Abstract
A new copper(II) complex was synthesized using chromone-2-carboxylic acid as the main ligand, and coordinated pyridine molecules. The complex was successfully crystallized and structurally characterized by single crystal X-ray diffraction. This revealed a mononuclear structure with a distorted square pyramidal geometry around the central Cu(II) ion. The coordination sphere comprises oxygen atoms from the chromone moiety and nitrogen atoms from pyridine, resulting in a five-coordinate complex. A comprehensive physicochemical characterization was performed using Fourier transform infrared spectroscopy (FT-IR), UV–Vis spectroscopy, elemental (C, H, N), electrochemical (CV) and thermal analysis (TGA/DSC) to confirm the coordination environment and thermal stability of the compound. The complex exhibits distinct spectroscopic features indicative of ligand–metal charge transfer and d–d transitions typical of Cu(II) species. In addition, the synthesized complex was subjected to antimicrobial screening against Gram-positive and Gram-negative bacteria. The compound showed promising antibacterial activity, particularly against Escherichia coli, indicating its potential as a bioactive coordination compound. These results contribute to the growing body of research on metal-based chromone derivatives and emphasize the importance of copper complexes for the development of new antibacterial agents with defined crystal structures.
1. Introduction
The global rise in antimicrobial resistance (AMR) has led to an urgent need for the development of novel antibacterial agents. AMR represents a serious threat to public health, complicating the treatment of infectious diseases and leading to prolonged hospitalization, more intensive care, and increased mortality. In response to this challenge, transition metal complexes, particularly copper-based ones, have shown significant promise due to their enhanced antibacterial activity when coordinated with organic ligands such as pyridine and chromone derivatives [1]. These metal-organic complexes not only exhibit potent antimicrobial properties but also offer the potential for designing compounds with specific biological activities. Copper, being a transition metal with versatile coordination chemistry, is an ideal candidate for this purpose as it can form stable complexes with various ligands, modulating its electronic properties and biological interactions.
Pyridine (C5H5N), a heterocyclic aromatic compound, plays a central role in coordination chemistry due to nitrogen atom containing a lone pair of electrons. This allows pyridine to act as a highly effective ligand, forming coordination bonds with metal centers. Pyridine is frequently used as a solvent in crystallization and synthesis and as a ligand in metal complex formation [2,3]. The nitrogen atom in pyridine participates in coordination with the metal, stabilizing metal complexes through various interactions such as hydrogen bonding, π–π stacking, and hydrophobic forces. Pyridine’s dual role both as a solvent and ligand enables it to be an essential component of crystalline frameworks, enhancing the stability and bioactivity of metal complexes. This versatility is especially significant in designing metal-based antibacterial agents, as pyridine’s ability to coordinate with metals like copper contributes to the stability and efficacy of these compounds [4].
In copper(II) coordination chemistry, pyridine typically coordinates as a monodentate ligand through the nitrogen atom. Depending on the coordination preferences of the Cu(II) center, pyridine-containing complexes can adopt square-planar or octahedral geometries. For instance, in dimeric complexes such as [Cu2(C6H5COO)4(py)2], the two copper(II) centers are bridged by carboxylate groups, while pyridine coordinates as a terminal monodentate ligand [5,6]. Pyridine’s ability to influence lattice architecture and crystal packing is crucial for the stability and functionality of metal–organic frameworks. Furthermore, pyridine-containing copper(II) complexes have been studied for their antimicrobial properties, often outperforming uncoordinated ligands. These complexes show improved antibacterial activity compared to free pyridine or copper salts [7]. A study by Islam et al. [8] demonstrated that Cu(II)–pyridine complexes significantly inhibited both Gram-positive and Gram-negative bacteria and various fungal strains. Similarly, copper–pyridine–carboxylate compounds have shown inhibition of biofilm formation by Staphylococcus aureus, with minimal inhibitory concentrations (MICs) in the low µg cm−3 range [9]. Heterocyclic carboxylic acids, such as chromone-2-carboxylic acid, have also attracted attention as ligands capable of forming stable complexes with transition metals like copper [10,11]. The chromone scaffold supports delocalized π-electron systems and can chelate metal centers via carboxylate and keto oxygen atoms. While copper complexes with chromone derivatives are less studied compared to pyridine analogs, several reports indicate strong biological activity, including antibacterial and cytotoxic effects [12,13,14,15]. Chromone-based ligands play a crucial role in modulating the electronic properties of metal centers, influencing their redox behavior and interactions with biological targets [16,17].
In this study, we focus on the synthesis and characterization of a novel copper(II) complex with chromone-2-carboxylic acid, crystallized from pyridine solution. Pyridine not only acts as a solvent but also integrates into the crystal lattice, coordinating to the copper(II) center. This integration of pyridine into the structure has significant implications on the stability, redox behavior, and biological activity [18]. Copper(II) complexes with substituted pyridines, such as copper–2,6-diaminopyridine, display strong antimicrobial activity against S. aureus and E. coli [19,20,21,22,23,24]. This suggests significant role of pyridine in the enhancement of the antibacterial properties of copper–chromone complexes.
Although copper–chromone complexes are well known for anticancer and antioxidant effects, their antibacterial potential in pyridine-based frameworks has been less explored. This research investigates the structure–activity relationship of a copper–chromone-2-carboxylate complex with integrated pyridine [25,26,27]. Our work is novel in two key aspects: first, we present a copper(II)–chromone complex in which pyridine plays a dual role, functioning both as a crystallization solvent and as a monodentate ligand coordinating through its nitrogen atom. Second, we examine its antibacterial activity against selected Gram-positive and Gram-negative bacteria, hypothesizing that pyridine stabilizes the copper center and enhances its interaction with bacterial membranes, improving efficacy. We describe the synthesis, structural characterization of the copper–chromone complex, comparing it to benchmark copper–pyridine complexes. Our findings contribute to the field of bioactive crystals, aiming to develop more effective antimicrobial agents.
2. Experimental Section
2.1. Physicochemical Characterization
The synthesized ligand precursors and copper(II) complex were characterized using a combination of elemental analysis, infrared and UV–Vis spectroscopy, thermogravimetric analysis, X-ray diffraction, and electrochemical measurements. Elemental analysis (C, H, N) was conducted using an Elementar vario MACRO cube analyzer (Quantum Analytics, Woodlands, TX, USA). The detection ranges were as follows: carbon (C), 0.002–100%; hydrogen (H), 0.015–100%; nitrogen (N), 0.004–100%. Fourier-transform infrared (FT-IR) spectroscopy was performed using a Shimadzu FTIR 8400S spectrometer (Kyoto, Japan) equipped with a Diffuse Reflectance Spectroscopy (DRS) 8000 accessory. Spectra were recorded in the 4000–400 cm−1 range. Thermal analysis was carried out on a Mettler–Toledo TGA/DSC1 instrument (Greifensee, Switzerland), which simultaneously records thermogravimetric (TGA) and differential scanning calorimetric (DSC) data. Approximately 10–20 mg of each sample was weighed into an alumina crucible (volume: 7.0 × 10−5 dm3) and heated from ambient temperature to 700 °C under an oxygen atmosphere at a constant heating rate of 10 °C min−1. The STARe Software [28] version 10.0 (Mettler Toledo) was used for data acquisition and interpretation. Powder X-ray diffraction (PXRD) patterns were obtained using a Panalytical Aeris diffractometer (Malvern, Worcestershire, UK) employing monochromatized CuKα radiation (λ = 1.5406 Å) operated at 40 kV and 15 mA. Data were collected at 295 K in Bragg–Brentano θ–θ geometry with a step size of 0.02° over a 2θ range of 5–50°. XRDMP version 1.2.0.0 was used for data acquisition, and HighScore Plus version 5.0.109 [29] was employed for data processing.
Electrochemical experiments were performed using a PalmSens potentiostat/galvanostat (PalmSens BV, Utrecht, The Netherlands) operated with PSTrace 4.2 software [30]. A conventional three-electrode configuration was employed, consisting of a glassy carbon working electrode, a platinum wire counter electrode, and an Ag/AgCl reference electrode. Prior to each measurement, the surface of the glassy carbon electrode was polished with α-Al2O3 (0.05 µm, ALS, Tokyo, Japan). Cyclic voltammetry measurements were carried out at scan rates between 100 and 300 mV s−1. Stock solutions (c = 1 × 10−2 mol dm−3) of ligands (L (chromone-2-carboxylic acid) and py (pyridine)), and the corresponding metal complex were prepared in DMSO. Immediately before each measurement, aliquots of the stock solutions were diluted with 0.1 mol dm−3 KNO3 to achieve the desired working concentrations. UV–Vis absorption spectra were recorded using a Shimadzu UV-1900 spectrophotometer (Kyoto, Japan). Measurements were performed in quartz cuvettes with a volume of 2 cm3. The investigated spectral range extended from 350 to 1000 nm. The analyzed samples consisted of aqueous solutions (prepared with ultrapure water) of the copper(II) complex, the corresponding ligands (L (chromone-2-carboxylic acid) and py (pyridine), and the copper(II) salt (S), each at a concentration of 10−2 mol dm−3. Spectra were collected at 24-h intervals over a period of five days to monitor the stability of the complex in solution.
