Abstract
For efficient utilization of carbide slag (CS) waste to high-value building materials, in this study, CS and ground granulated blast furnace slag (GBFS) were used as primary raw materials to prepare alkali-activated cementitious systems under strong alkaline excitation. Multiscale mechanisms involving macroscopic mechanical property development were investigated. Microstructural characterization elucidated how raw material composition affected mineral crystal formation and transformation while revealing enhancement mechanisms governing micro-gel network structure formation and evolution dynamics. The results indicate that excessive calcium components coupled with deficient Si–Al sources in CS severely inhibit the formation of C-S-H and C-A-S-H gel phases, consequently impeding mechanical performance development. Also, GBFS incorporation offsets inherent silicon–aluminum deficiencies. Active [SiO4]4− and [AlO4]5− released from GBFS drive polycondensation reactions toward advanced polymerization states. Compressive strength has a nonlinear growth kinetics characterized by rapid initial ascent, followed by asymptotic plateauing as GBFS content increases. Optimal comprehensive performance emerges at a 5:5 GBFS-to-CS mass ratio, where 28d compressive strength reaches 47.5 MPa.
1. Introduction
Carbide slag (CS) is an industrial byproduct generated from calcium carbide (CaC2) hydrolysis during acetylene production, consisting predominantly of Ca(OH)2 (70–90 wt.%) with strong alkalinity (pH 12–13) alongside trace amounts of SiO2, Al2O3, Fe2O3, and unreacted carbon particles [1]. The annual CS output in China is about 28–32 million tons, with production capacity heavily concentrated in Xinjiang, Inner Mongolia, Ningxia, and northern regions. Driven by the national “Dual Carbon” strategy, management paradigms for this solid waste have shifted fundamentally from passive stockpiling to systemic resource recovery [2]. CS is primarily utilized in cement production as a limestone substitute (acting as a calcium source); it is also used in chemical recycling (for soda ash synthesis and flue gas desulfurization), contaminated soil stabilization, and CO2 sequestration [3]. However, despite being calcium-rich, its high alkalinity and deficiency in silicon and aluminum limit its applicability. The high-value valorization, incurring huge costs, is another constraint. High-value stabilization of CS involves deep removal of chloride ions, which requires high energy consumption. These factors hinder its applicability and present obstacles to optimization in large-scale resource utilization [4].
CS faces significant challenges in resource utilization due to inherent limitations in its chemical composition, notably excessive free calcium oxide and chloride ions [5]. When used in cement production, these constituents readily cause process compatibility issues, posing risks of uncontrolled cement performance [6]. In the ammonia–soda process for synthetic soda ash, impurities such as silicon, aluminum, magnesium, and iron oxides present in the CS react with ammonia, generating insoluble precipitates [7]. These deposits rapidly form scale on the internal surfaces of ammonia still columns, heat exchangers, and pipelines, severely reducing heat transfer efficiency, increasing energy consumption, and clogging equipment [8]. More critically, these dissolved impurities combine with sulfate ions to form dense and complex precipitates under the acidic conditions of flue gas desulfurization slurry [9], which adhere to the internal walls and packing materials of key equipment (reaction towers, pipelines, pumps, and agitators) and are extremely difficult to remove [10]. Thus, the long-term, stable operation of desulfurization systems is negatively impacted. Consequently, whether utilized in cement kilns, soda ash production, or flue gas desulfurization, CA requires preprocessing, such as chloride washing, dewatering, aging, carbon particle screening, and fine grinding, to meet process specifications [11]. However, the preprocessing substantially increases the cost, presenting economic viability challenges [12].
The high-purity Ca(OH)2 in CS has an excellent alkali-activation potential, making it a low-cost calcium source for polycondensation reactions [13]. This effectively promotes C-(A)-S-H gel formation, establishing a fundamental basis for the resource utilization of alkali-activated building materials [14]. In such applications, the high-calcium content accelerates early-age condensation kinetics but suppresses the dissolution of aluminum-bearing phases [15]. Hence, optimizing the compositional design is critical to attaining desired mechanical properties and microstructural stability. The inherent impurities in CS do not significantly hinder the mechanical performance of alkali-activated materials; instead, these enhance CS reactivity by multi-ionic synergistic effects, facilitating the co-formation of different gel products and improving overall performance [16]. In order to address the challenges in CS resource utilization, high-strength, low-cost, and low-corrosion alkali-activated CS-based composites were developed in this study. Microstructural analyses were used to examine the influence mechanism of alkali composition design on activation efficacy, with particular emphasis on the effects of alkali environment on polycondensation reactions.
2. Materials and Methods
2.1. Raw Materials
The CS utilized in this study was sourced from an environmental technology company in Nanjing. Its specific surface area, measured by the nitrogen adsorption method, reached 725 m2/kg. The chemical composition determined by X-ray fluorescence spectroscopy (XRF) is presented in Table 1, indicating that CS has >70% CaO by mass, whereas combined amounts of secondary components, including SiO2, Al2O3, and Fe2O3, are <2%. Thus, CS is characterized as a high-calcium, low-silica-alumina solid waste. S95-grade ground granulated blast furnace slag (GBFS) with a specific surface area of 416 m2/kg, conforming to Chinese National Standard GB/T 18046-2017 [17], was obtained from a mineral products enterprise in Huainan. Its chemical composition data is given in Table 1. NaOH activator consisted of analytical-grade solid particles with purity ≥99%. Standard ISO sand with a specific gravity of 2.58 and a fineness modulus of 1.87 was used as fine aggregate. Ordinary tap water was used in the specimen preparation.

Table 1.
Chemical properties of CS and GBFS %.
2.2. Experimental Methods
2.2.1. Mix Proportions
Mix proportions for the alkali-activated CS pastes are given in Table 2. Mortars with a liquid-to-solid ratio of 0.5 were prepared for compressive strength evaluation. The control group comprised pure CS (designated C100). The mixes (C90G10 to C30G70) had a constant alkali-activator dosage while incorporating GBFS at 10% increments, correspondingly reducing CS proportion, to systematically investigate the influence of GBFS incorporation within this alkali-activated system.

Table 2.
Mix proportion of samples/g.
