Revisiting Mn4Al11: Growth of Stoichiometric Single Crystals and Their Structural and Magnetic Properties
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
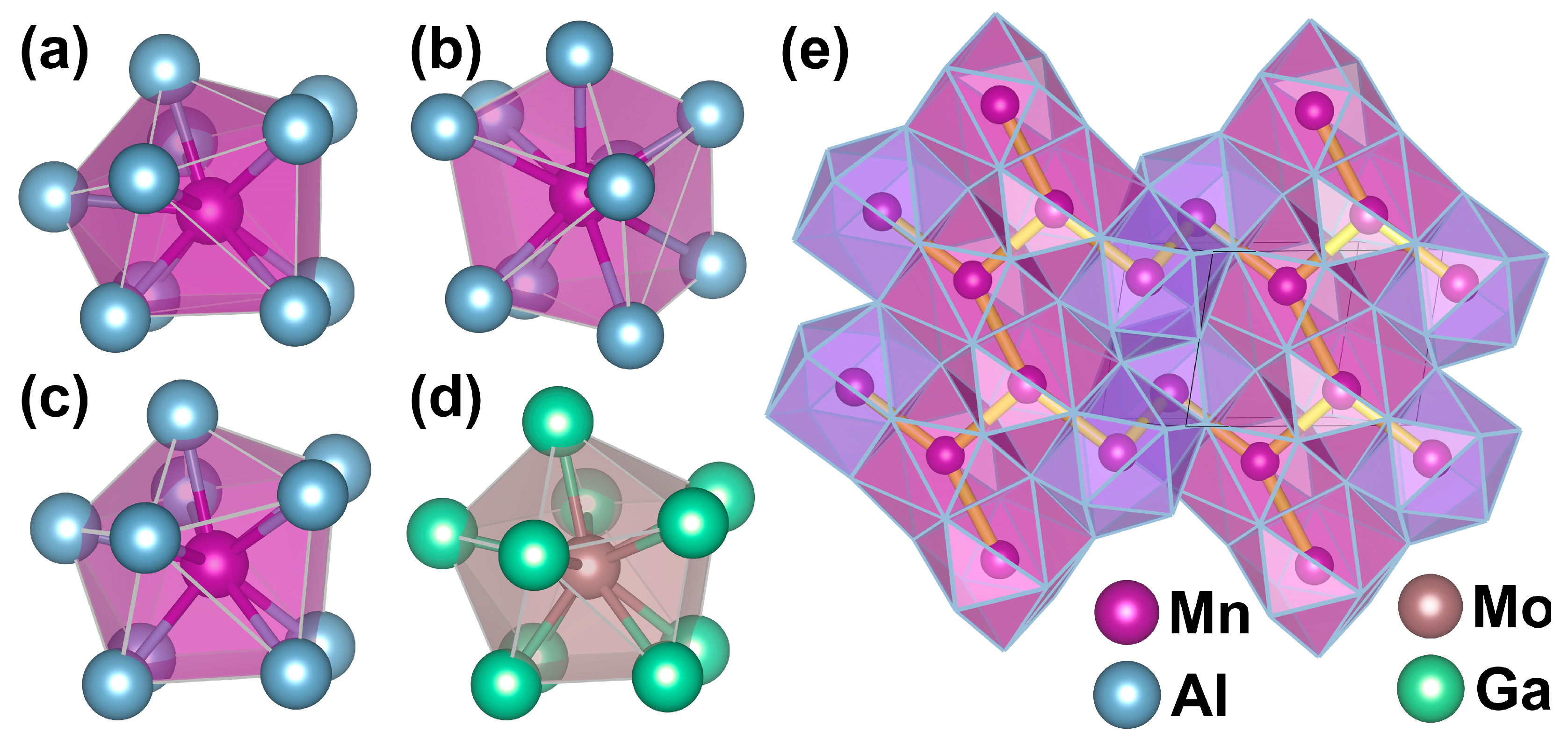
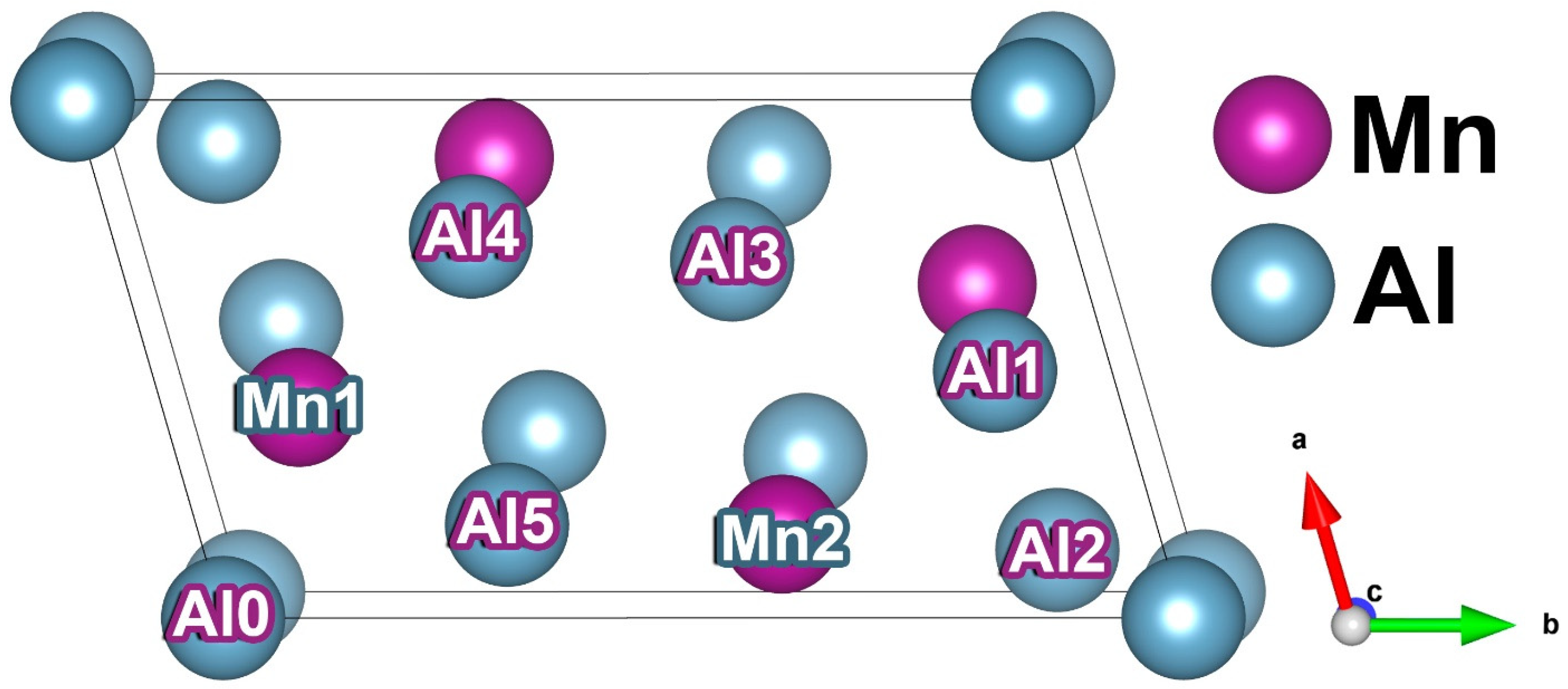
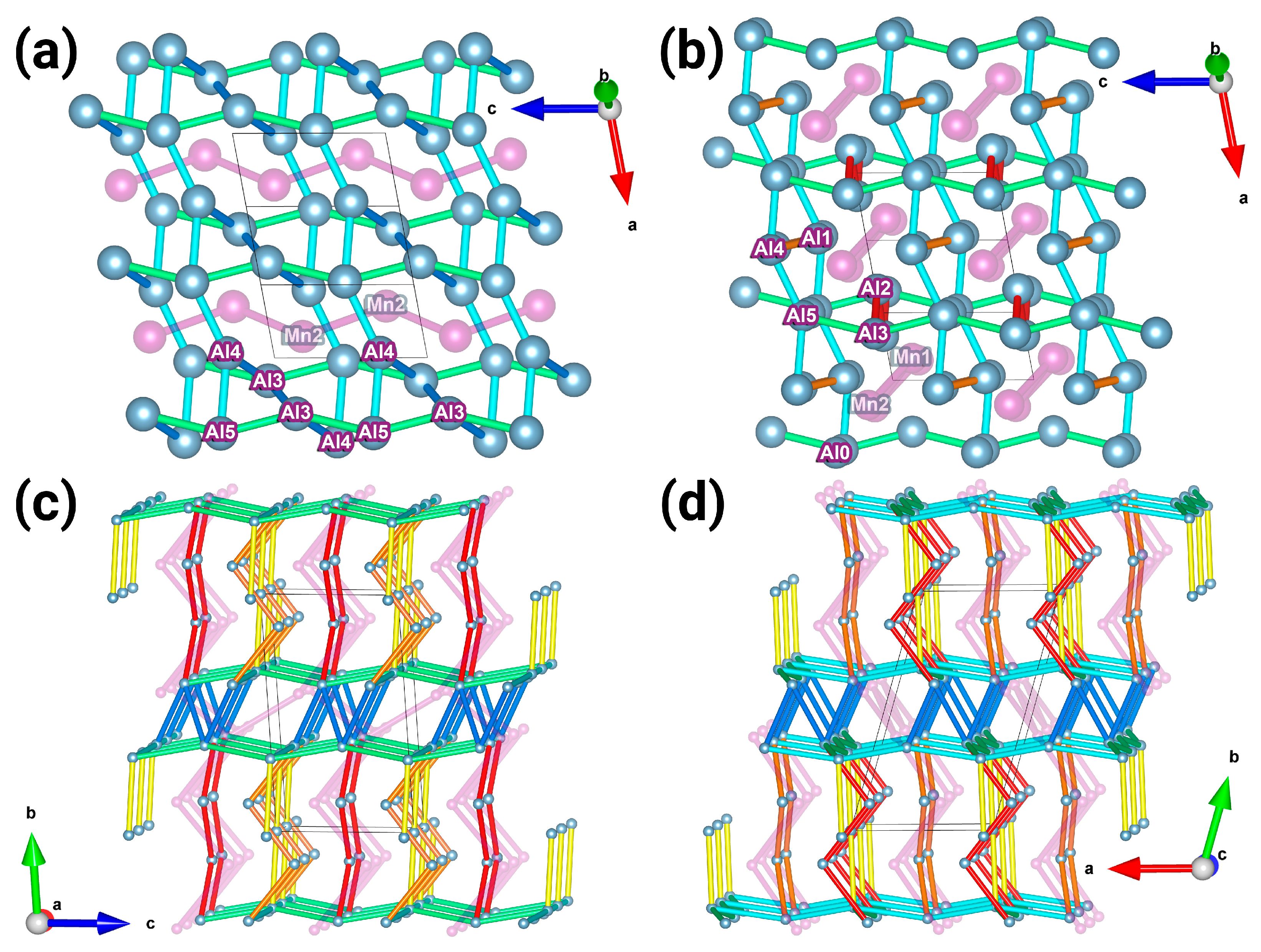
3.1. Crystal Structure of Mn4Al11
3.2. Magnetic Properties of Mn4Al11
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yannello, V.J.; Fredrickson, D.C. Generality of the 18−n rule: Intermetallic structural chemistry explained through isolobal analogies to transition metal complexes. Inorg. Chem. 2015, 54, 11385–11398. [Google Scholar] [CrossRef]
- Yannello, V.J.; Lu, E.; Fredrickson, D.C. At the limits of isolobal bonding: π-based covalent magnetism in Mn2Hg5. Inorg. Chem. 2020, 59, 12304–12313. [Google Scholar] [CrossRef]
- Häussermann, U.; Viklund, P.; Boström, M.; Norrestam, R.; Simak, S.I. Bonding and physical properties of Hume-Rothery compounds with the PtHg4 structure. Phys. Rev. B 2001, 63, 125118. [Google Scholar] [CrossRef]
- Khalaniya, R.A.; Shevelkov, A.V. When two is enough: On the origin of diverse crystal structures and physical properties in the Fe-Ge system. J. Solid State Chem. 2019, 270, 118–128. [Google Scholar] [CrossRef]
- Likhanov, M.S.; Shevelkov, A.V. Intermetallic compounds with non-metallic properties. Rus. Chem. Bull. 2020, 69, 2231–2255. [Google Scholar] [CrossRef]
- Rimmler, B.H.; Pal, B.; Parkin, S.S. Non-collinear antiferromagnetic spintronics. Nat. Rev. Mater. 2025, 10, 109–127. [Google Scholar] [CrossRef]
- Verchenko, V.Y.; Tsirlin, A.A. Semiconducting and Metallic Compounds within the IrIn3 Structure Type: Stability and Chemical Bonding. Inorg. Chem. 2022, 61, 3274–3280. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lei, H.; Qi, Y.; Felser, C. Topological quantum materials with Kagome lattice. Acc. Mater. Res. 2024, 5, 786–796. [Google Scholar] [CrossRef]
- Yin, J.X.; Lian, B.; Hasan, M.Z. Topological Kagome magnets and superconductors. Nature 2022, 612, 647–657. [Google Scholar] [CrossRef]
- Teng, X.; Oh, J.S.; Tan, H.; Chen, L.; Huang, J.; Gao, B.; Yin, J.-X.; Chu, J.-H.; Hashimoto, M.; Lu, D.; et al. Magnetism and charge density wave order in kagome FeGe. Nat. Phys. 2023, 19, 814–822. [Google Scholar] [CrossRef]
- Likhanov, M.S.; Khalaniya, R.A.; Verchenko, V.Y.; Gippius, A.A.; Zhurenko, S.V.; Tkachev, A.V.; Fazlizhanova, D.I.; Kuznetsov, A.N.; Shevelkov, A.V. ReGaGe2: An intermetallic compound with semiconducting properties and localized bonding. Chem. Comm. 2019, 55, 5821–5824. [Google Scholar] [CrossRef] [PubMed]