2.2. Synthesis of Pyridine-Integrated Copper(II) Complex, [CuL2(py)3]
All reagents used for the synthesis of the copper(II) complex were of analytical grade and employed without further purification. Copper(II) nitrate trihydrate (99%), ethanol, and sodium hydroxide were obtained from T.T.T. (Zagreb, Croatia). Chromone-2-carboxylic acid (ligand L) and pyridine (py) were purchased from Acros Organics (Waltham, MA, USA). Copper(II) nitrate trihydrate (Cu(NO3)2·3H2O, 0.1208 g; 0.5 mmol) was dissolved in 5 cm3 of high-purity water to yield a clear blue solution. Separately, chromone-2-carboxylic acid (0.095 g; 0.5 mmol) was dissolved in 10 cm3 of warm ethanol. The ethanolic ligand solution was added dropwise to the copper solution under continuous stirring at room temperature. The synthesis of the copper(II) complex was optimized by adjusting the pH to 7.2 using a 0.1 mol dm−3 sodium hydroxide solution to ensure complete complexation of the chromone ligand. A pale blue precipitate formed immediately [31]. The precipitate was filtered off, washed with 5 cm3 of cold ultrapure water, and air-dried to afford the crude product (m = 0.136 g; yield = 63.10%). To obtain crystalline material suitable for structural analysis, a portion of the dried product (0.1 g) was dissolved in 5 cm3 of pyridine. Upon slow evaporation of the solvent at room temperature over several days, deep blue single crystals were formed.
The diffuse reflectance infrared (DRIFT) spectrum of the bulk powder showed characteristic absorption bands (ν/cm−1): 3547 (s), 3473 (w − m), 1629 (s), 1510 (s), 1423 (s), 1346 (s), 680 (w), 511 (m). Elemental analysis of the isolated complex was consistent with the structure determined from single-crystal X-ray diffraction. The calculated values for carbon, hydrogen, and nitrogen content were 47.14%, 3.39%, and 4.29%, respectively, while the experimentally determined values were C (46.38%), H (3.43%) and N (4.25%), confirming the expected composition. The single crystals obtained from pyridine solution were used for X-ray diffraction analysis and revealed the inclusion of pyridine molecules in the crystal lattice.
2.3. Antibacterial Activity
The samples tested consisted of the active compound (copper(II) complex) and non-active substances (ligands (L and pyr) and copper(II) nitrate trihydrate. All were screened for antibacterial activity by disc-diffusion method on Mueller-Hinton agar. On autoclaved filter-paper discs (5 mm) all compounds were added on separate discs in 1 µL or 10 µL volume in two concentrations 1 µM and 1 mM. The test was repeated but with volumes of 2.5 and 5 µL. In both iterations, cultures of Escherichia coli and Staphylococcus aureus were used.
After screening, the antibiotic test was further conducted on all compounds by preparing fresh overnight bacterial culture (24 h) on nutrient agar (Biolife + NaCl). One loopful of culture was suspended by vortexing in 1.5 cm3 of sterile distilled water. 10 µL of bacterial suspension was added to 490 µL of sterile distilled water after which serial dilutions were prepared in water up to 10−3 after which further serial dilutions (450 µL + 50 µL) were made in 1 mM solution of each of the compounds up to 10−8 constantly using vortex between dilutions. The prepared dilutions were left on the bench for approximately 90 min before plating by the pour plate method (450 µL) in Mueller-Hinton agar. The agar was cooled before use, and the agar-sample mixture was thoroughly mixed by swirling. Controls were run in parallel by replacing the compound solution with sterile distilled water.
2.4. Crystallography
Diffraction data were collected on a XtaLAB Synergy, Dualflex, HyPix diffractometer using Cu-Kα radiation (λ = 1.54184 Å). The crystal was kept at 170 (1) K during data collection. The data reduction was performed using the CrysAlis software package (CrysAlis Pro) [32]. The collected diffraction data indicated a significant mismatch with the proposed lattice and peak overlap. Therefore, the data were refined using Ewald explorer, and data reduction was done to obtain an hklf5 file, which was used for the refinement process. The crystal was found to be a non-merohedral twin, and the final refinement resulted in a 0.9295(4): 0.0705(4) twin ratio. Using Olex2 suite [33], the structure was solved with the SHELXT [34] structure solution program using Intrinsic Phasing and refined with the olex2.refine [35] refinement package using Gauss-Newton minimisation. All non-hydrogen atoms were refined anisotropically. Geometrical calculations were done using PLATON [36] and the structure drawings with Olex2 and MERCURY [37] programs. The crystallographic data are summarized in Table 1.

Table 1.
Crystallographic data and structure refinement details for copper(II) complex.
3. Results and Discussion
3.1. Crystal Structure
The complex crystallizes in the monoclinic crystal system, space group P 2/n, Z = 4. The molecular structure of the compound is shown in Figure S1, and selected bond lengths and angles are given in Table 2. The Cu(II) ion is coordinated by 2 monodentate chromone-2-carboxylates and 3 pyridine molecules in the distorted square pyramidal geometry (Figure 1a). 2 chromone ligands and 2 pyridine ligands are located on the equatorial position and symmetry related by a 2-fold regular axis. The axial position is occupied by the third pyridine ligand. The τ5 value (Addison parameter) [38] can be used as a measure of five-coordinate geometry. This value indicates whether the coordination compound displays perfect square pyramidal geometry (τ5 = 0) or trigonal bipyramidal (τ5 = 1). The τ5 value for this compound is 0.23, indicating a distorted square pyramidal geometry. The largest deviation from regular geometry is found for N2−Cu1−N21 (159.46(11)°) angle. The bond lengths and angles are in good agreement with previously reported square pyramidal Cu(II) complexes with carboxylate and pyridine ligands [39,40]. The Cu(II) ion, coordinated and uncoordinated O atoms of the carboxylate group form a chelate-like “active site” suitable for substrate binding (Figure 1b).

Table 2.
Selected interatomic bond distances (Å) and valence angles (°) for the compound.
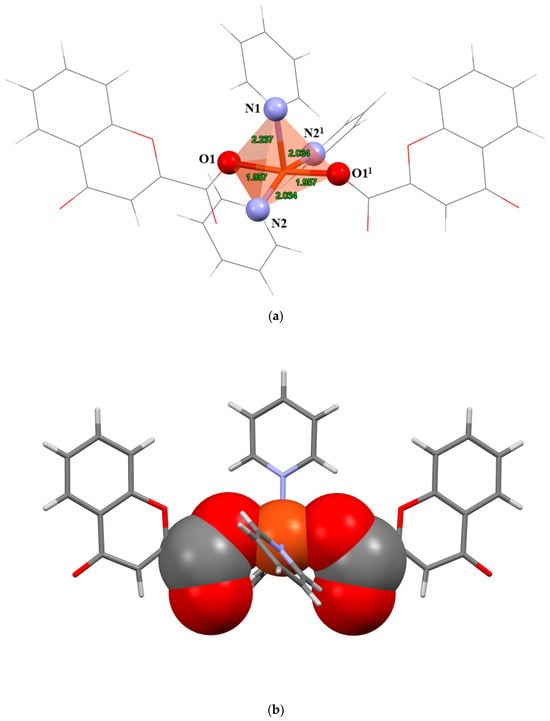
Figure 1.
(a) Coordination geometry of the compound. (b) Representation of chelate-like “active site” (spacefill model atoms). Symmetry operation (1) 3/2-x, +y, 1/2-z.
We were particularly interested in further investigating the structural and physicochemical properties of similar coordination compounds. Therefore, a CSD database [41] search for Cu(II) five-coordinated compounds with monodentately coordinated carboxylate and N-donor ligands was conducted. The search resulted in 48 relevant hits, among which 28 structures display discrete square pyramidal geometry (note: other compounds are either trigonal bypyramidal or polynuclear species). Interestingly, we were able to identify two main types of coordination motifs among these: (i) a chelate-like motif described above (Figure 1b and Figure 2a), and (ii) a non-chelate motif (Figure 2b). The (i) motif is observed only when the carboxylate ligand is bound in the trans position in the equatorial plane. The (ii) motif is observed when the apical position is occupied by one carboxylate ligand. Both of these motifs are almost equally represented in the database (16 compounds displaying (ii) and 12 displaying (i)), and found for different types of N-donor and carboxylate ligands. The compounds displaying (i) motif were previously reported as enantioselective host molecules (ZIRKAO) [42], acetylcholinesterase inhibitor (OTUBIR) [43], and oxygenolysis (LAQROK) [44]. On the other hand, compounds displaying motif (ii) are primarily reported as a part of structural investigations of coordination polymers (HUDHET) [45], and as promising magnetic materials (BAWROH) [46]. Based on structural aspects (steric hindrance of the copper ion), the motif (i) could be more suitable for selective catalysis of small chemical species (like diatomic gases).
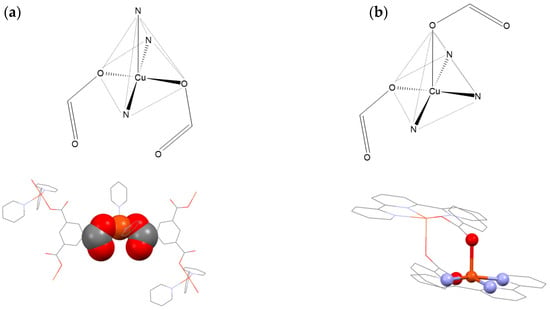
Figure 2.
