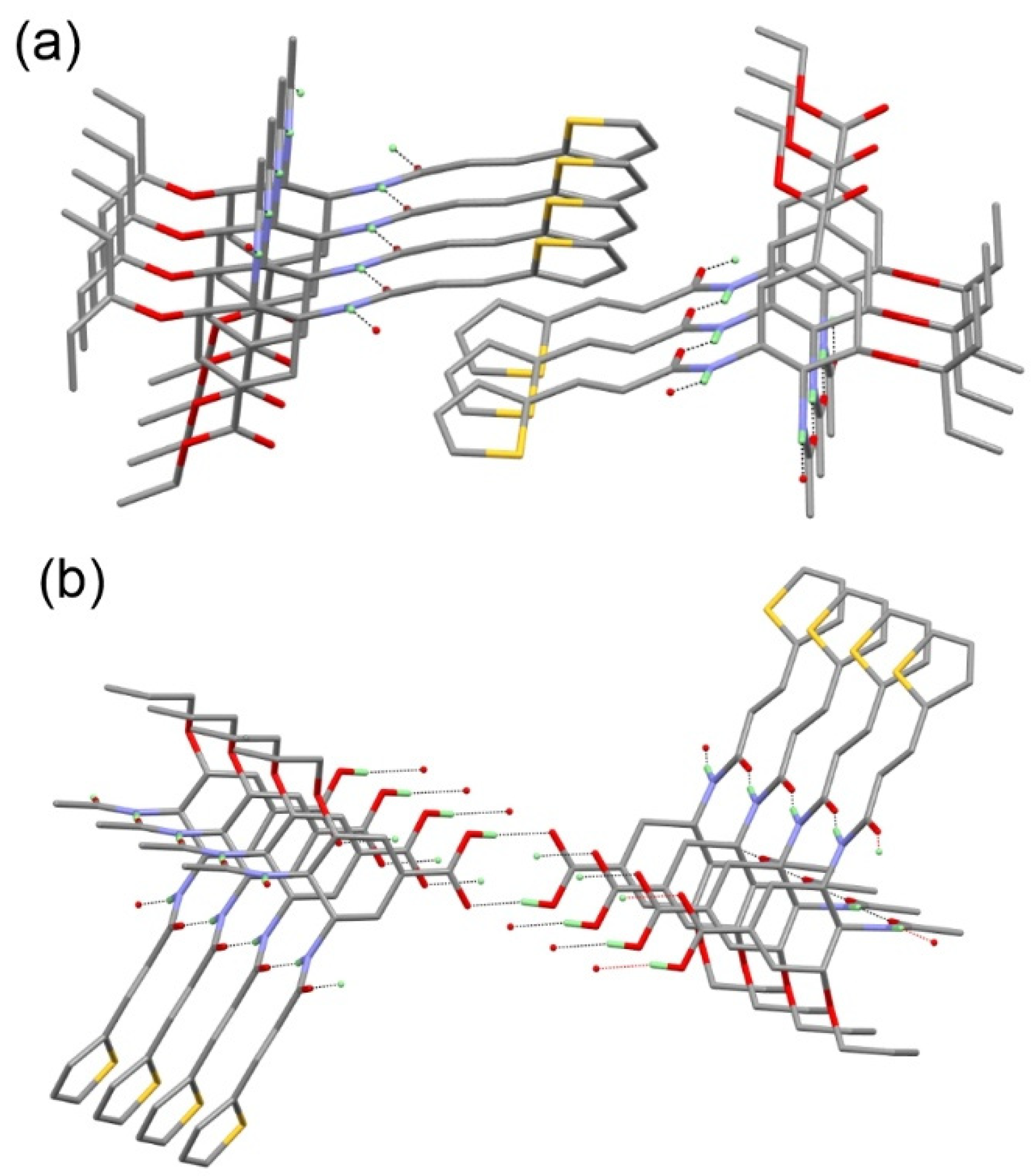
3.2. Crystallography
3.3. Evaluation of Phase Purity and Thermal Stability
3.4. Antiviral Investigation of the Tested Compounds Against Influenza Viruses In Vitro
3.5. QSAR/ADMET Investigation of the Tested Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DMF | Dimethylformamide |
| NAIs | Neuraminidase inhibitors |
| TA | 3-(2-Thienyl) acrylic acid or 3-(2-thienyl) propenoic acid |
| WHO | World Health Organization |
References
- Couch, R.B. Orthomyxoviruses. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; ISBN 978-0-9631172-1-2. [Google Scholar]
- Kumar, B.; Asha, K.; Khanna, M.; Ronsard, L.; Meseko, C.A.; Sanicas, M. The Emerging Influenza Virus Threat: Status and New Prospects for Its Therapy and Control. Arch. Virol. 2018, 163, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Asha, K.; Kumar, B. Emerging Influenza D Virus Threat: What We Know so Far! J. Clin. Med. 2019, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Javelle, E.; Raoult, D. COVID-19 Pandemic More than a Century after the Spanish Flu. Lancet Infect. Dis. 2021, 21, e78. [Google Scholar] [CrossRef]
- Lantis, P.M.; Evans, H.H. Caring for Pandemic Orphans: The Spanish Flu Experience. Pediatrics 2023, 151, e2021054525. [Google Scholar] [CrossRef]
- Ainslie, K.E.C.; Riley, S. Is Annual Vaccination Best? A Modelling Study of Influenza Vaccination Strategies in Children. Vaccine 2022, 40, 2940–2948. [Google Scholar] [CrossRef]
- Valkenburg, S.A.; Poon, L.L.M. Universal Influenza Vaccines Are Futile When Benchmarked against Seasonal Influenza Vaccines. Lancet Infect. Dis. 2022, 22, 750–751. [Google Scholar] [CrossRef]
- Hampson, A.W.; Mackenzie, J.S. The Influenza Viruses. Med. J. Aust. 2006, 185, S39–S43. [Google Scholar] [CrossRef]