- Armbrüster, M.; Schnelle, W.; Cardoso-Gil, R.; Grin, Y. Chemical bonding in compounds of the CuAl2 family: MnSn2, FeSn2 and CoSn2. Chem. A Eur. J. 2010, 16, 10357–10365. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Ouchi, H.; Takagiwa, Y.; Kimura, K. Glass-like lattice thermal conductivity and thermoelectric properties of incommensurate chimney-ladder compound FeGeγ. Chem. Mater. 2016, 28, 529–533. [Google Scholar] [CrossRef]
- Khalaniya, R.A.; Verchenko, V.Y.; Bogach, A.V.; Likhanov, M.; Shevelkov, A.V. Itinerant ferromagnet Re4−xMnxGe7−δ (x = 0.9 − 1.5, δ = 0.42 − 0.44) with incommensurate chimney-ladder structure stabilised at ambient pressure. Dalton Trans. 2023, 52, 10657–10661. [Google Scholar] [CrossRef]
- Elser, V.; Henley, C.L. Crystal and quasicrystal structures in Al-Mn-Si alloys. Phys. Rev. Lett. 1985, 55, 2883–2886. [Google Scholar] [CrossRef]
- Yamamoto, A.; Hiraga, K. Structure of an icosahedral Al-Mn quasicrystal. Phys. Rev. B 1988, 37, 6207–6215. [Google Scholar] [CrossRef]
- Kimura, K.; Hashimoto, T.; Suzuki, K.; Nagayama, K.; Ino, H.; Takeuchi, S. Structure and stability of quasicrystalline Al–Mn alloys. J. Phys. Soc. Jpn. 1986, 55, 534–543. [Google Scholar] [CrossRef]
- Li, H.; Ding, B.; Chen, J.; Li, Z.; Hou, Z.; Liu, E.; Zhang, H.; Xi, X.; Wu, G.; Wang, W. Large topological Hall effect in a geometrically frustrated Kagome magnet Fe3Sn2. Appl. Phys. Lett. 2019, 114, 192408. [Google Scholar] [CrossRef]
- Samanta, S.; Park, H.; Lee, C.; Jeon, S.; Cui, H.; Yao, Y.X.; Hwang, J.; Choi, K.-Y.; Kim, H.S. Emergence of flat bands and ferromagnetic fluctuations via orbital-selective electron correlations in Mn-based kagome metal. Nat. Comm. 2024, 15, 5376. [Google Scholar] [CrossRef]
- Banno, N. Low-temperature superconductors: Nb3Sn, Nb3Al, and NbTi. Superconductivity 2023, 6, 100047. [Google Scholar] [CrossRef]
- Verchenko, V.Y.; Shevelkov, A.V. Endohedral cluster intermetallic superconductors: At the frontier between chemistry and physics. Dalton Trans. 2021, 50, 5109–5114. [Google Scholar] [CrossRef]
- Xie, W.; Luo, H.; Phelan, B.F.; Klimczuk, T.; Cevallos, F.A.; Cava, R.J. Endohedral gallide cluster superconductors and superconductivity in ReGa5. Proc. Nat. Acad. Sci. USA 2015, 112, E7048–E7054. [Google Scholar] [CrossRef]