(a) Representation of motifs among Cu(II) five-coordinated compounds with monodentately coordinated carboxylate and N-donor ligands (figures above): (a) chelate-like motif (e.g., BUQKOP) [47]. (b) non-chelate motif (e.g., KEBHIL) [48] (figures below). BUQKOP and KEBHIL are CSD database Refcodes.
The crystal structure reveals a highly ordered network of interactions between complex molecules, with the copper centers separated by approximately 8.9 Å along the b-axis (far beyond any cuprophilic interactions). In the crystal, the molecules are connected through weak C–H···O, offset π···π, and C−H···π interactions. Adjacent molecules are connected by weak C–H···O interactions (d (C17–H17∙∙∙O2) = 3.105 Å, ∠ (C17–H17∙∙∙O2) = 122.9(2)°) into column-like motifs along b-axis. The adjacent column-like motifs are connected by weak C–H···O (d (C12–H12∙∙∙O4) = 3.117 Å, ∠(C12–H12∙∙∙O4) = 125.5(3)°) and C−H···π (C9–H9∙∙∙Cg3(N2→C15)) interactions into a sheet-like supramolecular assembly approximately along a-axis (Figure 3 and Table 3). The final 3D arrangement of molecules in the crystal is achieved by offset π···π interactions along the a-axis between adjacent benzene moieties of chromone ligands (Figure 3b and Table 3).
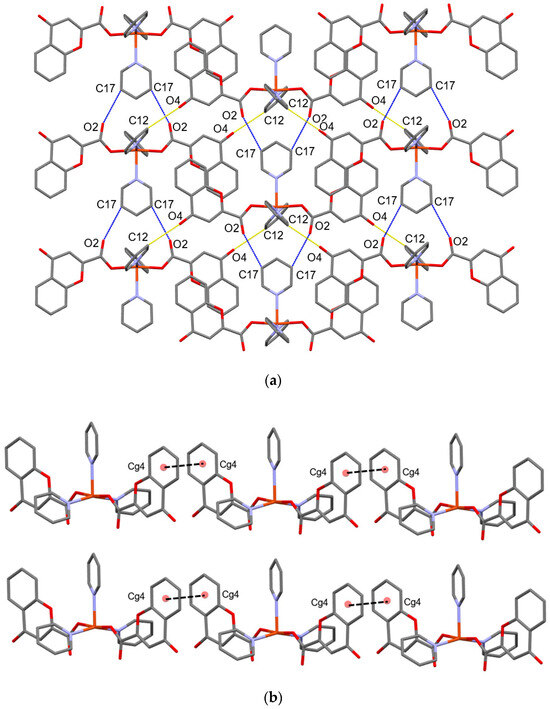
Figure 3.
(a) Representation of sheet-like motif and C–H···O interactions of the compound. Intracolumnar interactions are represented by blue-dashed lines and interactions between adjacent columns by yellow-dashed lines. (b) Representation of offset π···π interactions (black-dashed lines) along a-axis.

Table 3.
Hydrogen bond geometry (Å, °), C–H···π and π∙∙∙π interactions for compound.
These findings contribute to a better understanding of metal-ligand interactions in copper complexes, particularly those involving aromatic and heterocyclic ligands, which are critical for designing bioactive coordination compounds. The arrangement of ligands around the metal ion suggests potential applications in catalysis, drug design, and material science, where the combination of organic ligands and metal centers can impart unique properties. In drug design, such coordination compounds can be optimized for specific interactions with biological targets, potentially enhancing therapeutic efficacy [49,50].
3.2. FT-IR Spectroscopy
The IR spectrum (Figure 4) of the copper(II) complex with pyridine and chromone-2-carboxylic acid (Figure S2), as well as the copper(II) nitrate trihydrate (Figure S3), shows key characteristics that provide insight into the structure and bonding interactions of the complex. A prominent peak near 1700 cm−1 corresponds to the C=O stretching vibrations of the carboxyl group in chromone-2-carboxylic acid, confirming its coordination to the copper center. The frequency shift of the C=O stretch can be attributed to the metal-ligand interaction, consistent with observations in other carboxylate complexes [51]. The peak in the 1510 cm−1 region corresponds to aromatic C=C stretching vibrations within the pyridine ring, confirming the presence of pyridine in the coordination sphere of the copper ion. This is in agreement with literature observations of pyridine-based complexes [52]. A C–N stretching vibration observed at 1495 cm−1 is typical for pyridine ligands. The nitrogen atom in pyridine participates in coordination with the metal, and this band is consistent with C–N bond vibrations in pyridine-containing metal complexes [52]. In the region below 1000 cm−1, bands corresponding to metal-ligand interactions, particularly Cu–N and Cu–O vibrations, are observed. Although weaker, these bands are critical for identifying the coordination between the copper ion and the ligands, supporting the coordination of copper to the nitrogen atoms of pyridine and the oxygen atoms of the carboxylate groups [52]. The difference between the asymmetric (νasym = 1600 cm−1) and symmetric (νsym = 1346 cm−1) stretching vibrations of the carboxylate group (Δν) observed at approximately 254 cm−1 further confirms monodentate coordination of the copper ion to the oxygen atom of the chromone-2-carboxylate [53]. This Δν value, typical for monodentate complexes, supports the monodentate binding of the copper ion to a single oxygen atom in the carboxylate group. Finally, aromatic C–H stretching vibrations are detected in the 3000–3100 cm−1 region, confirming the presence of aromatic ligands, consistent with the IR spectra of aromatic coordination compounds [54]. The IR spectrum of the copper complex with pyridine and chromone-2-carboxylic acid ligands indicates the formation of the complex, highlighting key vibrations and metal-ligand interactions that provide insight into its structural characteristics.
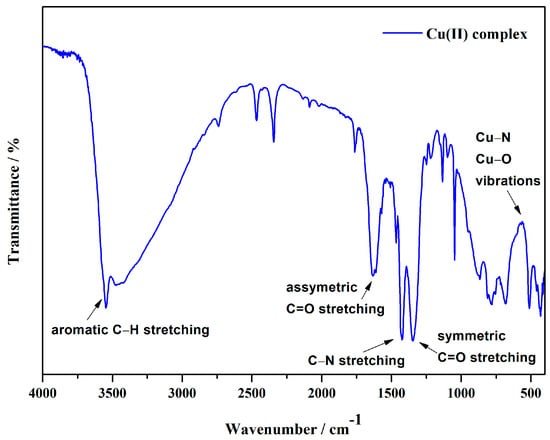
Figure 4.
IR spectrum of a copper(II) complex.
3.3. Thermal Analysis
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were used to evaluate the thermal stability of the copper(II) complex (Figure 5). This exothermic event corresponds to the thermal degradation of the organic ligands, especially the chromone-2-carboxylate and any pyridine residues. A further mass loss observed between 340 °C and 480 °C indicates the decomposition of the remaining organic components, which probably involves the degradation of the chromone-2-carboxylate ligand and other organic residues in the complex. The DSC curve confirms these results, with exothermic peaks at lower temperatures followed by a stabilisation phase at higher temperatures, reflecting the resistance of the complex to thermal degradation once the pyridine ligands have been removed. Using the TGA data, the composition of the complex was determined to be 9.33% copper, 34.8% pyridine and 55.9% chromone-2-carboxylate. These values agree well with the values calculated from the crystal structure (9.5% copper), which confirms the consistency between experimental and theoretical data. A comparison of the ligand contents determined by TGA analysis with the values derived from the crystal structure shows satisfactory agreement. The pyridine content determined by TGA analysis (34.8%) is slightly higher than the value calculated from the crystal structure (33.3%). The chromone-2-carboxylate content from the TGA analysis (55.9%) also agrees well with the value derived from the crystal structure (56.4%). These results indicate that the experimental data agree well with the theoretical ligand distribution derived from the crystal structure, despite minor discrepancies inherent in the TGA method, which are likely due to factors such as slow outgassing or limitations in the balance accuracy.
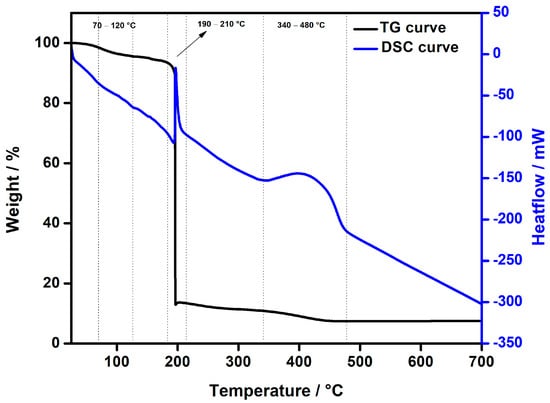
Figure 5.
TG/DSC curves of Cu(II) complex heated in an oxygen atmosphere to 700 °C at a rate of 10 °C min−1.