2.2.2. Specimen Preparation
Specimen preparation and curing were conducted following the standard test method for mortar strength (ISO method) GB/T 17671-2021 [18]. Taking mixture C90G10 as an example, according to Table 2, 20 g NaOH was dissolved in 225 g water and allowed to cool for 2 h. Subsequently, 405 g CS and 45 g GBFS were homogenized using a planetary mixer before transferring to the mixing container. The prepared NaOH solution was then introduced under slow mixing for 30 s, followed by 30 s of rapid mixing. Standard sand was subsequently incorporated with 60 s of high-speed mixing. The resulting mortar was cast into 40 mm × 40 mm × 160 mm steel molds and immediately subjected to compaction on a vibrating table for 30 s. All specimens were placed in a curing chamber immediately after molding, after which they were demolded (after 24 h of curing) and placed in the controlled environment until the desired testing age. The curing chamber maintained constant conditions at 20 ± 1 °C with relative humidity exceeding 95%.
2.2.3. Compressive Strength Testing
Compressive strength testing was conducted using a DYE-300S integrated testing machine (ISO method, GB/T 17671-2021) [18]. The compressive strength of specimens was determined at curing ages of 3d, 28d, and 60d, with reported results representing the average of six measurements.
2.2.4. Microscopic Analysis
The effects of GBFS incorporation ratio on mineral crystal evolution and micro-gel system formation were studied by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), and scanning electron microscopy (SEM). Through XRD and FT-IR, qualitative analyses of phase composition and crystallinity were carried out. SEM was performed to elucidate the formation of characteristic phases such as calcium silicate hydrate (C-S-H) and calcium aluminosilicate hydrate (C-(A)-S-H) under different mix proportions.
3. Results, Discussion, and Analysis
3.1. Compressive Strength
3.1.1. Analysis of Compressive Strength of Pure CS
The compressive strength evolution of CS specimens incorporating varying GBFS proportions after 3 d, 28 d, and 60 d standard curing is shown in Figure 1. C100 (pure CS, Figure 1a) exhibits significantly constrained early polycondensation, demonstrating merely 1.1 MPa compressive strength at 3 d, gradually increasing to 2.5 MPa by 28 d, and reaching only 3.2 MPa even after prolonged curing up to 60 d. This strength development indicates inadequate polymerization reactivity of CS under strong alkali activation, rendering it unsuitable for use as a construction material, as it does not meet the mechanical performance benchmarks [19]. Since the calcium-based compounds constitute approximately 80% of CS composition, while critical gel-forming elements such as silicon and aluminum (e.g., SiO2 and Al2O3) collectively account for <2% (Table 1), it lacks the essential chemical foundation for the formation of C-S-H, C-A-S-H, and amorphous gel networks even under optimized activation conditions [20]. Further, unlike Portland cement, which undergoes high-temperature clinkering, CS is produced as a byproduct in acetylene production, so it does not experience any energy-intensive atomic rearrangement [21]. This results in significantly restricted surface energy and dissolution–precipitation kinetics. The chemical composition imbalance and thermal history deficiency are the main reasons for the compromised strength development in the C100 system.
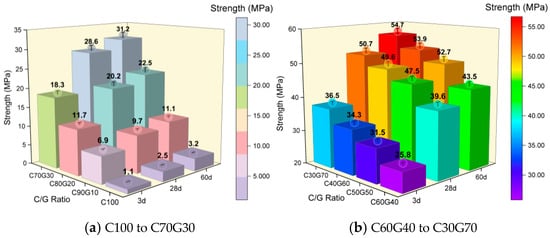
Figure 1.
Analysis of compressive strength.
3.1.2. Effect of GBFS Addition on Compressive Strength
At 10% GBFS incorporation (45 g), the C90G10 (Figure 1a) could achieve 6.9 MPa compressive strength after 3d curing, which further increased to 9.7 MPa and 11.1 MPa at 28d and 60d, respectively, significantly exceeding the performance of the C100. This demonstrates that GBFS incorporation effectively enhances the mechanical properties of CS-based alkali-activated cementitious materials. The strengthening mechanism is primarily attributed to the unique material characteristics of GBFS. GBFS consists mainly of a silicate–aluminate glassy network with core components including 30–45% CaO, 30–42% SiO2, and 7–15% Al2O3 (Table 1), forming a high-calcium, silicon–aluminum-enriched, low-crystallinity material that confers exceptional alkali-activated reactivity potential [22]. The metastable glassy structure formed through high-temperature melting, followed by water quenching, rapidly freezes atomic rearrangement dynamics within the silicate–aluminate melt. This process preserves numerous distorted [SiO4]4− and [AlO4]5− tetrahedral networks alongside high-density bond-breaking sites throughout the amorphous phase [23]. The amorphous structure releases reactive Si and Al ions that promote gel formation. Consequently, the high calcium content in CS (approximately 80%) is effectively utilized, facilitating accelerated formation of C-S-H and C-A-S-H gel phases through synergistic polycondensation.
Increasing GBFS amounts from C80G20 to C50G50 resulted in continuously enhanced compressive strength development. C50G50 (Figure 1b) could reach 31.5 MPa compressive strength after only 3d curing, further advancing to 47.5 MPa and 52.7 MPa at 28 d and 60 d, respectively. Strength increments for all curing ages consistently remained within 10–30%, signifying that elevated GBFS content optimizes polymerization reaction in CS-based systems. Primarily, GBFS introduces reactive silicon–aluminum components, modulating the Ca/Si molar ratio within CS–GBFS composites from 50.2 in pure CS (Table 1) to an optimal 3.02 range (C50G50). This adjustment achieves stoichiometric equilibrium in calcium, silicon, and aluminum at the atomic scale [24]. The optimized elemental proportion also significantly accelerates polycondensation efficiency between [SiO4]4−/[AlO4]5− tetrahedra and Ca2+, driving continuous nucleation and three-dimensional growth of high-density C-S-H and C-A-S-H gel phases [25]. Such microstructural evolution improves early-age strength development and establishes a percolating network skeleton through ionic bridging interactions among gel phases, ultimately realizing exponential enhancement in compressive strength throughout prolonged curing [26].