- Ryżyńska, Z.; Wiśniewski, P.; Kaczorowski, D.; Xie, W.; Cava, R.J.; Klimczuk, T.; Winiarski, M.J. Superconductivity in the endohedral Ga cluster compound PdGa5. J. Phys. Chem. C 2021, 125, 11294–11299. [Google Scholar] [CrossRef]
- Häussermann, U.; Boström, M.; Viklund, P.; Rapp, Ö.; Björnängen, T. FeGa3 and RuGa3: Semiconducting intermetallic compounds. J. Solid State Chem. 2002, 165, 94–99. [Google Scholar] [CrossRef]
- Khalaniya, R.A.; Verchenko, V.; Mironov, A.V.; Samarin, A.N.; Bogach, A.; Kulchu, A.N.; Polevik, A.O.; Wei, Z.; Dikarev, E.V.; Stern, R.; et al. Spin reorientation and magnetic frustration in Fe32+δGe35−xSix with a kagome lattice broken by crystallographic intergrowth. Dalton Trans. 2025, 54, 8317–8330. [Google Scholar] [CrossRef]
- Khalaniya, R.A.; Verchenko, V.Y.; Sobolev, A.V.; Presniakov, I.A.; Wei, Z.; Dikarev, E.V.; Shevelkov, A.V. Intricate magnetic behavior of Fe6Ge5 and its origin within a complex iron framework: The magnetic and 57Fe Mössbauer study. J. Alloy Compd. 2022, 902, 163759. [Google Scholar] [CrossRef]
- Kotur, B.; Babizhetskyy, V.; Bauer, E.; Kneidinger, F.; Danner, A.; Leber, L.; Michor, H. Metal Site Doping in the Narrow-Gap FeGa3 Semiconductor. Mater. Sci. 2013, 49, 211–219. [Google Scholar] [CrossRef]
- Ramachandran, B.; Syu, K.Z.; Kuo, Y.K.; Gippius, A.A.; Shevelkov, A.V.; Verchenko, V.Y.; Lue, C.S. Thermoelectric performance of intermetallic FeGa3 with Co doping. J. Alloys Compd. 2014, 608, 229–234. [Google Scholar] [CrossRef]
- Hammura, K.; Udono, H.; Ohsugi, I.J.; Aono, T.; De Ranieri, E. Magnetisation of bulk Mn11Si19 and Mn4Si7. Thin Solid Film. 2011, 519, 8516–8519. [Google Scholar] [CrossRef]
- Adams, T.; Mühlbauer, S.; Neubauer, A.; Münzer, W.; Jonietz, F.; Georgii, R.; Böni, P.; Pedersen, B.; Schmidt, M.; Rosch, A.; et al. Skyrmion lattice domains in Fe1−xCoxSi. Phys. Rev. B Cond. Matter Mater. Phys. 2010, 81, 041203. [Google Scholar] [CrossRef]
- Kitagawa, J.; Yakabe, G.; Nakayama, A.; Nishizaki, T.; Tsubota, M. Competition between ferromagnetic and antiferromagnetic states in Al8.5−xFe23Ge12.5+x (0 ≤ x ≤ 3). J. Solid State Chem. 2020, 284, 121188. [Google Scholar] [CrossRef]
- Venturini, G. Magnetic study of the HfFe6Ge6-type solid solutions ScMn6Ge6−xGax and LuMn6Ge6−xGax (0.25≤ x≤ 1.50). J. Alloys Compd. 2000, 309, 20–25. [Google Scholar] [CrossRef]
- Venturini, G. Antiferro-to ferromagnetic transition in HfFe6Ge6-type solid solution YMn6Ge6−xGax (0.25≤ x ≤ 2.00). J. Alloys Compd. 2000, 311, 101–108. [Google Scholar] [CrossRef]