3.4. Cyclic Voltammetry
Cyclic voltammetry was employed to analyze the electrochemical properties of the investigated compounds. Figure 6 presents the cyclic voltammograms of Cu(NO3)2·3H2O (Figure 6a), ligand L (chromone-2-carboxylic acid) (Figure 6b), pyridine (Figure 6c), and the complex (Figure 6d). The voltammogram of copper(II) nitrate trihydrate (Figure 6a) shows two oxidation peaks: the first at potential Ep,a,1 = 0.20 V (A1), which correspons to the oxidation of copper from Cu0 to Cu+, and the second at the potential Ep,a,2 = 0.30 V (A2), which corresponds to the oxidation of Cu+ to Cu2+. Two reduction peaks are observed at potentials Ep,c,1 = −0.05 V (C1), representing the reduction of Cu2+ to Cu+, and Ep,c,2 = −0.31 V (C2), indicating the reduction of Cu+ to Cu0 [55]. The cyclic voltammograms of ligand L (Figure 6b) and pyridine (Figure 6c) did not display any oxidation or reduction peaks. The voltammogram of the complex (Figure 6d) shows a single oxidation peak (A1) at the potential Ep,a,1 = 0.13 V, which corresponds to the oxidation of copper within the complex. Two reduction peaks are observed at potentials Ep,c,1 = −0.13 V, corresponding to the reduction of Cu2+ to Cu+, and Ep,c,2 = −0.41 V, corresponding to the reduction of Cu+ to Cu0 within the complex. The shift of oxidation and reduction peaks of copper in a complex, compared to oxidation and reduction peaks of copper salt is detected, which is caused by the interaction of copper with the ligand in a complex [55]. These results indicate the presence of Cu2+ cations in the complex.
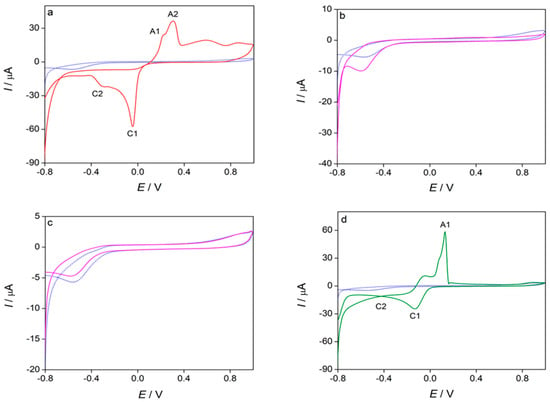
Figure 6.
Cyclic voltammograms of blank solution and investigated compounds (c = 1 × 10−3 mol dm−3): (a) Cu(NO3)2·3H2O, (b) ligand L (chromone-2-carboxylic acid), (c) pyridine and (d) complex, recorded at scan rate 100 mV/s (n = 3) in 0.1 mol dm−3 KNO3.
The effect of scan rate on the oxidation peak current and the oxidation peak potential of the investigated complex was analyzed in Figure 7. It was observed that the maximum current of the oxidation peak (A1) decreased as the scan rate increased, which may be due to complex adsorption on the surface of the glassy carbon electrode. Meanwhile, the oxidation peak potential shifted to higher values with increasing scan rate, potentially indicating slower electron transfer kinetics [56]. The peak currents of the two reduction peaks (C1 and C2) grew with higher scan rates, while their reduction peak potentials remained unchanged (Figure 7a). The anodic peak current (Ip,a) for the A1 peak in the complex showed a linear relationship with the square root of the scan rate (v1/2) (R2 = 0.8837) (Figure 7b), suggesting that the copper oxidation process in the complex is controlled by diffusion. The somewhat lower R2 value (R2 = 0.8837)), may indicate that the relationship between Ip,a and v1/2 is not perfectly linear, which could imply that the oxidation process is not purely diffusion-controlled but rather more complex process. The combined adsorption–diffusion control is shown in Figure 7c, where a linear relationship was observed between the logarithm of the anodic peak current (log Ip,a) and the logarithm of the scan rate (log v), with a slope approximately equal to 0.6 [57].
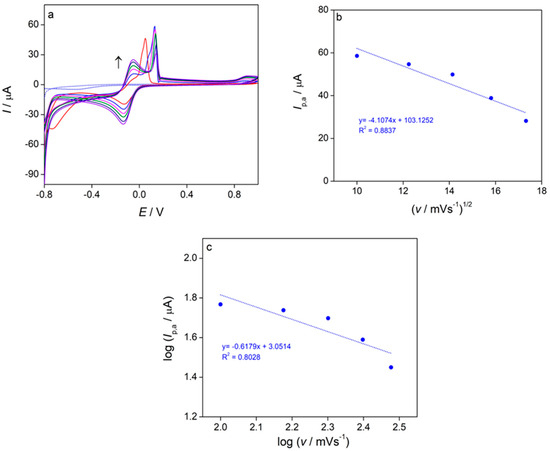
Figure 7.
(a) Cyclic voltammograms of the complex (c = 1 × 10−3 mol dm−3, Ic = 0.1 mol dm−3 KNO3) recorded at scan rates from 100–300 mV/s, (b) the anodic peak current (Ip,a) as a function of the square root of scan rate (v1/2) for the complex, (c) logarithm of the anodic peak current (log Ip,a) as a function of logarithm of scan rate (log v).
The first cathodic peak (C1), which corresponds to the reduction of Cu2+ to Cu+, is diffusion-controlled. This is confirmed by two key observations: the cathodic peak current (Ip,c) is a linear function of the square root of the scan rate (v1/2) with a high correlation coefficient (R2 = 0.9957) (Figure 8a), and the slope of the log Ip,c vs. log v plot is approximately 0.5 (Figure 8b) [57].
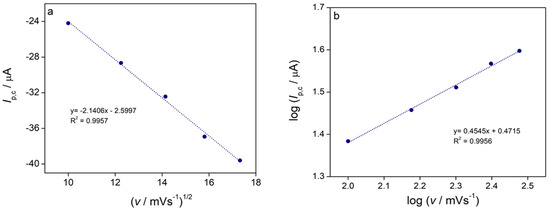
Figure 8.
(a) The cathodic peak current (Ip,c) of the first reduction peak C1 as a function of the square root of scan rate (v1/2) for the complex, (b) logarithm of cathodic peak current (log Ip,c) as a function of the logarithm of scan rate (log v).
The second cathodic peak (C2) showed that the reduction of Cu+ to Cu0 in the complex is diffusion-controlled process. The linear relationship was observed between the cathodic peak current (Ip,c) and the square root of scan rate (v1/2) (R2 = 0.9988) (Figure 9a) and the slope of the line plotting the logarithm of the cathodic peak current (log Ip,c) vs. the logarithm of the scan rate (log v) was approximately 0.5 (as shown in Figure 9b) [57].
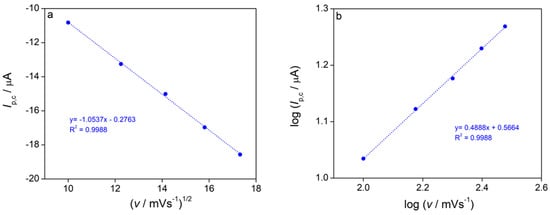
Figure 9.
(a) The cathodic peak current (Ip,c) of the second reduction peak C2 as a function of the square root of scan rate (v1/2) for the complex, (b) logarithm of cathodic peak current (log Ip,c) as a function of the logarithm of scan rate (log v).
The results of cyclic voltammetry have shown that in copper complex, one oxidation peak (A1) is observed, which corresponds to the oxidation of copper in the complex, and two reduction peaks, C1 and C2, which correspond to the reduction of Cu2+ to Cu+, and Cu+ to Cu0 in the complex, respectively. The oxidation of copper in the complex is under mixed adsorption-diffusion control, while the reduction of copper is a diffusion-controlled process. The obtained results indicate the presence of Cu2+ cation in the complex which enhances its stability and could enhance the antibacterial activity [58,59] of the complex. Since copper is in the Cu2+ state in this complex, it can undergo redox transitions, making the complex suitable for applications such as catalysis in various organic reactions [60] (e.g., serving as a homogeneous catalyst in oxidation [61], reduction, and epoxidation [62] processes), detection of biologically significant organic anions [63] and biomolecules [64], as well as enhancement of anticancer activity [65,66].
3.5. UV-Vis Spectroscopy
The UV-Vis spectra (Figure 10) provided valuable insights into the formation and stability of the copper(II) chromone-2-carboxylate-pyridine complex. In Figure 10, the UV–Vis absorption spectra of the individual components (S: copper(II) nitrate trihydrate, L: chromone-2-carboxylic acid, Py: pyridine) are compared with that of the resulting copper(II) complex. The spectrum of the complex (magenta line) exhibits pronounced differences relative to the free components, including distinct absorption maxima at around 700 nm. These spectral changes are indicative of coordination between the metal center and the ligands. The observed shifts indicate the successful formation of the copper(II) coordination complex, in which pyridine and chromone-2-carboxylate are bound to the copper(II) ion [67]. The inset in Figure 10 presents the UV–Vis spectra of the copper(II) complex recorded at different time intervals (0 h, 24 h, 48 h, 72 h, and 96 h). The spectra exhibit minimal changes in absorption characteristics over the five-day period. At 0 h, the complex displays an absorption maximum at 700 nm, and spectra recorded at subsequent time points (24 h, 48 h, 72 h, and 96 h) show almost identical peak positions and intensities. The negligible variations observed suggest that the complex maintains its structural integrity and stability in aqueous solution, with no significant degradation or ligand displacement occurring during prolonged exposure. This remarkable stability is crucial for potential biological applications, as it ensures the complex remains effective in in vitro and in vivo studies conducted in aqueous media [68].