- World Health Organization. Influenza (Seasonal). Available online: https://www.who.int/En/News-Room/Fact-Sheets/Detail/Influenza-(Seasonal) (accessed on 16 March 2020).
- Venkatesan, P. Influenza Deaths for the 2022–23 Season. Lancet Respir. Med. 2023, 11, e82. [Google Scholar] [CrossRef]
- WHO. The Burden of Influenza. Available online: https://www.who.int/news-room/feature-stories/detail/the-burden-of-influenza (accessed on 30 March 2024).
- Simonsen, L.; Fukuda, K.; Schonberger, L.B.; Cox, N.J. The Impact of Influenza Epidemics on Hospitalizations. J. Infect. Dis. 2000, 181, 831–837. [Google Scholar] [CrossRef]
- Englund, J.A. Antiviral Therapy of Influenza. Semin. Pediatr. Infect. Dis. 2002, 13, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Vijaykrishna, D.; Smith, G.J.D.; Pybus, O.G.; Zhu, H.; Bhatt, S.; Poon, L.L.M.; Riley, S.; Bahl, J.; Ma, S.K.; Cheung, C.L.; et al. Long-Term Evolution and Transmission Dynamics of Swine Influenza A Virus. Nature 2011, 473, 519–522. [Google Scholar] [CrossRef]
- Smith, G.J.D.; Bahl, J.; Vijaykrishna, D.; Zhang, J.; Poon, L.L.M.; Chen, H.; Webster, R.G.; Peiris, J.S.M.; Guan, Y. Dating the Emergence of Pandemic Influenza Viruses. Proc. Natl. Acad. Sci. USA 2009, 106, 11709–11712. [Google Scholar] [CrossRef]
- Guan, Y.; Vijaykrishna, D.; Bahl, J.; Zhu, H.; Wang, J.; Smith, G.J.D. The Emergence of Pandemic Influenza Viruses. Protein Cell 2010, 1, 9–13. [Google Scholar] [CrossRef]
- Klenk, H.D. Influenza Virology. In Influenza Virus Sialidase—A Drug Discovery Target; Von Itzstein, M., Ed.; Springer: Basel, Switzerland, 2012; pp. 1–29. ISBN 978-3-7643-8926-0. [Google Scholar]
- WHO. COVID-19 Deaths. Available online: https://data.who.int/dashboards/covid19/deaths (accessed on 10 August 2025).