- Dunlop, J.B.; Grüner, G. One-dimensional effects in the intermetallic compound Al11Mn4. Solid State Comm. 1976, 18, 827–829. [Google Scholar] [CrossRef]
- Singh, P.; Del Rose, T.; Mudryk, Y.; Pecharsky, V.K.; Johnson, D.D. Designed metal-insulator transition in low-symmetry magnetic intermetallics. Phys. Rev. B 2024, 109, 064207. [Google Scholar] [CrossRef]
- Noor, M.; Law, K.; Yasmin, N.; Hauser, A.; Besara, T. Ferrimagnetism and half-metallicity in Cr-substituted Mn4–xCrxAl11. J. Phys. Cond. Matter 2025, 37, 235704. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.A. Studies of aluminum-rich alloys with the transition metals manganese and tungsten. II. The crystal structure of δ (Mn-Al)-Mn4Al11. Acta Crystallogr. 1958, 11, 236–244. [Google Scholar] [CrossRef]
- Kontio, A.; Stevens, E.D.; Coppens, P.; Brown, R.D.; Dwight, A.E.; Williams, J.M. New investigation of the structure of Mn4Al11. Acta Crystallogr. Sect. B 1980, 36, 435–436. [Google Scholar] [CrossRef]
- Xian, J.W.; Peng, L.; Zeng, G.; Wang, D.; Gourlay, C.M. Al11Mn4 formation on Al8Mn5 during the solidification and heat treatment of AZ-series magnesium alloys. Materialia 2021, 19, 101192. [Google Scholar] [CrossRef]
- Yoshida, K. One-Dimensionally Disordered δ (Mn-Al) Structure in Vacuum Co-Deposited Film. J. Phys. Soc. Jpn. 1972, 32, 431–440. [Google Scholar] [CrossRef]
- Boato, G.; Gallinaro, G.; Rizzuto, C. Effect of transition-metal impurities on the critical temperature of superconducting Al, Zn, In, and Sn. Phys. Rev. 1966, 148, 353–361. [Google Scholar] [CrossRef]
- Petricek, V.; Dusek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Kristallogr.-Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-Ray Microanalysis, 4th ed.; Springer: New York, NY, USA, 2017; pp. 341–357. [Google Scholar]
- Fedotov, V.K.; Antonov, V.E.; Cornell, K.; Grosse, G.; Kolesnikov, A.I.; Sikolenko, V.V.; Sumin, V.V.; Wagner, F.E.; Wipf, H. Neutron scattering studies of the structure and lattice dynamics of a solid solution of hydrogen in-manganese. J. Phys. Cond. Matter 1998, 10, 5255–5266. [Google Scholar] [CrossRef]
- Shoemaker, C.B.; Shoemaker, D.P.; Hopkins, T.E.; Yindepit, S. Refinement of the structure of β-manganese and of a related phase in the Mn–Ni–Si system. Acta Crystallogr. Sect. B 1978, 34, 3573–3576. [Google Scholar] [CrossRef]
- Nicol, A.D.I. The structure of MnAl6. Acta Crystallogr. Sect. B 1953, 6, 285–293. [Google Scholar] [CrossRef]
