The Influence of Support Basicity on the Adsorption of Lead on the (100) Surface of Alkaline Earth Metal Oxide Crystals
Abstract
1. Introduction
2. Methods
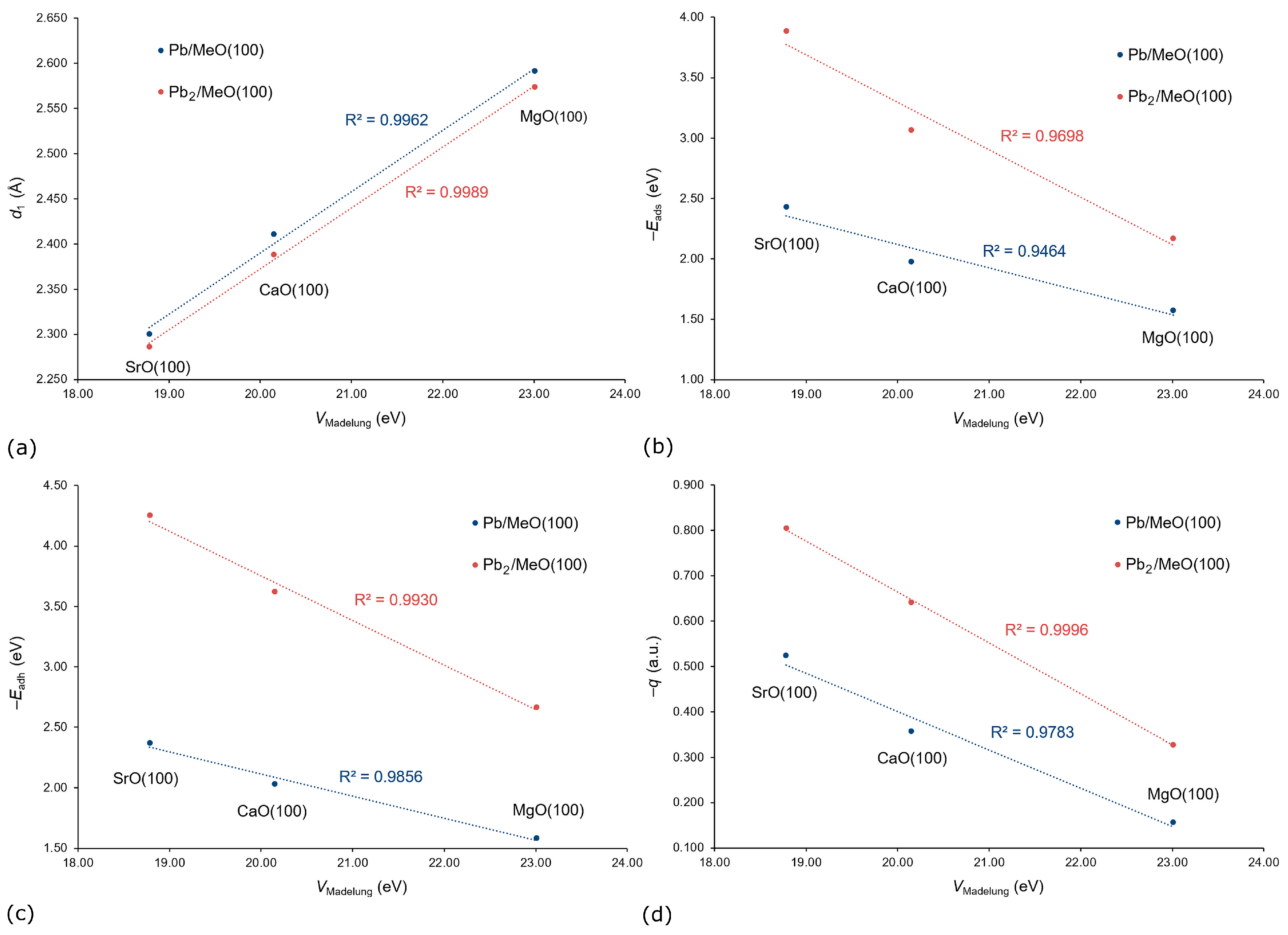
3. Results and Discussion
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sinfelt, J.H. Bimetallic Catalysis: Discoveries, Concepts and Applications; Wiley: New York, NY, USA, 1982. [Google Scholar]
- Chorkendorff, I.; Niemantsverdriet, J.W. Concepts of Modern Catalysis and Kinetics; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Lindlar, H. Ein Neuer Katalysator Für Selektive Hydrierungen. Helv. Chim. Acta 1952, 35, 446–450. [Google Scholar] [CrossRef]
- Garcıa-Mota, M.; Gomez-Dıaz, J.; Novell-Leruth, G.; Vargas-Fuentes, C.; Bellarosa, L.; Bridier, B.; Perez-Ramırez, J.; Bluchterova, N. A Density Functional Theory Study of the ‘Mythic’ Lindlar Hydrogenation Catalyst. Theor. Chem. Acc. 2011, 128, 663–673. [Google Scholar] [CrossRef]
- Furukawa, S.; Suga, A.; Komatsu, T. Mechanistic Study on Aerobic Oxidation of Amine over Intermetallic Pd3Pb: Concerted Promotion Effects by Pb and Support Basicity. ACS Catal. 2015, 5, 1214–1222. [Google Scholar] [CrossRef]
- Diao, Y.; Yang, P.; Yan, R.; Jiang, L.; Wang, L.; Zhang, H.; Li, C.; Li, Z.; Zhang, S. Deactivation and Regeneration of the Supported Bimetallic Pd–Pb Catalyst in Direct Oxidative Esterification of Methacrolein with Methanol. Appl. Catal. B 2013, 142–143, 329–336. [Google Scholar] [CrossRef]
- Jiang, L.; Diao, Y.; Han, J.; Yan, R.; Zhang, X.; Zhang, S. MgO–SBA-15 Supported Pd–Pb Catalysts for Oxidative Esterification of Methacrolein with Methanol to Methyl Methacrylate. Chin. J. Chem. Eng. 2014, 22, 1098–1104. [Google Scholar] [CrossRef]
- Shesterkina, A.A.; Kirichenko, O.A.; Tkachenko, O.P.; Kustov, A.L.; Kustov, L.M. Liquid-Phase Partial Hydrogenation of Phenylacetylene at Ambient Conditions Catalyzed by Pd-Fe-O Nanoparticles Supported on Silica. Nanomaterials 2023, 13, 2247. [Google Scholar] [CrossRef]
- Wang, P.; Ma, Y.; Shi, Y.; Duan, F.; Wang, M. Application of Metal-Based Catalysts for Semi-Hydrogenation of Alkynol: A Review. Materials 2023, 16, 7409. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.R. Surface Studies of Supported Model Catalysts. Surf. Sci. Rep. 1998, 31, 235–325. [Google Scholar] [CrossRef]
- Anderson, J.A.; García, M.F. Supported Metals in Catalysis, 2nd ed.; Catalytic Science Series; Imperial College Press: London, UK, 2012; Volume 11. [Google Scholar]
- Gatou, M.-A.; Skylla, E.; Dourou, P.; Pippa, N.; Gazouli, M.; Lagopati, N.; Pavlatou, E.A. Magnesium Oxide (MgO) Nanoparticles: Synthetic Strategies and Biomedical Applications. Crystals 2024, 14, 215. [Google Scholar] [CrossRef]