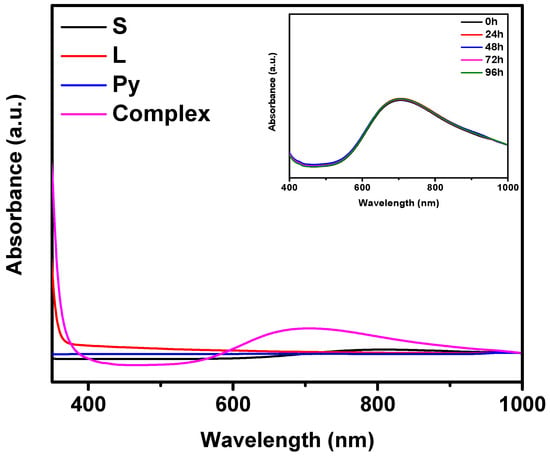
Figure 10.
UV-Vis absorption spectra of the individual components (S: copper(II) nitrate trihydrate, L: chromone-2-carboxylic acid, Py: pyridine) and the resulting copper(II) complex, with all components at a concentration of 10−2 mol dm−3, recorded over time. The inset shows the stability of the complex in aqueous solution at different time intervals (0 h, 24 h, 48 h, 72 h, and 96 h). Characteristic absorption maxima for the complex occur at 700 nm.
In comparison to other studies on copper(II) complexes with chromone or related ligands, where instability or significant changes in spectral properties have been reported upon contact with water or biological fluids [69], the copper(II) complex in this study demonstrates an encouraging degree of stability. This enhanced stability is consistent with previous findings that pyridine can play a crucial role in stabilizing copper(II) complexes in aqueous environments. Pyridine acts as a monodentate coordinating ligand, binding to the copper(II) center and enhancing the ligand field, which contributes to the overall stability of the complex [70,71]. This coordination reduces the lability of the metal center, limiting unwanted dissociation or degradation over time. The observed stability and unique spectral properties of the complex are therefore consistent with its potential for biological applications. Copper(II) complexes with carboxylate-based ligands, including chromone derivatives, are known for their biological activity, such as antimicrobial or anticancer effects [69]. The stability of the complex in aqueous media suggests that it could retain its bioactivity over extended periods, making it a suitable candidate for further biological testing. Furthermore, the minimal changes in UV-Vis spectra over time indicate that the complex is likely to exhibit consistent behavior in biological assays, which is a significant advantage for its potential therapeutic applications. UV-Vis analysis sugests the successful formation of the copper(II) chromone-2-carboxylate pyridine complex and its stability in aqueous solution. This stability, combined with the favorable coordination chemistry, paves the way for further studies, including biological testing and detailed characterization, to comprehensively evaluate the therapeutic potential of the complex [68].
3.6. X-Ray Powder Difraction
Accurate identification and structural characterization of the sample are essential for ensuring the reliability of biological testing. In this study, a comparative analysis was performed between the experimental powder X-ray diffraction (PXRD) pattern and the simulated XRD pattern of a monocrystal to confirm their structural equivalence (Figure 11). The highly similar peak positions between the two patterns suggest that the powder sample shares the same crystal structure as the monocrystal. The experimental diffraction (black line) shows broader peaks, typical of polycrystalline materials, while the simulated diffraction (red line) exhibits sharper peaks, characteristic of ideal single crystal diffraction. Despite differences in peak sharpness, the congruence in peak positions confirms structural identity between the two forms of the compound. This structural confirmation is vital for biological assays. If the powder sample were structurally different, such as showing polymorphism or phase mixtures, it could introduce uncertainty regarding the biological activity observed. Polymorphs of the same compound may exhibit differences in molecular packing, solubility, and stability, potentially altering biological properties [72]. Therefore, confirming the powder’s structural equivalence to the monocrystal ensures that the biological effects are reliably attributed to the intended compound. Furthermore, this analysis rules out contamination or structural heterogeneity, which could affect the biological results. The structural consistency is crucial in bioinorganic research, particularly in correlating a compound’s molecular structure with its biological activity [73]. By confirming that the powder is identical to the monocrystal, we ensure robust and reproducible results, which are critical for the development of therapeutic agents.
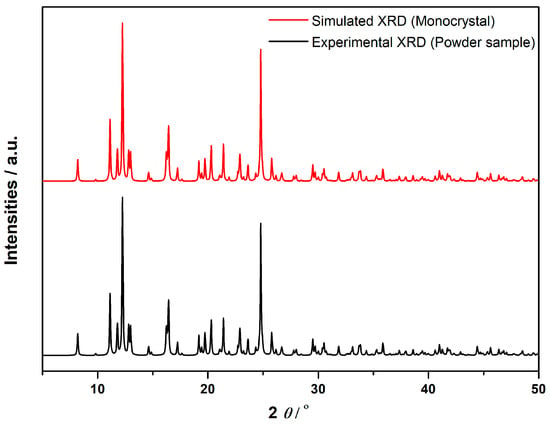
Figure 11.
Comparison of Simulated XRD (Monocrystal) and Experimental XRD (Powder Sample).
3.7. Antibacterial Activity
The antibacterial activity of the synthesised copper(II) complex was evaluated using standard microbiological tests. Initially, the disc diffusion method was used to investigate the activity against both Gram-negative and Gram-positive bacteria. Both iterations of the disc-diffusion assay yielded similar results showing a narrow (2–3 mm) but clearly defined inhibition zone only around the active compound on plates inoculated with Escherichia coli. The compound was not effective against Staphylococcus aureus. Consequently, subsequent quantitative tests of antibacterial activity were conducted exclusively with E. coli. These experiments demonstrated a reduction in the number of colony-forming units (CFU) by up to 7.3 x compared to the controls (Figure 12).
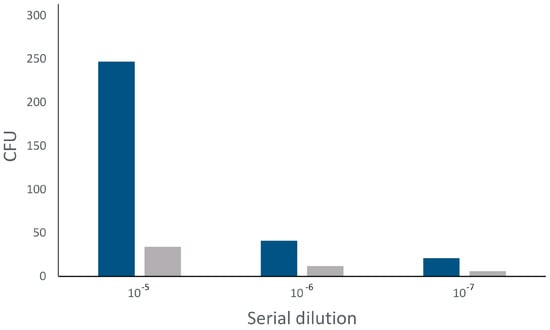
Figure 12.
Abundance of colony forming units after incubation of E. coli with active compound. Blue = controls, grey = copper(II) complex.
The crystallographic data of the copper complex [CuL2(py)3] reveal a distorted square pyramidal geometry with a potentially labile axial position at the Cu(II) center. This vacant coordination site may facilitate interactions with biomolecules inside bacterial cells, particularly with thiol- and amine-rich residues, such as cysteine and histidine, found in essential enzymes. In E. coli, potential targets for these interactions include phosphate groups in nucleic acids and metal-binding sites in enzymes. Similar mechanisms have been reported for other Cu(II) complexes, which can inhibit DNA gyrase and topoisomerase IV, intercalate into DNA, and generate reactive oxygen species (ROS), leading to oxidative DNA cleavage [74,75]. Additionally, copper complexes have been shown to disrupt bacterial membranes and proteins by binding to thiols and carboxylates, which may contribute to bacterial cell death. The enhanced antibacterial activity observed against E. coli suggests that [CuL2(py)3] may exert its effects through a combination of mechanisms, including coordination with copper-handling proteins and metal-dependent enzymes, alongside ROS generation that damages cellular components. Interestingly, S. aureus did not show susceptibility, likely due to the protective role of its thick peptidoglycan layer and efficient copper resistance mechanisms, such as specialized chaperones and efflux systems. These findings indicate that the antibacterial effect against E. coli may stem from both Cu2+ ions and specific coordination interactions, as supported by the literature [74,76]. To put these findings into perspective, the antibacterial activity of the copper complex was compared to that of erythromycin, a commonly used antibiotic, to assess its potential as an antimicrobial agent. In preliminary tests, the concentrations used were comparable to erythromycin plasma levels observed in clinical therapy with 500 mg erythromycin taken four times daily, but were higher than typical tissue concentrations. A 1 mM concentration of the copper complex was used in subsequent quantitative assays, while erythromycin plasma concentrations are generally in the µM range. Despite showing slightly lower antibacterial activity compared to erythromycin, the copper complex demonstrates promise as a metal ion donor and may serve as a precursor for the development of more effective antibacterial agents [77].
When compared to other copper complexes [51,78,79] [CuL2(py)3] exhibited comparable, albeit slightly reduced, antibacterial activity against E. coli. The observed differences may stem from variations in the coordination environments, which affect their interactions with bacterial membranes and enzymatic targets. Although slightly less potent than some other Cu(II) complexes, [CuL2(py)3] shows potential as a novel antimicrobial agent. Further optimization of its coordination environment could enhance its antibacterial potency and provide a basis for the development of alternative copper-based antibacterial agents.