- Mtambo, S.E.; Amoako, D.G.; Somboro, A.M.; Agoni, C.; Lawal, M.M.; Gumede, N.S.; Khan, R.B.; Kumalo, H.M. Influenza Viruses: Harnessing the Crucial Role of the M2 Ion-Channel and Neuraminidase toward Inhibitor Design. Molecules 2021, 26, 880. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Lou, K.; Wang, W. New Small-Molecule Drug Design Strategies for Fighting Resistant Influenza A. Acta Pharm. Sin. B 2015, 5, 419–430. [Google Scholar] [CrossRef]
- Lee, J.K.H.; Lam, G.K.L.; Shin, T.; Samson, S.I.; Greenberg, D.P.; Chit, A. Efficacy and Effectiveness of High-Dose Influenza Vaccine in Older Adults by Circulating Strain and Antigenic Match: An Updated Systematic Review and Meta-Analysis. Vaccine 2021, 39 (Suppl. S1), A24–A35. [Google Scholar] [CrossRef]
- Flannery, B.; Chung, J.R.; Thaker, S.N.; Monto, A.S.; Martin, E.T.; Belongia, E.A.; McLean, H.Q.; Gaglani, M.; Murthy, K.; Zimmerman, R.K.; et al. Interim Estimates of 2016–17 Seasonal Influenza Vaccine Effectiveness—United States, February 2017. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Meseko, C.; Sanicas, M.; Asha, K.; Sulaiman, L.; Kumar, B. Antiviral Options and Therapeutics against Influenza: History, Latest Developments and Future Prospects. Front. Cell. Infect. Microbiol. 2023, 13, 1269344. [Google Scholar] [CrossRef] [PubMed]
- McKimm-Breschkin, J.L. Influenza Neuraminidase Inhibitors: Antiviral Action and Mechanisms of Resistance. Influenza Other Respir. Viruses 2013, 7 (Suppl. S1), 25–36. [Google Scholar] [CrossRef]
- Pinto, L.H.; Lamb, R.A. The M2 Proton Channels of Influenza A and B Viruses. J. Biol. Chem. 2006, 281, 8997–9000. [Google Scholar] [CrossRef]
- Pinto, L.H.; Holsinger, L.J.; Lamb, R.A. Influenza Virus M2 Protein Has Ion Channel Activity. Cell 1992, 69, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Chizhmakov, I.V.; Geraghty, F.M.; Ogden, D.C.; Hayhurst, A.; Antoniou, M.; Hay, A.J. Selective Proton Permeability and pH Regulation of the Influenza Virus M2 Channel Expressed in Mouse Erythroleukaemia Cells. J. Physiol. 1996, 494 Pt 2, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Magid, A.F.; Maryanoff, C.A.; Mehrman, S.J. Synthesis of Influenza Neuraminidase Inhibitors. Curr. Opin. Drug Discov. Devel. 2001, 4, 776–791. [Google Scholar]
- Glanz, V.Y.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Inhibition of Sialidase Activity as a Therapeutic Approach. Drug Des. Devel. Ther. 2018, 12, 3431–3437. [Google Scholar] [CrossRef]
- Moscona, A. Neuraminidase Inhibitors for Influenza. N. Engl. J. Med. 2005, 353, 1363–1373. [Google Scholar] [CrossRef]
- Takashita, E.; Meijer, A.; Lackenby, A.; Gubareva, L.; Rebelo-de-Andrade, H.; Besselaar, T.; Fry, A.; Gregory, V.; Leang, S.-K.; Huang, W.; et al. Global Update on the Susceptibility of Human Influenza Viruses to Neuraminidase Inhibitors, 2013–2014. Antiviral Res. 2015, 117, 27–38. [Google Scholar] [CrossRef]