- Henrich, V.E.; Cox, P.A. The Surface Science of Metal Oxides; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Tasker, P.W. The Stability of Ionic Crystal Surfaces. J. Phys. C Solid State Phys. 1979, 12, 4977–4984. [Google Scholar] [CrossRef]
- Wander, A.; Bush, I.J.; Harrison, N.M. Stability of Rocksalt Polar Surfaces: An Ab Initio Study of MgO(111) and NiO(111). Phys. Rev. B 2003, 68, 233405. [Google Scholar] [CrossRef]
- Kramer, J.; Ernst, W.; Tegenkamp, C.; Pfnur, H. Mechanism and Kinetics of Color Center Formation on Epitaxial Thin Films of MgO. Surf. Sci. 2002, 517, 87–97. [Google Scholar] [CrossRef]
- Pacchioni, G. Theory of Metal Clusters on the MgO Surface: The Role of Point Defects. In Nanocatalysis. Nanoscience and Technology; Heiz, U., Landman, U., Eds.; Springer: Heidelberg, Germany, 2007; pp. 193–243. [Google Scholar]
- Matveev, A.V.; Neyman, K.M.; Yudanov, I.V.; Rosch, N. Adsorption of Transition Metal Atoms on Oxygen Vacancies and Regular Sites of the MgO(001) Surface. Surf. Sci. 1999, 426, 123–139. [Google Scholar] [CrossRef]
- Fuente, S.A.; Belelli, P.G.; Ferullo, R.M.; Castellani, N.J. Adsorption of NO on Au Atoms and Dimers Supported on MgO(100): DFT Studies. Surf. Sci. 2008, 602, 1669–1676. [Google Scholar] [CrossRef]
- Ferrari, A.M.; Pacchioni, G. Electronic Structure of F and V Centers on the MgO Surface. J. Phys. Chem. 1995, 99, 17010–17018. [Google Scholar] [CrossRef]
- Garrone, E.; Zecchina, A.; Stone, F.S. CO Adsorption on MgO and CaO. Spectroscopic Investigations of Stages Prior to Cyclic Anion Cluster Formation. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1988, 84, 2843–2854. [Google Scholar] [CrossRef]
- Horiuchi, T.; Hidaka, H.; Fukui, T.; Kubo, Y.; Horio, M.; Suzuki, K.; Mori, T. Effect of Added Basic Metal Oxides on CO2 Adsorption on Alumina at Elevated Temperatures. Appl. Catal. A 1998, 167, 195–202. [Google Scholar] [CrossRef]
- Chiesa, M.; Giamello, E.; Di Valentin, C.; Pacchioni, G.; Sojka, Z.; Van Doorslaer, S. Nature of the Chemical Bond between Metal Atoms and Oxide Surfaces: New Evidences from Spin Density Studies of K Atoms on Alkaline Earth Oxides. J. Am. Chem. Soc. 2005, 127, 16935–16944. [Google Scholar] [CrossRef]
- Doig, J.A.; Sayle, T.X.T.; Sayle, D.C. The Influence of a Material Microstructure on the Behaviour of Dopants. J. Mater. Chem. 2004, 14, 2380–2388. [Google Scholar] [CrossRef]
- Lopez, N. Effect of the Basicity of the Support on the Properties of Deposited Metal Atoms. J. Chem. Phys. 2001, 114, 2355–2361. [Google Scholar] [CrossRef]