- Kontio, A.; Coppens, P. New study of the structure of MnAl6. Acta Crystallogr. Sect. B 1981, 37, 433–435. [Google Scholar] [CrossRef]
- Onishi, T.; Nakatani, Y. Crystal Structures of MnAl6 and MnAl4. J. Japan Inst. Light Metals 1975, 25, 253–258. [Google Scholar] [CrossRef]
- Kreiner, G.; Franzen, H.F. The crystal structure of λ-Al4Mn. J. Alloys Compd. 1997, 261, 83–104. [Google Scholar] [CrossRef]
- Shoemaker, C.B.; Keszler, D.A.; Shoemaker, D.P. Structure of μ-MnAl4 with composition close to that of quasicrystal phases. Acta Crystallogr. Sect. B 1989, 45, 13–20. [Google Scholar] [CrossRef]
- Pavlyuk, V.V.; Yanson, T.I.; Bodak, O.I.; Černý, R.; Gladyshevskii, R.E.; Yvon, K.; Stepien-Damm, J. Structure refinement of orthorhombic MnAl3. Cryst. Struct. Comm. 1995, 51, 792–794. [Google Scholar] [CrossRef]
- Hiraga, K.; Kaneko, M.; Matsuo, Y.; Hashimoto, S. The structure of Al3Mn: Close relationship to decagonal quasicrystais. Phil. Mag. B 1993, 67, 193–205. [Google Scholar] [CrossRef]
- Shi, N.C.; Li, X.Z.; Ma, Z.S.; Kuo, K.H. Crystalline phases related to a decagonal quasicrystal. I. A single-crystal X-ray diffraction study of the orthorhombic Al3Mn phase. Acta Crystallogr. Sect. B 1994, 50, 22–30. [Google Scholar] [CrossRef]
- Thimmaiah, S.; Tener, Z.; Lamichhane, T.N.; Canfield, P.C.; Miller, G.J. Crystal structure, homogeneity range and electronic structure of rhombohedral γ-Mn5Al8. Z. Kristallogr.-Cryst. Mater. 2017, 232, 601–610. [Google Scholar] [CrossRef]
- Moze, O.; Pareti, L.; Ermakov, A.E. Neutron diffraction and magnetic investigations of Ga substituted MnAl permanent magnet materials. J. Appl. Phys. 1988, 63, 4616–4619. [Google Scholar] [CrossRef]
- Bandyopadhyay, J.; Gupta, K.P. Low temperature lattice parameters of Al and Al-Zn alloys and Grüneisen parameter of Al. Cryogenics 1978, 18, 54–55. [Google Scholar] [CrossRef]
- Schnepf, A.; Schenk, C. Na6[Ge10{Fe(CO)4}8]∙18THF: A Centaur Polyhedron of Germanium Atoms. Angew. Chem. Int. Ed. 2006, 45, 5373–5376. [Google Scholar] [CrossRef]
- Barlock, J.G.; Mondolfo, L.F. Structure of some aluminium-iron-magnesium-manganese-silicon alloys. Int. J. Mater. Res. 1975, 66, 605–611. [Google Scholar] [CrossRef]
- Wells, A.F. Structural Inorganic Chemistry, 4th ed.; Oxford University Press: Oxford, UK, 1975; pp. 1008–1059. [Google Scholar]
- Gross, N.; Kotzyba, G.; Künnen, B.; Jeitschko, W. Binary Compounds of Rhodium and Zinc: RhZn, Rh2Zn11, and RhZn13. Z. Anorg. Allg. Chem. 2001, 627, 155–163. [Google Scholar] [CrossRef]