- Ma, L.-L.; Ge, M.; Wang, H.-Q.; Yin, J.-Q.; Jiang, J.-D.; Li, Y.-H. Antiviral Activities of Several Oral Traditional Chinese Medicines against Influenza Viruses. Evid.-Based Complement. Altern. Med. ECAM 2015, 2015, 367250. [Google Scholar] [CrossRef]
- Mohammed, F.S.; Uysal, İ.; Sevindik, M. A review on antiviral plants effective against different virus types. Prospects Pharm. Sci. 2023, 21, 1–21. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, Y.; Li, P.; Wu, Z.; Li, R.; Cai, J.; Zhang, Y.; Wang, G.; Li, Y.; Tang, W.; et al. Discovery of Cyperenoic Acid as a Potent and Novel Entry Inhibitor of Influenza A Virus. Antivir. Res. 2024, 223, 105822. [Google Scholar] [CrossRef]
- Sevindik, M.; Bal, C.; Eraslan, E.C.; Uysal, İ.; Mohammed, F.S. Medicinal Mushrooms: A Comprehensive Study on Their Antiviral Potential. Prospect. Pharm. Sci. 2023, 21, 42–56. [Google Scholar] [CrossRef]
- Seo, D.J.; Choi, C. Antiviral Bioactive Compounds of Mushrooms and Their Antiviral Mechanisms: A Review. Viruses 2021, 13, 350. [Google Scholar] [CrossRef] [PubMed]
- Bharate, S.B.; Lindsley, C.W. Natural Products Driven Medicinal Chemistry. J. Med. Chem. 2024, 67, 20723–20730. [Google Scholar] [CrossRef] [PubMed]
- Shibnev, V.A.; Garaev, T.M.; Finogenova, M.P.; Shevchenko, E.S.; Burtseva, E.I. New Adamantane Derivatives Can Overcome Resistance of Influenza A(H1N1)Pdm2009 and A(H3N2) Viruses to Remantadine. Bull. Exp. Biol. Med. 2012, 153, 233–235. [Google Scholar] [CrossRef]
- Chayrov, R.; Parisis, N.A.; Chatziathanasiadou, M.V.; Vrontaki, E.; Moschovou, K.; Melagraki, G.; Sbirkova-Dimitrova, H.; Shivachev, B.; Schmidtke, M.; Mitrev, Y.; et al. Synthetic Analogues of Aminoadamantane as Influenza Viral Inhibitors—In Vitro, In Silico and QSAR Studies. Molecules 2020, 25, 3989. [Google Scholar] [CrossRef]
- Stankova, I.; Chuchkov, K.; Chayrov, R.; Mukova, L.; Galabov, A.; Marinkova, D.; Danalev, D. Adamantane Derivatives Containing Thiazole Moiety: Synthesis, Antiviral and Antibacterial Activity. Int. J. Pept. Res. Ther. 2020, 26, 1781–1787. [Google Scholar] [CrossRef]
- Thakur, S.; Kumar, D.; Jaiswal, S.; Goel, K.K.; Rawat, P.; Srivastava, V.; Dhiman, S.; Jadhav, H.R.; Dwivedi, A.R. Medicinal Chemistry-Based Perspectives on Thiophene and Its Derivatives: Exploring Structural Insights to Discover Plausible Druggable Leads. RSC Med. Chem. 2024, 16, 481–510. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Sharma, S.; Kashid, S.; Verma, P.K.; Sapra, A. Therapeutic Potential of Thiophene Compounds: A Mini-Review. Mini Rev. Med. Chem. 2023, 23, 1514–1534. [Google Scholar] [CrossRef]
- Roman, G. Thiophene-Containing Compounds with Antimicrobial Activity. Arch. Pharm. 2022, 355, e2100462. [Google Scholar] [CrossRef]
- Archna; Pathania, S.; Chawla, P.A. Thiophene-Based Derivatives as Anticancer Agents: An Overview on Decade’s Work. Biorgan. Chem. 2020, 101, 104026. [Google Scholar] [CrossRef]