- Abdel Halim, W.S.; Abdel Aal, S.; Shalabi, A.S. CO Adsorption on Pd Atoms Deposited on MgO, CaO, SrO and BaO Surfaces: Density Functional Calculations. Thin Solid Films 2008, 516, 4360–4365. [Google Scholar] [CrossRef]
- Abdel Halim, W.S.; Shalabi, A.S.; Soliman, K.A. Transition Metal Atoms on Oxide Supports Density Functional Calculations. Int. J. Quantum Chem. 2009, 109, 1094–1102. [Google Scholar] [CrossRef]
- Pacchioni, G.; Sousa, C.; Illas, F.; Parmigiani, F.; Bagus, P.S. Measures of Ionicity of Alkaline-Earth Oxides from the Analysis of Ab Initio Cluster Wave Functions. Phys. Rev. B 1993, 48, 11573–11582. [Google Scholar] [CrossRef]
- Di Valentin, C.; Locati, C.; Pacchioni, G. Probing the Basicity of Regular and Defect Sites of Alkaline Earth Metal Oxide Surfaces by BF3 Adsorption: A Theoretical Analysis. ChemPhysChem 2004, 5, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Mallat, T.; Baiker, A.; Patscheider, J. Liquid Phase Oxidation of 1-Methoxy-2-Propanol with Air: II. Structure and Chemical Properties of Lead-Promoted Palladium Catalysts. Appl. Catal. A 1991, 79, 59–75. [Google Scholar] [CrossRef]
- Stachurski, J.; Thomas, J.M. Structural Aspects of the Lindlar Catalyst for Selective Hydrogenation. Catal. Lett. 1988, 1, 67–72. [Google Scholar] [CrossRef]
- Matczak, P. Effect of Surface Vacancies on the Adsorption of Pd and Pb on MgO(100). Monatshefte Chem.-Chem. Mon. 2018, 149, 1009–1015. [Google Scholar] [CrossRef]
- Starr, D.E.; Bald, D.J.; Musgrove, J.E.; Ranney, J.T.; Campbell, C.T. Microcalorimetric Measurements of the Heat of Adsorption of Pb on Well-Defined Oxides: MgO(100) and p(2×1)-Oxide on Mo(100). J. Chem. Phys. 2001, 114, 3752–3764. [Google Scholar] [CrossRef]
- Starr, D.E.; Campbell, C.T. Low-Temperature Adsorption Microcalorimetry: Pb on MgO(100). J. Phys. Chem. B 2001, 105, 3776–3782. [Google Scholar] [CrossRef]
- Starr, D.E.; Diaz, S.F.; Musgrove, J.E.; Ranney, J.T.; Bald, D.J.; Nelen, L.; Ihm, H.; Campbell, C.T. Heat of Adsorption of Cu and Pb on Hydroxyl-Covered MgO(100). Surf. Sci. 2002, 515, 13–20. [Google Scholar] [CrossRef]
- Campbell, C.T.; Starr, D.E. Metal Adsorption and Adhesion Energies on MgO(100). J. Am. Chem. Soc. 2002, 124, 9212–9218. [Google Scholar] [CrossRef]
- Starr, D.E.; Campbell, C.T. Large Entropy Difference between Terrace and Step Sites on Surfaces. J. Am. Chem. Soc. 2008, 130, 7321–7327. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Misra, N. Ab Initio Investigations on Planar (MgO)n Clusters (n = 1–5) and Their Hydrogen Adsorption Behaviour. Mol. Simul. 2016, 42, 208–214. [Google Scholar] [CrossRef]