- Sidorenko, F.; Kotov, A.P.; Zelenin, L.P.; Gel’d, P.V. Physical properties of mutual FeAl-CoAl solid solutions. Fiz. Met. Metalloved. 1973, 35, 209–211. [Google Scholar]
- Feschotte, P.; Bass, P. A new, very shy intermetallic compound: AgGa. J. Less-Common Met. 1991, 171, 157–162. [Google Scholar] [CrossRef]
- Lecocq, P.; Michel, A. Proprietes magnetiques et structurales des solutions solides de substitution de germanium dans le fer α. Bull. Soc. Chim. Fr. 1962, 7, 1412–1416. [Google Scholar]
- Dutchak, Y.I.; Chekh, V.G. High temperature x-ray diffraction study of the lattice dynamics of the compounds AlCo and AlNi. Russ. J. Phys. Chem. 1981, 55, 1326–1328. [Google Scholar]
- Bogner, J.; Steiner, W.; Reissner, M.; Mohn, P.; Blaha, P.; Schwarz, K.; Krachler, R.; Ipser, H.; Sepiol, B. Magnetic order and defect structure of FexAl1−x alloys around x = 0.5: An experimental and theoretical study. Phys. Rev. B 1998, 58, 14922. [Google Scholar] [CrossRef]
- Cascio, D.L.; Bakker, H. Change in magnetisation of Co50Ga50 due to atomic disorder induced by quenching from high temperatures. Phys. Status Solidi 1993, 135, 611–619. [Google Scholar] [CrossRef]
- Whittle, G.L.; Clark, P.E.; Cywinski, R.A. Mössbauer effect study of the structural and magnetic properties of Co-Ga alloys. J. Magn. Magn. Mater. 1982, 28, 64–76. [Google Scholar] [CrossRef]
- Straumanis, M.E.; Chopra, K.S. Lattice Parameters, Expansion Coefficients and Extent of the Al2Au Phase. Z. Phys. Chem. 1964, 42, 344–350. [Google Scholar] [CrossRef]
- Verchenko, V.Y.; Zubtsovskii, A.O.; Plenkin, D.S.; Bogach, A.V.; Wei, Z.; Tsirlin, A.A.; Dikarev, E.V.; Shevelkov, A.V. Family of Mo4Ga21-based superconductors. Chem. Mater. 2020, 32, 6730–6735. [Google Scholar] [CrossRef]
- Pchelkina, Z.V.; Mazurenko, V.V.; Volkova, O.S.; Deeva, E.B.; Morozov, I.V.; Shutov, V.V.; Troyanov, S.I.; Werner, J.; Koo, C.; Klingeler, R.; et al. Electronic structure and magnetic properties of the strong-rung spin-1 ladder compound Rb3Ni2(NO3)7. Phys. Rev. B 2018, 97, 144420. [Google Scholar] [CrossRef]
- Vorobyova, A.A.; Shilov, A.I.; Spiridonov, F.M.; Knotko, A.V.; Danilovich, I.L.; Vasiliev, A.N.; Morozov, I.V. One-dimensional magnet basic copper (ii) dihydroxoborate Cu2{BO(OH)2}(OH)3: Synthesis and properties. Russ. Chem. Bull. 2020, 69, 704–711. [Google Scholar] [CrossRef]