4. Conclusions
The successful synthesis, structural characterization, and antibacterial evaluation of a novel ternary copper(II) complex with pyridine and chromone-2-carboxylic acid are presented. The crystallographic analysis revealed a distorted square–pyramidal geometry, with pyridine playing a dual role as both a solvent and a coordinating ligand. Spectroscopic, electrochemical, and thermal analyses further supported the stability and coordination environment of the complex. Antibacterial screening showed selective activity against Escherichia coli, with the complex exhibiting reduced activity against Staphylococcus aureus, highlighting its potential for targeted bacterial inhibition. The observed antibacterial activity is attributed to the coordination dynamics, particularly the labile axial site around the copper(II) ion, which facilitates interactions with bacterial biomolecules. These results suggest that this copper-based coordination complex may serve as a valuable foundation for the development of new antibacterial agents. Future research could focus on optimizing the coordination environment to enhance antibacterial potency and broaden the range of bacterial targets.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cryst15100870/s1: Figure S1: ORTEP plot of compound with displacement ellipsoids of non-hydrogen atoms drawn at the 50% probability level Symmetry operation (1) 3/2-X, +Y, 1/2-Z. Figure S2. IR spectrum of a ligand L. Figure S3. IR spectrum of a copper(II) nitrate trihydrate. Figure S4. X-ray diffraction pattern of ligand L (chromone-2-carboxylic acid). Figure S5. X-ray diffraction pattern of copper(II) nitrate trihydrate.
Author Contributions
Conceptualization, N.F. and T.B.; methodology, T.B., N.F., S.Š., A.S., G.P., M.M.-K. and D.G. software, M.M.-K., D.G., T.B., N.F. and S.Š.; validation, T.B., A.S., N.F. and G.P.; formal analysis, N.F., M.M.-K., D.G., A.S., T.B., S.Š. and G.P.; investigation, N.F., M.M.-K., D.G., A.S., T.B., S.Š. and G.P., curation, N.F., M.M.-K., D.G., T.B. and G.P., writing—review and editing, N.F., M.M.-K., D.G., A.S., T.B., S.Š. and G.P., visualization, N.F., M.M.-K., D.G., A.S., T.B., S.Š. and G.P.; supervision, T.B. All authors have read and agreed to the published version of the manuscript.
Funding
Microbiological analyses were supported by the Department of Biology grant VFZ310520.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors would like to thank Ivica Đilović from the Faculty of Science, University of Zagreb, for his assistance in solving the crystal structure of the complex. His expertise and support were invaluable to the success of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AMR | Antimicrobial Resistance |
| C, H, N | Carbon, Hydrogen, Nitrogen (Elemental analysis) |
| CV | Cyclic Voltammetry |
| DSC | Differential Scanning Calorimetry |
| DRS | Diffuse Reflectance Spectroscopy |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| IR | Infrared |
| MIC | Minimum Inhibitory Concentration |
| PXRD | Powder X-ray Diffraction |
| TGA | Thermogravimetric Analysis |
| UV–Vis | Ultraviolet–Visible |
| XRD | X-ray Diffraction |
References
- Ngece, K.; Khwaza, V.; Paca, A.M.; Aderibigbe, B.A. The Antimicrobial Efficacy of Copper Complexes: A Review. Antibiotics 2025, 14, 516. [Google Scholar] [CrossRef]
- De Ruiter, G.; Lahav, M.; van der Boom, M.E. Pyridine Coordination Chemistry for Molecular Assemblies on Surfaces. Acc. Chem. Res. 2014, 12, 3407–3416. [Google Scholar] [CrossRef]
- Bendi, A.; Bhathiwal, A.S.; Chanchal, V.S.; Tiwari, A.; Raghav, N. Exploration of pyridine-based self-assembled complexes—An overview. J. Mol. Struct. 2024, 1312, 138568. [Google Scholar] [CrossRef]
- Costa, J.; Delgado, R. Metal complexes of macrocyclic ligands containing pyridine. Inorg. Chem. 1993, 32, 5257–5265. [Google Scholar] [CrossRef]
- Santana, F.S.; Briganti, M.; Cassaro, R.A.A.; Totti, F.; Ribeiro, R.R.; Hughes, D.L.; Nunes, G.G.; Reis, D.M. An Oxalate-Bridged Copper(II) Complex Combining Monodentate Benzoate, 2,2′-bipyridine and Aqua Ligands: Synthesis, Crystal Structure and Investigation of Magnetic Properties. Molecules 2020, 25, 1898. [Google Scholar] [CrossRef]
- Bártová, M.; Liška, A.; Studená, V.; Vojtíšek, P.; Kašpar, M.; Mikysek, T.; Česlová, L.; Švancara, I.; Sýs, M. Dinuclear Copper(II) Complexes of 2,6-Bis[(N-Methylpiperazine-1-yl)methyl]-4-Formyl Phenol Ligand: Promising Biomimetic Catalysts for Dye Residue Degradation and Drug Synthesis. Int. J. Mol. Sci. 2025, 26, 1603. [Google Scholar] [CrossRef]
- Beyeh, N.K.; Puttreddy, R. Methylresorcinarene: A reaction vessel to control the coordination geometry of copper(ii) in pyridine N-oxide copper(ii) complexes. Dalton. Trans. 2015, 44, 9881–9886. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Al Foisal, J.; Rahman, M.; Islam, M.Z.; Mimi, M.A.; Habib, R.; Hossain, M.A.; Chowdhury, L.; Bhattacharjee, S.C.; Cui, D. Antimicrobial Activity of Cu(II) and Fe(III) with Pyridine Complexes As Ligands Contrary to Clinical Strains of Bacteria and Fungi Species. Asian J. Chem. 2019, 31, 2323–2326. [Google Scholar] [CrossRef]
- Bilal, H.; Zhang, C.X. Copper(II) carboxylate complexes inhibit Staphylococcus aureus biofilm formation by targeting extracellular proteins. J. Inorg. Biochem. 2025, 266, 112835. [Google Scholar] [CrossRef]
- Yan, Y.-L.; Miller, M.T.; Chao, Y.; Cohen, M.S. Synthesis of Hydroxypyrone-Based Matrix Metalloproteinase Inhibitors: Developing a Structure-Activity Relationship. Bioorg. Med. Chem. Lett. 2009, 19, 1970–1976. [Google Scholar] [CrossRef]
- Toso, L.; Crisponi, G.; Nurchi, V.M.; Crespo-Alonso, M.; Lachowicz, J.I.; Santos, M.A.; Marques, S.M.; Niclós-Gutiérrez, J.; González-Pérez, J.M.; Domínguez-Martín, A.; et al. A Family of Hydroxypyrone Ligands Designed and Synthesized as Iron Chelators. J. Inorg. Biochem. 2013, 127, 220–231. [Google Scholar] [CrossRef]
- Thompson, K.H.; Barta, C.A.; Orvig, C. Metal Complexes of Maltol and Close Analogues in Medicinal Inorganic Chemistry. Chem. Soc. Rev. 2006, 35, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Kandioller, W.; Kurzwernhart, A.; Hanif, M.; Meier, S.M.; Henke, H.; Keppler, K.B.; Hartinger, C.G. Pyrone Derivatives and Metals: From Natural Products to Metal-Based Drugs. J. Organomet. Chem. 2011, 696, 999–1010. [Google Scholar] [CrossRef]
- Livingstone, R. Rodd’s Chemistry of Carbon Compounds: Six Membered Ring Compounds with One Heteroatom; Elsevier: Amsterdam, The Netherlands, 1975; Chapter 20; pp. 1–397. [Google Scholar]
- Zhou, T.; Kong, X.-L.; Hider, R.C. Synthesis and Iron Chelating Properties of Hydroxypyridinone and Hydroxypyranone Hexadentate Ligands. Dalton Trans. 2019, 48, 3459–3466. [Google Scholar] [CrossRef]
- Zalevskaya, O.A. Recent Studies on the Antimicrobial Activity of Copper Complexes. Russ. J. Coord. Chem. 2021, 47, 861–880. [Google Scholar] [CrossRef]
- Filipović, N.; Balić, T.; Medvidović-Kosanović, M.; Goman, D.; Marković, B.; Tatar, D.; Roca, S.; Mišković Špoljarić, K. Chromone-Based Copper(II) Complexes as Potential Antitumour Agents: Synthesis, Chemical Characterisation and In Vitro Biological Evaluation. Crystals 2025, 15, 389. [Google Scholar] [CrossRef]
- Aldabaldetrecu, M.; Parra, M.; Soto, S.; Arce, P.; Tello, M.; Guerrero, J.; Modak, B. New Copper(I) Complex with a Coumarin as Ligand with Antibacterial Activity against Flavobacterium psychrophilum. Molecules 2020, 25, 3183. [Google Scholar] [CrossRef] [PubMed]