3.1.3. Effect of Excessive GBFS Addition
Although increasing GBFS dosage improves compressive strength, excessive GBFS addition is not that useful. When GBFS incorporation exceeds the 50% threshold, C40G60 and C30G70 (Figure 1b) exhibit merely 1–3% strength gain, elucidating a well-defined saturation point in the GBFS enhancement effect and an optimal stoichiometric ratio within the CS–GBFS binary system. Excessive GBFS introduction leads to surplus reactive silicon–aluminum components (C30G70, SiO2 + Al2O3 content > 37%, Table 1), whereas proportional CS reduction leads to insufficient calcium supply (CaO content < 45%, Table 1). Consequently, the Ca/(Si + Al) molar ratio decreases disproportionally from 3.02 in C50G50 to 1.2 in C30G70 (Table 1). Such calcium-deficient conditions hinder gel formation, as excess [SiO4]4− and [AlO4]5− tetrahedra lack sufficient Ca2+ for three-dimensional network crosslinking, while inadequate Ca2+ concentration substantially impedes the nucleation barrier-crossing process for C-(A)-S-H gel formation [27]. Therefore, the 1:1 GBFS-to-CS mass ratio (C50G50) satisfies stoichiometric balance among calcium–silicon–aluminum and fulfills ion migration kinetics requirements. This equilibrium enables continuous gel formation and synergistic optimization of macro-mechanical properties.
3.2. X-Ray Diffraction (XRD) Analysis
3.2.1. XRD Analysis of GBFS and CS
Figure 2a shows the XRD patterns for CS and GBFS. GBFS exhibits characteristic features of amorphous material, notably the absence of sharp crystalline diffraction peaks. Instead, a prominent and broad diffuse scattering hump, often termed a “broad hump,” is observed within 20° to 35° (2θ). This broad hump shows that GBFS consists predominantly of a glassy phase, i.e., an amorphous structure [28]. This phenomenon originates from the rapid quenching applied to GBFS following high-temperature calcination, which effectively arrests the crystallization process, preventing the transformation of the molten slag into stable crystalline phases and consequently “freezing” its structure into a disordered, non-crystalline state long term. The resulting high glass-phase content constitutes the fundamental material basis for the high reactivity potential inherent in GBFS.
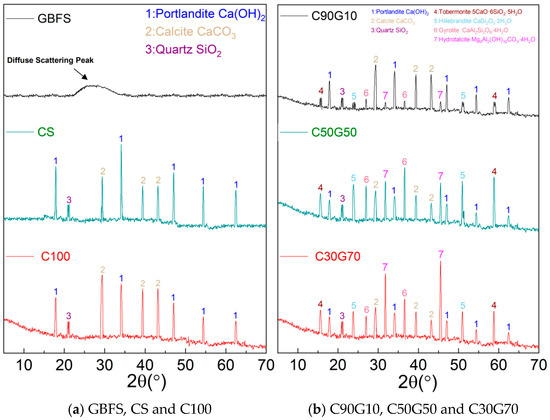
Figure 2.
XRD analysis.
In contrast, the XRD pattern of CS has distinctly different mineralogical characteristics. Well-defined diffraction peaks are readily identifiable, with the most intense ones corresponding to portlandite (Ca(OH)2, PDF#44-1481) and calcite (CaCO3, PDF#05-0586), indicating the high-calcium nature of CS. The presence of calcite does not represent an original phase; rather, it is the product of carbonation reactions, which occur when calcium-bearing minerals within CS, such as portlandite (Ca(OH)2), react with atmospheric carbon dioxide (CO2) during prolonged exposure to the natural environment. Further, the XRD shows a weak diffraction peak attributed to quartz (SiO2, PDF#46-1045). The attenuated intensity and broadened profile of this peak indicate poor crystallinity of the quartz phase, signifying its limited content in CS and an imperfect crystalline state. This result closely aligns with the known chemical profile of CS, characterized by high calcium content and lower silicon and aluminum amounts, corroborating its phase dominated by calcium-rich minerals with minimal contributions from silico-aluminous phases [29].
3.2.2. Influence of GBFS Doping on XRD Analysis
Figure 2a shows the XRD analysis of C100. Compared to the CS, the C100 exhibits three main characteristics, i.e., a pronounced reduction in the portlandite characteristic diffraction peak intensity, a substantial increase in calcite characteristic peak intensity with maintained stability in quartz-phase diffraction peak intensity, and the absence of detectable new peaks corresponding to calcium silicate or aluminosilicate mineral phases. This indicates the structural reconfiguration of Ca(OH)2 crystals under strong alkaline conditions. Elevated hydroxyl ion (OH−) concentrations disrupt hydrogen bonding networks within the layered lattice of Ca(OH)2, promoting its transformation into soluble calcium tetrahydroxide complex ions [30]. This process drastically enhances Ca2+ solubility, causing a reduction in diffraction signals from the portlandite phase. Simultaneously, continuous atmospheric CO2 ingress into the strong alkaline system initiates anisotropic carbonation. Dissolved Ca2+ and CO32− undergo preferential complexation catalyzed by OH−, yielding calcite crystals with high purity and density. The newly formed calcite crystals participate in synergistic crystallization with inherent CaCO3 particles present in the raw CS, establishing a rigid skeleton framework through nanoscale interlocking [31]. However, lower reactive silica and alumina phases within the system suppress polycondensation reactions involving calcium silicate oxygen anions and aluminosilicate oxygen anions. Consequently, critical cementitious phases, calcium aluminosilicate hydrate gel, and geopolymer networks fail to develop [32]. This binder architecture, dominated by mineral crystals without gel phase reinforcement, ultimately restricts C100 to exhibiting only marginal mechanical strength.
A 10% GBFS addition significantly changes the XRD pattern of the C90G10, as shown in Figure 2b. The diffraction peaks of tobermorite (5CaO·6SiO2·5H2O, PDF#45-1480) and hillebrandite (CaSi2O5·2H2O, PDF#29-0332) are identified; although these phases are of low intensity, they confirm the formation of calcium silicate mineral phases within the system. The diminished peak intensity and broadened peak profiles indicate insufficient crystallinity and limited formation of these crystals, directly attributed to inadequate supply of reactive silica sources resulting from the low GBFS dosage. Further, the appearance of a gismondine characteristic peak (CaAl2Si2O8∙4H2O, PDF#35-0755) reveals the crystallization process of calcium aluminosilicate minerals. Also, the formation of hydrotalcite (Mg6Al2(OH)16CO3·4H2O, PDF#41-1428) is observed. As a layered double hydroxide, its presence elucidates the critical role of GBFS in enhancing silica sources and confirms the successful introduction of reactive Mg2+ and Al3+ into the alkali-activated reaction system [33]. The Mg2+ and Al3+ ions liberated through dissolution from the GBFS glassy phase undergo polymerization with continuously dissolved Ca2+ from CS in the strong alkaline medium [34] and participate in developing a network of aluminosilicate tetrahedra, ultimately promoting the growth of aluminosilicate mineral crystals. The persistent strength development in later stages is attributed to this phenomenon. These collective phenomena reveal the dual contribution of GBFS incorporation to the mineral evolution within the system. It provides the essential nucleation for calcium silicate and calcium aluminosilicate minerals, while simultaneously triggering a synergistic effect through the release of highly reactive ions [35]. The substantial quantity of dissolved Ca2+ from CS exhibits high mobility in the alkaline environment, actively binding to dissociated silicate tetrahedra and aluminate tetrahedra from GBFS. This facilitates efficient condensation and crystallization of C-S-H and C-A-S-H phases. This synergistic action significantly optimizes the phase composition and microstructure of the polymerization products, ultimately leading to mechanical enhancement.