- Mishra, R.; Kumar, N.; Sachan, N. Thiophene and Its Analogs as Prospective Antioxidant Agents: A Retrospective Study. Mini Rev. Med. Chem. 2022, 22, 1420–1437. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, K.; Rong, B.; Wen, Y.; Li, G.; Li, S.; Guo, D.; Zhou, Q.; Liu, S.; Zhang, X. Discovery of Novel Thiophene-Based Baloxavir Derivatives as Potent Cap-Dependent Endonuclease Inhibitors for Influenza Treatment. J. Med. Chem. 2024, 67, 22039–22054. [Google Scholar] [CrossRef]
- Zhong, Z.J.; Hu, X.T.; Cheng, L.P.; Zhang, X.Y.; Zhang, Q.; Zhang, J. Discovery of Novel Thiophene Derivatives as Potent Neuraminidase Inhibitors. Eur. J. Med. Chem. 2021, 225, 113762. [Google Scholar] [CrossRef]
- Howl, J. (Ed.) Peptide Synthesis and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; Volume 298. [Google Scholar]
- Knorr, R.; Trzeciak, A.; Bannwarth, W.; Gillessen, D. New Coupling Reagents in Peptide Chemistry. Tetrahedron Lett. 1989, 30, 1927–1930. [Google Scholar] [CrossRef]
- Chochkova, M.; Rusew, R.; Kalfin, R.; Tancheva, L.; Lazarova, M.; Sbirkova-Dimitrova, H.; Popatanasov, A.; Tasheva, K.; Shivachev, B.; Petek, N.; et al. Synthesis, Molecular Docking, and Neuroprotective Effect of 2-Methylcinnamic Acid Amide in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP)—An Induced Parkinson’s Disease Model. Crystals 2022, 12, 1518. [Google Scholar] [CrossRef]
- Diffraction, Rigaku Oxford. Single crystal diffraction software: CrysAlisPro. Rigaku J. 2016, 32, 31–34. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spek, A.L. Structure Validation in Chemical Crystallography. Acta Crystallogr. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Rajasekaran, D.; Palombo, E.A.; Chia Yeo, T.; Lim Siok Ley, D.; Lee Tu, C.; Malherbe, F.; Grollo, L. Identification of Traditional Medicinal Plant Extracts with Novel Anti-Influenza Activity. PLoS ONE 2013, 8, e79293. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Shi, S.; Yi, J.; Wang, N.; He, Y.; Wu, Z.; Peng, J.; Deng, Y.; Wang, W.; Wu, C.; et al. ADMETlab 3.0: An Updated Comprehensive Online ADMET Prediction Platform Enhanced with Broader Coverage, Improved Performance, API Functionality and Decision Support. Nucleic Acids Res. 2024, 52, W422–W431. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An Integrated Online Platform for Accurate and Comprehensive Predictions of ADMET Properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. admetSAR: A Comprehensive Source and Free Tool for Assessment of Chemical ADMET Properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef]
- Singh, A.; Singh, G.; Bedi, P.M.S. Thiophene Derivatives: A Potent Multitargeted Pharmacological Scaffold. J. Heterocycl. Chem. 2020, 57, 2658–2703. [Google Scholar] [CrossRef]
- Pettit, G.R.; Taylor, S.R. Synthesis of the Marine Sponge Cycloheptapeptide Stylopeptide 11. J. Org. Chem. 1996, 61, 2322–2325. [Google Scholar] [CrossRef]