- Reckien, W.; Janetzko, F.; Peintinger, M.F.; Bredow, T. Implementation of Empirical Dispersion Corrections to Density Functional Theory for Periodic Systems. J. Comput. Chem. 2012, 33, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Bajdich, M.; Nørskov, J.K.; Vojvodic, A. Surface Energetics of Alkaline-Earth Metal Oxides: Trends in Stability and Adsorption of Small Molecules. Phys. Rev. B 2015, 91, 155401. [Google Scholar] [CrossRef]
- Varjovi, M.J.; Tosoni, S. DFT Investigation of X55 (X = Ni, Pd, and Pt) Clusters on Ultrathin Supported MgO Films: Evidence of Oxygen Spillover and Relevance for Catalytic Model Studies. J. Phys. Chem. C 2024, 128, 21331–21342. [Google Scholar] [CrossRef]
- Xue, R.; Wang, F.-F.; Chen, D.-L.; Zhang, F.; Zhu, W. Theoretical Insights on the Synergistic Effect of Dual Metal Sites Supported on MgO(100) Promoting the Hydrogenation Reaction. J. Phys. Chem. C 2025, 129, 359–368. [Google Scholar] [CrossRef]
- Tada, K.; Kawakami, T.; Hinuma, Y. Model Calculations for the Prediction of the Diradical Character of Physisorbed Molecules: p-Benzyne/MgO and p-Benzyne/SrO. Phys. Chem. Chem. Phys. 2023, 25, 29424–29436. [Google Scholar] [CrossRef]
- Ončák, M.; Włodarczyk, R.; Sauer, J. Hydration Structures of MgO, CaO, and SrO (001) Surfaces. J. Phys. Chem. C 2016, 120, 24762–24769. [Google Scholar] [CrossRef]
- Matczak, P. First-Principles Study of Sn Dimer Adsorbed on MgO Surface. Crystals 2025, 15, 410. [Google Scholar] [CrossRef]
- Evjen, H.M. On the Stability of Certain Heteropolar Crystals. Phys. Rev. 1932, 39, 675–687. [Google Scholar] [CrossRef]
- Matczak, P. Theoretical Study of Sn Adsorbed on the MgO(100) Surface with Defects. C. R. Chim. 2018, 21, 669–675. [Google Scholar] [CrossRef]
- Labello, N.P.; Ferreira, A.M.; Kurtz, H.A. An Augmented Effective Core Potential Basis Set for the Calculation of Molecular Polarizabilities. J. Comput. Chem. 2005, 26, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Stevens, W.J.; Basch, H.; Krauss, M. Compact Effective Potentials and Efficient Shared-Exponent Basis Sets for the First- and Second-Row Atoms. J. Chem. Phys. 1984, 81, 6026–6033. [Google Scholar] [CrossRef]
- Stevens, W.J.; Krauss, M.; Basch, H.; Jasien, P.G. Relativistic Compact Effective Potentials and Efficient, Shared-Exponent Basis Sets for the Third-, Fourth-, and Fifth-Row Atoms. Can. J. Chem. 1992, 70, 612–630. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Matczak, P. Assessment of B3LYP Combined with Various ECP Basis Sets for Systems Containing Pd, Sn, and Pb. Comput. Theor. Chem. 2012, 983, 25–30. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The Calculation of Small Molecular Interactions by the Differences of Separate Total Energies. Some Procedures with Reduced Errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Del Vitto, A.; Pacchioni, G.; Delbecq, F.; Sautet, P. Au Atoms and Dimers on the MgO(100) Surface: A DFT Study of Nucleation at Defects. J. Phys. Chem. B 2005, 109, 8040–8048. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Baldes, A. Segmented Contracted Basis Sets for One- and Two-Component Dirac–Fock Effective Core Potentials. J. Chem. Phys. 2010, 133, 174102. [Google Scholar] [CrossRef] [PubMed]
- Sierka, M.; Burow, A.; Döbler, J.; Aldridge, J. Point Defects in CeO2 and CaF2 Investigated Using Periodic Electrostatic Embedded Cluster Method. J. Chem. Phys. 2009, 130, 174710. [Google Scholar]
- Mulliken, R.S. Electronic Population Analysis on LCAO-MO Molecular Wave Functions. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Clarendon: Oxford, UK, 1990. [Google Scholar]
- Mayer, I.; Salvador, P. Overlap Populations, Bond Orders and Valences for ‘Fuzzy’ Atoms. Chem. Phys. Lett. 2004, 383, 368–375. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Ahlrichs, R.; Armbruster, M.K.; Bachorz, R.A.; Bahmann, H.; Baldes, A.; Bär, M.; Baron, H.; Bauernschmitt, R.; Bischof, F.A.; Böcker, S.; et al. TURBOMOLE 7.9; University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007; TURBOMOLE GmbH: Karlsruhe, Germany, 2024. [Google Scholar]
- Franzke, Y.J.; Holzer, C.; Andersen, J.H.; Begušić, T.; Bruder, F.; Coriani, S.; Della Sala, F.; Fabiano, E.; Fedotov, D.A.; Fürst, S.; et al. TURBOMOLE: Today and Tomorrow. J. Chem. Theory Comput. 2023, 19, 6859–6890. [Google Scholar] [CrossRef]
- Yu, M.; Trinkle, D.R. Accurate and Efficient Algorithm for Bader Charge Integration. J. Chem. Phys. 2011, 134, 064111. [Google Scholar] [CrossRef]
- Arnaldsson, A.; Tang, W.; Chill, S.; Chai, W.; Anselm, R.; Henkelman, G. Bader 1.04; The University of Texas at Austin, College of Natural Sciences: Austin, TX, USA, 2020. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T. A Comprehensive Electron Wavefunction Analysis Toolbox for Chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef]
- Pacchioni, G.; Ricart, J.M.; Illas, F. Ab Initio Cluster Model Calculations on the Chemisorption of CO2 and SO2 Probe Molecules on MgO and CaO (100) Surfaces. A Theoretical Measure of Oxide Basicity. J. Am. Chem. Soc. 1994, 116, 10152–10158. [Google Scholar] [CrossRef]
- Giamello, E.; Ugliengo, P.; Garrone, E. Superoxide Ions Formed on MgO through the Agency of Presorbed Molecules. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1989, 85, 1373–1382. [Google Scholar]