- Kootahmeshki, T.; Souldozi, A. Antibacterial Activity of 2,6-Diaminopyridine Metal Complexes and Corresponding Metal Salts Against Some Pathogenic Bacteria. J. Biotechnol. Bioprocess. 2025, 6, 146. [Google Scholar] [CrossRef]
- Soleimani, E. Synthesis, characterization and anti-microbial activity of a novel macrocyclic ligand derived from the reaction of 2,6-pyridinedicarboxylic acid with homopiperazine and its Co(II), Ni(II), Cu(II), and Zn(II) complexes. J. Mol. Struct. 2011, 995, 1–8. [Google Scholar] [CrossRef]
- Andrejević, T.P.; Aleksic, I.; Počkaj, M.; Kljun, J.; Milivojevic, D.; Stevanović, N.L.; Nikodinovic-Runic, J.; Turel, I.; Djuran, M.I.; Glišić, B.Đ. Tailoring copper(II) complexes with pyridine-4,5-dicarboxylate esters for anti-Candida activity. Dalton Trans. 2021, 50, 2627–2638. [Google Scholar] [CrossRef]
- Çolak, A.T.; Çolak, F.; Akduman, D.; Yeşilel, O.Z.; Büyükgüngör, O. Syntheses, crystal structures, spectral and thermal analysis and biological activities of copper(II)-pyridine-2,5-dicarboxylate complexes with 4-methylimidazole, imidazole, and 3,4-dimethylpyridine. Solid. State Sci. 2009, 11, 1908–1918. [Google Scholar] [CrossRef]
- Abalintsina, S.A.; Conde, M.A.; Eni, D.B.; Agwara, M.O. Synthesis, characterization and antimicrobial properties of Cobalt(III), Nickel(III), Copper(II) and Zinc(II) complexes with pyridine and thiocyanate. Sci. Afr. 2022, 18, e01402. [Google Scholar] [CrossRef]
- Marinescu, M.; Popa, C.-V. Pyridine Compounds with Antimicrobial and Antiviral Activities. Int. J. Mol. Sci. 2022, 23, 5659. [Google Scholar] [CrossRef] [PubMed]
- Pahonțu, E.; Ilieș, D.-C.; Shova, S.; Paraschivescu, C.; Badea, M.; Gulea, A.; Roșu, T. Synthesis, Characterization, Crystal Structure and Antimicrobial Activity of Copper(II) Complexes with the Schiff Base Derived from 2-Hydroxy-4-Methoxybenzaldehyde. Molecules 2015, 20, 5771–5792. [Google Scholar] [CrossRef] [PubMed]
- Hangan, A.C.; Lucaciu, R.L.; Turza, A.; Dican, L.; Sevastre, B.; Páll, E.; Oprean, L.S.; Borodi, G. New Copper Complexes with Antibacterial and Cytotoxic Activity. Int. J. Mol. Sci. 2023, 24, 13819. [Google Scholar] [CrossRef]
- Qian, W.; Wang, Z.; Xia, J.; Wang, H.; Dong, S.; Lou, S.; Ding, P.; Li, L. Synthesis, Structural Characterization, EPR Analysis and Antimicrobial Activity of a Copper(II) Thiocyanate Complex Based on 3,7-Di(3-pyridyl)-1,5-dioxa-3,7-diazacyclooctane. Symmetry 2025, 17, 791. [Google Scholar] [CrossRef]
- Available online: https://www.mt.com/hr/hr/home/products/Laboratory_Analytics_Browse/TA_Family_Browse/TA_software_browse.html?cmp=als_ta-software (accessed on 4 September 2025).
- Available online: https://www.malvernpanalytical.com/en/products/category/software/x-ray-diffraction-software/highscore (accessed on 4 September 2025).
- Available online: https://www.palmsens.com/software/ps-trace/ (accessed on 4 September 2025).
- Marinova, P.; Tamahkyarova, K. Synthesis and Biological Activities of Some Metal Complexes of Peptides: A Review. BioTech 2024, 13, 9. [Google Scholar] [CrossRef]
- Agilent. CrysAlis PRO; Agilent Technologies Ltd.: Oxfordshire, UK, 2014. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H.J. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment-Olex2 dissected. Acta Cryst. 2015, A71, 59–75. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148–155. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J.J. Mercury: Visualization and Analysis of Crystal Structures. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Blackmanm, A.G.; Schenk, E.B.; Jelley, R.E.; Krensked, E.H.; Gahan, L.R. Five-coordinate transition metal complexes and the value of τ5: Observations and caveats. Dalton Trans. 2020, 49, 14798–14806. [Google Scholar] [CrossRef]
- Tsymbal, L.V.; Andriichuk, I.L.; Lampeka, Y.D.; Arion, V.B. Two-dimensional coordination polymers based on pyridine-containing cations of cu(II) and Ni(II) and 1,3,5-benzenetricarboxylate anion and their supramolecular structure. J. Struct. Chem. 2014, 55, 1466. [Google Scholar] [CrossRef]
- Golobič, A.; Kristl, M.; Podnar, T.M.; Jagličić, Z.; Dojer, B. Mixed-Ligand Copper(II) Complexes Derived from Pyridinecarbonitrile Precursors: Structural Features and Thermal Behavior. Inorganics 2025, 13, 287. [Google Scholar] [CrossRef]
- Tanaka, K.; Kikumoto, Y.; Hota, N.; Takahashi, H. Homochiral Coordination Polymers with Nanotubular Channels for Enantioselective Sorption of Chiral Guest Molecules. New J. Chem. 2014, 38, 1519–1524. [Google Scholar] [CrossRef]
- Barmpa, A.; Hatzidimitriou, A.G.; Psomas, G. Copper(II) Complexes with Meclofenamate Ligands: Structure, Interaction with DNA and Albumins, Antioxidant and Anticholinergic Activity. J. Inorg. Biochem. 2021, 217, 111357. [Google Scholar] [CrossRef]
- Balogh-Hergovich, É.; Kaizer, J.; Speier, G.; Argay, G.; Párkányi, L. Kinetic Studies on the Copper(II)-Mediated Oxygenolysis of the Flavonolate Ligand. Crystal Structures of [Cu(fla)2] (fla = Flavonolate) and [Cu(O-bs)2(py)3] (O-bs = O-Benzoylsalicylate). Dalton Trans. 1999, 2110, 3847–3854. [Google Scholar] [CrossRef]
- Chui, S.S.; Lo, S.M.; Charmant, J.P.; Orpen, A.G.; Williams, I.D. A Chemically Functionalizable Nanoporous Material. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Hu, D.-H.; Huang, W.; Gou, S.-H.; Fang, J.-L.; Fun, H.-K. Synthesis, Characterization, and Magnetic Properties of a Dinuclear Complex [Cu(2,2′-bpy)(HL)(L)2(NO3)2·(H2O)3/2] and a 1D Chain {Cu(2,2′-bpy)(4,4′-bpy)1/2(L) ·(1/2H2O)}ₙ (L is p-Aminobenzoate). Polyhedron 2003, 22, 2661–2667. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Althobaiti, I.O.; Soliman, K.A.; Asiri, Y.M.; Alenezy, E.K.; Alrashdi, S.; Gad, E.S. Copper(II) Complex with a 3,3′-Dicarboxy-2,2′-Dihydroxydiphenylmethane-Based Carboxylic Ligand: Synthesis, Spectroscopic, Optical, Density Functional Theory, Cytotoxic, and Molecular Docking Approaches for a Potential Anti-Colon Cancer Control. Inorganics 2025, 13, 151. [Google Scholar] [CrossRef]
- Walczak, A.; Kurpik, G.; Stefankiewicz, A.R. Intrinsic Effect of Pyridine-N-Position on Structural Properties of Cu-Based Low-Dimensional Coordination Frameworks. Int. J. Mol. Sci. 2020, 21, 6171. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Wackerbarth, I.; Widhyadnyani, N.N.A.T.; Schmitz, S.; Stirnat, K.; Butsch, K.; Pantenburg, I.; Meyer, G.; Klein, A. CuII Complexes and Coordination Polymers with Pyridine or Pyrazine Amides and Amino Benzamides—Structures and EPR Patterns. Inorganics 2020, 8, 65. [Google Scholar] [CrossRef]
- Filopoulou, A.; Vlachou, S.; Boyatzis, S.C. Fatty Acids and Their Metal Salts: A Review of Their Infrared Spectra in Light of Their Presence in Cultural Heritage. Molecules 2021, 26, 6005. [Google Scholar] [CrossRef]
- Al-Fakeh, M.S.; Allazzam, G.A.; Yarkandi, N.H. Ni(II), Cu(II), Mn(II), and Fe(II) Metal Complexes Containing 1,3-Bis(diphenylphosphino)propane and Pyridine Derivative: Synthesis, Characterization, and Antimicrobial Activity. Int. J. Biomater. 2021, 12, 4981367. [Google Scholar] [CrossRef]
- Patel, R.N.; Kumhar, D.; Patel, S.K.; Patel, A.K.; Patel, N.; Butcher, R.J. Copper(II) Mononuclear Complexes Incorporating Pyridine Derivatives: Synthesis, Structural Characterization, and Unusual X-Band EPR Spectra. J. Chem. Crystallogr. 2022, 52, 378–393. [Google Scholar] [CrossRef]
- Bissantz, C.; Kuhn, B.; Stahl, M. A medicinal chemist’s guide to molecular interactions. J. Med. Chem. 2010, 53, 5061–5084. [Google Scholar] [CrossRef]