- Bodansky, M.; Bodansky, A. The Practice of Peptide Synthesis; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1984. [Google Scholar]



| Compounds | TC50 μg/mL | A/Fort Monmouth/1/1947 | A/Jinnan/15/2009 | A/Wuhan/359/1995 | |||
|---|---|---|---|---|---|---|---|
| IC50 (μg/mL) | SI | IC50 (μg/mL) | SI | IC50 (μg/mL) | SI | ||
| TA-Am (1) | 12.84 | 3.56 | 3.6 | 4.79 | 2.7 | 4.79 | 2.7 |
| TA-Rim (2) | 6.17 | 2.06 | 3.0 | 2.06 | 3.0 | 2.06 | 3.0 |
| TA-Os-OEt (3) | 80.12 | >18.52 | - | >18.52 | - | >18.52 | - |
| TA-OsC (4) | >210.26 | NT | NT | 40.47 | >5.2 | ||
| Boc-Os-Hda-TA (7) | >500 | 115.56 | >4.3 | >166.67 | - | 115.56 | >4.3 |
| TA-Hda-AdA (8) | 240.37 | 32.08 | 7.5 | >18.52 | - | 18.52 | 13.0 |
| OsP | 577.35 | 2.47 | 233.7 | 115.47 | 5.0 | 3.12 | 185.0 |
| OsC | 14.22 | NT | NT | 0.034 | 416.74 | ||
| Am.HCl | 39.22 | 0.32 | 122.6 | 2.86 | 13.71 | 2.86 | 13.71 |
| 1 | 2 | 3 | 4 | 7 | 8 | Am | Rim | Os | |
|---|---|---|---|---|---|---|---|---|---|
| Rat oral Acute Toxicity | 0.441 | 0.366 | 0.072 | 0.115 | 0.346 | 0.49 | 0.323 | 0.484 | 0.25 |
| Caco-2 permeability (log values) | −4.979 | −5.313 | −5.234 | −5.369 | −4.811 | −5.435 | −5.073 | −5.038 | −5.545 |
| MDCK permeability | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Human hepatotoxicity | 0.52 | 0.8 | 0.383 | 0.334 | 0.748 | 0.885 | 0.777 | 0.69 | 0.681 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chochkova, M.; Stoykova, B.; Angelova, M.; Sbirkova-Dimitrova, H.; Rusew, R.; Li, Y.; Popatanasov, A.; Petek, N.; Štícha, M.; Shivachev, B. Thienyl-Based Amides of M2 and Neuraminidase Inhibitors: Synthesis, Structural Characterization, and In Vitro Antiviral Activity Against Influenza a Viruses. Crystals 2025, 15, 772. https://doi.org/10.3390/cryst15090772
Chochkova M, Stoykova B, Angelova M, Sbirkova-Dimitrova H, Rusew R, Li Y, Popatanasov A, Petek N, Štícha M, Shivachev B. Thienyl-Based Amides of M2 and Neuraminidase Inhibitors: Synthesis, Structural Characterization, and In Vitro Antiviral Activity Against Influenza a Viruses. Crystals. 2025; 15(9):772. https://doi.org/10.3390/cryst15090772
Chicago/Turabian StyleChochkova, Maya, Boyka Stoykova, Magdalena Angelova, Hristina Sbirkova-Dimitrova, Rusi Rusew, Yuhuan Li, Andrey Popatanasov, Nejc Petek, Martin Štícha, and Boris Shivachev. 2025. "Thienyl-Based Amides of M2 and Neuraminidase Inhibitors: Synthesis, Structural Characterization, and In Vitro Antiviral Activity Against Influenza a Viruses" Crystals 15, no. 9: 772. https://doi.org/10.3390/cryst15090772
APA StyleChochkova, M., Stoykova, B., Angelova, M., Sbirkova-Dimitrova, H., Rusew, R., Li, Y., Popatanasov, A., Petek, N., Štícha, M., & Shivachev, B. (2025). Thienyl-Based Amides of M2 and Neuraminidase Inhibitors: Synthesis, Structural Characterization, and In Vitro Antiviral Activity Against Influenza a Viruses. Crystals, 15(9), 772. https://doi.org/10.3390/cryst15090772