- Vorobyova, A.; Danilovich, I.; Morozov, I.; Ovchenkov, Y.; Vasiliev, A.; Volkova, O.; Iqbal, A.; Rahaman, B.; Saha-Dasgupta, T. Square lattice antiferromagnets (NO)M(NO3)3 (M= Co, Ni): Effects of anisotropy. J. Alloys Compd. 2022, 929, 167197. [Google Scholar] [CrossRef]
- Khalaniya, R.A.; Mironov, A.V.; Verchenko, V.Y.; Jesche, A.; Tsirlin, A.A.; Shevelkov, A.V. Nontrivial recurrent intergrowth structure and unusual magnetic behavior of intermetallic compound Fe32+δGe33As2. Inorg. Chem. 2016, 55, 12953–12961. [Google Scholar] [CrossRef]
- Khalaniya, R.A.; Verchenko, V.Y.; Wei, Z.; Dikarev, E.V.; Heinmaa, I.; Stern, R.; Jesche, A.; Tsirlin, A.A.; Shevelkov, A.V. From Fe32+δGe35-xPx to Fe32+δGe35-x-yPxAsy: Fine geometry optimization in new intergrowth structures. J. Alloys Compd. 2019, 779, 229–236. [Google Scholar] [CrossRef]
- Khalaniya, R.A.; Sobolev, A.V.; Verchenko, V.Y.; Tsirlin, A.A.; Senyshyn, A.; Damay, F.; Presniakov, I.A.; Shevelkov, A.V. Magnetic structures of Fe32+δGe33As2 and Fe32+δ′Ge35−xPx intermetallic compounds: A neutron diffraction and 57Fe Mössbauer spectroscopy study. Dalton Trans. 2021, 50, 2210–2220. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, U.; Sulpice, A.; Lambert-Andron, B.; Laborde, O. Magnetic properties of single crystalline Mn4Si7. J. Alloys Compd. 2003, 361, 13–18. [Google Scholar] [CrossRef]
- Wagner, F.R.; Cardoso-Gil, R.; Boucher, B.; Wagner-Reetz, M.; Sichelschmidt, J.; Gille, P.; Baenitz, M.; Grin, Y. On Fe–Fe dumbbells in the ideal and real structures of FeGa3. Inorg. Chem. 2018, 57, 12908–12919. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.K.; Rabello, A.F.; Martelli, V.; Zivkovic, I.; Fogh, E.; Rønnow, H.M.; Cornejo, D.; Jiménez, J.L. Electrical and Magnetic Properties of FeGa3 with Antisite Disorder. New Phys. Sae Mulli 2023, 73, 1149–1154. [Google Scholar] [CrossRef]
- Verchenko, V.Y.; Wei, Z.; Tsirlin, A.A.; Callaert, C.; Jesche, A.; Hadermann, J.; Dikarev, E.V.; Shevelkov, A.V. Crystal growth of the Nowotny chimney ladder phase Fe2Ge3: Exploring new Fe-based narrow-gap semiconductor with promising thermoelectric performance. Chem. Mater. 2017, 29, 9954–9963. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Y.; Cao, H.; Guo, S.; Yan, J.; Chen, X. Single crystal growth and thermoelectric properties of Nowotny chimney-ladder compound Fe2Ge3. Phys. Rev. Mater. 2023, 7, 125404. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Wu, W.B.; He, Y.X.; Zhang, J.Y.; He, T.; Li, Z.; Xiao, Q.-L.; Ge, J.Y. Extremely large magnetoresistance in high quality magnetic Fe2Ge3 single crystals. Commun. Phys. 2025, 8, 116. [Google Scholar] [CrossRef]