The XRD pattern of the C50G50 (i.e., when the GBFS dosage increases to 50%) indicates a continuous optimization trend, as shown in Figure 2b. The characteristic peak intensities of both calcite and portlandite exhibit significant reduction, whereas the distinctive peaks of tobermorite and hillebrandite display significantly sharpened intensity. The characteristic peak intensities of gismondine and hydrotalcite also show substantial enhancement compared to those in C90G10. Thus, it is inferred that increased GBFS content leads to a progressive increase in the highly reactive silicon–aluminum components within the vitreous phase of the blended system. Under strong alkaline conditions, these components undergo accelerated dissociation, releasing significantly elevated concentrations of silicate tetrahedra and aluminate tetrahedra, providing abundant nucleation sites for calcium ions dissolved from CS. As GBFS incorporation reaches the 50% threshold, the molar ratio of calcium to silicon to aluminum within the system reaches an optimal range, driving the condensation reaction away from discrete mineral crystallization pathways, such as calcite formation, towards a three-dimensionally networked calcium aluminosilicate gel-dominated mode, e.g., gismondine [36]. Simultaneously, the polymerization process involving magnesium and aluminum elements from GBFS intensifies. The resulting layered double hydroxides, such as hydrotalcite phases, capture free ions through interlayer anion exchange and reduce liquid-phase ionic strength, thereby enhancing the condensation rates of silico-aluminate oxygen anions. Further, Al3+ released from GBFS selectively integrates into the C-S-H gel framework. Thus, the gel density increases by the isomorphous substitution, where aluminate tetrahedra replace silicate tetrahedra. This transforms the structure of the reaction product from low-polymerization-degree chain-like configurations to high-polymerization-degree layered or framework architectures [37]. Such microstructural densification increases compressive strength beyond 60 MPa and overcomes the inherent brittle fracture in pure CS systems by forming a continuous matrix rich in gel phases.
3.2.3. XRD Analysis of Mixes with Excessive GBFS Addition
When the GBFS incorporation increases to 70%, the XRD pattern of the C30G70 composite system does not exhibit a continuous optimization trend, as shown in Figure 2b. Compared to C50G50, the characteristic diffraction peak intensities of tobermorite and hillebrandite in C30G70 do not increase with increasing GBFS content but rather are weakened. This indicates a reduction in the crystallinity of calcium silicate minerals. Similarly, the characteristic peak intensity of gismondine also weakens, confirming that the crystallization process of calcium aluminosilicate minerals is suppressed [38]. However, the characteristic peak intensity of hydrotalcite increases, signifying that excessively high GBFS content (>60%) substantially inhibits CS reactivity. The fundamental mechanism lies in the reaction pathway disorder induced by multi-ion competition. Excess GBFS releases silicate tetrahedra and aluminate tetrahedra that form localized supersaturation within the strong alkaline environment, promoting homogeneous condensation among silico-aluminate oxygen anions and generating discrete magnesium aluminate crystals, such as hydrotalcite and amorphous aluminosilicate colloids, rather than enabling effective heterogeneous bonding with dissolved Ca2+ from CS [39]. This ionic imbalance has dual negative effects. On the one hand, the limited quantity of Ca2+ within the system becomes encapsulated by the excessive silico-aluminate network, hindering access to nucleation sites, which causes a sharp decline in the nucleation efficiency of calcium silicate minerals and calcium aluminosilicate minerals. Also, excess silicon and aluminum elements accelerate the crystallization of layered double hydroxides, such as hydrotalcite phases. The layer growth process of these phases consumes substantial amounts of Ca2+ and OH− within the system, leading to reduced reactant concentrations. Thus, a significant reduction in the compressive strength development rate is observed.
3.3. FT-IR Analysis
3.3.1. FT-IR Analysis of C100
The infrared spectrum of C100 is shown in Figure 3a, exhibiting a prominent characteristic peak at 3442 cm−1, which corresponds to free O-H stretching vibrations. This indicates abundant alkaline substances within the system, likely originating from the NaOH activator or the Ca(OH)2 component inherent in CS. This further signifies low efficiency of CS in the polycondensation reaction of C100, as both the activator and the precursor (CS) failed to participate effectively in polymerization. Also, the appearance of a CO32− group stretching vibration peak at 1440 cm−1 corroborates XRD results, elucidating substantial formation of CaCO3 crystals. The H-O-H bending vibration peak of water molecules at 1640 cm−1 reveals the presence of crystalline water. The complete absence of the Si-O-T (T = Si or Al) asymmetric stretching vibration characteristic peak within the 800–1200 cm−1 range demonstrates that no C-S-H or C-A-S-H gel phases are formed. The lack of the aluminosilicate framework characteristic peak (Al-O vibration) at 670 cm−1 further signifies a severe deficiency in the formation of silicon–aluminum structures, so there were no significant aluminosilicate gelation reactions. These findings are consistent with the XRD results.
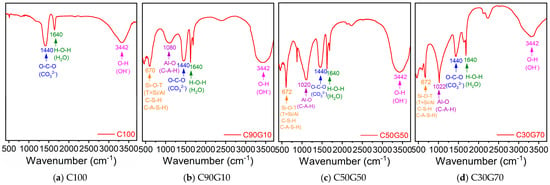
Figure 3.
FT-IR analysis.