- Al-Harazie, A.G.; Gomaa, E.A.; Zaky, R.R.; El-Hady, M.N.A. Spectroscopic Characterization, Cyclic Voltammetry, Biological Investigations, MOE, and Gaussian Calculations of VO(II), Cu(II), and Cd(II) Heteroleptic Complexes. ACS Omega 2023, 8, 13605–13625. [Google Scholar] [CrossRef] [PubMed]
- Sandford, C.; Edwards, M.A.; Klunder, K.J.; Hickey, P.D.; Min, L.; Barman, K.; Sigman, M.S.; White, H.S.; Minteer, S.D. A synthetic chemist’s guide to electroanalytical tools for studying reaction mechanisms. Chem. Sci. 2019, 10, 6404–6422. [Google Scholar] [CrossRef] [PubMed]
- Masood, Z.; Muhammad, H.; Tahiri, I.A. Comparison of Different Electrochemical Methodologies for Electrode Reactions: A Case Study of Paracetamol. Electrochem 2024, 5, 57–69. [Google Scholar] [CrossRef]
- Fiore, C.; Lekhan, A.; Bordignon, S.; Chierotti, M.R.; Gobetto, R.; Grepioni, F.; Turner, R.J.; Braga, D. Mechanochemical Preparation, Solid-State Characterization, and Antimicrobial Performance of Copper and Silver Nitrate Coordination Polymers with L- and DL-Arginine and Histidine. Int. J. Mol. Sci. 2023, 24, 5180. [Google Scholar] [CrossRef]
- Alem, M.B.; Desalegn, T.; Damena, T.; Bayle, E.A.; Koobotse, M.O.; Ngwira, J.K.; Ombito, J.O.; Zachariah, M.; Demissie, T.B. Cytotoxicity and antibacterial potentials of mixed ligand Cu (II) and Zn (II) complexes: A combined experimental and computational study. ACS Omega 2023, 8, 13421–13434. [Google Scholar] [CrossRef]
- Barwiolek, M.; Jankowska, D.; Kaczmarek-Kędziera, A.; Lakomska, I.; Kobylarczyk, J.; Podgajny, R.; Popielarski, P.; Masternak, J.; Witwicki, M.; Muzioł, T.M. New Dinuclear Macrocyclic Copper(II) Complexes as Potentially Fluorescent and Magnetic Materials. Int. J. Mol. Sci. 2023, 24, 3017. [Google Scholar] [CrossRef] [PubMed]
- Rajabimoghadam, K.; Darwish, Y.; Bashir, U.; Pitman, D.; Eichelberger, S.; Siegler, M.A.; Swart, M.; Garcia-Bosch, I. Catalytic aerobic oxidation of alcohols by copper complexes bearing redox-active ligands with tunable H-bonding groups. J. Am. Chem. Soc. 2018, 140, 16625–16634. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Maity, T.; Dey, T.; Koner, S. One-dimensional chain copper (II) complex: Synthesis, X-ray crystal structure and catalytic activity in the epoxidation of styrene. Polyhedron 2012, 35, 55–61. [Google Scholar] [CrossRef]
- Hontz, D.; Hensley, J.; Hiryak, K.; Lee, J.; Luchetta, J.; Torsiello, M.; Venditto, M.; Lucent, D.; Terzaghi, W.; Mencer, D.; et al. A copper (II) macrocycle complex for sensing biologically relevant organic anions in a competitive fluorescence assay: Oxalate sensor or urate sensor? ACS Omega 2020, 5, 19469–19477. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Sethi, P.; Kumar, S.; Rathi, P.; Umar, A.; Kumar, R.; Chaudhary, S.; Alkhanjaf, A.A.M.; Ibrahim, A.A.; Baskoutas, S. Synthesis and biomedical applications of macrocyclic complexes. J. Mol. Struct. 2024, 1317, 139098. [Google Scholar] [CrossRef]
- Boga, S.; Bouzada, D.; Lopez-Blanco, R.; Sarmiento, A.; Salvadó, I.; Gil, D.A.; Brea, J.; Loza, M.I.; Barreiro-Piñeiro, N.; Martínez-Costas, J.; et al. Copper (II) Cyclopeptides with High ROS-Mediated Cytotoxicity. Bioconjugate Chem. 2025, 36, 500–509. [Google Scholar] [CrossRef]
- Mucha, P.; Hikisz, P.; Gwoździński, K.; Krajewska, U.; Leniart, A.; Budzisz, E. Cytotoxic effect, generation of reactive oxygen/nitrogen species and electrochemical properties of Cu (II) complexes in comparison to half-sandwich complexes of Ru (II) with aminochromone derivatives. RSC Adv. 2019, 9, 31943–31952. [Google Scholar] [CrossRef]
- Balewski, Ł.; Inkielewicz-Stępniak, I.; Gdaniec, M.; Turecka, K.; Hering, A.; Ordyszewska, A.; Kornicka, A. Synthesis, Structure, and Stability of Copper(II) Complexes Containing Imidazoline-Phthalazine Ligands with Potential Anticancer Activity. Pharmaceuticals 2025, 18, 375. [Google Scholar] [CrossRef]
- Sarvepalli, S.; Pasika, S.R.; Verma, V.; Thumma, A.; Bolla, S.; Nukala, P.K.; Butreddy, A.; Bolla, P.K. A Review on the Stability Challenges of Advanced Biologic Therapeutics. Pharmaceutics 2025, 17, 550. [Google Scholar] [CrossRef] [PubMed]
- Murekhina, A.E.; Yarullin, D.N.; Sovina, M.A.; Kitaev, P.A.; Gamov, G.A. Copper (II)-Catalyzed Oxidation of Ascorbic Acid: Ionic Strength Effect and Analytical Use in Aqueous Solution. Inorganics 2022, 10, 102. [Google Scholar] [CrossRef]
- Shen, J.; Wang, M.; Zhang, P.; Jiang, J.; Sun, L. Electrocatalytic water oxidation by copper(ii) complexes containing a tetra- or pentadentate amine-pyridine ligand. Chem. Commun. 2017, 21, 4374–4377. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Zhang, X. A special case of copper(II) complex having monodentate and uncoordinated 4-aminopyridine molecules stabilized by highly cooperative supramolecular interactions. Inorg. Chim. Acta. 2012, 392, 465–468. [Google Scholar] [CrossRef]
- Kowalik, M.; Masternak, J.; Łakomska, I.; Kazimierczuk, K.; Zawilak-Pawlik, A.; Szczepanowski, P.; Khavryuchenko, O.V.; Barszcz, B. Structural Insights into New Bi(III) Coordination Polymers with Pyridine-2,3-Dicarboxylic Acid: Photoluminescence Properties and Anti-Helicobacter pylori Activity. Int. J. Mol. Sci. 2020, 21, 8696. [Google Scholar] [CrossRef]
- Marinova, P.E.; Tamahkyarova, K.D. Synthesis, Investigation, Biological Evaluation, and Application of Coordination Compounds with Schiff Base—A Review. Compounds 2025, 5, 14. [Google Scholar] [CrossRef]
- Wu, S.; Wang, M.; Liu, Z.; Fu, C. Mechanisms Operating in the Use of Transition Metal Complexes to Combat Antimicrobial Resistance. Microorganisms 2025, 7, 1570. [Google Scholar] [CrossRef]
- Moreira Martins, P.M.; Gong, T.; de Souza, A.A.; Wood, T.K. Copper Kills Escherichia coli Persister Cells. Antibiotics 2020, 9, 506. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Narváez, M.E.; González-Sebastián, L.; Colorado-Peralta, R.; Reyes-Márquez, V.; Franco-Sandoval, L.O.; Romo-Pérez, A.; Cruz-Navarro, J.A.; Mañozca-Dosman, I.V.; Aragón-Muriel, A.; Morales-Morales, D. Anticancer and Antimicrobial Activity of Copper(II) Complexes with Fluorine-Functionalized Schiff Bases: A Mini-Review. Inorganics 2025, 13, 38. [Google Scholar] [CrossRef]
- Božić Cvijan, B.; Korać Jačić, J.; Bajčetić, M. The Impact of Copper Ions on the Activity of Antibiotic Drugs. Molecules 2023, 28, 5133. [Google Scholar] [CrossRef] [PubMed]
- Krasniqi, S.; Matzneller, P.; Kinzig, M.; Sörgel, F.; Hüttner, S.; Lackner, E.; Müller, M.; Zeitlinger, M. Blood, tissue, and intracellular concentrations of erythromycin and its metabolite anhydroerythromycin during and after therapy. Antimicrob. Agents Chemother. 2012, 56, 1059–1064. [Google Scholar] [CrossRef]
- Premkumar, M.; Kaleeswaran, D.; Kaviyarasan, G.; Prasanth, D.A.; Venkatachalam, G. Mono and Dinuclear Cu(II) Carboxylate Complexes with Pyridine and 1-methylimidazole as Co-Ligands: Synthesis, Structure, Antibacterial Activity, and Catalytic Nitroaldol Reactions. ChemistrySelect 2019, 4, 7507–7511. [Google Scholar] [CrossRef]
- Hijazi, A.K.; El-Khateeb, M.; Taha, Z.A.; Alomari, M.I.; Khwaileh, N.M.; Alakhras, A.I.; Al-Momani, W.M.; Elrashidi, A.; Barham, A.S. Anti-Bacterial and Anti-Fungal Properties of a Set of Transition Metal Complexes Bearing a Pyridine Moiety and [B(C6F5)4]2 as a Counter Anion. Molecules 2025, 30, 3121. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).