- Gingerich, K.A.; Cocke, D.L.; Miller, F. Thermodynamic Investigation of the Lead Molecules Pb2, Pb3, and Pb4 by Mass Spectrometry. J. Chem. Phys. 1976, 64, 4027–4033. [Google Scholar] [CrossRef]
- Rappoport, D.; Furche, F. Property-Optimized Gaussian Basis Sets for Molecular Response Calculations. J. Chem. Phys. 2010, 133, 134105. [Google Scholar] [CrossRef] [PubMed]



| MeO | Center | Spin State | d1 a | ΔE | Eads | Eadh |
|---|---|---|---|---|---|---|
| MgO | O2− | LS | 2.429 | 0.25 | −1.32 | −2.35 |
| HS | 2.431 | 0.00 | −1.57 | −1.58 | ||
| Fs | LS | 2.368 | 0.18 | −2.66 | −3.64 | |
| HS | 2.383 | 0.00 | −2.84 | −2.79 | ||
| CaO | O2− | LS | 2.376 | 0.20 | −1.78 | −2.85 |
| HS | 2.386 | 0.00 | −1.98 | −2.03 | ||
| Fs | LS | 2.448 | 0.19 | −2.96 | −3.86 | |
| HS | 2.461 | 0.00 | −3.15 | −3.02 | ||
| SrO | O2− | LS | 2.323 | 0.20 | −2.23 | −3.22 |
| HS | 2.328 | 0.00 | −2.43 | −2.37 | ||
| Fs | LS | 2.043 | 0.19 | −3.08 | −3.70 | |
| HS | 2.046 | 0.00 | −3.27 | −2.86 |
| MeO | Center | Spin State | d1 a | d2 b | d3 | ΔE | Eads | Eadh |
|---|---|---|---|---|---|---|---|---|
| MgO | O2− | LS | 2.456 | 2.536 | 2.940 | 0.00 | −2.17 | −2.66 |
| HS | 2.584 | 2.911 | 2.967 | 0.68 | −1.49 | −1.35 | ||
| Fs | LS | 2.360 | 2.680 | 2.854 | 0.00 | −3.69 | −4.24 | |
| HS | 2.373 | 2.667 | 3.114 | 0.94 | −2.75 | −2.70 | ||
| CaO | O2− | LS | 2.418 | 2.436 | 2.969 | 0.00 | −3.07 | −3.62 |
| HS | 2.414 | 2.440 | 3.263 | 0.76 | −2.31 | −2.48 | ||
| Fs | LS | 2.379 | 2.568 | 2.889 | 0.00 | −4.37 | −4.79 | |
| HS | 2.357 | 2.522 | 3.159 | 0.84 | −3.53 | −3.43 | ||
| SrO | O2− | LS | 2.355 | 2.355 | 2.989 | 0.00 | −3.89 | −4.25 |
| HS | 2.350 | 2.366 | 3.294 | 0.71 | −3.18 | −3.18 | ||
| Fs | LS | 2.035 | 2.421 | 2.912 | 0.00 | −4.91 | −4.90 | |
| HS | 1.844 | 2.371 | 3.013 | 0.60 | −4.31 | −3.74 |
| Pb2/MeO or Pb2 | Center | FBO |
|---|---|---|
| MgO | O2− | 1.68 |
| Fs | 1.71 | |
| CaO | O2− | 1.65 |
| Fs | 1.68 | |
| SrO | O2− | 1.49 |
| Fs | 1.62 | |
| Pb2 | 2.33 |
| MeO | Center | Spin State | Pb/MeO | Pb2/MeO | |||
|---|---|---|---|---|---|---|---|
| q(Pb) | Nspin(Pb) | q(Pb2) | Nspin(Pb1) | Nspin(Pb2) | |||
| MgO | O2− | LS | −0.020 (−0.156) | 0.000 | −0.266 (−0.328) | 0.000 | 0.000 |
| HS | −0.057 (−0.156) | 1.806 | −0.107 (−0.248) | 0.745 | 1.033 | ||
| Fs | LS | −0.377 (−1.698) | 0.000 | −0.507 (−1.811) | 0.000 | 0.000 | |
| HS | −0.427 (−1.693) | 1.627 | −0.601 (−1.807) | 0.786 | 0.908 | ||
| CaO | O2− | LS | −0.097 (−0.375) | 0.000 | −0.393 (−0.642) | 0.000 | 0.000 |
| HS | −0.137 (−0.357) | 1.767 | −0.508 (−0.635) | 0.880 | 0.927 | ||
| Fs | LS | −0.483 (−1.926) | 0.000 | −0.711 (−2.131) | 0.000 | 0.000 | |
| HS | −0.539 (−1.920) | 1.707 | −0.840 (−2.156) | 0.821 | 0.916 | ||