3.3.2. FT-IR Analysis of C90G10, C50G50, and C30G70
Incorporating GBFS led to improved spectral features in the infrared spectrum of C90G10 (Figure 3b). A weak characteristic peak corresponding to Si-O-T (T = Si/Al) asymmetric stretching vibration was observed at 1080 cm−1, confirming formation of calcium silicate and calcium aluminosilicate gel within the system. However, due to the low GBFS content (i.e., 10%), the total silicon and aluminum introduced into the system were insufficient, resulting in limited gel formation, which is the direct cause of the low peak intensity. Further, the emergence of an Al-O vibration characteristic peak at 670 cm−1, together with the peak at 1080 cm−1, validates the role of GBFS in enhancing silicon and aluminum supply to the system. The low intensity of these characteristic peaks is due to the limited GBFS content. These findings indicate that GBFS incorporation effectively compensates for the deficiency of silicon and aluminum components in CS. It significantly enhances the reactivity of the system, promotes the formation of high high-strength gel phase and consequently improves mechanical performance. Thus, phase analysis by XRD and spectral features from FT-IR are consistent in ascertaining the mechanical enhancement of alkali-activated cementitious systems.
Increasing the GBFS incorporation to 50% resulted in enhanced intensity and significantly broadened width of the Si-O-T (T = Si/Al) characteristic peak at 1020 cm−1 for C50G50 (Figure 3c) compared to C90G10. This indicates an expanded distribution range of chemical bond vibration modes, reduced local structural ordering, and diminished uniformity in atomic arrangement. It also confirms the transition of the system toward an amorphous C-A-S-H gel phase. The peak shifted towards left, corresponding to movement toward higher wavenumbers, reflecting the incorporation of Al3+ into the silicate network structure through substitution of [SiO4]4− tetrahedra by [AlO4]5− tetrahedra. This process effectively increased the quantity of C-A-S-H gel generated, substantially increasing the compressive strength of C50G50. This mechanism is further strongly supported by the enhanced intensity of the Al-O vibration characteristic peak observed at 672 cm−1. This result also shows high consistency with the increased formation of calcium aluminosilicate mineral crystals identified in XRD analysis.
When the GBFS dosage is increased to 70%, the Si-O-T (T = Si/Al) characteristic peak intensity at 1022 cm−1 for C30G70 (Figure 3d) did not exhibit the anticipated increase; instead, the peak width narrowed significantly, indicating that the C-A-S-H gel generated did not increase with higher GBFS amount and that the system crystallinity increased. It also confirms that the transition toward the amorphous gel phase was hindered. Further, a distinct attenuation in the intensity of the Al-O vibration characteristic peak at 672 cm−1 was observed, signifying reduced capacity for Al3+ incorporation into the silicate network structure, resulting in a reduced structural substitution rate of [SiO4]4− tetrahedra by [AlO4]5− tetrahedra. This weakening in aluminum incorporation efficiency suppressed C-A-S-H gel formation, explaining the lack of significant compressive strength improvement despite a continued increase in GBFS dosage.
3.4. SEM Imaging
3.4.1. SEM Analysis of CS and C100
The SEM images of CS and C100 are shown in Figure 4. The microscopic morphology of CS (Figure 4a) exhibits distinct multiscale characteristics. The dominant features consist of irregularly sized spherical or ellipsoidal particles, which commonly show agglomeration or mutual adhesion. This spherical morphology is typically associated with rapid carbonation and calcination. Irregular flaky particles are also present, which originate from the layered crystallization of Ca(OH)2, where interlaminar stacking forms an abundant open-pore network, leading to a high specific surface area. Driven by surface forces and capillary effects, micron-scale particle clusters further aggregate, generating loose porous flocculent blocks. Their internal and external surfaces feature interconnected channels spanning nanoscale to micron-scale dimensions. The complex structure comprising flaky crystals, spherical particles, and porous agglomerates regulates the physical properties and chemical reactivity of the material [40].

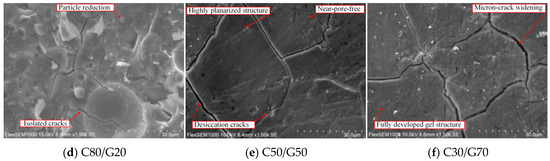
Figure 4.
Microstructure of CS alkali-activated paste specimen (×1500).
As shown in Figure 4b, strong alkali activation significantly affects the microstructure of C100. Characteristic spherical and ellipsoidal particles within CS exhibit densely etched grooves and pores on their surfaces, with edges displaying distinct passivation. The layered Ca(OH)2 crystals undergo profound dissociation, and their lamellar frameworks progressively disintegrate in the alkaline environment, generating a typically amorphous gel phase within particle interstices and across surfaces. This gel phase and particulate matter are loosely arranged with weak interparticle bonding cohesion. The hydrated C100 system shows pronounced polymerization inhibition characteristics, including incomplete polycondensation and structurally loose products, heterogeneous gel with bonding defects, and crack networks developing extensively. These structural defects are due to porosity and fissure propagation originating from the elemental imbalance of CS [41]. Specifically, excessive Ca2+ induces precipitation of low-cohesion Ca(OH)2 recrystallized phases, whereas deficient [SiO4]4− and [AlO4]5− supply restricts formation of highly polymerized C-S-H and C-A-S-H gels. This nucleation and growth restriction constrains the enhancement of microstructural density and mechanical strength, consistent with the XRD results, validating the underlying causes of reduced mechanical performance in C100.
3.4.2. Microstructure Analysis of GBFS Improves CS
As shown in Figure 4c, adding GBFS induces systematic microstructural optimization within CS. Compared to C100, the microstructure of the C90/G10 composite shows fewer unreacted spherical particles, with residual particles showing partial integration into the matrix. Although surface pores and cracks are observed, the original loose honeycomb pore network is replaced by a dense continuous gel phase. Micron-scale cracks are effectively filled by newly formed gels, leading to a significant reduction in density. Pore dimensions become refined to nanoscale levels by progressive gel growth, enhancing structural integrity and overall compactness [42]. This microstructural evolution confirms that GBFS effectively improves polycondensation reactions within CS particles. Active silicon and aluminum components released from GBFS precisely compensate for the inherent calcium–silicon imbalance, optimizing the Ca/Si molar ratio to 14.6 (C100, 50.2). This process forms highly stable gels through dual mechanisms—supplementation of [SiO4]4− and [AlO4]5− enables construction of highly polymerized gel frameworks and moderation of alkalinity suppresses Ca(OH)2 recrystallization. The resulting particle gel structure becomes compact and densified, thereby increasing the macroscopic mechanical properties.