| refined composition | Mn4Al11 |
| EDX composition | Mn4.18(2)Al10.82(2) |
| molar weight | 516.6 |
| structure type | Mn4Al11 |
| crystal system | Triclinic |
| space group | P-1 |
| a, Å | 5.0876(12) |
| b, Å | 8.8438(17) |
| c, Å | 5.0562(11) |
| A | 89.800(16) |
| β, ° | 100.548(16) |
| Γ | 105.358(16) |
| V, Å3 | 215.42(8) |
| Z | 1 |
| ρcalc, g/cm3 | 3.9817 |
| crystal size | 0.21 × 0.13 × 0.03 |
| radiation | Ag Kα |
| wavelength, Å | 0.56083 Å |
| temperature, K | 293 |
| 2θ range, ° | 3.24–25.95 |
| number of parameters | 71 |
| number of reflections | 1700 |
| GoF | 1.03 |
| Δρmax/min, e−/Å3 | 0.86/−0.66 |
| R[F2 > 3σ(F2)]/Rw(F2) | 0.0304/0.0436 |
| Atom | Wyck. | x | Y | z | Ueq, Å2 |
|---|---|---|---|---|---|
| Mn1 | 2i | 0.38941(8) | 0.13305(5) | 0.33559(9) | 0.00564(11) |
| Mn2 | 2i | 0.85296(9) | 0.40131(5) | 0.70820(9) | 0.00707(12) |
| Al0 | 1a | 0 | 0 | 0 | 0.0103(3) |
| Al1 | 2i | 0.53059(18) | 0.12593(10) | 0.84570(19) | 0.0087(2) |
| Al2 | 2i | 0.89478(17) | 0.12552(10) | 0.4907(2) | 0.0088(2) |
| Al3 | 2i | 0.33000(17) | 0.37389(10) | 0.57031(19) | 0.0082(3) |
| Al4 | 2i | 0.72648(18) | 0.36785(11) | 0.1925(2) | 0.0093(2) |
| Al5 | 2i | 0.17738(18) | 0.32225(11) | 0.05353(19) | 0.0104(3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalaniya, R.A.; Mironov, A.V.; Samarin, A.N.; Bogach, A.V.; Kulchu, A.N.; Shevelkov, A.V. Revisiting Mn4Al11: Growth of Stoichiometric Single Crystals and Their Structural and Magnetic Properties. Crystals 2025, 15, 714. https://doi.org/10.3390/cryst15080714
Khalaniya RA, Mironov AV, Samarin AN, Bogach AV, Kulchu AN, Shevelkov AV. Revisiting Mn4Al11: Growth of Stoichiometric Single Crystals and Their Structural and Magnetic Properties. Crystals. 2025; 15(8):714. https://doi.org/10.3390/cryst15080714
Chicago/Turabian StyleKhalaniya, Roman A., Andrei V. Mironov, Alexander N. Samarin, Alexey V. Bogach, Aleksandr N. Kulchu, and Andrei V. Shevelkov. 2025. "Revisiting Mn4Al11: Growth of Stoichiometric Single Crystals and Their Structural and Magnetic Properties" Crystals 15, no. 8: 714. https://doi.org/10.3390/cryst15080714
APA StyleKhalaniya, R. A., Mironov, A. V., Samarin, A. N., Bogach, A. V., Kulchu, A. N., & Shevelkov, A. V. (2025). Revisiting Mn4Al11: Growth of Stoichiometric Single Crystals and Their Structural and Magnetic Properties. Crystals, 15(8), 714. https://doi.org/10.3390/cryst15080714