| SrO | O2− | LS | −0.420 (−0.540) | 0.000 | −0.869 (−0.805) | 0.000 | 0.000 |
| HS | −0.438 (−0.523) | 1.787 | −0.947 (−0.799) | 0.910 | 0.930 | ||
| Fs | LS | −1.240 (−2.045) | 0.000 | −1.588 (−2.281) | 0.000 | 0.000 | |
| HS | −1.234 (−1.980) | 1.873 | −1.582 (−1.992) | 1.088 | 0.132 | ||
| MeO | Center | Spin State | ||
|---|---|---|---|---|
| MgO | O2− | LS | −3.07 | −1.49 |
| HS | −2.39 | −0.82 | ||
| Fs | LS | −3.32 | −1.75 | |
| HS | −2.38 | −0.81 | ||
| CaO | O2− | LS | −3.56 | −1.58 |
| HS | −2.80 | −0.83 | ||
| Fs | LS | −3.69 | −1.72 | |
| HS | −2.85 | −0.88 | ||
| SrO | O2− | LS | −3.93 | −1.50 |
| HS | −3.22 | −0.78 | ||
| Fs | LS | −4.11 | −1.68 | |
| HS | −3.51 | −1.07 |
| MeO | Center | Pb/MeO | Pb2/MeO | ||
|---|---|---|---|---|---|
| Scalar | Scalar + SO | Scalar | Scalar + SO | ||
| MgO | O2− | −1.70 | −1.07 | −3.09 | −2.03 |
| Fs | −3.02 | −2.08 | −4.71 | −3.46 | |
| CaO | O2− | −2.21 | −1.44 | −4.12 | −2.86 |
| Fs | −3.62 | −2.44 | −5.30 | −4.00 | |
| SrO | O2− | −3.03 | −1.92 | −4.94 | −3.52 |
| Fs | −3.74 | −3.02 | −5.45 | −4.40 | |
| MeO | Spin State | d1 | ΔE | Eads | Eadh |
|---|---|---|---|---|---|
| MgO | LS | 2.429 (2.403) [2.395] | 0.25 (0.21) [0.19] | −1.32 (−1.39) [−1.36] | −2.35 (−2.41) [−2.37] |
| HS | 2.431 (2.412) [2.403] | 0.00 (0.00) [0.00] | −1.57 (−1.60) [−1.55] | −1.58 (−1.58) [−1.57] | |
| CaO | LS | 2.376 (2.336) [2.313] | 0.20 (0.20) [0.19] | −1.78 (−1.81) [−1.78] | −2.85 (−2.90) [−2.84] |
| HS | 2.386 (2.345) [2.321] | 0.00 (0.00) [0.00] | −1.98 (−2.01) [−1.96] | −2.03 (−2.06) [−2.04] | |
| SrO | LS | 2.323 (2.166) [2.146] | 0.20 (0.40) [0.17] | −2.23 (−2.29) [−2.20] | −3.22 (−3.55) [−3.90] |
| HS | 2.328 (2.166) [2.147] | 0.00 (0.00) [0.00] | −2.43 (−2.69) [−2.67] | −2.37 (−3.20) [−3.39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matczak, P. The Influence of Support Basicity on the Adsorption of Lead on the (100) Surface of Alkaline Earth Metal Oxide Crystals. Crystals 2025, 15, 748. https://doi.org/10.3390/cryst15090748
Matczak P. The Influence of Support Basicity on the Adsorption of Lead on the (100) Surface of Alkaline Earth Metal Oxide Crystals. Crystals. 2025; 15(9):748. https://doi.org/10.3390/cryst15090748
Chicago/Turabian StyleMatczak, Piotr. 2025. "The Influence of Support Basicity on the Adsorption of Lead on the (100) Surface of Alkaline Earth Metal Oxide Crystals" Crystals 15, no. 9: 748. https://doi.org/10.3390/cryst15090748
APA StyleMatczak, P. (2025). The Influence of Support Basicity on the Adsorption of Lead on the (100) Surface of Alkaline Earth Metal Oxide Crystals. Crystals, 15(9), 748. https://doi.org/10.3390/cryst15090748