As shown in Figure 4d, increasing GBFS content to 20% improves C80/G20. Unreacted free particles decrease continuously while surface flatness improves substantially. Primary macropores within the initial defective structure are eliminated, and the nanoscale pore density is further reduced. Microcracks with widths < 800 nanometers diminished and crack networks transitioned from continuous interconnected patterns to localized isolated distributions. Gel uniformity is also significantly enhanced as C-A-S-H gels develop interpenetrating networks covering over 90% of the matrix area. This densification and homogenization are because of the active components in GBFS and CS, stabilizing the Ca/Si ratio to 8.1 [43].
Figure 4e shows the microstructure of C50/G50. It is observed that increasing GBFS content to 50% triggers phase transitions in the C50/G50. Original CS particles are completely dissolved into the gel matrix with unreacted cores disappearing entirely. The pore structure is refined as the surface root mean square roughness and porosity significantly decrease markedly relative to C90/G10. The microstructural refinement validates the development of polycrystalline phases observed by XRD analysis and explains the compressive strength enhancement. However, increased GBFS content increases the proportion of aluminum-rich C-A-S-H phases, increasing the drying shrinkage rate substantially. Differential shrinkage between phases induces micron-scale cracks (1–5-micron width). These cracks propagate along gel-phase interfaces, forming grid-like damage skeletons that partially offset densification gains from pore refinement. Hence, it is inferred that the critical GBFS dosage is 40–50%. Exceeding this threshold activates microdefect regeneration governed by shrinkage stresses.
3.4.3. Influence of Excessive GBFS Dosage on Microstructure
When GBFS content increases to 70%, the C30/G70 (Figure 4f) microstructure exhibits significant degradation. Compared to C50/G50, C30/G70 shows no deterioration in gel structural integrity, matrix compactness, or phase distribution uniformity. CS particle participation in polycondensation reactions is high, and bulk gel networks maintain continuous skeletal characteristics. However, the microdamage pattern is transformed. Average microcrack width increases to 10–15 microns, i.e., 3.2 times, relative to C50/G50. Microcrack density increases concurrently, forming interconnected and penetrating networks. The accelerated crack propagation is due to the phase composition imbalance caused by excessive GBFS content. When GBFS content is >50% (threshold value), the aluminum-rich C-A-S-H gel proportion significantly increases [44]. The layered silicate tetrahedral structure generates intense in-plane shrinkage anisotropy during dehydration, causing increased drying shrinkage and microcrack propagation. Ultimately, the strain energy dissipation capacity of crack networks reduces, leading to stagnation in compressive strength enhancement. This structural degradation confirms that, beyond 50% GBFS content, shrinkage stresses dominated by aluminum-rich phases counter the densification effects from pore refinement. Thus, mix design requires controlling GBFS dosage within 40% to 50% to achieve an optimal balance between shrinkage stress and gel strengthening effects.
4. Conclusions
In this study, the multiscale mechanisms governing alkali activation and GBFS synergistic modulation of CS condensation are investigated, and the design principles based on compressive strength development, phase transformation, and microstructure evolution are put forward. The following conclusions are drawn from the obtained results:
(1) Under strong alkali activation of pure CS, the C100 remains constrained by inherent high-calcium, low-silicon–aluminum characteristics. Reaction becomes restricted to metastable calcite-phase crystallization processes, while recrystallized Ca(OH)2 phases are generated. This phase combination prevents the formation of spatially continuous high-strength C-S-H or C-A-S-H gel networks within C100. The resulting compressive strength is developed only through weak bonding mechanisms, reaching merely 2.5 MPa at 28 days.
(2) Incorporating GBFS compensates for inherent silicon–aluminum deficiencies in CS. The released active [SiO4]4− and [AlO4]5− anions drive polycondensation reactions to high polymerization degrees. XRD analysis confirms synergistic formation of multiple cementitious phases, optimizing aluminosilicate interfaces. At microscopic scales, CS reaction extent substantially increases, transforming gel structures from discrete heterogeneous states into 3D covalent-bonded interlocking networks. This phase reorganization elevates 28-day compressive strength to 42.6 MPa, attributed to high-polymerization-degree gel networks replacing weak crystalline phases.
(3) Further increasing GBFS content progressively optimizes calcium–silicon–aluminum elemental ratios within the composite. The compressive strength shows a nonlinear trend, i.e., rapid initial ascent followed by gradual stabilization. At a 5:5 mass ratio of GBFS to CS, the C50G50 achieves optimal comprehensive performance with 28-day compressive strength reaching 47.5 MPa. Microscopic characterization verifies significant sharpening of characteristic XRD peaks for calcium silicate and aluminum calcium silicate phase minerals. Enhanced CS particle reaction transforms the microstructure to a 3D skeletal network dominated by C-A-S-H gel, facilitating strength enhancement.
(4) Increasing GBFS content to 70% degrades calcium–silicon–aluminum elemental ratios, not further increasing compressive strength. Microscopic analysis reveals intensified hydrotalcite phases alongside attenuated peak intensities and crystallinity reduction for calcium silicate and aluminum calcium silicate phases. This phase composition imbalance may lead to drying shrinkage, consequently expanding average microcrack widths and increasing crack density. These phenomena confirm that, beyond the 50% GBFS critical threshold, shrinkage stresses dominate and cause microdefect regeneration, which counters structural densification attained by GBFS.
Author Contributions
Formal analysis, Y.H.; Investigation, F.Z.; Resources, Z.H.; Data curation, M.L. and J.H.; Writing—review & editing, G.H. All authors have read and agreed to the published version of the manuscript.
Funding
The research described in this paper was financially supported by Anhui ProvincialNatura Science Foundation “Design and Collaborative Enhancement Mechanism of Cement lmproved CoaGangue Alkali Activated Double Cementitious Material” (2308085ME184) and Researchand Development Special Project of Environmentally Friendly Materials and Occupational HealthResearch Institute (Wuhu), Anhui University of Science and Technology “Research and applicationof compatible coupling and synergistic enhancement mechanism of alkali activated cementitiousmaterials (ALW2021YF01); 2023 Wuhu Science and Technology Bureau Applied Basic Research Project” Designand Collaborative Enhancement Mechanism of Cement Improved Waste Incineration BottomAsh Alkali Activated Double Cementitious Materials”(No. 2023jc01); Anhui Provincial Department of Education’s 2023 New Era Education Quality Project “Wuhu OiuhuaThermal Insulation Materials Co., Ltd. Graduate Enterprise Workstation” (2023qygzz021).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Zhenghu Han was employed by the company China ConstructionSecond Engineering Bureau Co., Ltd. Author Fengan Zhang was employed by the Special Equipment Inspection Institute of Anhui. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Sun, D.; Yin, F.; Deng, Y.; Liu, K.; Tang, J.; Shen, C.; Sun, Y.; Wang, A.; Huang, N.; Hu, C. Utilization of carbide slag in autoclaved aerated concrete (CS-AAC) and optimization: Foaming, hydration process, and physic-mechanical properties. Case Stud. Constr. Mater. 2023, 19, e02354. [Google Scholar] [CrossRef]
- Yang, J.; Dong, S.; Xie, L.; Cen, Q.; Zheng, D.; Ma, L.; Dai, Q. Analysis of hydrogen-rich syngas generation in chemical looping gasification of lignite: Application of carbide slag as the oxygen carrier, hydrogen carrier, and in-situ carbon capture agent. Energy 2023, 283, 128499. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, Y.; Zhu, H.; Zhou, Q. Performance activation and strength evolution mechanism of carbide slag on anhydrous phosphogypsum backfill material. Constr. Build. Mater. 2024, 419, 135503. [Google Scholar] [CrossRef]
- Li, H.; Wang, R.; Wei, M.; Lei, N.; Wei, T.; Liu, F. Characteristics of carbide-slag-activated GGBS-fly ash materials: Strength, hydration mechanism, microstructure, and sustainability. Constr. Build. Mater. 2024, 422, 135796. [Google Scholar] [CrossRef]
- Alnahhal, M.F.; Kim, T.; Hajimohammadi, A. Waste-derived activators for alkali activated materials: A review. Cem. Concr. Compos. 2021, 118, 103980. [Google Scholar] [CrossRef]
- Chen, T.; Gao, Y.; Li, Y.; Zhu, J.; Cheng, Z.; Xiong, H. The strength; reaction mechanism, sustainable potential of full solid waste alkali-activated cementitious materials using red mud and carbide slag. Constr. Build. Mater. 2024, 449, 138454. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, J.; Yan, C.; Yin, L.; Wang, X.; Liu, S. Hydration characteristics of low carbon cementitious materials with multiple solid wastes. Constr. Build. Mater. 2022, 322, 126366. [Google Scholar] [CrossRef]
- Sun, X.; Liu, J.; Qiu, J.; Wu, P.; Zhao, Y. Alkali activation of blast furnace slag using a carbonate-calcium carbide residue alkaline mixture to prepare cemented paste backfill. Constr. Build. Mater. 2022, 320, 126234. [Google Scholar] [CrossRef]
- Gao, X.; Yao, X.; Yang, T.; Zhou, S.; Wei, H.; Zhang, Z. Calcium carbide residue as auxiliary activator for one-part sodium carbonate-activated slag cements: Compressive strength, phase assemblage and environmental benefits. Constr. Build Mater. 2021, 308, 125015. [Google Scholar] [CrossRef]
- Seo, J.; Park, S.; Yoon, H.N.; Jang, J.G.; Kim, S.H.; Lee, H.K. Utilization of calcium carbide residue using granulated blast furnace slag. Materials 2019, 12, 3511. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, Q.; Xue, C.; Jia, Y.; Guo, W.; Zhang, Y.; Qiu, Y. Preparation and curing method of red mud-calcium carbide slag synergistically activated fly ash-ground granulated blast furnace slag based eco-friendly geopolymer. Cem. Concr. Compos. 2023, 139, 104999. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; He, X.; Su, Y.; Zeng, J.; Xiong, L.; Zeng, L.; Yu, X.; Tan, H. Low-carbon wet-ground fly ash geopolymer activated by single calcium carbide slag. Constr. Build. Mater. 2022, 353, 129084. [Google Scholar] [CrossRef]
- Yang, J.; Bai, H.; Zeng, J.; Su, Y.; Wang, X.; Zhao, H.; Mao, C. Performances and microstructure of one-part fly ash geopolymer activated by calcium carbide slag and sodium metasilicate powder. Constr. Build. Mater. 2023, 367, 130303. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Li, C.; Wang, J.; Lyu, X. Synthesis and optimization of green one-part geopolymer from mine tailings and slag: Calcium carbide residue and soda residue as supplementary alkali sources. Constr. Build. Mater. 2022, 353, 129013. [Google Scholar] [CrossRef]
- An, O.; Pan, H.; Zhao, Q.; Wang, D. Strength development and microstructure of sustainable geopolymers made from alkali-activated ground granulated blast-furnace slag, calcium carbide residue, and red mud. Constr. Build. Mater. 2022, 356, 129279. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, W.; Jia, Y.; Xue, C.; Qiu, Y.; Zhao, Q.; Wang, D. Preparation of non-sintered lightweight aggregate ceramsite based on red mud-carbide slag-fly ash: Strength and curing method optimization. J. Clean. Prod. 2022, 372, 133788. [Google Scholar] [CrossRef]
- GB/T 18046-2017; Granulated Blast Furnace Slag Used for Cement Production. AQSIQ: Beijing, China, 2017.
- GB/T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). AQSIQ: Beijing, China, 2021.
- Wang, S.; Pan, H.; Xiao, C.; Zhao, Q.; Wang, J. Preparation and mix proportion optimization of red mud-fly ash-based cementitious material synergistic activated by carbide slag and MSWIFA. Constr. Build. Mater. 2024, 415, 135032. [Google Scholar] [CrossRef]
- Bai, Y.; Guo, W.; Wang, X.; Pan, H.; Zhao, Q.; Wang, D. Utilization of municipal solid waste incineration fly ash with red mud-carbide slag for eco-friendly geopolymer preparation. J. Clean Prod. 2022, 340, 130820. [Google Scholar] [CrossRef]
- Li, M.; Tan, H.; Zhang, J.; Deng, X.; Kong, X.; Chen, P.; Jian, S.; He, X.; Yang, J. Enhancement in compressive strength of carbide slag activated ground granulated blast furnace slag by introducing CaCl2 and NaCl. Constr. Build. Mater. 2023, 385, 131071. [Google Scholar] [CrossRef]
- Duana, K.; Wang, J.; Liu, Z.; Li, X.; Zhang, J.; Wang, X.; Wang, D. Flowability and in-situ phase evolution of Na2CO3-carbide slag-activated blast furnace slag and fly ash. Constr. Build. Mater. 2025, 466, 140341. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, X.; Liu, M.; Fang, B.; Wang, C.; Mi, H. Compatibility of sodium hydroxide, sodium silicate and calcium-enriched additives in alkali-activated materials: From the perspectives of flowability, strength and microstructure. Constr. Build. Mater. 2023, 403, 133102. [Google Scholar] [CrossRef]
- Huang, G.; Yang, K.; Sun, Y.; Lu, Z.; Zhang, X.; Zuo, L.; Feng, Y.; Qian, R.; Qi, Y.; Ji, Y.; et al. Influence of NaOH content on the alkali conversion mechanism in MSWI bottom ash alkali-activated mortars. Constr. Build. Mater. 2020, 248, 118582. [Google Scholar] [CrossRef]
- Zhu, X.P.; Qian, C.; He, B.; Chen, Q.; Jiang, Z.W. Experimental study on the stability of C-S-H nanostructures with varying bulk CaO:SiO2 ratio under cryogenic attack. Cem. Concr. Res. 2020, 135, 106114. [Google Scholar] [CrossRef]
- Liu, M.; Yang, D.; Chen, L.; Chen, G.; Ma, Z. Effect of silicate modulus and alkali content on the microstructure and macroscopic properties of alkali-activated recycled powder mortar. Constr. Build. Mater. 2023, 397, 132365. [Google Scholar] [CrossRef]
- Kabay, N.; Miyan, N.; Ozkan, H. Basic oxygen furnace and ground granulated blast furnace slag based alkali-activated pastes: Characterization and optimization. J. Clean. Prod. 2021, 327, 129483. [Google Scholar] [CrossRef]
- Cao, R.; Zhang, S.; Banthia, N.; Zhang, Y.; Zhang, Z. Interpreting the early-age reaction process of alkali-activated slag by using combined embedded ultrasonic measurement, thermal analysis, XRD, FTIR and SEM. Compos. Part B Eng. 2020, 186, 107840. [Google Scholar] [CrossRef]
- Huang, G.; Yuan, L.; Ji, Y.; Liu, B.; Xu, Z. Cooperative action and compatibility between Portland cement and MSWI bottom ash alkali-activated double gel system materials. Constr. Build. Mater. 2019, 209, 445–453. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Zhang, L.; Liu, X.; Liu, B. Effect of activated silica on polymerization mechanism and strength development of MSWI bottom ash alkali-activated mortars. Constr. Build. Mater. 2019, 201, 90–99. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, F.; Zhao, L.; Duan, X.; Feng, C.; Su, F. Effect of biomineralization on the early mechanical properties and microstructure of fly-ash cement-based materials. Constr. Build. Mater. 2022, 359, 129422. [Google Scholar] [CrossRef]
- Dai, X.; Aydin, S.; Yardımcı, M.Y.; Qiang, R.; Lesage, K.; De Schutter, G. Rheology, early-age hydration and microstructure of alkali-activated GGBFS-Fly ashlimestone mixtures. Cem. Concr. Compos. 2021, 124, 104244. [Google Scholar] [CrossRef]
- Du, S.; Zhao, Q.; Shi, X. Quantification of the reaction degree of fly ash in blended cement systems. Cem. Concr. Res. 2023, 167, 107121. [Google Scholar] [CrossRef]
- Li, N.; Shi, C.; Zhang, Z. Understanding the roles of activators towards setting and hardening control of alkali-activated slag cement. Comp. Part B Eng. 2019, 171, 34–45. [Google Scholar] [CrossRef]
- Shen, Y.; Kang, S.; Cheng, G.; Wang, J.; Wu, W.; Wang, X.; Zhao, Y.; Li, Q. Effects of silicate modulus and alkali dosage on the performance of one-part electric furnace nickel slag-based geopolymer repair materials. Case Stud. Constr. Mater. 2023, 19, e02224. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Hou, Z.; Dong, Z. Improving strength of calcinated coal gangue geopolymer mortars via increasing calcium content. Constr. Build. Mater. 2018, 166, 760–768. [Google Scholar] [CrossRef]
- Dai, X.; Aydın, S.; Yardımcı, M.Y.; Lesage, K.; De Schutter, G. Effects of activator properties and GGBFS/FA ratio on the structural build-up and rheology of AAC. Cem. Concr. Res. 2020, 138, 106253. [Google Scholar] [CrossRef]
- Cao, R.; Zhang, S.; Jia, Z.; Chen, C.; Zhang, Z.; Banthia, N.; Gao, Y.; Zhang, Y. Influence of ferronickel slag on the reaction kinetics and microstructure of alkali-activated slag. Cem. Concr Comp 2023, 142, 105173. [Google Scholar] [CrossRef]
- Irbe, L.; Beddoe, R.E.; Heinz, D. The role of aluminium in C-A-S-H during sulfate attack on concrete. Cem. Concr. Res. 2019, 116, 71–80. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhang, Y.; Gu, X. Research on hydration characteristics of OSRGGBFS-FA alkali-activated materials. Constr. Build. Mater. 2024, 411, 134321. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Hou, Z.; Jin, C. Use of slaked lime and Portland cement to improve the resistance of MSWI bottom ash-GBFS geopolymer concrete against carbonation. Constr. Build. Mater. 2018, 166, 290–300. [Google Scholar] [CrossRef]
- Gijbels, K.; Pontikes, Y.; Samyn, P.; Schreurs, S.; Schroeyers, W. Effect of NaOH content on hydration, mineralogy, porosity and strength in alkali/sulfate-activated binders from ground granulated blast furnace slag and phosphogypsum. Cem. Concr. Res. 2020, 132, 106054. [Google Scholar] [CrossRef]
- Zhu, Y.; Longhi, M.A.; Wang, A.; Hou, D.; Wang, H.; Zhang, Z. Alkali leaching features of 3-year-old alkali activated fly ash-slag-silica fume: For a better understanding of stability. Compos. B Eng. 2022, 230, 109469. [Google Scholar] [CrossRef]
- Huang, G.; Yang, K.; Chen, L.; Lu, Z.; Sun, Y.; Zhang, X.; Feng, Y.; Ji, Y.; Xu, Z. Use of pretreatment to prevent expansion and foaming in highperformance MSWI bottom ash alkali-activated mortars. Constr. Build. Mater. 2020, 245, 118471. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).